Abstract
Ischemia reperfusion injury results from tissue damage during ischemia and ongoing inflammation and injury during reperfusion. Liver reperfusion injury is reduced by lymphocyte depletion or activation of adenosine A2A receptors (A2ARs) with the selective agonist 4- {3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]- prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester (ATL146e). We show that NKT cells are stimulated to produce interferon (IFN)-γ by 2 h after the initiation of reperfusion, and the use of antibodies to deplete NK1.1-positive cells (NK and NKT) or to block CD1d-mediated glycolipid presentation to NKT cells replicates, but is not additive to, the protection afforded by ATL146e, as assessed by serum alanine aminotransferase elevation, histological necrosis, neutrophil accumulation, and serum IFN-γ elevation. Reduced reperfusion injury observed in RAG-1 knockout (KO) mice is restored to the wild-type (WT) level by adoptive transfer of NKT cells purified from WT or A2AR KO mice but not IFN-γ KO mice. Additionally, animals with transferred A2AR−/− NKT cells are not protected from hepatic reperfusion injury by ATL146e. In vitro, ATL146e potently inhibits both anti-CD3 and α-galactosylceramide–triggered production of IFN-γ by NKT cells. These findings suggest that hepatic reperfusion injury is initiated by the CD1d-dependent activation of NKT cells, and the activation of these cells is inhibited by A2AR activation.
Reperfusion injury after hepatic ischemia is associated with inflammation and ongoing necrosis that is amplified by deletion of the adenosine A2A receptor (A2AR) (1). The activity of most inflammatory cells, including but not limited to macrophages, monocytes, T lymphocytes, platelets, and polymorphonuclear leukocytes, is inhibited by the activation of the antiinflammatory Gs-coupled A2AR, resulting in reduced proinflammatory cytokine production and diminished endothelial adhesion molecule expression (2–7). Accumulating evidence suggests that hepatic reperfusion injury is triggered by lymphocyte activation (1) and that the activation of A2ARs on bone marrow–derived cells mediates liver protection (8). These findings, and studies establishing that the activation of the A2AR on CD4+ T cells inhibits TCR-mediated IFN-γ production in vitro (3), suggest that treatment with the selective A2AR agonist 4-{3-[6-Amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan- 2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohex anecarboxylic acid methyl ester (ATL146e) may mediate protection from hepatic ischemia reperfusion injury (IRI) by inhibiting the activation of CD4+ T lymphocytes. However, the rapidity of reperfusion injury is not consistent with the timeframe required for activation and differentiation of conventional CD4+ T cell responses, suggesting it is mediated by a rapidly activated T cell subset.
The majority of mouse CD4+NK1.1+ NKT cells express the invariant TCR, Vα14Jα18, and are dependent on CD1d for positive selection in the thymus and subsequent activation in the periphery (9, 10). CD1d is expressed by hepatocytes, gut epithelial cells, and APCs and presents either self-glycolipid, such as isoglobotrihexosylceramide (11), or foreign glycolipid, such as the marine sponge–derived α-galactosylceramide (α-Gal-Cer) (12), to NKT cells. The rapid release of IFN-γ or IL-4 after activation of invariant NKT (iNKT) cells by CD1d glycolipid presentation to TCRs has been attributed to preformed cytokine transcripts (13). Although NKT cells compose only 0.1–3% of the T lymphocyte population in blood and spleen, in the mouse liver NKT cells account for as much as 30% of the total lymphocyte population and as much as 50% of total αβ TCR+ T cells (14). The high abundance of NKT cells in the liver and their rapid response to activation suggests that they might play a role in hepatic reperfusion injury. In this study we sought to better characterize the effects of hepatic reperfusion injury and A2AR activation on NKT cell activity. We show that NKT cells are involved in the pathogenesis of hepatic IRI and that they comprise a subset of CD4+ T lymphocytes through which ATL146e mediates liver protection.
RESULTS
Blockade of NKT cell activation reduces hepatic IRI
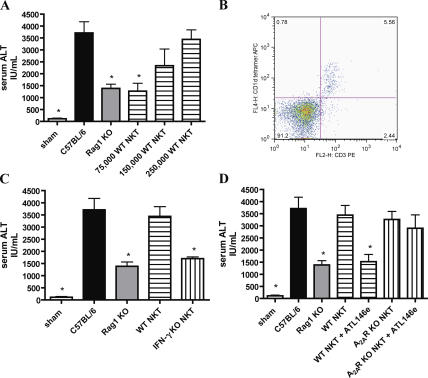
Clamping the hepatic triad of WT C57BL/6 mice for various times and reperfusing for 24 h induces considerable time-dependent liver damage. Deletion of the A2AR exacerbates reperfusion injury, implicating endogenous adenosine in liver protection (1). Protection, as manifested by reduced serum alanine aminotransferase (ALT) levels and lessened necrotic area, is produced in WT mice by administration of the synthetic A2AR agonist ATL146e immediately after the initiation of reperfusion. Serum ALT levels in ATL146e-treated mice are reduced by ∼58% versus vehicle-treated controls, and the necrotic area is 6.1 ± 0.8% as opposed to 79.3 ± 3% in vehicle-treated animals (lightly stained areas are necrotic; Fig. 1). RAG-1 KO mice, which lack mature lymphocytes, also exhibit reduced ALT and necrosis when compared with age- and sex-matched WT C57BL/6 mice (63% reduction in serum ALT levels and 4.5 ± 1% reduced necrotic area; Fig. 1). ATL146e treatment of C57BL/6 mice and lymphocyte deficiency in RAG-1 KO mice result in similar reductions in serum ALT levels and liver necrosis. To test the hypothesis that NKT cell activity contributes to liver IRI, we examined the effects of depleting these cells or blocking their CD1d-dependent activation. Treatment of WT C57BL/6 mice with anti-NK1.1 (PK136) 2 d before liver IRI substantially depletes NKT and NK cells in the spleen and liver as assessed by FACS analysis (Fig. 2 A) while leaving conventional CD4+ and CD8+ T cell number intact (not depicted). This depletion results in ∼60% reduction in serum ALT levels 24 h after reperfusion and a large reduction in necrotic area (8.2 ± 2% necrotic area; Fig. 2 B). The administration of a CD1d-blocking antibody 24 h before injury elicits a similar reduction in serum ALT levels and necrosis as does PK136 treatment (Fig. 2 C). Co-treatment with either antibody in conjunction with ATL146e affords no additional protection beyond that achieved by NK1.1 cell depletion or CD1d blockade alone. These results are consistent with CD1d-restricted NKT cell involvement in hepatic IRI.
