Abstract
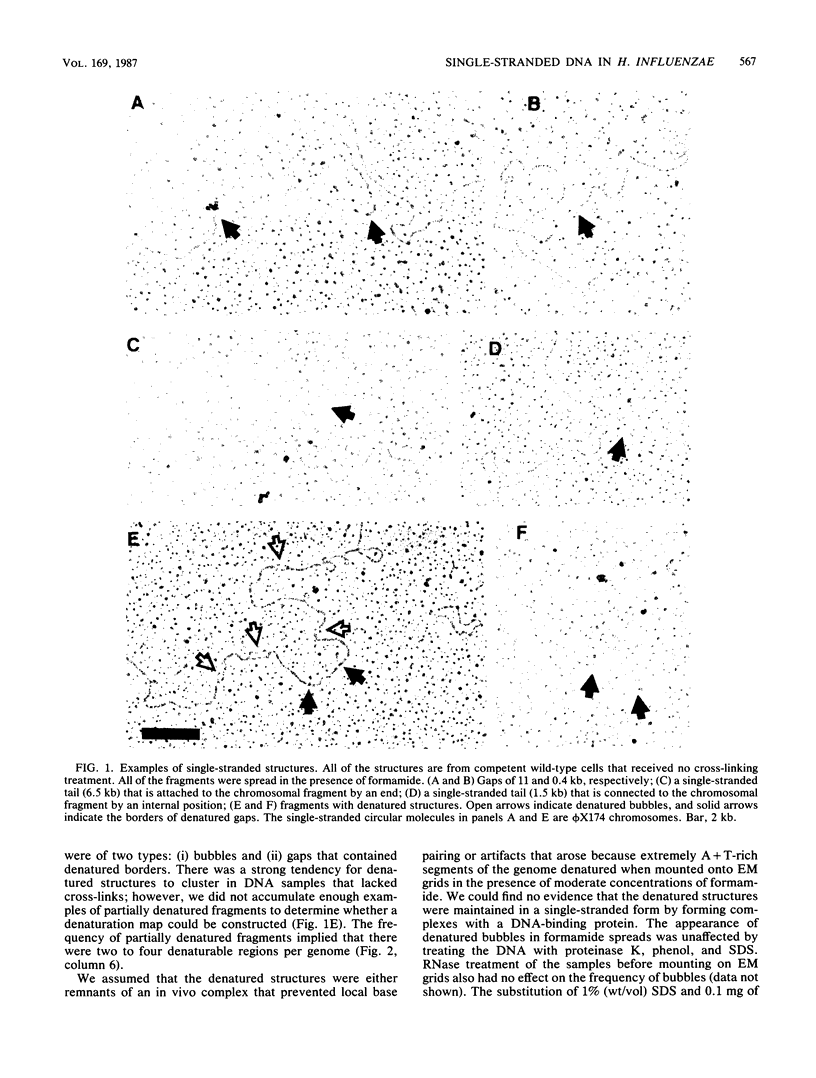
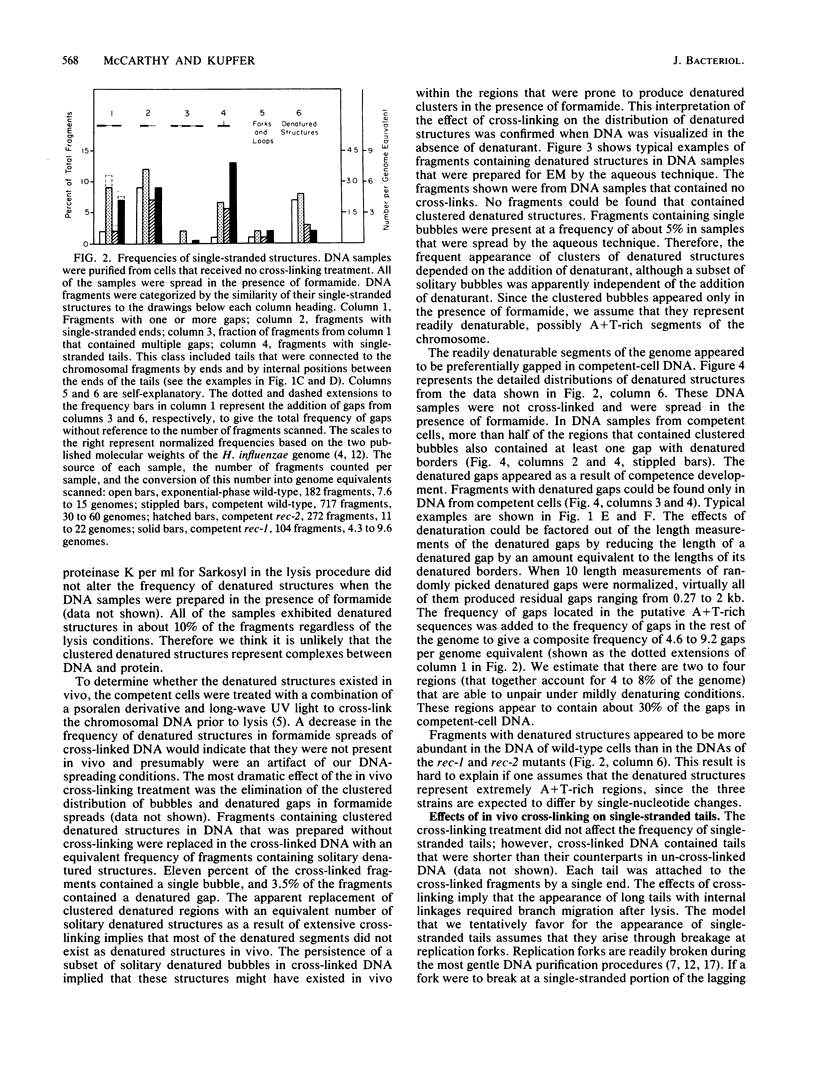
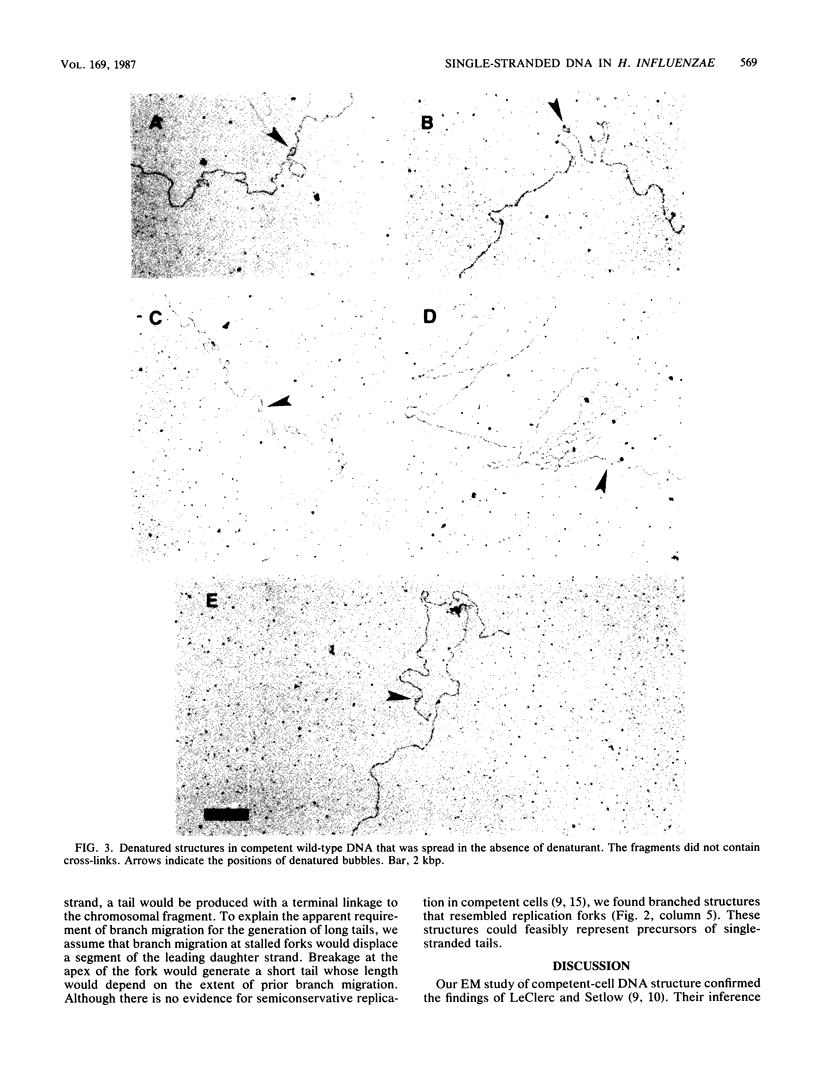
Chromosomal DNAs from exponential-phase and competent cells of Haemophilus influenzae were examined by electron microscopy to determine whether the chromosome undergoes structural changes during competence development. Single-stranded gaps and single-stranded tails formed in chromosomal DNA during competence development. The generation of gaps was dependent on the rec-2 function. Since the rec-2 mutant is defective in the translocation of donor DNA, it was inferred that the gaps were involved in the translocation step of transformation. The generation of single-stranded tails was independent of the rec-1 and rec-2 genes. Therefore, these structures were assumed to play no direct role in the interaction of donor and recipient DNAs during transformation. Gaps were preferentially associated with a readily denaturable, possibly A + T-rich fraction of the genome. This finding raised the possibility that hot spots for transformation might