Abstract
An important feature of atopic asthma is the T cell–driven late phase reaction involving transient bronchoconstriction followed by development of airways hyperresponsiveness (AHR). Using a unique rat asthma model we recently showed that the onset and duration of the aeroallergen-induced airway mucosal T cell activation response in sensitized rats is determined by the kinetics of functional maturation of resident airway mucosal dendritic cells (AMDCs) mediated by cognate interactions with CD4+ T helper memory cells. The study below extends these investigations to chronic aeroallergen exposure. We demonstrate that prevention of ensuing cycles of T cell activation and resultant AHR during chronic exposure of sensitized rats to allergen aerosols is mediated by CD4+CD25+Foxp3+LAG3+ CTLA+CD45RC+ T cells which appear in the airway mucosa and regional lymph nodes within 24 h of initiation of exposure, and inhibit subsequent Th-mediated upregulation of AMDC functions. These cells exhibit potent regulatory T (T reg) cell activity in both in vivo and ex vivo assay systems. The maintenance of protective T reg activity is absolutely dependent on continuing allergen stimulation, as interruption of exposure leads to waning of T reg activity and reemergence of sensitivity to aeroallergen exposure manifesting as AMDC/T cell upregulation and resurgence of T helper 2 cytokine expression, airways eosinophilia, and AHR.
Aeroallergen challenge of atopic asthmatics triggers an initial short-lived bronchoconstriction reaction mediated predominantly by IgE-armed mast cells, followed by a later and more sustained reaction associated with T helper (Th)2 cell activation in the airway mucosa (1). Indirect evidence (2) suggests that this late asthmatic reaction may be elicited directly by the products of activated allergen-reactive T cells. Ensuing damage to airway mucosal tissues during this response, in particular by infiltrating eosinophils activated via Th2 cell–derived cytokines such as IL-5 (1), results in many asthmatics in the subsequent development of airways hyperresponsiveness (AHR) to inhaled irritants. The duration of AHR after asthma exacerbation is highly variable and is one of the most important determinants of disease severity. In the most severe forms of chronic asthma, AHR can develop into an essentially continuous state, resulting in markedly reduced respiratory function.
The most important aeroallergens involved in asthma pathogenesis are ubiquitous “indoor” allergens, which are present continuously in the airborne environment (3). This begs the questions of why only a relatively small subset of atopics sensitized to this class of allergens manifest wheezing symptoms and/or AHR (4), and why individual late phase reactions (LPRs) typically terminate within a few hours.
Recent reports have highlighted the potentially important role of CD4+CD25+ T reg cells in control of T cell–mediated inflammation (5). There are two major categories of T reg cells. First, the naturally occurring thymically derived CD4+CD25+Foxp3+ cells and, second, antigen-specific T reg cells, which can be induced in vitro or in vivo under particular conditions of antigenic stimulation. Antigen-induced T reg cells include a heterogeneous mix of phenotypes and include Th1 and Th2 types, which produce the antiinflammatory cytokines IL-10 and/or TGF-β (5). Recent studies suggest that naturally occurring T reg cells and antigen-induced IL-10–secreting T reg cells have a role in protecting against human allergic disease. A mutation in the gene encoding Foxp3, which confers suppressive activity on T reg cells, results in IPEX syndrome, which involves multiple pathologies including allergic symptoms (5). Additionally, the frequency of allergen-specific IL-10–secreting T reg cells is reportedly reduced in atopics relative to nonatopics (6), and therapies beneficial for treatment of asthma/allergy (glucocorticoids and immunotherapy) have been shown to induce antiinflammatory cytokine production by T cells and to modify T reg cells function (5, 7).
In animal models, several lines of indirect evidence also argue for a role for T reg cells in control of asthma/allergy. First, naturally occurring T reg cells have been shown to limit experimental airway inflammation and to modulate allergen-driven AHR (8). A similar role has been proposed for IL-10 and TGF-β producing adaptive T reg cells based on studies in mouse asthma models (9–14). However, a limitation in the majority of these studies has been their focus on T reg cells generated in vitro or at systemic lymphoid sites such as spleen, and as such they do not address issues relating directly to microenvironmental regulation at the site of aeroallergen challenge. A more restricted series of studies (9, 11, 12) have attempted to bridge this gap by focusing on T reg cell–associated cellular functions in whole lung digests or in lymph nodes draining the peripheral lung as surrogate models for events occurring at the asthma lesional site in the mucosa of the conducting airways. As we have reported recently (15, 16), there are considerable differences in the functional phenotype of cell populations involved in control of T cell activation in these different tissue compartments, which question the validity of such extrapolations.