Figure 1.
Protection from hepatic IRI by A2AR activation or lymphocyte deficiency. WT or Rag-1 KO C57BL/6 mice were subjected to 72 min of hepatic ischemia followed by 24 h of reperfusion, or sham surgeries. Immediately after the initiation of reperfusion, animals received ATL146e or vehicle control. Animals were killed by cervical dislocation after 24 h of reperfusion, blood was collected via retro-orbital bleed, and serum ALT was measured. Additionally, livers were perfused, and left liver lobes were collected and placed immediately into 4% paraformaldehyde. Necrosis was measured via H &E staining. Data shown are from three independent experiments (n = 9); error bars indicate SEM. *, P < 0.01 versus C57BL/6 vehicle control as assessed by one-way ANOVA, followed by Dunnett's multiple comparison test. H &E staining shown is representative of five 10× fields of view photographed for each of nine animals in three independent experiments. Bar, 200 μm.
Figure 2.
Involvement of NKT cells in the pathogenesis of hepatic IRI. WT C57BL/6 mice were subjected to hepatic IRI or sham surgeries. Immediately after the initiation of reperfusion, select animals received ATL146e or vehicle control. Additionally, animals received either a single i.p. injection of 200 μg PK136 or vehicle control 2 d before surgery (A and B) or a single i.p. injection of 300 μg of a CD1d blocking antibody or vehicle control 24 h before surgery (C). Animals were killed by cervical dislocation after 24 h of reperfusion, blood was collected via retro-orbital bleed, and serum ALT was measured. Livers were perfused, and left liver lobes were collected and placed immediately into 4% paraformaldehyde. Necrosis was measured via H &E staining. Cell depletion by PK136 was assessed via the FACS analysis of spleen and liver tissue harvested from PK136-treated mice after 24 h of reperfusion (A). Numbers indicate the percentages of CD3+CD1d tetramer+ (A, top) and CD45+DX5+ (A, bottom) cells in the boxed regions. Data shown are from three independent experiments (n = 9); error bars indicate SEM. *, P < 0.01 versus vehicle control as assessed by one-way ANOVA, followed by Dunnett's multiple comparison test. H &E staining shown is representative of five 10× fields of view photographed for each of nine animals in three independent experiments. Bar, 200 μm.
The adoptive transfer of NKT cells restores liver injury to RAG-1 KO mice
Adoptive transfer of CD4+NK1.1+ NKT cells collected from the spleens of WT C57BL/6 mice into RAG-1 KO mice 4 d before surgery was found to reconstitute hepatic injury after IRI. This effect is cell number–dependent, with WT levels of injury restored by the adoptive transfer of 250,000 NKT cells and intermediate injury by 150,000 cells (Fig. 3 A). Approximately 75% of the CD4+NK1.1+ cells transferred expressed the invariant Vα14Jα18 TCR, as indicated by binding of an α-Gal-Cer–loaded CD1d tetramer (not depicted), and FACS analysis confirmed that the adoptively transferred NKT cells reach the livers of reconstituted animals (Fig. 3 B). Although the adoptive transfer of WT NKT cells reconstitutes liver injury after IRI, the transfer of 250,000 NKT cells collected from IFN-γ KO mice fails to do so; serum ALT levels are not significantly different from RAG-1 KO controls (Fig. 3 C). The adoptive transfer of 250,000 NKT cells from A2AR KO mice restores injury to RAG-1 KO mice to an extent similar to transfer of WT NKT cells, but treatment with ATL146e protects from tissue damage only when WT cells are transferred; A2AR deletion on the NKT cells abolishes the effect of agonist administration (Fig. 3 D). These findings suggest that NKT cells play a pivotal role in hepatic reperfusion injury, that this activity is dependent on the production of IFN-γ, and that the protection elicited by ATL146e treatment is dependent on the expression of functional A2ARs on NKT cells.
Figure 3.
Adoptive transfer of NKT cells restores injury to RAG-1 KO mice. WT or RAG-1 KO C57BL/6 mice were subjected to 72 min of hepatic IRI or sham surgeries. Immediately after the initiation of reperfusion, select animals received ATL146e or vehicle control. Purified CD4+NK1.1+ T cells from WT (A and B), IFN-γ KO (C), or A2AR KO (D) C57BL/6 mice were adoptively transferred into select RAG-1 KO mice 4 d before hepatic IRI. Successful reconstitution of CD4+NK1.1+ T cells into the livers of recipient animals was confirmed by FACS analysis of leukocytes collected from liver tissue after 24 h of reperfusion; numbers indicate the percentages of cells in each quadrant (B). Animals were killed by cervical dislocation after 24 h of reperfusion, blood was collected via retro-orbital bleed, and serum ALT was measured. Data shown are from three independent experiments (n = 9); error bars indicate SEM. *, P < 0.01 versus WT C57BL/6 control as assessed by one-way ANOVA, followed by Dunnett's multiple comparison test.
