Abstract
Aberrant cytokine expression has been proposed as an underlying cause of psoriasis, although it is unclear which cytokines play critical roles. Interleukin (IL)-23 is expressed in human psoriasis and may be a master regulator cytokine. Direct intradermal administration of IL-23 in mouse skin, but not IL-12, initiates a tumor necrosis factor–dependent, but IL-17A–independent, cascade of events resulting in erythema, mixed dermal infiltrate, and epidermal hyperplasia associated with parakeratosis. IL-23 induced IL-19 and IL-24 expression in mouse skin, and both genes were also elevated in human psoriasis. IL-23–dependent epidermal hyperplasia was observed in IL-19−/− and IL-24−/− mice, but was inhibited in IL-20R2−/− mice. These data implicate IL-23 in the pathogenesis of psoriasis and support IL-20R2 as a novel therapeutic target.
Psoriasis is a common skin disorder that affects ∼2% of the worldwide population. Lesions are typically well-demarcated erythematous, scaly plaques. Histologically, there is marked epidermal hyperplasia (acanthosis) accompanied by parakeratosis (retention of keratinocyte nuclei in the stratum corneum) and a mixed dermal infiltrate, including CD4+ T cells, dendritic cells, macrophages, and mast cells. Neutrophilic exudates are often seen (Munro microabscesses) and CD8+ T cells are present in the epidermis. Dermal papillary blood vessels are dilated and tortuous, and there is increased expression of angiogenesis-associated genes (1).
The pathogenesis of psoriasis is unclear, but the effectiveness of cyclosporine and other newer agents implicates an important role for T cells. No clear-cut autoantigens, however, have been described, and it is likely that the innate immune system is also involved (2). Bacterial infections and physical trauma (Köebner's phenomenon) often precede lesion formation and neutrophil accumulation in the dermis, and an influx into the epidermis is observed in initial pre-pinpoint lesions (3). Plasmacytoid pre–dendritic cells initiate psoriasis in a xenograft animal model (4), and other cells, including dermal dendritic cells and NK T cells, have also been implicated (5, 6). Given their ability to link the innate and acquired immune systems, dysregulated cytokine production (elevated levels of TNF, IFN-γ, IL-1α, IL-1β, TGF-α, IL-6, and other cytokines in lesional skin) is postulated to establish chronic lesions by providing persistent proinflammatory signals in the skin (7). Immune deviation from a Th1 to Th2 cytokine milieu can ameliorate disease, which also supports the cytokine hypothesis (8). Additionally, a novel family of IL-10–related cytokines consisting of IL-19, IL-20, and IL-24 has recently been associated with psoriasis, but their roles in disease pathogenesis are unclear (9).
IL-23 is a recently described, IL-12–related heterodimeric cytokine (10). IL-23 and IL-12 both contain the IL-12p40 subunit, which is paired with IL-23p19 and IL-12p35, respectively. The heterodimeric receptors for IL-23 and IL-12 consist of IL-23R paired with IL-12Rβ1 and IL-12Rβ2 paired with IL-12Rβ1, respectively (11, 12). IL-23 has a dominant role over IL-12 in driving animal models of chronic autoimmune disease (13, 14), and IL-23 transgenic mice have systemic inflammation involving multiple organs, including the skin (15, 16). IL-23 is produced by dendritic cells and macrophages in response to bacteria, including Bordetella pertussis (17), Mycobacterium tuberculosis (18), Streptococcus pyogenes (19), and Klebsiella pneumoniae (20). IL-23 gene expression is increased in psoriatic lesions compared with uninvolved skin (21), but downstream consequences of IL-23 dysregulation are unclear. We demonstrate that intradermal delivery of IL-23 protein in mice results in acute skin histopathology that shares many features of psoriasis. We also show a role for the IL-19 family of cytokines, via the shared receptor IL-20R2, in IL-23–dependent epidermal hyperplasia, supporting IL-20R2 as a novel therapeutic target.
RESULTS
Elevated IL-23 is associated with human psoriasis
IL-23p19 mRNA expression was increased in lesional psoriatic skin compared with nonlesional skin from psoriatic and normal donors (Fig. 1 A, left), in agreement with a previous report (21). Similar results were observed for IL-12p40, including increased expression in nonlesional psoriatic skin compared with normal controls (Fig. 1 A, middle). In contrast, IL-12p35 expression was not increased but actually decreased in lesional psoriatic skin compared with nonlesional and normal skin (Fig. 1 A, right).
Figure 1.
IL-23 is associated with human psoriasis and drives epidermal hyperplasia in mice. Expression of IL-23p19, IL-12p40, and IL-12p35 mRNA in normal, nonlesional (NL), and lesional (L) human psoriatic skin. Bar represents median (A). Representative photographs (B) of 129SvEv mice at day 4 treated with daily injections of saline (left) or 1 μg IL-23 (right). Representative frozen sections stained for CD31. Samples were taken at day 4 from 129SvEv mice treated daily with saline (top) or 1 μg IL-23 (bottom). Red arrows indicate CD31 staining in the dermal papillary. Bar, 100 μm (C). Representative histological sections at day 4 of littermate wild-type C57BL/6 mice treated daily with saline (left) or 1 μg IL-23 (middle), and IL-23R−/− mice treated daily with 1 μg IL-23 (right). Red arrows indicate parakeratosis. Green arrows indicate neutrophilic exudates. Bar, 100 μm (D). Epidermal thickness was measured in 129SvEv mice treated daily with saline (◯) or 1 μg IL-23 (•). Data was pooled together from six independent experiments performed in quadruplicate. Mean ± SD is shown. *, P < 0.001 (E). Epidermal thickness was measured at day 4 in 129SvEv mice treated daily with saline, 1 μg IL-23, 1 μg IL-12, or 1 μg TNF. Data was pooled together from two independent experiments (F).
IL-23 drives inflammation and epidermal hyperplasia in mouse skin
Because IL-23 is elevated in human psoriasis, we injected IL-23 protein intradermally into mice to explore the downstream consequences of aberrant cutaneous IL-23 exposure. Wild-type mice treated daily with IL-23, but not saline, developed visually apparent erythema and induration (Fig. 1 B) and associated prominent dermal papillary blood vessels (Fig. 1 C). Induration was apparent by day 2 and persisted to day 7. Erythema was also apparent at day 2 but began to recede at day 5. Histological evaluation of IL-23–treated skin showed epidermal (and follicular) hyperplasia accompanied by parakeratosis at day 4 (Fig. 1 D, middle, red arrows). No rete ridges were observed, although hair follicles cut in the appropriate plane may falsely suggest otherwise. Focal neutrophilic exudates, reminiscent of Munro microabscesses, were often observed (Fig. 1 D, middle, green arrows). Markedly less parakeratosis and fewer focal neutrophilic exudates were observed by day 7 (unpublished data).