This study instead focuses directly on effector and regulatory cell populations extracted from the conducting airway mucosa itself and its major draining lymph nodes (DLNs), avoiding contamination with cells from other parts of the lung which are not directly involved in the asthma disease process. We demonstrate that the restoration and subsequent maintenance of immunological and physiological homeostasis in the airway mucosa after an experimental asthma exacerbation is a dynamic allergen-driven process involving interplay between allergen-specific Th2 cells, airway mucosal dendritic cells (AMDCs), and CD4+CD25+ T reg cells. Moreover, ongoing protection of airway tissues in sensitized animals via T reg cells relies absolutely on continuation of allergen exposure, as withdrawal of stimulation restores susceptibility to the Th2-dependent AHR-inducing effects of aeroallergen challenge.
RESULTS
These studies used a rat model developed in our laboratory to study cellular events in the airway mucosa triggered by exposure of sensitized animals to aerosolized OVA, focusing in particular on the initiation phase of the T cell activation response after challenge. Our forerunner studies (15) have demonstrated that OVA aerosol exposure of sensitized rats leads to concomitant up-regulation of CD86 expression and APC activity within the AMDC compartment by 2 h after exposure, and is followed by local activation of Th cells and development of AHR to inhaled methacholine (MCh), which is a common feature of asthma exacerbation in humans. This experimental system was further modified in this study to encompass repeated (daily) OVA aerosol challenge to model the effects of exposure of atopic asthmatics to aeroallergens present continuously within the airborne environment. Fig. 1 illustrates the density and phenotype of AMDCs and adjacent T cells in the tracheal mucosa, assessed at 2 h after the first OVA-aerosol exposure of sensitized animals, and ensuing responses in other animals exposed repeatedly for up to 10 consecutive days. As shown in Fig. 1, A and B, the number of TcRαβ+ cells and those expressing CD25 increase markedly during the first two days of exposure, and remain elevated thereafter as exposure continues. Examination of AMDCs from the same tracheal samples (gating strategy shown in Fig. 1 E) revealed a qualitatively different response. Notably, AMDCs increased transiently in number within the 24 h after initiation of aerosol exposure, subsequently returning slowly to baseline (Fig. 1 C), whereas the proportion of AMDCs expressing CD86 increased explosively after a single exposure (Fig. 1, D, H, and J) as reported previously (15). However, unexpectedly, and in marked contrast to that seen with the T cells, this latter AMDC response disappeared entirely after 24 h and could not be elicited again despite repeated exposure (Fig. 1, D, K, and L). Fig. 1 (F–L) illustrates this transient up-regulation of CD86 on AMDCs, showing representative FACS plots of CD86 expression (Fig. 1, G, J, and L) and isotype control mAb OX21 binding (Fig. 1, F, H, and K) for naive control animals (Fig. 1, F and G) and for animals which have been sensitized and exposed to either a single (OVA x1; Fig. 1, H and J) or multiple (mOVA; Fig. 1, K and L) OVA aerosols.
Figure 1.
Cellular responses in the tracheal mucosa in sensitized animals during OVA aerosol exposure. Cell numbers as percentage of tracheal digest population: (A) TcRαβ+; (B) CD25+ TcRαβ+; (C) % MHC Class II+; (D) CD86+ AMDC. Data shown as mean ± SE for days 0–5 and 10 inclusive (n ≥ 3 separate experiments, using pools of >10 animals each), or representative of at least two experiments for days 6–9. Tracheal digests were prepared 2 h after OVA aerosol exposure on designated day. After exposure > day 0: *, P < 0.05; **, P <0.01; ***, P < 0.001; ****, P < 0.0001. (E) Gate settings used for selection of high MHC class II+ AMDCs (R1) in digest after macrophage and B cell depletion as per reference 15. (F–L) Staining profiles of MHC class II versus isotype control mAb OX21 (F, H, and K) or CD86 (G, J, and L) surface expression on tracheal digest AMDC (as defined in R1) after either no (F and G), a single (H and J), or multiple (mOVA; K and L) OVA aerosol exposures of presensitized animals. Data shown are representative FACS plots from a series of at least three separate experiments.
We next questioned whether maintenance of resistance on the part of AMDCs to the CD86-inductive effects of OVA required continuation of allergen exposure. To address this issue we compared groups of OVA-sensitized animals exposed repeatedly to cycles of multiple (5–8) OVA aerosols with intervening 14-d exposure-free rest periods. Fig. 2 illustrates data representative of this experimental series. Consistent with the results in Fig. 1, the markedly up-regulated CD86 response in the airway mucosa preceding the initial LPR was shut down by repeated exposure (Fig. 2 A), whereas total and CD25+ T cell numbers remained elevated (Fig. 2, B and C). The 14-d rest period was accompanied by a decline in levels of local T cells toward baseline. Rechallenge with OVA aerosol at the end of the rest period elicited a vigorous but again transient CD86 response (Fig. 2 A) equivalent to that observed during the initial challenge cycle, accompanied again by a persistent T cell response (Fig. 2, B and C, and Fig. 1). In a single series of follow-up experiments we have also extended observations to encompass a third post-rest exposure cycle, again demonstrating comparably rapid CD86 up-regulation on AMDCs at 24 h after reinitiation of aerosol exposure (unpublished data).