IFN-γ production and neutrophil accumulation after hepatic IRI is dependent on NKT cell activation
NKT cells isolated from postischemic mouse liver and liver- draining lymph nodes after 2 h of reperfusion display an activated phenotype, as indicated by an increase in intracellular IFN-γ expression as compared with sham surgery controls. Treatment with ATL146e at the initiation of reperfusion significantly inhibits this activation. (Fig. 4 A). Because activated NKT cells are known to release large amounts of IFN-γ and to stimulate IFN-γ release from bystander cells, we also examined plasma levels of IFN-γ 24 h after reperfusion injury. IRI substantially increased plasma IFN-γ concentrations at 24 h, and treatment with ATL146e, PK136, or anti-CD1d antibodies all diminished this elevation to a similar extent (Fig. 4 B). The large accumulation of neutrophils that is observed in the postischemic liver of WT C57BL/6 mice after 24 h of reperfusion was also reduced substantially in RAG-1 KO mice and to a similar extent in mice pretreated with PK136 or CD1d blocking antibody (Fig. 4 C). These findings indicate that NKT cells are activated rapidly after the initiation of reperfusion, that ATL146e inhibits this activation, and that the large accumulation of both serum IFN-γ and hepatic neutrophils that occurs 24 h after liver reperfusion is secondary to NKT cell activation.
Figure 4.
Effect of NKT cell depletion or blockade on downstream events in reperfusion injury. WT or Rag-1 KO C57BL/6 mice were subjected to 72 min of partial hepatic ischemia and 2 or 24 h of reperfusion. Select animals received an i.p. injection of 200 μg PK136 2 d before surgery, an i.p. injection of 300 μg CD1d blocking antibody 24 h before surgery, or ATL146e immediately after the initiation of reperfusion. (A) Intracellular IFN-γ production by CD3+/CD4+/CD1d-tetramer labeled NKT cells collected from postischemic tissue was assessed by FACS. Data shown are from three independent experiments (n = 9); error bars indicate SEM. *, P < 0.05 versus vehicle control as assessed by one-way ANOVA, followed by Dunnett's multiple comparison test. (B) Blood was collected by retro-orbital bleed 24 h after the initiation of reperfusion, and serum IFN-γ levels were measured by ELISA. Data shown are from three independent experiments (n = 9); error bars indicate SEM. *, P < 0.01 versus vehicle control as assessed by one-way ANOVA, followed by Dunnett's multiple comparison test. (C) Mice were killed by cervical dislocation 24 h after the initiation of reperfusion, livers were perfused, and the left liver lobes were collected and placed immediately into 4% paraformaldehyde fixative. Immunostaining of neutrophils was performed with rat anti–mouse neutrophil primary antibody. Data shown are from a single experiment representative of three independent experiments (n = 9). Bar, 200 μm.
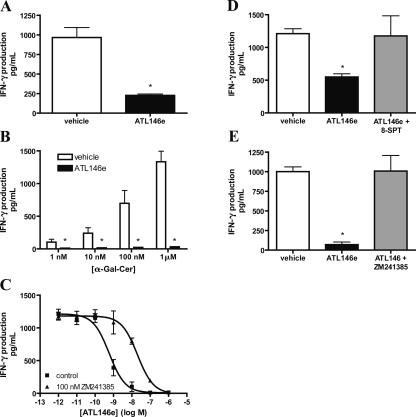
ATL146e treatment inhibits IFN-γ production by purified NKT cells
CD4+NK1.1+ NKT cells purified from spleens of WT C57BL/6 mice were activated on immobilized anti-CD3 mAb to stimulate the release of IFN-γ, as measured in cell supernatants after 24 h of incubation. TCR-stimulated IFN-γ production is inhibited by ∼73% by coincubation with 100 nM ATL146e (Fig. 5 A). iNKT cells in a mixed splenocyte culture were selectively activated in a dose-dependent manner by α-Gal- Cer, and this activation stimulated the production of IFN-γ, which is inhibited competently by 100 nM ATL146e (Fig. 5 B). The iNKT-mediated production of IFN-γ that is stimulated by 1 μM α -Gal-Cer is inhibited by ATL146e with an EC50 value of 0.58 nM. The addition of 100 nM of the selective A2AR antagonist 4-(2-[7-amino-2-[2-furyl][1,2,4]triazolo [2,3-a][1,3,5]triazin-5-yl-amino]ethyl)phenol (ZM241385) causes a right shift in the ATL146e dose-response curve that is characteristic of competitive A2AR blockade (Fig. 5 C). Co-treatment with 1 μM of the charged sulfonic acid adenosine receptor antagonist 8-sulfophenyltheophylline (8-SPT) also blocks the inhibitory effects of ATL146e on α-Gal-Cer–mediated IFN-γ production by a mixed splenocyte culture (Fig. 5 D). Because 8-SPT cannot cross the cell membrane, this indicates that the effects of ATL146e are mediated by A2ARs expressed on the cell surface. It is possible that some of the IFN-γ produced by mixed splenocytes might be derived from the transactivation of conventional lymphocytes or NK cells secondary to NKT cell activation. To eliminate these possible sources of IFN-γ, we also measured the release of IFN-γ from purified CD4+ NK1.1+ NKT cells activated with 1 μM α-Gal-Cer in the presence of lymphocyte-deficient, NK cell–depleted RAG-1 KO splenocytes as a source of APCs. IFN-γ derived from NKT cell activation in this experiment was reduced 93% by 100 nM ATL146e, and this effect was blocked by co-treatment with 100 nM ZM241385 (Fig. 5 E). These findings demonstrate for the first time that the production of IFN-γ by NKT cells in response to CD1d-dependent activation is inhibited by activation of the A2AR. Blockade of ATL146e activity by ZM241385 and 8-SPT indicate that this activity is dependent on functional cell surface expression of the A2AR.
Figure 5.