The specificity of this observation was confirmed using IL-23R–deficient mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20060244/DC1), which did not respond to intradermal IL-23 treatment (Fig. 1 D, right). IL-23–induced epidermal thickening was maximal by day 4 and began to decrease by day 7 (Fig. 1 E). IL-12 treatment, in comparison, did not induce significant changes in epidermal thickness. No changes in epidermal thickness were observed with TNF treatment either (Fig. 1 F), in agreement with a previous report (22). Bioactivity of these cytokines was confirmed by examining expression of IL-12–dependent (IFN-γ, CXCL9, and CXCL10) and TNF-dependent (VCAM-1) genes in skin samples (Fig. S2). Immunohistochemical analysis demonstrated a mixed dermal infiltrate consisting of CD4+ T cells, CD11c+ dendritic cells, F4/80+ macrophages, and neutrophils that began 1 d after IL-23 treatment and was markedly increased by day 4 (Fig. S3). No CD8+ T cells or increased numbers of mast cells were detected at either time point, in contrast to psoriasis (unpublished data). Aside from the obvious local changes in skin appearance, the IL-23 treatment regimen was well tolerated because the mice appeared healthy and did not lose weight during the course of the experiment. Serum cytokine levels, including GM-CSF, IL-1β, IL-2, IL-4, IL-5, IL-6, TNF, and IFN-γ, remained unchanged compared with saline-treated controls (unpublished data).
Epidermal thickening associated with IL-23 exposure was examined in detail using transmission electron microscopy. Hyperplastic skin was selected from animals without histopathologic evidence of suppurative dermatitis (day 4) for the purpose of defining ultrastructural features of the model in the absence of cellular infiltrates. Acanthosis (increased thickness of the stratum spinosum) was a predominant feature and accounted for the majority of the epidermal thickening and was characterized by spinous cell hyperplasia, hypertrophy, and spongiosis (intercellular edema) (Fig. 2 A). Spinous cells, although enlarged, maintained normal cytoplasmic contents and membrane structure. The granular cell layer (stratum granulosum) was more prominent in IL-23–treated treated skin when compared with untreated control skin. However, it was still very sparse, even in severely thickened skin, and often showed retained “end-stage” nuclei (i.e., nuclei with condensed and marginated chromatin). Nuclear fragmentation was not observed within this layer. The stratum corneum was characterized by altering areas of hyperparakeratosis (cells with retained epithelioid nuclei, keratohyalin granules, and compacted keratin filaments) and orthohyperkeratosis (Fig. 2 B). These results are discussed in the context of psoriasis keratinocyte biology in the Discussion. Additional transmission electron microscopy pictures are available at http://www.jem.org/cgi/content/full/jem.20060244/DC1.
Figure 2.
IL-23–stimulated epidermal thickening is due to keratinocyte hyperplasia in the spinous layer and altered granular layer differentiation. Low magnification (bar, 8.4 μm) of skin illustrating the major ultrastructural features of IL-23–induced epidermal hyperplasia, including hypertrophy and hyperplasia of the spinous layer, with spongiosis and retained nuclei as well as compacted keratin filaments in the keratin layer (A). Transmission electron photomicrograph of skin from an IL-23–treated mouse showing retained nuclei (single arrows) within the stratum corneum. Note the division between the cornified and granular layers (double arrow). Bar, 9.7 μm (B).
IL-17A is not required for IL-23–dependent epidermal hyperplasia
IL-17A is a proinflammatory cytokine that lies downstream of IL-23 in the pathogenesis of experimental autoimmune encephalomyelitis and whose presence is correlated with the progression of collagen-induced arthritis (13, 23). Human psoriatic skin has elevated IL-17A gene expression in lesions relative to nonlesional skin and normal controls (Fig. 3 A). IL-17A mRNA expression was increased in IL-23–treated mouse skin at day 1 and remained elevated at day 4 (Fig. 3 C). We used a blocking monoclonal antibody to IL-17A to determine whether IL-17A is required for IL-23–dependent epidermal thickening. Anti–IL-17A did not inhibit IL-23– stimulated acanthosis (Fig. 3 E), nor did it affect IL-23–dependent erythema, induration, and parakeratosis (unpublished data). Anti–IL-17A treatment inhibited IL-23–stimulated G-CSF (Fig. 3 G) and MMP-13 expression (unpublished data), genes previously shown to be IL-17A regulated (24, 25). This anti–IL-17A antibody is efficacious at inhibiting experimental autoimmune encephalomyelitis, where IL-17A expressed in the central nervous system is a key pathogenic factor that contributes to paralysis (26), and also in inhibiting collagen-induced joint inflammation models, where IL-17A is a key pathogenic factor driving cartilage and bone destruction (unpublished data). Intradermal IL-17A delivery caused significant keratinocyte hypertrophy, but minimal if any hyperplasia by day 4 (unpublished data).
Figure 3.
IL-17A is not required for IL-23–dependent epidermal hyperplasia. Expression of IL-17A (A) or TNF (B) mRNA in human psoriatic skin. Expression of IL-17A (C) or TNF (D) mRNA in mice treated daily with saline (open bars) or 1 μg IL-23 (filled bars). Data are mean ± SEM of three independent experiments using 129SvEv, C57BL/6, or B6 × 129 mice, where mRNA was pooled together from four mice per group. *, P < 0.05. Epidermal thickness at day 4 in 129SvEv mice treated daily with saline or 1 μg IL-23 in the presence of anti–IL-17A (E) or anti-TNF (F) blocking monoclonal antibody or isotype control. Sum of two independent experiments for each antibody is shown. Expression of G-CSF (G) at day 4 in 129SvEv mice (n = 4, mRNA was pooled together) treated daily with saline or IL-23 in the presence of blocking anti–IL-17A monoclonal antibody (filled bars) or isotype control (open bars). One of two representative experiments is shown.