Figure 2.
Fluctuations in tracheal mucosal cellular responses during repeated versus intermittent aerosol exposure. Animals were exposed to single (OVA × 1) versus multiple (mOVA) aerosol exposures, and rested for 14 d ([r14]) before a second exposure cycle. Data shown are representative analyses of airway mucosa (tracheal digest for groups of n ≥ 15) from one of two time course studies illustrating: (A) % CD86+ AMDCs in tracheal digest at 2 h after OVA aerosol exposure as indicated; (B) TCRαβ+ cells as the percentage of tracheal digest in the same preparations; (C) CD25+ TCRαβ+ cells as the percentage of tracheal digest in the same preparations.
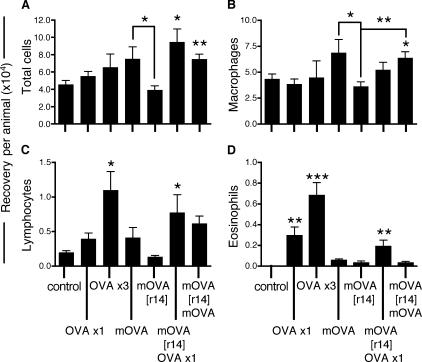
These cellular responses in the airway mucosa were mirrored in the BAL (Fig. 3) by biphasic changes in total cell numbers (A) and in populations of BAL macrophages (B), lymphocytes (C) and eosinophils (D) in response to comparable cycles of repeated exposure/rest/rechallenge. Serum OVA-specific IgE titers followed the same cyclic pattern (unpublished data).
Figure 3.
BAL responses in OVA-sensitized animals during repeated OVA exposure and rest/challenge cycles. After the first cycle of repeated OVA exposures (1, 3, or multiple), animals were rested for 14 d ([r14]) before reexposure to either a single aerosol or multiple aerosols; BAL was performed at the time points indicated. Cell numbers per BAL per animal: (A) total number of cells recovered; (B) macrophages per BAL per animal; (C) lymphocytes per BAL per animal; (D) eosinophils per BAL per animal. Data are from a sequential series and are expressed as mean ± SE (n = 5 per group) taken at 24 h after final OVA aerosol exposure. After exposure > day 0 or as indicated by adjoining lines: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As shown in Fig. 4 A, OVA-sensitized animals exhibited marked AHR at 24 h after the initial OVA aerosol challenge compared with naive controls exposed to the same OVA aerosol protocol, as illustrated by the reduced MCh dose required to increase airway resistance to ≥200% saline control (EC200). Consistent with the cellular responses, further continuation of exposure resulted in a return to baseline levels of MCh responsiveness (illustrated in Fig. 4 B by the first mOVA group shown), inferring the onset of a form of tolerance. Moreover, withdrawal of exposure for 14 d restored sensitivity to aerosol challenge, as evidenced in this case by reestablishment of AHR to MCh in the second exposure cycle (Fig. 4 B). In parallel with the pattern observed during the initial cycle of multiple OVA aerosol exposures this resurgence in AHR was again transient, returning to baseline levels when the animals were exposed to multiple OVA aerosols.
Figure 4.
AHR to inhaled MCh. (A) AHR after exposure to aerosolized OVA of nonimmunized versus OVA-sensitized animals. Shown are representative dose–response curves to MCh challenge measured at 24 h after a single OVA aerosol. Note the shift in the curve of the sensitized animals displaying marked AHR. OVA ×1 > naive control; *, P = 0.003 by student's t test. (B) EC200 Raw (the concentration of MCh required to double airway resistance) was determined in OVA-sensitized animals after one or multiple aerosol challenges as detailed in reference 15 and in groups of animals which had undergone the challenge/rest/rechallenge protocol. At each observation point, individual sets of naive animals exposed to a single OVA aerosol (negative/baseline controls) and sensitized animals exposed to a single OVA aerosol (positive AHR controls) were included. Data shown are expressed relative to naive controls (mean EC200 4.49 ± 0.41 mg/ml). Figures represent mean (n ≥ 8 individual animals for each group) absolute reduction or increase in EC200 (in mg/ml MCh) relative to the mean control value (set at zero). OVA ×1 > mOVA, P, < 0.001; OVA ×1 > mOVA [r14], P = 0.021; OVA ×1 > mOVA, [r14], OVA ×1, P = 0.738; OVA ×1 > mOVA [r14] mOVA, P = 0.041, by ANOVA (Holm-Sidak after hoc test).