Inhibition by ATL146e of IFN-γ production by activated NKT cells. (A) Purified NKT cells (200,000 per well) were incubated on immobilized anti-CD3 mAb in the presence of vehicle or 100 nM ATL146e. Supernatants were collected after 24 h, and IFN-γ concentrations were measured by ELISA. Data are shown as the mean ± SEM from three independent experiments performed in triplicate. *, P < 0.01 versus vehicle control as assessed by an unpaired t test. (B) Bulk splenocytes (300,000 cells per well) were incubated with 1 U/ml ADA and 1 nM-1 μM α-Gal-Cer in the presence of 100 nM ATL146e or vehicle. *, P < 0.01 versus corresponding vehicle control as assessed by an unpaired t test. (C) Bulk splenocytes (300,000 cells per well) were incubated with 1 U/ml ADA and 1 μM α-Gal-Cer in the presence or absence of varying concentrations of ATL146e ± 100 nM ZM241385 or vehicle. (D) Bulk splenocytes (300,000 cells per well) were incubated with 1 U/ml ADA and 1 μM α-Gal-Cer in the presence or absence of 1 nM ATL146e ± 1 μM 8-SPT or vehicle. (E) Purified CD4+NK1.1+ T cells (150,000 cells per well) were incubated with lymphocyte- and NK cell–deficient splenocytes (300,000 cells per well), 1 U/ml ADA, and 1 μM α-Gal-Cer in the presence or absence of 100 nM ATL146e ± 100 nM ZM241385. NK1.1-expressing cells were depleted from RAG-1 KO splenocytes via a FACSVantage SE Turbo Sorter. Supernatants were collected after 48 h, and IFN-γ concentrations were determined by ELISA. Data shown are from a single experiment performed in triplicate, representative of three independent experiments. *, P < 0.01 versus vehicle control as assessed by one-way ANOVA, followed by Dunnett's multiple comparison test (D and E); error bars indicate SEM (A–E).
DISCUSSION
IRI is characterized by initial tissue damage during the ischemic period followed by progressive injury during the reperfusion period. Reperfusion is a trigger for the generation of reactive oxygen species (ROS), release of cytokines, induction of adhesion molecules on vascular endothelial cells, and the adhesion and extravasation of leukocytes into postischemic tissue. We and others have found that treatment with agonists of A2ARs or depletion of CD4+ lymphocytes effectively reduces inflammatory processes and the amount of tissue damage that occurs during reperfusion (8, 15–18). Of the total tissue necrosis that occurs in models of heart, kidney, skin, and liver IRI, 30–75% of the tissue injury occurs during reperfusion and can be prevented by treatment with A2AR agonists (19). In this study we show that the activation of NKT cells by a CD1d-dependent mechanism plays a central role in initiating the inflammatory cascade responsible for reperfusion injury in the liver and that these cells are key targets of A2AR agonists (Fig. 6). Based on adoptive transfer experiments of NKT cells into RAG-1 KO mice, we show that NKT cells are sufficient to cause reperfusion injury even in the absence of other lymphocytes. Additionally, we show that the activity of NKT cells to mediate liver reperfusion injury is dependent on the production of IFN-γ and that activation of the Gs-coupled A2AR markedly inhibits the production of IFN-γ by NKT cells both in vitro and in vivo. Although cAMP elevation has been found to inhibit CD8+ NKT cell cytotoxic activity (20), the current study is the first to demonstrate inhibition of CD4+ NKT cell cytokine production by a cAMP-elevating A2AR agonist.
Figure 6.
Hypothetical scheme of reperfusion-induced inflammatory injury in the liver. Reperfusion results in the generation of ROS and H2O2, and also in the CD1d-dependent activation of NKT cells. NKT cells are activated to produce IFN-γ early after the initiation of reperfusion, and this activation is inhibited by A2AR activation. As a consequence of this inhibition, downstream events in the reperfusion-induced inflammatory cascade (including neutrophil accumulation and tissue necrosis) are considerably reduced by ATL146e treatment.
Liver-resident NKT cells are known to play a role in tumor surveillance and protection from hepatitis B viral infection (12, 21–23). The selective activation of NKT cells with i.p. or i.v. injection of α-Gal-Cer results in an elevation of serum IFN-γ and ALT levels and induces liver tissue damage (24). The involvement of TCR activation in reperfusion injury is supported by previous work demonstrating that blockade of TCR signaling with cyclosporine treatment reduces hepatic reperfusion injury (25, 26). Additionally, CD1d−/− mice demonstrate considerably reduced liver reperfusion injury as compared with WT controls (27). The activity of CD1d to activate NKT cells during reperfusion implicates host glycolipid antigens, possibly derived or released from necrotic cells, in the rapid activation of the innate immune system. When activated, NKT cells rapidly release large amounts of both IL-4 and IFN-γ, which has been demonstrated to act via a STAT-1–dependent mechanism to activate Kupffer cells, as well as hepatocytes and sinusoidal endothelial cells, to produce chemokines and up-regulate adhesion molecules responsible for promoting the infiltration of leukocytes (28). IFN-γ also induces the generation of ROS and endoplasmic reticulum stress proteins in hepatocytes (29). Although mediators such as FasL have been shown to play a role in lymphocyte-mediated liver injury (30, 31), we show that NKT cell–initiated reperfusion injury is dependent on the production of IFN-γ. Although it is unlikely that conventional CD4+ T lymphocytes release large amounts of IFN-γ rapidly after exposure to activating stimuli, this is a characteristic response of CD4+NK1.1+ NKT cells (32, 33), and we show that NKT cells in the liver and liver draining lymph nodes have been stimulated to produce IFN-γ by 2 h after the initiation of reperfusion. Moreover, the mouse liver contains more NKT cells than any other immune organ (14), and based on these considerations and the data shown in this study, we propose that liver reperfusion injury results from an inflammatory cascade initiated by the release of IFN-γ from NKT cells. This in turn may stimulate the release of TNF-α and other cytokines from Kupffer cells, driving chemotaxis and activation of neutrophils and culminating in secondary liver injury (Fig. 6).