Role of TNF in IL-23–dependent epidermal hyperplasia
TNF is a validated target for various inflammatory diseases, including psoriasis (27, 28). TNF mRNA was elevated in human psoriatic skin relative to normal controls (Fig. 3 B) and was also induced in mouse skin by intradermal IL-23 treatment at day 1, but not at day 4 (Fig. 3 D). TNF protein was also detected by ELISA in IL-23–treated skin (unpublished data). We used a blocking monoclonal antibody to TNF to determine whether TNF is involved in IL-23–dependent epidermal thickening. In contrast to anti–IL-17A, anti-TNF treatment partially inhibited IL-23–dependent acanthosis (Fig. 3 F), and the degree of erythema and induration was decreased.
IL-23 indirectly stimulates keratinocyte proliferation
Immunohistochemical analysis of IL-23–treated skin at day 1 showed increased Ki67+ cells in the epidermis, suggesting that keratinocytes had received an IL-23–stimulated proliferative signal by day 1 (Fig. 4 A). This observation was confirmed by increased keratin 16 (K16) gene expression, which is associated with keratinocyte hyperplasia in psoriasis (29). Elevated K16 was evident at day 1, before changes in epidermal thickness, and was further increased at day 4 (Fig. 4 B). Increased expression of K16 was induced by IL-23, but not IL-12 or TNF (Fig. 4 C). Gene expression analysis of skin samples at day 1 suggested that the effects of IL-23 treatment on epidermal hyperproliferation were not due to increased gene expression of a panel of keratinocyte growth factors (Fig. 4 D). Furthermore, in vitro assays indicated that IL-23 did not have a direct effect on keratinocyte proliferation (Fig. 4 E).
Figure 4.
IL-23 stimulates keratinocyte proliferation in mice. Representative immunohistochemical staining for Ki67 in mice at day 1 (A) after treatment with saline (top) or 1 μg IL-23 (bottom). Bar, 20 μm. Expression of K16 mRNA (B) in mice treated daily with saline (open bars) or 1 μg IL-23 (filled bars). Data are mean ± SEM of three independent experiments using 129SvEv, C57BL/6, or B6 × 129 mice, where mRNA was pooled together from four mice per group. *, P < 0.05. K16 mRNA expression in mice at day 4 treated daily with saline, 1 μg IL-23, 1 μg IL-12, or 1 μg TNF. Data are mean ± STD, n = 4. One of two representative experiments is shown (C). mRNA expression at day 1 for a panel of keratinocyte growth factors (D) in mice treated with saline (open bars) or IL-23 (filled bars). Data are mean ± SEM of three independent experiments using 129SvEv, C57BL/6, or B6 × 129 mice, where mRNA was pooled together from four mice per group. Keratinocyte proliferation assay (E) in which normal human keratinocytes were treated in triplicate with vehicle or IL-23 (1, 10, or 100 ng/ml). 10 ng/ml KGF was used as a positive control. One of three representative experiments is shown.
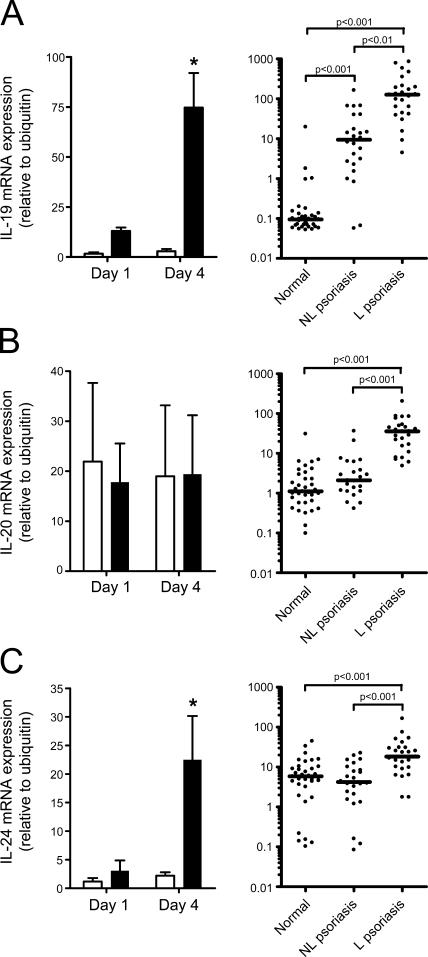
IL-19 family members are stimulated by IL-23 and expressed in human psoriatic skin
Because IL-23 did not stimulate the expression of any growth factors tested at day 1 (a time point before visual epidermal thickening, but after a keratinocyte proliferative signal had been received based on Ki67 staining and K16 expression), we examined the novel IL-19 family of cytokines, which consists of IL-19, IL-20, and IL-24. These cytokines all bind the heterodimeric receptor IL-20R1/IL-20R2. IL-20 and IL-24 can also bind IL-22R1/IL-20R2 (30). These receptors are highly expressed in epithelial cells, including keratinocytes (31, 32), and are all elevated in human psoriatic skin (9), suggesting a possible role in disease pathology. IL-23 delivery to mouse skin elevated IL-19 and IL-24, but not IL-20, gene expression (Fig. 5, left). IL-23 did not increase IL-20R1, IL-20R2, or IL-22R1 gene expression in mouse skin (unpublished data). IL-19, IL-20, and IL-24 mRNA was also increased in human psoriatic skin relative to normal controls (Fig. 5, right). IL-20R1 and IL-22R1 were elevated in psoriatic skin compared with normal controls, whereas no significant changes were observed for IL-20R2 (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20060244/DC1).
Figure 5.
IL-19 family members are expressed in human psoriatic skin and stimulated by IL-23 in mouse skin. IL-19 (A), IL-20 (B), and IL-24 (C) mRNA expression was measured in mice (left) treated daily with saline (open bars) or 1 μg IL-23 (filled bars), and in human psoriatic skin (right). Mouse data are mean ± SEM of three independent experiments using 129SvEv, C57BL/6, or B6 × 129 mice, where mRNA was pooled together from four mice per group. *, P < 0.05. Bar represents median for human samples.