The experiments in Fig. 5 illustrate aspects of the DC regulatory functions of cells present in the tracheal mucosa of multiaerosol exposed animals, using an in vitro model developed in our early studies (15). The top panel illustrates changes in in vitro CD86 expression on tracheal DC in the presence of different cell populations from the same tissue site, and the bottom panel displays paired FACS plots from selected cultures. As shown previously (15), the OVA-aerosol–induced up-regulation of CD86 on resting AMDCs can be mimicked in vitro by brief co-culture of naive control AMDCs with OVA plus a source of OVA-specific Th cells (Fig. 5, culture A vs. culture C), modeling the effects of cognate interactions between transiting memory T cells and antigen-bearing resident AMDCs within the tracheal mucosa during the first few hours after allergen challenge. The introduction into these cultures of unfractionated tracheal mucosal cells (culture D) or purified tracheal TcRαβ+ cells (culture J) from animals exposed on 8 consecutive days to OVA, markedly reduced Th cell–induced CD86 up-regulation on AMDCs. This inhibitory activity was not present in tracheal cell preparations from unexposed animals (unpublished data). Prior depletion of the tracheal cell preparation of TCRαβ+ cells (culture E), CD4+ cells (culture F), or CD25+ cells (culture G) before introduction into the culture system abrogated this inhibitory activity, suggesting the presence of functional CD4+CD25+ T reg cells. Additionally, we observed that depletion of MHC II+ cells from the tracheal preparation abolished this regulatory activity and instead promoted the CD86-inductive effects of OVA-specific T cell–DC interaction (culture H). This suggested that depletion of MHC class II+ cells had unmasked an additional cellular source of CD86-inductive signals capable of driving AMDC activation/maturation. Supporting this possibility, follow-up experiments performed as per culture H but without the inclusion of the exogenous OVA-specific Th cells demonstrated comparable levels of ensuing CD86 up-regulation on AMDC (unpublished data).
Figure 5.
Modulation of Th cell–induced CD86 expression on AMDCs by immunoregulatory cells from the tracheal mucosa. Purified AMDCs from naive animals were cultured alone (A) or in the presence of T cell–enriched OVA immune lymph node cells (C) at a density of 20:1 (T cells to DCs). 3 h after addition of 50 μg/ml OVA, CD86 staining was examined and MFI and proportion of AMDCs expressing CD86 determined after accounting for nonspecific binding of the isotype control (C versus A). Parallel cultures of DC/OVA-specific T cells/OVA were further supplemented with tracheal digest cells from sensitized animals exposed to eight OVA aerosols at a ratio of 1:4 (tracheal cells to DCs), either unfractionated (B and D) or after depletion of cells expressing TcRαβ (E), CD4 (F), IL-2R (G), or MHC class II (H); resulting CD86 expression at 3 h was then determined and normalized against that in the positive control culture (C). Note that cultures depleted of MHC class II+ cells gave identical results with/without addition of exogenous OVA (+/−). Purified TcRαβ+ tracheal cells selected by multiparameter sorting (culture J) were added to other cultures at a ratio of 1:2 (TcRαβ+ to DCs), and resulting CD86 expression levels again normalized against culture C. Data shown are group means ± SE, except for culture J (representative of two experiments; individual group sizes ≥20). CD86 expression on AMDCs significantly different from culture C; *, P < 0.0001 by paired t test; CD86 expression significantly different from culture D; *, P < 0.1; **, P < 0.05; ***, P < 0.01 by paired t test.
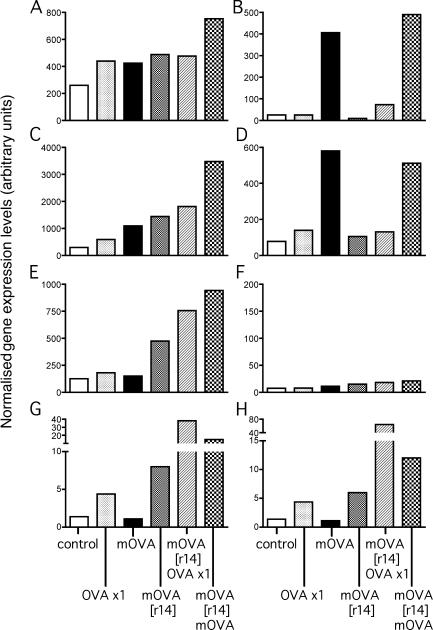
Fig. 6 (A–H) illustrates the results of quantitative RT-PCR analyses of expression of selected marker genes in CD4+CD25+ cells sorted from tracheal mucosa of resting OVA-sensitized controls and those exposed to single or multiple OVA aerosols over two exposure cycles. Foxp3 and LAG-3 expression were not seen in CD4+CD25− or CD4−CD25− populations sorted from the same digests (unpublished data). Of note is the stepwise up-regulation in tracheal mucosal CD4+CD25+ of the transcriptional regulator Foxp3 (Fig. 6 A) which is a marker of T reg cells activity (5), over the course of the successive rechallenge protocols. Up-regulation of the additional T reg cell–associated markers LAG3 (Fig. 6 B; reference 17) CTLA4 (Fig. 6 C; reference 18) and IL-10 (Fig. 6 D) was also observed in the tracheal CD4+CD25+ population from animals exposed to multiple aerosols, both before and after the rest period. Levels of TGF-β (Fig. 6 F) remained consistently low in all groups. We additionally compared levels of expression of the Th2-trophic transcription factor GATA-3 and its Th1 counterpart T-bet. This mucosal CD4+CD25+ population was enriched for GATA-3–expressing cells and even higher expression was observed in the overall unfractionated mucosal TCRαβ+ population (unpublished data), indicating strong Th2 polarity, reflecting the Th2-skewing properties of the OVA priming protocol used. Levels of T-bet were consistently low for all preparations examined (<20 arbitrary units; unpublished data). The Th2 genes typically associated with allergic airways inflammation, IL-4, IL-5, and IL-13, were also examined. A small transient spike in expression of IL-5 (Fig. 6 G) and IL-13 (Fig. 6 H) was observed after a single OVA aerosol challenge of sensitized animals. Of note, this response was seen to be substantially boosted after the rest period and a single further OVA aerosol challenge. IL-4 expression was not detected in any of the preparations examined (<10 arbitrary units; unpublished data). Expression levels of CD127 were also found to be consistently low (<10 arbitrary units; unpublished data).