The C-type lectin receptor, NK1.1, is expressed on NKT cells and NK cells (34), and both cell types can be depleted competently by anti-NK1.1 antibodies as assessed by FACS analysis of splenocytes and liver leukocytes. The protective effect of PK136, therefore, indicates that NK cells, NKT cells, or both are involved in tissue damage after IRI. CD1d, however, acts specifically to prevent glycolipid antigen presentation to NKT cells (35), so the observation that the blockade of CD1d protects from hepatic IRI to a similar extent as does PK136 treatment indicates that NKT cells are the NK1.1-expressing cell type predominantly responsible for the induction of reperfusion injury. It is possible, however, that NK cells are involved in the later stages of injury, owing to their transactivation by NKT cell–released cytokines (36, 37). The role of NK cells in hepatic IRI warrants further investigation. Previous studies have implicated T cells in reperfusion injury (15–18), but a T cell– activating stimulus has not previously been clearly identified. Our data implicate CD1d-dependent antigen presentation as a key early event in the inflammatory cascade, but it is probably not the only stimulus. H2O2 derived from ROS is produced early during reperfusion and is known to facilitate activation of T cells through the oxidation of cysteine residues on protein tyrosine phosphatases that dephosphorylate activated TCRs (38, 39). In addition, H2O2 directly activates NF-κB (40), resulting in widespread activation of inflammatory cells. Thus, NKT cell activation and ROS may collaborate to trigger reperfusion injury.
The results of this study implicate NKT cells as predominant mediators of hepatic reperfusion injury that are sensitive to regulation by A2AR activation. Residual injury that is observed after blockade of NKT activation may be due to damage caused in an inflammatory cell–independent manner during the ischemic period. The majority of, but not all, mouse CD4+NK1.1+ NKT cells express an invariant Vα14Jα18 TCR, and we show that these cells are activated to produce IFN-γ early after the initiation of reperfusion. Moreover, this activation is inhibited by ATL146e treatment, resulting in substantial protection from injury. These data suggest that Vα14Jα18 iNKT cells play a pivotal role in reperfusion injury. Nevertheless, there are CD1d-dependent mouse NKT cells with diverse TCRs that may also be activated during reperfusion injury if CD1d-dependent ligands for these cells are generated. Protection from iNKT cell–mediated injury by A2AR activation may be relevant in humans because an analogous Vα24 NKT cell population exists (41), and these and similar cells in other mammalian species are activated by glypolipid antigens (42). It is notable that NKT cells with the invariant TCR are considerably less abundant in human than in mouse liver, and it remains to be seen whether this reduced cell number diminishes the contribution of iNKT cells to human liver IRI. Interestingly, a subpopulation of CD1d-reactive, non-iNKT cells has been identified in human liver (43). These intrahepatic cells are Th1 cell polarized and display similar activity as their invariant counterparts. It is feasible that if the reduced numbers of iNKT cells found in human liver are insufficient to induce hepatic injury after reperfusion, the specialized subset of CD1d-restricted non-iNKT cells may be poised to act in their stead or in addition to invariant cells; this possibility merits further investigation. Human NKT cells have been implicated in the pathophysiology of primary biliary cirrhosis, suggesting that these cells are physiologically important in man (44).
The results of this work suggest a paradigm shift in the way we view the role of T lymphocytes in IRI. Whereas myeloid cells have previously been thought of as the major facilitators of reperfusion injury, this study indicates that the initiation of the reperfusion-induced inflammatory cascade is dependent on CD1d-mediated IFN-γ production by NKT cells. Furthermore, profound protection is imparted when this early event in the inflammatory cascade is inhibited by A2AR activation; through this mechanism, the release of adenosine from injured tissue may serve as an endogenous regulator of NKT cell activity. Therapeutic agents that inhibit the activity of NKT cells may therefore hold promise in the treatment of IRI. Clinicians have historically attempted to limit the by-products of reperfusion-induced inflammation via the use of neutralizing antibodies to cytokines or free radical scavengers, but it may be possible to reduce the production of these mediators more substantially by targeting an upstream event in the cascade (i.e., NKT cell activation). The activities of ATL146e to potently inhibit the production of IFN-γ by CD1d-activated NKT cells and to dramatically protect the liver from reperfusion injury indicate that A2AR-selective agonists may be useful tools in the treatment of IRI. Moreover, there is no evidence of severe toxicity evoked by the use of A2AR agonists as antiinflammatory agents. It will be interesting to see if the inhibition of NKT cell activity by A2AR activation proves to be a clinically viable treatment for hepatic IRI or transplantation. It also will be of interest in future studies to define the role of the NKT cells in reperfusion injury in other tissue where NKT cells are less abundant.
MATERIALS AND METHODS
Animals.
WT, RAG-1 KO, and IFN-γ KO C57BL/6 mice were purchased from the Jackson Laboratory. A2AR KO mice on a mixed genetic background were provided by J.-F. Chen (Boston University, Boston, MA). All animal studies were approved by the University of Virginia Animal Care and Use Committee.
Creating A2AR KO mice congenic to C57BL/6.
The KO locus of B6;129P-adora2atm1chen mice with an ablated A2AR gene on a mixed genetic background (45) was moved onto a C57BL/6 background by monitoring 96 microsatellites for five generations of marker-assisted breeding. In the resulting mouse line, DNA derived from the 129 strain can be detected only in an 8-cM region between D10Mit31 and D10Mit42 surrounding the Adora2a locus on chromosome 10.
NKT cell purification.