Role of IL-20R2 in IL-23–dependent epidermal hyperplasia
IL-23 was delivered into the skin of IL-20R2−/− mice (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20060244/DC1) to simultaneously examine the role of all three IL-19 family cytokines. Epidermal thickening was significantly inhibited in IL-23–treated IL-20R2−/− mice compared with IL-23–treated wild-type controls (Fig. 6 A), despite the induction of IL-19 and IL-24 gene expression (Fig. 6 B). IL-23 was delivered into the skin of IL-19−/− and IL-24−/− mice (Fig. S6 and S7, respectively) to determine if IL-19 or IL-24 was required for IL-23–dependent epidermal hyperplasia. IL-23–stimulated epidermal thickening was observed in both IL-19−/− and IL-24−/− mice (Fig. 6 A). These data suggest that these cytokines are redundant in function because IL-24 and IL-19 gene expression was induced in IL-19−/− and IL-24−/− mice, respectively (Fig. 6, C and D). IL-20 gene expression was not significantly altered by IL-23 in skin from either IL-19−/− or IL-24−/− mice compared with wild-type controls (unpublished data). Importantly, in vitro stimulation of keratinocytes with increasing quantities of IL-19, IL-20, or IL-24 failed to stimulate proliferation, suggesting that IL-20R2 ligands are required but not sufficient for IL-23–stimulated epidermal hyperplasia (unpublished data).
Figure 6.
Role of IL-20R2 in IL-23–dependent epidermal hyperplasia. Epidermal thickness was measured at day 4 in wild-type, IL-20R2−/−, IL-19−/−, or IL-24−/− mice treated daily with saline (open symbols) or 1 μg IL-23 (filled symbols). Data from different wild-type strains were pooled together because no differences in IL-23–stimulated epidermal thickening were observed. Data are the aggregate of seven independent experiments (each strain of mouse was used in at least two separate experiments) (A). IL-19 and IL-24 gene expression in IL-20R2−/− mice at day 4 (B). IL-24 gene expression in IL-19−/− mice at day 4 (C). IL-19 gene expression in IL-24−/− mice at day 4 (D). Data are representative of two independent experiments where mRNA (n = 4) was pooled together. Representative immunohistochemical staining for CD4+ T cells (E), CD11c+ dendritic cells (F), F4/80+ macrophages (G), and neutrophils (H) at day 4 in IL-20R2−/− mice treated daily with saline (left) or 1 μg IL-23 (middle), or littermate wild-type mice at day 4 treated daily with 1 μg IL-23 (right). Bar, 100 μm.
Impaired leukocyte recruitment in IL-23–treated IL-20R2−/− mice
We performed immunohistochemical analysis on IL-23–treated IL-20R2−/− skin to determine whether impaired signaling through IL-20R2 affected the cellular composition of the dermal infiltrate. Although epidermal thickening was not apparent in IL-23–treated IL-20R2−/− mice, there were elevated numbers of CD4+ T cells and CD11c+ dendritic cells compared with saline-treated control mice, which was similar to IL-23–treated wild-type mice (Fig. 6, E and F, respectively). In contrast, there were reduced numbers of dermal F4/80+ macrophages and neutrophils in IL-20R2−/− mice (Fig. 6, G and H). Table I summarizes the differences between human psoriasis and wild-type or IL-20R2−/− mice in their responses to intradermal IL-23 treatment.
Table I.
Comparison of clinical and histological features of human psoriasis with IL-23–treated wild-type and IL-20R2−/− mouse skin
| Human psoriasis | IL-23–treated wild-type skin | IL-23–treated IL-20R2−/− skin | |
|---|---|---|---|
| Clinical | |||
| Erythematous | yes | yes | minimal |
| Well-demarcated from symptomless skin | yes | no | n/a |
| White-silvery scale | yes | no, although small areas of flaky skin | no, no evidence of flaky skin |
| Histological | |||
| Acanthosis | yes | yes | significantly reduced |
| Granular layer | no | yes, but no evidence of nuclei disintegration | no |
| Parakeratosis | yes | yes | no |
| Elongation of rete ridges | yes | no | n/a |
| Follicular hyperplasia | no | yes | no |
| Epidermal spongiosis | no | yes | no |
| Intracorneal pustules (Munro microabscesses) | yes | yes | no |
| Recruitment to dermis of: | |||
| CD4+ lymphocytes | yes | yes | yes |
| Dendritic cells | yes | yes | yes |
| Macrophages | yes | yes | reduced |
| Neutrophils | yes | yes | no |
| Mast cells | yes | no | n/a |
| CD8+ cells in epidermis | yes | no | n/a |
| Prominent dermal papillary blood vessels | yes | yes | no |
DISCUSSION
Because IL-23p19 and IL-12p40 gene expression was elevated in lesional psoriatic skin compared with nonlesional psoriatic skin and normal controls, we tested the hypothesis that IL-23 was involved in disease pathogenesis by injecting IL-23 intradermally into mice. IL-23 induced changes in mouse skin that share many features with human psoriasis, as summarized in Table I. The most striking similarity to psoriasis microscopically was the acanthosis (epithelial hyperplasia) and parakeratosis (keratinocyte nuclei in the stratum corneum).
Histological analysis of psoriatic skin reveals the absence of the normal granular layer where normal keratinocyte differentiation starts to give rise to a highly cross-linked, lipid-rich anuclear structure that provides the first barrier to the environment (i.e., stratum corneum). In normal skin, the granular layer is defined by numerous keratinocyte nuclei undergoing condensation and disintegration. Psoriatic skin, however, has little to no keratinocyte nuclei disintegration, which ultimately results in a modified stratum corneum with retained keratinocyte nuclei (parakeratosis). One difference between the acute IL-23–induced skin hyperplasia response and the chronic psoriatic lesional skin, however, is that a modified granular layer is present. IL-23–stimulated granular layer keratinocytes have nuclei condensation but no evidence of nuclei disintegration. This gives rise to parakeratosis, similar to psoriasis, by a slightly different mechanism than observed in psoriasis. Whether this is due to the IL-23–stimulated hyperplasia being an acute self-limiting condition versus the chronic nature of psoriatic lesions is under investigation. Reversible edema in the epidermis (spongiosis) was also observed in the acute model, which is not commonly seen in chronic psoriatic skin lesions. A rare disease, granular parakeratosis, is characterized by parakeratosis but in combination with a granular layer. IL-23 may also play a role in this disease due to the similarities between it and our observations in this mouse model.