Figure 6.
qRT-PCR profiling of tracheal cells. (A–H) Data from a representative experiment shows relative expression levels of Foxp3 (A), LAG-3 (B), CTLA4 (C), IL-10 (D), GATA-3 (E), TGF-β (F), IL-5 (G), and IL-13 (H) genes in sorted CD4+CD25+ tracheal cells from groups of ≥15 OVA-primed animals exposed to 0, 1, or multiple aerosol exposures (mOVA) over successive exposure cycles as indicated. Data are expressed as arbitrary RT-PCR units derived by normalization against the housekeeping gene Eef1α1.
Next, we identified the T reg cells in the airway mucosa via immunofluorescent staining of tracheal digest preparations, as illustrated in Fig. 7 (A–J) by representative FACS plots from a series of experiments. The CD25+ gated population (Fig. 7, A–H) in tracheal digest preparations from sensitized controls or animals sensitized and exposed to a single or multiple OVA aerosols are shown, depicting expression of CD4 and Foxp3 (Fig. 7, B, E, and H) relative to isotype controls (Fig. 7, A, D, and G). Surface expression of CD4 and CD25 on tracheal digest cells gated for Foxp3 are shown for the same groups of animals (Fig. 7, C, F, and J). We also determined that these Foxp3+ cells expressed surface CD45RC illustrative of the memory phenotype in the rat but did not express CD103 or CD127 (unpublished data). The total number of Foxp3+ cells on a per trachea basis as determined via intracellular staining is shown in Fig. 7 K. Of particular note is the large scale increase in the total number of Foxp3+ cells per trachea after the initial cycle of repeated OVA aerosol exposures compared with controls or animals having experienced only a single exposure. The maintenance of elevated numbers of Foxp3+ cells in the airway mucosa is dependent on continuing OVA aerosol exposure as animals receiving only a single aerosol returned to baseline levels over the subsequent 6 d (unpublished data). Moreover, after cessation of exposure of the mOVA group and the ensuing rest period, the number of Foxp3+ cells per trachea also returned to baseline levels (Fig. 7 K). It is of interest to note that despite the major reduction in Foxp3+ population size in this group, the cells that persisted maintained elevated levels of Foxp3 gene expression (Fig. 6 A). A further cycle of multiple OVA exposures resulted in a resurgence in the numbers of Foxp3+ cells in the tracheal mucosa. PCR analysis of sorted CD4+CD25+ T reg cells from additional groups of animals undergoing this second cycle of challenge after resting indicated that this resurgent T reg cells response was accompanied by additional up-regulation of Foxp3, LAG-3, CTLA-4, and IL-10 expression (Fig. 6, A–D). It is noteworthy that although the numerical increase in Foxp3+ cell numbers in the second exposure cycle was less than that observed in the first cycle (Fig. 7 K), a reciprocal pattern was observed with respect to Foxp3 gene expression (Fig. 6 A). As noted already these cells express the memory phenotype, and these observations may collectively indicate progressive selection for smaller numbers of more potent T reg cells over time, but additional comparative experiments will be required to test this possibility.
Figure 7.