WT, A2AR KO, or IFN-γ KO C57BL/6 mice were killed, and spleens were removed. Splenocytes were passed through a 40-mm nylon cell strainer (BD Biosciences) and collected in phosphate-buffered saline. Red blood cells were lysed, and CD4+ T lymphocytes were isolated with mouse CD4 subset column kits (R&D Systems), resulting in >92% pure CD4+ T cells. The column-purified cells were stained for 30 min with FITC-conjugated anti–mouse CD4 and PE-conjugated anti–mouse NK1.1 (eBioscience) and sorted using a FACSVantage SE Turbo Sorter (Becton Dickinson) to produce cell populations of ≥99.8% pure CD4+NK1.1+ T lymphocytes.
In vitro activation of NKT cells.
Cells were washed and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotic-antimycotic (Invitrogen). In vitro activation of NKT cells was achieved by co-culture for 48 h with splenocytes and 1 nM-1 μM α-Gal-Cer (KRN7000; obtained from K. Miyayama, Kirin Brewery Company, Tokyo, Japan) at 37°C in 5% CO2. Alternately, NKT cells were activated by incubation for 24 h in 96-well plates coated with 2–10 μg/ml of immobilized anti-CD3 mAb (BD Biosciences) at 37°C in 5% CO2. All in vitro T cell activation experiments were performed with the addition of 1 U/ml adenosine deaminase (ADA; Roche) to remove endogenous adenosine produced by the cells that may partially activate the A2AR. For select experiments, cells were co-cultured with ATL146e (obtained from J. Rieger, Adenosine Therapeutics, Charlottesville, VA) in the presence or absence of 100 nM of the selective A2AR antagonist ZM241385 (Tocris) or 1 μM of the cell-impermeable AR antagonist 8-SPT (Research Biochemicals International).
Hepatic IRI.
Mice were anesthetized by i.p. injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Ambient temperature was controlled in the range of 24–26°C, and mice were placed on a heating pad at 37°C. The core body temperature of selected mice was monitored with a monitoring thermometer (TH-8 Thermalert; Physitemp) and ranged from 35–36°C. After midline laparotomy, a microaneurysm clip was applied to the hepatic triad above the bifurcation to clamp the flow of the hepatic artery, portal vein, and bile duct. After superfusion of the liver with warm saline, the peritoneum was closed during 72 min of ischemia. The peritoneum was then reopened, and the microaneurysm clip was removed. For select experiments, animals received an i.p. loading dose of 1 μg/kg ATL146e or vehicle control immediately after the onset of reperfusion, and a primed Alzet osmotic minipump was implanted i.p. 10 ng/kg/min ATL146e or vehicle was placed in the pumps and delivered until the experiment was terminated. The peritoneum was sutured, and the surgical wound was closed with metal staples. Animals were killed by cervical dislocation at various time points after the initiation of reperfusion, and blood was collected via retro-orbital bleed. Additionally, livers were perfused, and left liver lobes were collected.
NK1.1 cell depletion and CD1d blockade.
NK1.1-expressing cells were depleted via a single i.p. injection of 200 μg PK136 (a gift from M. Brown, University of Virginia, Charlottesville, VA) (46) 2 d before hepatic IRI. Successful depletion was confirmed by FACS analysis of splenocytes and liver leukocytes collected at the termination of reperfusion. CD1d was blocked by a single i.p. injection of 300 μg of anti–mouse CD1d mAb clone 1B1 (a gift from M. Kronenberg, La Jolla Institute for Allergy and Immunology, San Diego, CA) (47) 24 h before hepatic IRI. Anti-NK1.1 (PK136) and anti-CD1d (clone 1B1) were purified from hybridomas in the University of Virginia hybridoma core.
Adoptive transfer of NKT cells.
CD4+NK1.1+ NKT cells were purified from WT, A2AR KO, or IFN-γ KO C57BL/6 mice and adoptively transferred into RAG-1 KO mice via jugular vein injection 4 d before hepatic IRI. Successful reconstitution was confirmed by FACS analysis. Control animals received vehicle injections.
Serum ALT determination.
After liver ischemia, blood was collected via retro-orbital bleed 24 h after the initiation of reperfusion. Serum ALT was measured with a transaminase kit according to the manufacture's protocol (Pointe Scientific). In brief, 20 μl of undiluted or 10×-diluted serum was added to 200 μl of a preheated (37°C) mix of 500 mM l-alanine and 15 mM α-ketoglutaric acid in a 96-well plate. The plate was placed in a spectrophotometer preheated to 37°C, and the absorbance at 304 nm was measured every minute for 10 min. The slope of the linear portion of the change in absorbance over time was used to calculate IU/L of ALT.
Flow cytometry of cell surface T cell markers.
Spleens were harvested, passed through a 40-mm nylon cell strainer (BD Biosciences) and collected in phosphate-buffered saline. Red blood cells were lysed. Alternately, livers were harvested, passed through a 40-μm cell strainer, and leukocyte fractions were isolated via Percol density gradient. Cells were washed and resuspended at 5 × 106 cells/ml in PBS supplemented with 5% FBS and 0.1% NaN3. 0.1-ml aliquots were placed on ice and labeled for 30 min in the dark with anti–mouse CD45, anti–mouse CD3, anti–mouse CD4, anti–mouse CD8, anti–mouse NK1.1, anti–mouse DX5 (eBioscience), and/or α-Gal-Cer–loaded CD1d tetramer (National Institute of Allergy and Infectious Disease; Tetramer Facility). Control samples were labeled with isotype-matched control antibodies. Stained cells were washed with 1 ml iced PBS and resuspended in PBS containing 1% paraformaldehyde. The fluorescence intensity was measured with a dual laser benchtop flow cytometer (FACSCalibur; Becton Dickinson) with a minimum of 10,000 events being collected. An excitation wavelength of 488 nm and an emission wavelength of 530 nm were used for FITC-stained cells; an excitation wavelength of 488 nm and an emission wavelength of 585 nm were used for PE-stained cells; an excitation wavelength of 635 nm and an emission wavelength of 661 nm were used for APCs and Alexa 647–stained cells; and an excitation wavelength of 488 nm and an emission wavelength of 670 nm were used for PE-Cy5.5–stained cells. Analysis was performed with FlowJo software (Tree Star, Inc.), and CD45+ cells were gated on for analysis.