An IL-12p40 antagonist that blocks IL-23 and IL-12p70 simultaneously was successful in a phase I psoriasis clinical trial (33), and IL-23p19, but not IL-12p35, gene expression was associated with human psoriasis (21). These data plus the observation that intradermal injections of IL-12 did not cause psoriasiform lesions in mice are consistent with IL-23 being the relevant target of anti–IL-12p40 therapy in psoriasis. It is noteworthy that IL-12 treatment did not induce psoriasiform lesions despite enhanced IFN-γ expression because human psoriatic lesions have a distinct signature of IFN-γ–regulated genes (34). This raises the possibility that IL-23 (dys)regulation may trigger psoriatic lesion formation and that IFN-γ–dependent processes may contribute to the chronicity of disease. This notion is consistent with IL-23 production early in an innate immune response to bacteria (17–20) that may initiate psoriatic lesions and with the multigenic nature of this disease (35).
IL-23 is a potent regulator of memory T cell development and/or expansion that is skewed to IL-17A secretion (36), and IL-23 has been implicated as a key disease driver in autoimmune disease models (13, 23). The human psoriatic data demonstrate that IL-17A gene expression is also associated with disease. Intradermal IL-23 injections stimulated elevated IL-17A expression; however, pretreatment with an antagonistic antibody did not ameliorate psoriasiform lesion formation. These data and the observation that direct IL-17A delivery has minimal effect on epidermal hyperplasia suggest that IL-17A, although downstream of IL-23 and associated with human psoriasis, is not required for disease pathogenesis. It is possible that IL-17F, whose expression is also skewed by IL-23 during memory T cell development (36) and is driven by IL-23 in this model (unpublished data), may compensate in some way for IL-17A because they both can bind IL-17RC (37). Interestingly, psoriatic patients are relatively resistant to cutaneous infections (38). IL-17A and IL-17F may play key roles in host defense to cutaneous infections because they stimulate neutrophil recruitment (39, 40), and IL-17A directly stimulates the anti-microbial gene β-defensin 2 in epithelial cells (41).
IL-23 stimulates macrophage TNF production (14) and IL-23p19 transgenic mice have elevated serum TNF levels (16). Neutralizing TNF partially inhibited epidermal hyperplasia induced by IL-23 in our study. This observation is consistent with the efficacy of anti-TNF–directed therapies in psoriasis (27, 28). TNF inhibition breaks the self-sustaining nature of psoriatic lesions by rapidly down-regulating several proinflammatory genes, including IL-23p19 and IL-19 (42). TNF alone, however, was not sufficient to induce epidermal hyperplasia, indicating that IL-23 stimulates other signaling pathways that synergize with TNF for disease pathogenesis.
The physiologic functions of the structurally related cytokines IL-19, IL-20, and IL-24 appear to be quite diverse despite their shared receptor profile. IL-19 may be involved in the induction of Th2 responses (43). IL-20 appears to play a key role in skin biology because IL-20 transgenic mice have aberrant epidermal differentiation with no immune cell infiltrates (32). Adenoviral delivery of IL-24 (MDA-7/mob-5/c49a) induces apoptosis in tumor cells (44), and IL-24 gene expression is increased during wound healing in the rat (45). The common link between IL-19, IL-20, and IL-24 is their use of heterodimeric receptors that share the IL-20R2 subunit: IL-20R1/IL-20R2 heterodimer and IL-22R1/IL-20R2 heterodimer (30). These receptors are primarily expressed in epithelial cells such as keratinocytes (31, 32) and are elevated in human psoriatic lesions (9, 32). Our human psoriatic data confirm that IL-20R1 and IL-22R2 are increased in psoriatic skin, although we did not detect a statistically significant increase in IL-20R2 mRNA compared with normal skin. Administration of IL-23 to IL-20R2−/− mice, but not IL-19−/− or IL-24−/− mice, resulted in significantly decreased acanthosis and parakeratosis. This suggests that either IL-19 or IL-24 may be sufficient to mediate epidermal hyperplasia via IL-20R2–containing receptors, although it is unclear whether these cytokines directly stimulate keratinocyte proliferation or alter the keratinocyte differentiation program.
Interestingly, cutaneous IL-23 exposure also drives high-level expression of IL-22, a cytokine structurally related to IL-19, IL-20, and IL-24 (unpublished data). IL-22 binds the heterodimeric receptor IL-22R1/IL-10RB, but it does not use IL-20R2. IL-22 activation of keratinocytes stimulates a variety of antimicrobial activities, such as psoriasin (S100A7), calgranulin A (S100A8), calgranulin B (S100A9), β-defensin 2, and β-defensin 3 (46–48). Therefore, cutaneous IL-23 expression in psoriatic skin drives both IL-22–dependent and IL-17A/IL-17F–dependent antimicrobial responses that protect the host.
The cellular mechanisms by which IL-23 drives IL-20R2–dependent epidermal hyperplasia and psoriasiform lesions remain to be investigated. Analysis of the inflammatory cell infiltrate in IL-23–treated IL-20R2−/− mice revealed decreased numbers of neutrophils and F4/80+ macrophages in the dermis, suggesting that these recruited cells may contribute to the lesion development in our model. In support of this possibility, neutrophil depletion inhibits acanthosis in the fsn/fsn mouse, which spontaneously develops flaky skin (49). Infiltrating myeloid cells can produce several factors associated with psoriasis, including iNOS, IL-19, MMP-12, and IL-23 (42).
T cells, monocytes, and NK cells express IL-23R (11), which raises the possibility that IL-23 may act directly on these cells to produce IL-19 and IL-24. Published data show that the major hematopoietic sources of IL-19 and IL-24 are monocytes and monocytes and T cells, respectively (50). IL-23–treated bone marrow macrophages do not express IL-19, IL-20, or IL-24 (unpublished data), suggesting that IL-19 and IL-24 expression may be indirectly stimulated by IL-23. This notion is supported by our data that keratinocytes do not respond to IL-23 and that IL-19 is expressed by keratinocytes in psoriatic lesions (9). TNF may be important here as IL-23 stimulates TNF expression in macrophages (14) and TNF directly stimulates IL-19 expression in normal human epidermal keratinocytes (unpublished data).