Characterization of mucosal T reg cells. (A–J) Representative staining profiles of surface CD4 expression and intracellular Foxp3 (B, E, and H) on pooled tracheal digest preparations from groups of ≥15 animals gated for CD25 from control animals (A and B) and sensitized animals exposed to a single (B and E) or multiple (G and H) OVA aerosols. Isotype controls are shown in (A, D, and G). Tracheal digest cells gated for Foxp3 from the same groups of animals express surface CD4 and CD25 as illustrated in C, F, and J, respectively. (K) Total number of Foxp3 cells per trachea in OVA-sensitized animals during repeated OVA aerosol exposure/rest/reexposure cycles. Data are representative (n ≥ 2) experiments at each time point and derived from intracellular staining profiles and total digest yields after accounting for isotype control staining. (L) Mean proliferative responses of LN cells obtained from OVA-sensitized animals in the presence or absence of CD4+CD25+ cells derived from the tracheal mucosa of sensitized animals exposed to multiple OVA aerosols. Data shown are from one representative experiment CD4+CD25+ were added at ratios of 1:10 (LN to CD4+CD25+, shaded bars) and 1:20 (checked bars). Addition of CD4+CD25+ cells from unexposed controls (1:10) was without effect (not shown). (M) Proliferative responses of LN CD4+ T cells from OVA/CFA-sensitized animals in the presence of OVA (50 μg/ml) and DCs sorted from tracheal digest of naive animals (▪) or animals sensitized and exposed to a single (◯) or multiple (•) OVA aerosols. Proliferation is shown as mean CPM of triplicate cultures and is representative of at least two individual experiments. (N) Proliferative responses of LN CD4+ T cells from OVA/CFA-sensitized animals in the presence of AMDCs sorted from animals sensitized and exposed to multiple OVA aerosol (no additional OVA added to culture). Proliferation in L, M, and N is shown as mean CPM of triplicate cultures and is representative of two or three experiments in each case. *, P < 0.001; **, P < 0.0005.
The in vitro suppressive activity of sorted CD4+CD25+ cells from the tracheal mucosa of animals exposed to multiple consecutive OVA aerosols is formally demonstrated in Fig. 7 L, which shows >80% suppression of the proliferative response of OVA-primed LN cells at T reg cells to LN ratios of 1:10 and 1:20. In a further experiment (unpublished data), further titration of the CD4+CD25+ population revealed that a 1:40 ratio of T reg cells to OVA-primed LN cells still achieved >50% suppression.
To gain additional insight into the nature of the in vivo down-regulation of AMDC activation (Fig. 1 C) by T reg cells induced as a result of multiple OVA aerosol exposure, we next sorted AMDC populations from these animals and determined their in vitro APC capacity, once removed from the suppressive milieu of the repeatedly exposed airway mucosa. Fig. 7 (M and N) illustrate representative proliferative responses of OVA-primed CD4+ T cells in the presence of various AMDC populations with and without the addition of exogenous OVA. Fig. 7 M demonstrates that with the addition of OVA to the cultures, AMDCs isolated from naive animals or those previously sensitized to OVA and exposed to either a single or multiple OVA aerosols have comparable APC activity. More importantly, AMDCs derived from animals sensitized and exposed to multiple OVA aerosols were capable of driving an OVA-specific proliferation without the addition of OVA to the cultures. This finding mirrors our original report on the T memory cell–stimulating activity of AMDCs harvested from OVA- sensitized animals after a single OVA aerosol exposure (15).
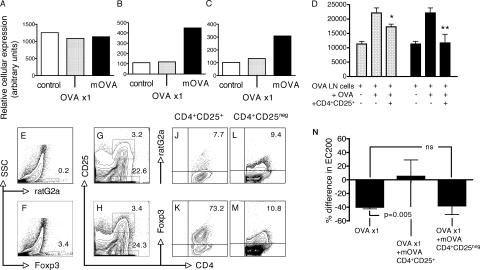
Finally, we addressed questions relating to the presence and characteristics of T reg cells in the airway DLN. The gene expression profiles of CD4+CD25+ cells derived from DLN of controls (sensitized only), and animals sensitized and exposed to a single or multiple OVA aerosols, is shown in Fig. 8 (A–C). As in tracheal CD4+CD25+ populations (Fig. 6 A) Foxp3 expression in DLN CD4+CD25+ cells (Fig. 8 A) remained relatively consistent during this initial cycle of exposure, whereas other T reg cells markers LAG-3 (Fig. 8 B) and IL-10 (Fig. 8 C) were unequivocally elevated as a consequence of multiple OVA aerosol challenge. TGF-β expression in this cell population was minimal and independent of the number of OVA aerosol exposures (unpublished data) consistent with the pattern in CD4+CD25+ cells from the airway mucosa (Fig. 6 F). GATA-3 expression, but not T-bet, was again elevated in all samples (unpublished data) as per the airway mucosa, whereas Th2 cytokine expression (IL-4, IL-5, and IL-13) was not detected. The in vitro suppressive activity of CD4+CD25+ cells sorted from the DLN is demonstrated in Fig. 8 D. In particular, DLN cells from mOVA animals achieve close to complete suppression of Th cell proliferation at a 1:20 ratio. Characteristic staining profiles of DLN cell preparations derived from animals sensitized to OVA and exposed to multiple OVA aerosols are shown in Fig. 8 (E–M). Relevant Foxp3 expression data for total DLN is shown in Fig. 8 (E and F). Gates used to define populations of CD4+CD25+ and CD4+CD25− populations within the total DLN population are illustrated in Fig. 8 (G and H). Foxp3 expression versus isotype control for the CD4+CD25+ population is shown in Fig. 8 (J and K) and Fig. 8 (L and M) for the CD4+CD25− population. Note the predominant localization of Foxp3 expression in the CD4+CD25+ population. These two populations of cells were sorted from airway DLN of other animals exposed to multiple OVA aerosols and adoptively transferred into sensitized animals. 24 h after aerosol exposure of the recipients' airways, responsiveness to MCh was determined and compared with that of aerosol-exposed sensitized and naive animals (Fig. 8 N). CD4+CD25+ T reg cells derived from the mOVA group protected against development of AHR induced in sensitized animals by a single OVA aerosol challenge (Fig. 8 N), reproducing the effects observed with multiple OVA aerosol exposures (Fig. 4).