be associated with A + T-rich DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Barouki R., Smith H. O. Reexamination of phenotypic defects in rec-1 and rec-2 mutants of Haemophilus influenzae Rd. J Bacteriol. 1985 Aug;163(2):629–634. doi: 10.1128/jb.163.2.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie K. L., Setlow J. K. Transformation-defective strains of Haemophilus influenzae. Nat New Biol. 1971 Jun 9;231(23):177–179. doi: 10.1038/newbio231177a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C., RAY D. S. ISOLATION OF THE GROWING POINT IN THE BACTERIAL CHROMOSOME. Proc Natl Acad Sci U S A. 1964 Jul;52:125–132. doi: 10.1073/pnas.52.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Setlow J. K. Single-strand regions in the deoxyribonucleic acid of competent Haemophilus influenzae. J Bacteriol. 1975 Jun;122(3):1091–1102. doi: 10.1128/jb.122.3.1091-1102.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHATTIE L. A., BERNE K. I., THOMAS C. A., Jr ELECTRON MICROSCOPY OF DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:648–649. doi: 10.1016/s0022-2836(65)80019-5. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Minner C., Bernstein H., Bernstein C. DNA elongation rates and growing point distributions of wild-type phage T4 and a DNA-delay amber mutant. J Mol Biol. 1976 Oct 5;106(4):963–981. doi: 10.1016/0022-2836(76)90346-6. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Huang P. C. Identification of competence-repressing factors during log-phase growth of Haemophilus influenzae. J Bacteriol. 1972 Feb;109(2):560–564. doi: 10.1128/jb.109.2.560-564.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J. J., Habersat M. Synchronous division and rates of macromolecular synthesis in Haemophilus influenzae competent for genetic transformation. J Bacteriol. 1978 Sep;135(3):961–967. doi: 10.1128/jb.135.3.961-967.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A., Poonian M., Bartl P. Concatemers in DNA replication: electron microscopic studies of partially denatured intracellular lambda DNA. J Mol Biol. 1972 Mar 14;64(3):541–550. doi: 10.1016/0022-2836(72)90081-2. [DOI] [PubMed] [Google Scholar]