In summary, our data propose a molecular mechanism by which IL-23 dysregulation is one of the causative factors in psoriasis pathogenesis. Dysregulated cutaneous IL-23 production sets into motion several independent pathways. IL-23 contributes to the antimicrobial nature of psoriatic lesions by stimulating IL-17A (and IL-17F) and neutrophil recruitment. In parallel, IL-23 stimulates IL-19 and IL-24, which may directly act on keratinocytes in a TNF-regulated manner resulting in epidermal hyperplasia and/or altered keratinocyte differentiation. These functionally different arms suggest that IL-23 may have evolved as a “response to danger” cytokine invoking the body to protect itself by rapidly mobilizing antimicrobial components and instructing the epidermis to proliferate to provide additional protection from the environment. Given that IL-23–dependent epidermal hyperplasia was inhibited in IL-20R2−/− mice, targeting IL-20R2 and/or its associated receptors may be a novel therapeutic strategy for the treatment of this disease.
MATERIALS AND METHODS
Human skin specimens.
Psoriasis patients (n = 45) and normal volunteers (n = 30) consented under a protocol approved by the Stanford Panel on Human Subjects. Psoriasis patients needed to have a Psoriasis Area Severity Index of at least 8 and a typical lesion at least 1 cm in size suitable for biopsy. The target lesion and the surrounding 5-cm area could not have been treated with any medicated topical formulation for at least 2 wk before obtaining the biopsy. Patients treated with systemic immunosuppressives including corticosteroids were excluded. One 4-mm biopsy from a lesional and an adjacent nonlesional site was collected from psoriatic patients. One 4-mm biopsy was obtained from each normal volunteer. Specimens were flash frozen in liquid nitrogen.
Reagents and antibodies.
Generation of IL-23R−/−, IL-20R2−/−, IL-19−/−, and IL-24−/− mice are described in Online supplemented material. Recombinant human and murine IL-23 have been described (10). Recombinant murine IL-12 was generated using approaches described previously (10). Murine TNF and human keratinocyte growth factor 1 (KGF) were purchased (PeproTech). Rat anti–IL-17A (JL7.1D10) (26), rat anti-TNF (MP6-XT22) (51), and isotype control antibody (25D2) were purified from hybridoma culture supernatants. Rat anti-Ki67 (TEC-3; DakoCytomation), rat anti-neutrophils (7/4; Serotec), rat anti-CD4 (L3T4; BD Biosciences), rat anti-F4/80 (A3-1; Serotec), hamster anti-CD11c (HL3; BD Biosciences), and rat anti-CD31 (MEC 13.3; BD Biosciences) were purchased.
IL-23–dependent skin inflammation model.
All animal protocols were approved by DNAX/Schering-Plough Biopharma's Institutional Animal Care and Use Committee. Hair was removed from the back of mice with electric clippers and a cream depilatory (Nair). 3 d later, mice were injected intradermally with IL-23, vehicle control, or other cytokines (IL-12 or TNF) in two locations on either side of the back for a total of 1 μg protein per mouse using a 29.5-gauge needle. Sterile saline was used as a vehicle control. Injections were performed daily until mice were killed as per the objective of the experiment. For antibody blocking studies, mice were treated subcutaneously with 0.2–1.0 mg anti–IL-17A, anti-TNF, or isotype control 2 d before the first cytokine injection. Mice were killed using carbon dioxide and blood was collected by cardiac puncture. Skin samples were removed from the prepared area, keeping at least 5 mm away from the hair boundary. Skin samples were frozen directly in liquid nitrogen for mRNA extraction, fixed in 10% neutral buffered formalin for histology, or embedded and frozen in OCT for immunohistochemistry.
Electron microscopy.
Skin samples were fixed in a solution containing 2.5% glutaraldehyde and 2% formaldehyde with 0.12 M sodium cacodylate followed by secondary fixation in buffered 1% OsO4. The samples were then dehydrated and embedded in epoxy resin by standard methods. Thin sections were stained with 5% uranyl acetate in 50% methanol and 1% aqueous lead citrate, and images were captured using an electron microscope (CM10; Philips).
RNA extraction and real-time quantitative PCR.
RNA isolation was performed by standard techniques, and gene expression was calculated using the Δ-ΔCt method (using the mean cycle threshold value for ubiquitin and the gene of interest for each sample. The equation 1.8e (Ct ubiquitin − Ct gene of interest) × 104 was used to obtain the normalized values.
Histology and immunohistochemistry.
Skin sections were stained with hematoxylin and eosin, and epidermal thickness was determined by measuring the average interfollicular distance from the basal lamina to the bottom of the stratum corneum in a blinded manner. Similar results were obtained for wild-type mice on 129SvEv, C57BL/6, or mixed B6 × 129 genetic backgrounds. For immunohistochemistry, 8-μm sections were fixed with 75% acetone/25% ethanol. Endogenous peroxidase was quenched with Peroxidase Blocking Reagent (DakoCytomation) and endogenous biotin was blocked with SP-2001 (Vector Laboratories). Primary antibodies were incubated for 30 min at room temperature and visualized using VECTASTAIN elite ABC kit (Vector Laboratories). Positive staining developed as a brown reaction precipitate.
In vitro keratinocyte proliferation assay.
In vitro keratinocyte proliferation assays were performed as described previously (52), with minor modification. Normal human epidermal keratinocytes (Cambrex) were seeded at 2,000/well in a 24-well plate in keratinocyte basal medium (Cambrex) supplemented with hydrocortisone (Cambrex) and 5 μg/ml human recombinant insulin (Roche Diagnostics). During seeding, cells were treated with vehicle control, 1–100 ng/ml human IL-23, or 10 ng/ml KGF as a control. After 6 d, cells were washed and incubated with 0.2% crystal violet (Sigma-Aldrich). Cells were lysed with 1% SDS and absorbance was read at 565 nm. The total number of cells per well was determined by reference to a standard curve. All conditions were performed in triplicate.
Statistical analyses.
Human data was analyzed by Kruskal-Wallis with Dunn's Multiple Comparison after test. One-way or two-way ANOVA with Bonferroni after test were used where appropriate. P < 0.05 was considered to be statistically significant.
Online supplemented material.