Detection of intracellular IFN-γ.
Intracellular IFN-γ was detected in liver NKT cells by FACS analysis using FIX & PERM cell permeabilization reagents (Caltag Laboratories) according to the manufacturer's protocol.
Histology.
Mice were killed, and livers were perfused with saline via the portal vein at various times after the initiation of reperfusion. Left liver lobes were harvested, fixed in 4% paraformaldehyde in PBS, pH 7.4, and embedded in paraffin. 4-μm sections were subjected to standard hematoxylin and eosin (H&E) staining. Necrotic area was quantified using Photoshop software (Adobe).
Measurement of IFN-γ.
IFN-γ concentrations in cell culture supernatants or serum samples were measured by ELISA according to the manufacturer's protocol (eBioscience).
Statistics.
Prism software (GraphPad) was used for all statistical analyses. Unpaired t tests or one-way analysis of variance (ANOVA) with post-hoc Dunnett's multiple comparison were used to compare experimental groups with a control group.
Acknowledgments
The authors gratefully acknowledge Joanne Lannigan and Michael Solga of the University of Virginia Flow Cytometry Core Facility for their invaluable assistance; Dr. William Sutherland, Director of the University of Virginia Hybridoma Facility, for production and purification of antibodies; and Dr. Peter Ernst for helpful discussion.
This research was supported by grant R01 HL37942 from the National Institutes of Health.
J. Linden owns equity in Adenosine Therapeutics, LLC, Charlottesville, VA, a biopharmaceutical company developing ATL146e for clinical applications. The authors have no other conflicting financial interests.
Abbreviations used: 8-SPT, 8-sulfophenyltheophylline; α-Gal-Cer, α-galactosylceramide; ADA, adenosine deaminase; ALT, alanine aminotransferase; ANOVA, analysis of variance; A2AR, adenosine A2A receptor; ATL146e, 4-{3-[6-amino-9- (5-ethylcarbamoyl-3,4-dihydroxy- tetrahydro-furan-2-yl)-9H- purin-2-yl]-prop-2-ynyl}- cyclohexanecarboxylic acid methyl ester; H&E, hematoxylin and eosin; IRI, ischemia reperfusion injury; iNKT, invariant NKT; ROS, reactive oxygen species; ZM241385, 4-(2-[7-amino-2-[2-furyl][1,2,4] triazolo[2,3-a][1,3,5]triazin-5-yl- amino]ethyl)phenol.
References
- 1.Day, Y.J., M.A. Marshall, L. Huang, M.J. McDuffie, M.D. Okusa, and J. Linden. 2004. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G285–G293. [DOI] [PubMed] [Google Scholar]
- 2.Cronstein, B.N. 1994. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 76:5–13. [DOI] [PubMed] [Google Scholar]
- 3.Lappas, C.M., J.M. Rieger, and J. Linden. 2005. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 174:1073–1080. [DOI] [PubMed] [Google Scholar]
- 4.Linden, J. 2001. Molecular approach to adenosine receptors: receptor- mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41:775–787. [DOI] [PubMed] [Google Scholar]
- 5.Ohta, A., and M. Sitkovsky. 2001. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 414:916–920. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan, G.W., J. Linden, E.L. Hewlett, H.T. Carper, J.B. Hylton, and G.L. Mandell. 1990. Adenosine and related compounds counteract tumor necrosis factor-alpha inhibition of neutrophil migration: implication of a novel cyclic AMP-independent action on the cell surface. J. Immunol. 145:1537–1544. [PubMed] [Google Scholar]
- 7.Sullivan, G.W., J.M. Rieger, W.M. Scheld, T.L. Macdonald, and J. Linden. 2001. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br. J. Pharmacol. 132:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day, Y.J., Y. Li, J.M. Rieger, S.I. Ramos, M.D. Okusa, and J. Linden. 2005. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J. Immunol. 174:5040–5046. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey, D.I., H.R. MacDonald, M. Kronenberg, M.J. Smyth, and K.L. Van. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4:231–237. [DOI] [PubMed] [Google Scholar]
- 11.Zhou, D., J. Mattner, C. Cantu III, N. Schrantz, N. Yin, Y. Gao, Y. Sagiv, K. Hudspeth, Y.P. Wu, T. Yamashita, et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science. 306:1786–1789. [DOI] [PubMed] [Google Scholar]
- 12.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, H. Sato, E. Kondo, M. Harada, H. Koseki, T. Nakayama, et al. 1998. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA. 95:5690–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronenberg, M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23:877–900. [DOI] [PubMed] [Google Scholar]
- 14.Hammond, K.J., D.G. Pellicci, L.D. Poulton, O.V. Naidenko, A.A. Scalzo, A.G. Baxter, and D.I. Godfrey. 2001. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167:1164–1173. [DOI] [PubMed] [Google Scholar]
- 15.Day, Y.J., L. Huang, H. Ye, L. Li, J. Linden, and M.D. Okusa. 2006. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J. Immunol. 176:3108–3114. [DOI] [PubMed] [Google Scholar]
- 16.Yang, Z., Y.J. Day, M.C. Toufektsian, S.I. Ramos, M. Marshall, X.Q. Wang, B.A. French, and J. Linden. 2005. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 111:2190–2197. [DOI] [PubMed] [Google Scholar]
- 17.Savransky, V., R.R. Molls, M. Burne-Taney, C.C. Chien, L. Racusen, and H. Rabb. 2006. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 69:233–238. [DOI] [PubMed] [Google Scholar]