Figure 8.
T reg cell–mediated changes in AHR. (A–C) Data from a representative experiment using group sizes of n = 5 showing relative gene expression levels of Foxp3 (A), LAG-3 (B), and IL-10 (C) in sorted CD4+CD25+ cells from airway DLNs of sensitized animals exposed to 0, 1, or multiple OVA aerosols as indicated. Data are expressed as arbitrary RT-PCR units derived by normalization against the housekeeping gene Eef1α1. (D) Proliferative responses of OVA-primed LN cells obtained from OVA/CFA-sensitized animals in the presence of CD4+CD25+ cells isolated from airway DLN preparations of sensitized animals exposed to a single (dotted bars), or multiple (shaded bars) OVA aerosols. CD4+CD25+ cells were added at ratios (CD4+CD25+ to LN cells) of 1:20. Proliferation is shown as mean CPM from triplicate cultures. Addition of CD4+CD25+ cells from unexposed controls was without effect (not shown). Data are representative of three separate experiments. *, P < 0.05; **, P < 0.01. (E–M) Representative staining profiles for rat G2a isotype control (E) and Foxp3 (F) expression in airway DLN digest preparations from animals sensitized and exposed to multiple OVA aerosols. CD4 versus CD25 profiles of same DLN preparations (G and H) depict CD4+CD25+ and CD4+CD25− populations of cells. Staining profiles of ratG2a isotype controls and Foxp3 for CD4+CD25+ cells (J and K, respectively) and CD4+CD25− cells (L and M, respectively) are also shown. (N) EC200 was determined in OVA-sensitized animals after adoptive transfer of CD4+CD25+ or CD4+CD25neg cells sorted from pooled airway DLN of groups of ≥5 animals sensitized to OVA and exposed to multiple OVA aerosols or control animals (no adoptive transfer) and naive animals, on four different occasions. Data are expressed relative to naive controls. Figures represent mean absolute reduction or increase in EC200 (in mg/ml MCh) relative to the mean control value (set at zero). OVA ×1 > OVA ×1 + mOVA CD4+CD25+; P = 0.005; OVA ×1 > CD4+CD25neg, P = 0.873.
DISCUSSION
The LPR in asthma is associated with the local activation of allergen-specific Th2 memory cells in the airway mucosa. We have previously identified networks of DCs within the conducting airway epithelium and underlying submucosa of humans (19) and experimental animals (20), and as the sole professional APC population resident in these tissues, AMDCs are ideally positioned to drive activation of the Th2 cells, which are associated with the LPR component of asthma exacerbations. However, in the resting state these AMDCs are functionally immature, being specialized only for uptake and processing of antigen but lacking capacity for efficient presentation (21), thus questioning how allergen-specific T cells are initially activated in situ during an asthma attack.
Our recent studies (15) have provided a plausible mechanism for this T cell activation process, by demonstrating a sequence of cellular interactions which occur in situ within the airway mucosa during the first few hours after challenge of sensitized animals with aerosolized allergen. The crucial observation in these studies was the demonstration that resident AMDCs are in virtually continuous communication (via short-term clustering) with Th memory cells transiting through the airway mucosa. We demonstrated that when AMDCs from aerosol-exposed animals acquire antigen of equivalent specificity to (some of) these transiting T cells, the ensuing cognate interactions between these two cell populations trigger CD86 expression on AMDCs and accompanying up-regulation of the full range of APC functions. This in turn stimulates their mobilization and emigration to DLN over the ensuing 12–24 h, during which further interactions occur within the mucosa with other incoming Th memory cells, resulting in their activation in situ, and thus in development of AHR (15, 22). This emigration process, which involves translocation of all detectable allergen-bearing AMDCs with APC activity to the draining lymph nodes within 24 h of the exposure event, effectively terminates the response. It is likely that costimulator molecules in addition to CD86 are also directly involved in this process. We have previously shown that CD80 is not part of this response (15), but follow-up studies will be required to examine other possibilities.
In the present study we have further adapted this model to study the sequelae of a more realistic mode of allergen challenge which mimics that confronting human atopic asthmatics, i.e., exposure to perennial aeroallergen which occurs not as a single pulse but instead on a daily basis. A key feature of the model is initial sensitization of animals in a manner to selectively prime recirculating Th2-polarized memory cells, analogous to those observed in the circulation and the airway mucosa of human atopic asthmatics. The dominance of expression of GATA-3 in the airway mucosal T cell population of these animals attests to the effectiveness of this priming protocol.