Fig. S1 is the gene deletion strategy for the IL-23R knockout mouse. Fig. S2 shows the in vivo bioactivity of intradermally injected IL-12 and TNF. Fig. S3 shows the IL-23–dependent inflammatory cell infiltrate into mouse skin. Fig. S4 shows the IL-19 family receptor subunits in human psoriasis. Figs. S5–S7 show the gene deletion strategy for IL-20R2, IL-19, and IL-24 knockout mice, respectively. Fig. S8 is a high magnification hematoxylin and eosin–stained section showing general features of an IL-23–mediated model. Fig. S9 shows a high magnification hematoxylin and eosin–stained section illustrating an epidermal microabscess. Fig. S10 is a high magnification electron micrograph (EM) of spinous (bottom) and granular (top) layers (note presence of intercellular edema). Fig. S11 is a medium-high EM of junction between spinous and granular layers (note the presence of nuclear degeneration [condensed chromatin] in a granular cell [red arrow] vs. a more normal nucleus in a spinous cell [blue arrow]). Fig. S12 is a low magnification EM from a control animal illustrating the “normal” appearance of the epidermis (note that the granular layer is present, but not prominent, and there is little keratin). Fig. S13 is a high magnification EM from a control animal illustrating the “normal” appearance of the corneum (note the absence of nucleated keratinocytes and sparse keratohyalin granules). Table S1 is genotyping primers for analyzing knockout mice. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20060244/DC1.
Supplemental Material
Acknowledgments
The authors would like to acknowledge Kevin Keane for reviewing histology; Don Cook and Sergio Lira for expertise in generating IL-19−/− mice; and Gil Asio and Sharon Osborn for histology assistance.
Schering-Plough Biopharma (formerly DNAX Research, Inc.) is a division of Schering-Plough. All authors, except A.B. Kimball, were employees of Schering-Plough at the time of these studies, which could be considered to pose a financial conflict of interest regarding the submitted manuscript.
A.B. Kimball's present address is Massachusetts General and Brigham and Women's Hospitals, Harvard Medical School, Boston, MA 02114.
Abbreviations used: EM, electron micrograph; K16, keratin 16; KGF, keratinocyte growth factor 1.
References
- 1.Schon, M.P., and W.H. Boehncke. 2005. Psoriasis. N. Engl. J. Med. 352:1899–1912. [DOI] [PubMed] [Google Scholar]
- 2.Nickoloff, B.J., and F.O. Nestle. 2004. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113:1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, J.D., M.A. de Rie, M.B. Teunissen, and G. Piskin. 2005. Psoriasis: dysregulation of innate immunity. Br. J. Dermatol. 152:1098–1107. [DOI] [PubMed] [Google Scholar]
- 4.Nestle, F.O., C. Conrad, A. Tun-Kyi, B. Homey, M. Gombert, O. Boyman, G. Burg, Y.J. Liu, and M. Gilliet. 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickoloff, B.J., B. Bonish, B.B. Huang, and S.A. Porcelli. 2000. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J. Dermatol. Sci. 24:212–225. [DOI] [PubMed] [Google Scholar]
- 6.Boyman, O., C. Conrad, C. Dudli, E. Kielhorn, B.J. Nickoloff, and F.O. Nestle. 2005. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 152:1211–1218. [DOI] [PubMed] [Google Scholar]
- 7.Nickoloff, B.J. 1991. The cytokine network in psoriasis. Arch. Dermatol. 127:871–884. [PubMed] [Google Scholar]
- 8.Ghoreschi, K., P. Thomas, S. Breit, M. Dugas, R. Mailhammer, W. van Eden, R. van der Zee, T. Biedermann, J. Prinz, M. Mack, et al. 2003. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 9:40–46. [DOI] [PubMed] [Google Scholar]
- 9.Romer, J., E. Hasselager, P.L. Norby, T. Steiniche, J. Thorn Clausen, and K. Kragballe. 2003. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J. Invest. Dermatol. 121:1306–1311. [DOI] [PubMed] [Google Scholar]
- 10.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 11.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K.P. Singh, F. Vega, et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699–5708. [DOI] [PubMed] [Google Scholar]
- 12.Presky, D.H., H. Yang, L.J. Minetti, A.O. Chua, N. Nabavi, C.Y. Wu, M.K. Gately, and U. Gubler. 1996. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA. 93:14002–14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy, C.A., C.L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R.A. Kastelein, J.D. Sedgwick, and D.J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 15.Kopp, T., P. Lenz, C. Bello-Fernandez, R.A. Kastelein, T.S. Kupper, and G. Stingl. 2003. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J. Immunol. 170:5438–5444. [DOI] [PubMed] [Google Scholar]
- 16.Wiekowski, M.T., M.W. Leach, E.W. Evans, L. Sullivan, S.C. Chen, G. Vassileva, J.F. Bazan, D.M. Gorman, R.A. Kastelein, S. Narula, and S.A. Lira. 2001. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166:7563–7570. [DOI] [PubMed] [Google Scholar]
- 17.Fedele, G., P. Stefanelli, F. Spensieri, C. Fazio, P. Mastrantonio, and C.M. Ausiello. 2005. Bordetella pertussis-infected human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization and interleukin-23 expression. Infect. Immun. 73:1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verreck, F.A., T. de Boer, D.M. Langenberg, M.A. Hoeve, M. Kramer, E. Vaisberg, R. Kastelein, A. Kolk, R. de Waal-Malefyt, and T.H. Ottenhoff. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA. 101:4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veckman, V., M. Miettinen, J. Pirhonen, J. Siren, S. Matikainen, and I. Julkunen. 2004. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J. Leukoc. Biol. 75:764–771. [DOI] [PubMed] [Google Scholar]
- 20.Happel, K.I., M. Zheng, E. Young, L.J. Quinton, E. Lockhart, A.J. Ramsay, J.E. Shellito, J.R. Schurr, G.J. Bagby, S. Nelson, and J.K. Kolls. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, E., W.L. Trepicchio, J.L. Oestreicher, D. Pittman, F. Wang, F. Chamian, M. Dhodapkar, and J.G. Krueger. 2004. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 199:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson, T., D.N. Cook, R.J. Nibbs, A. Rot, C. Nixon, P. McLean, A. Alcami, S.A. Lira, M. Wiekowski, and G.J. Graham. 2005. The chemokine receptor D6 limits the inflammatory response in vivo. Nat. Immunol. 6:403–411. [DOI] [PubMed] [Google Scholar]