- 18.Yokota, N., F. Daniels, J. Crosson, and H. Rabb. 2002. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation. 74:759–763. [DOI] [PubMed] [Google Scholar]
- 19.Linden, J. 2005. Adenosine in tissue protection and tissue regeneration. Mol. Pharmacol. 67:1385–1387. [DOI] [PubMed] [Google Scholar]
- 20.Scheffold, C., M. Kornacker, Y.C. Scheffold, C.H. Contag, and R.S. Negrin. 2002. Visualization of effective tumor targeting by CD8+ natural killer T cells redirected with bispecific antibody F(ab')(2)HER2xCD3. Cancer Res. 62:5785–5791. [PubMed] [Google Scholar]
- 21.Grubor-Bauk, B., A. Simmons, G. Mayrhofer, and P.G. Speck. 2003. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 170:1430–1434. [DOI] [PubMed] [Google Scholar]
- 22.Kakimi, K., L.G. Guidotti, Y. Koezuka, and F.V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth, M.J., K.Y. Thia, S.E. Street, E. Cretney, J.A. Trapani, M. Taniguchi, T. Kawano, S.B. Pelikan, N.Y. Crowe, and D.I. Godfrey. 2000. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa, T., H. Kobayashi, T. Nonami, A. Harada, A. Nakao, S. Sugiyama, T. Ozawa, and H. Takagi. 1992. Beneficial effects of cyclosporine on postischemic liver injury in rats. Transplantation. 53:308–311. [DOI] [PubMed] [Google Scholar]
- 26.Sakr, M.F., and A.N. Abdel-Aal. 1996. Protective effect of cyclosporine A (CyA) against the hepatic injury associated with ischemia and reperfusion. Int. Surg. 81:180–183. [PubMed] [Google Scholar]
- 27.Shimamura, K., H. Kawamura, T. Nagura, T. Kato, T. Naito, H. Kameyama, K. Hatakeyama, and T. Abo. 2005. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell. Immunol. 234:31–38. [DOI] [PubMed] [Google Scholar]
- 28.Jaruga, B., F. Hong, W.H. Kim, and B. Gao. 2004. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am. J. Physiol. Gastrointest. Liver Physiol. 287:G1044–G1052. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe, Y., O. Suzuki, T. Haruyama, and T. Akaike. 2003. Interferon- gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell. Biochem. 89:244–253. [DOI] [PubMed] [Google Scholar]
- 30.Kondo, T., T. Suda, H. Fukuyama, M. Adachi, and S. Nagata. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3:409–413. [DOI] [PubMed] [Google Scholar]
- 31.Li, M., and G.T. Liu. 2004. Inhibition of Fas/FasL mRNA expression and TNF-alpha release in concanavalin A-induced liver injury in mice by bicyclol. World J. Gastroenterol. 10:1775–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii, S., K. Shimizu, M. Kronenberg, and R.M. Steinman. 2002. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat. Immunol. 3:867–874. [DOI] [PubMed] [Google Scholar]
- 33.Hansen, D.S., and L. Schofield. 2004. Regulation of immunity and pathogenesis in infectious diseases by CD1d-restricted NKT cells. Int. J. Parasitol. 34:15–25. [DOI] [PubMed] [Google Scholar]
- 34.Mercer, J.C., M.J. Ragin, and A. August. 2005. Natural killer T cells: rapid responders controlling immunity and disease. Int. J. Biochem. Cell Biol. 37:1337–1343. [DOI] [PubMed] [Google Scholar]
- 35.Yu, K.O., and S.A. Porcelli. 2005. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol. Lett. 100:42–55. [DOI] [PubMed] [Google Scholar]
- 36.Carnaud, C., D. Lee, O. Donnars, S.H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647–4650. [PubMed] [Google Scholar]
- 37.Eberl, G., and H.R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985–992. [DOI] [PubMed] [Google Scholar]
- 38.Ginn-Pease, M.E., and R.L. Whisler. 1998. Redox signals and NF-kappaB activation in T cells. Free Radic. Biol. Med. 25:346–361. [DOI] [PubMed] [Google Scholar]
- 39.Reth, M. 2002. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 3:1129–1134. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar, S., B. Lamothe, and B.B. Aggarwal. 2002. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J. Immunol. 168:2644–2651. [DOI] [PubMed] [Google Scholar]
- 41.Dellabona, P., E. Padovan, G. Casorati, M. Brockhaus, and A. Lanzavecchia. 1994. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 180:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brossay, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Exley, M.A., Q. He, O. Cheng, R.J. Wang, C.P. Cheney, S.P. Balk, and M.J. Koziel. 2002. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J. Immunol. 168:1519–1523. [DOI] [PubMed] [Google Scholar]
- 44.Kita, H., O.V. Naidenko, M. Kronenberg, A.A. Ansari, P. Rogers, X.S. He, F. Koning, T. Mikayama, J. van de Water, R.L. Coppel, et al. 2002. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 123:1031–1043. [DOI] [PubMed] [Google Scholar]
- 45.Chen, J.F., Z. Huang, J. Ma, J. Zhu, R. Moratalla, D. Standaert, M.A. Moskowitz, J.S. Fink, and M.A. Schwarzschild. 1999. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 19:9192–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smart, Y.C., K.L. Stevenson, R.F. Thorne, W.D. Thomas, L.H. Hsu, and R.C. Burton. 1989. Expression of natural killer (NK) cell-specific alloantigens on a mouse NK-like cell line. Immunol. Cell Biol. 67:239–242. [DOI] [PubMed] [Google Scholar]
- 47.Brossay, L., D. Jullien, S. Cardell, B.C. Sydora, N. Burdin, R.L. Modlin, and M. Kronenberg. 1997. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J. Immunol. 159:1216–1224. [PubMed] [Google Scholar]