- 23.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenders, M.I., J.K. Kolls, B. Oppers-Walgreen, L. van den Bersselaar, L.A. Joosten, J.R. Schurr, P. Schwarzenberger, W.B. van den Berg, and E. Lubberts. 2005. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52:3239–3247. [DOI] [PubMed] [Google Scholar]
- 25.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J.J. Pin, P. Garrone, E. Garcia, S. Saeland, et al. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, Y., C.L. Langrish, B. McKenzie, B. Joyce-Shaikh, J.S. Stumhofer, T. McClanahan, W. Blumenschein, T. Churakovsa, J. Low, L. Presta, et al. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonardi, C.L., J.L. Powers, R.T. Matheson, B.S. Goffe, R. Zitnik, A. Wang, and A.B. Gottlieb. 2003. Etanercept as monotherapy in patients with psoriasis. N. Engl. J. Med. 349:2014–2022. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhari, U., P. Romano, L.D. Mulcahy, L.T. Dooley, D.G. Baker, and A.B. Gottlieb. 2001. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 357:1842–1847. [DOI] [PubMed] [Google Scholar]
- 29.Leigh, I.M., H. Navsaria, P.E. Purkis, I.A. McKay, P.E. Bowden, and P.N. Riddle. 1995. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol. 133:501–511. [DOI] [PubMed] [Google Scholar]
- 30.Parrish-Novak, J., W. Xu, T. Brender, L. Yao, C. Jones, J. West, C. Brandt, L. Jelinek, K. Madden, P.A. McKernan, et al. 2002. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J. Biol. Chem. 277:47517–47523. [DOI] [PubMed] [Google Scholar]
- 31.Wang, M., Z. Tan, R. Zhang, S.V. Kotenko, and P. Liang. 2002. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J. Biol. Chem. 277:7341–7347. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg, H., D. Conklin, W.F. Xu, A. Grossmann, T. Brender, S. Carollo, M. Eagan, D. Foster, B.A. Haldeman, A. Hammond, et al. 2001. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 104:9–19. [DOI] [PubMed] [Google Scholar]
- 33.Kauffman, C.L., N. Aria, E. Toichi, T.S. McCormick, K.D. Cooper, A.B. Gottlieb, D.E. Everitt, B. Frederick, Y. Zhu, M.A. Graham, et al. 2004. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J. Invest. Dermatol. 123:1037–1044. [DOI] [PubMed] [Google Scholar]
- 34.Lew, W., A.M. Bowcock, and J.G. Krueger. 2004. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 25:295–305. [DOI] [PubMed] [Google Scholar]
- 35.Bowcock, A.M. 2004. Psoriasis genetics: the way forward. J. Invest. Dermatol. 122:xv–xvii. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 37.McAllister, F., A. Henry, J.L. Kreindler, P.J. Dubin, L. Ulrich, C. Steele, J.D. Finder, J.M. Pilewski, B.M. Carreno, S.J. Goldman, et al. 2005. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 175:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henseler, T., and E. Christophers. 1995. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 32:982–986. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi, M., F. Kokubu, S. Matsukura, K. Ieki, M. Odaka, S. Watanabe, S. Suzuki, M. Adachi, and S.K. Huang. 2003. Induction of C-X-C chemokines, growth-related oncogene alpha expression, and epithelial cell-derived neutrophil-activating protein-78 by ML-1 (interleukin-17F) involves activation of Raf1-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase 1/2 pathway. J. Pharmacol. Exp. Ther. 307:1213–1220. [DOI] [PubMed] [Google Scholar]
- 40.Laan, M., Z.H. Cui, H. Hoshino, J. Lotvall, M. Sjostrand, D.C. Gruenert, B.E. Skoogh, and A. Linden. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347–2352. [PubMed] [Google Scholar]
- 41.Kao, C.Y., Y. Chen, P. Thai, S. Wachi, F. Huang, C. Kim, R.W. Harper, and R. Wu. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173:3482–3491. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb, A.B., F. Chamian, S. Masud, I. Cardinale, M.V. Abello, M.A. Lowes, F. Chen, M. Magliocco, and J.G. Krueger. 2005. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 175:2721–2729. [DOI] [PubMed] [Google Scholar]
- 43.Gallagher, G., J. Eskdale, W. Jordan, J. Peat, J. Campbell, M. Boniotto, G.P. Lennon, H. Dickensheets, and R.P. Donnelly. 2004. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int. Immunopharmacol. 4:615–626. [DOI] [PubMed] [Google Scholar]
- 44.Su, Z.Z., M.T. Madireddi, J.J. Lin, C.S. Young, S. Kitada, J.C. Reed, N.I. Goldstein, and P.B. Fisher. 1998. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc. Natl. Acad. Sci. USA. 95:14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soo, C., W.W. Shaw, E. Freymiller, M.T. Longaker, C.N. Bertolami, R. Chiu, A. Tieu, and K. Ting. 1999. Cutaneous rat wounds express c49a, a novel gene with homology to the human melanoma differentiation associated gene, mda-7. J. Cell. Biochem. 74:1–10. [PubMed] [Google Scholar]
- 46.Wolk, K., S. Kunz, E. Witte, M. Friedrich, K. Asadullah, and R. Sabat. 2004. IL-22 increases the innate immunity of tissues. Immunity. 21:241–254. [DOI] [PubMed] [Google Scholar]
- 47.Boniface, K., F.X. Bernard, M. Garcia, A.L. Gurney, J.C. Lecron, and F. Morel. 2005. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 174:3695–3702. [DOI] [PubMed] [Google Scholar]
- 48.Wolk, K., E. Witte, E. Wallace, W.D. Docke, S. Kunz, K. Asadullah, H.D. Volk, W. Sterry, and R. Sabat. 2006. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36:1309–1323. [DOI] [PubMed] [Google Scholar]
- 49.Schon, M., D. Denzer, R.C. Kubitza, T. Ruzicka, and M.P. Schon. 2000. Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice. J. Invest. Dermatol. 114:976–983. [DOI] [PubMed] [Google Scholar]
- 50.Nagalakshmi, M.L., E. Murphy, T. McClanahan, and R. de Waal Malefyt. 2004. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int. Immunopharmacol. 4:577–592. [DOI] [PubMed] [Google Scholar]
- 51.Abrams, J.S., M.G. Roncarolo, H. Yssel, U. Andersson, G.J. Gleich, and J.E. Silver. 1992. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol. Rev. 127:5–24. [DOI] [PubMed] [Google Scholar]
- 52.Krane, J.F., D.P. Murphy, D.M. Carter, and J.G. Krueger. 1991. Synergistic effects of epidermal growth factor (EGF) and insulin-like growth factor I/somatomedin C (IGF-I) on keratinocyte proliferation may be mediated by IGF-I transmodulation of the EGF receptor. J. Invest. Dermatol. 96:419–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.