The salient findings from this study are that after the triggering of an initial round of AMDC activation and a subsequent burst of local Th cell activation, exposed animals rapidly become refractory to further aeroallergen exposure. In particular, a second round of OVA aerosol exposure as early as 24 h after the initial (day 1) aerosol exposure does not elicit an ensuing burst of CD86 expression on AMDCs. This “desensitized” state is maintained in the face of at least 10 consecutive daily aerosol challenges and is paralleled by decreased Th2 cytokine gene expression and airways' eosinophilia, and waning of AHR which returns to baseline beyond day 3. Our earlier experiments (15) indicated that AMDCs, which acquired OVA during the initial aerosol exposure and emigrated from the airway mucosa by the 24-h time point, are replaced in the mucosa by incoming immature DC precursors. The failure of the latter to respond (by CD86 up-regulation) to the second round of OVA aerosol exposure suggests that either the airway mucosal OVA-specific Th2 memory cell population in these animals had become anergized, or that a down-regulatory control mechanism which prevents AMDCs responding to activation signal(s) from CD4+ Th2 memory cells had been triggered within the mucosa.
The experiments in Fig. 5 addressed these two possibilities. Importantly, they demonstrate that OVA-specific T cell–induced up-regulation of CD86 expression on AMDCs can be inhibited by a population of cells which appear in the tracheal mucosa of OVA-sensitized animals after repeated aerosol exposure. The depletion experiments in this series (Fig. 5, F and G) identify these inhibitory cells as TcRαβ+, CD4+, and CD25+, and follow-up experiments extend this phenotype to include expression of (inter alia) Foxp3 (Fig. 7 K), LAG3 (Fig. 6 B), IL-10 (Fig. 6 D), and CTLA4 (Fig. 6 C), as well as Th cell suppressive activity in a standard lymphoproliferation assay (Fig. 7 L), properties which are collectively suggestive of T reg cells. Expression of the LAG3 and IL-10 markers in CD4+CD25+ cells in the airway mucosa display a particularly strong association with repeated aerosol exposures, and this pattern was reiterated in the local DLN (Fig. 7, B and C). T reg cells activity developed in parallel at this regional lymphoid site over a similar time frame to that observed in the airway mucosa (Fig. 8 D), possibly in response to incoming OVA-bearing DCs which we have shown previously in this model to rapidly translocate from the tracheal mucosa to DLN after aerosol challenge (15).
These findings are consistent with other in vitro and in vivo studies demonstrating that T reg cells can modify DC to down-regulate expression of costimulatory molecules (8, 23), and with recent imaging studies showing that T reg cells can directly interfere with Th cell–DC interactions (24). However, they do not provide a direct link with the physiological in vivo endpoint of the T cell–dependent experimental LPR, i.e., the development of AHR. This latter issue was addressed directly in the studies in Fig. 8 (E–N), which collectively demonstrate that adoptive transfer of CD4+CD25+ DLN cells (of which 73% were Foxp3+) from repeatedly exposed animals into sensitized animals before their first aerosol challenge, is capable of abrogating ensuing AHR development.
An additional aspect of our in vitro DC activation model was the experiments involving selective depletion of MHC class II+ cells from the tracheal preparation before their addition to the cultures, which resulted in striking enhancement of CD86 up-regulation on the AMDCs. Moreover, comparable levels of CD86 expression on AMDCs were achieved when the only source of OVA-specific T cells in these cultures were those present in the tracheal preparation itself. This suggests that MHC class II depletion removes residual T reg cells activity which had survived depletion of CD4+ or CD25+ cells, thus unmasking the activity of endogenous OVA-specific Th cells in the tracheal population. It is feasible that the latter T reg cells may themselves express MHC class II (as is the case for activated Th cells in the rat; [25]). Alternatively, they may have been clustered with MHC class II+ AMDCs during the depletion process. In this context, we have previously demonstrated by confocal microscopy that in resting airway mucosal tissues, ≥65% of local Th cells are in intimate contact with local AMDCs, and this clustering process is further enhanced after local antigen stimulation (15). It is pertinent to note that active T reg cells–DC clustering has recently been reported in a lymph node explant model, and moreover was demonstrated to inhibit ensuing activation of Th cells present in the same microenvironment (26).
A key additional feature of our present data is the establishment of a link between the waning of the T reg cells response after withdrawal of the stimulatory (aerosolized) antigen, and the resurgence of sensitivity to a subsequent round of aerosol challenge. Thus, by the end of the 2-wk antigen-fee rest period, putative T reg cell levels in the tracheal mucosa had declined >10-fold to baseline levels (Fig. 7 K), and rechallenge with OVA aerosol at this time triggered rapid resurgence of Th cell–dependent CD86 expression on AMDCs (Fig. 2 A), accompanied by accumulation over the ensuing 24 h of a second wave of CD25+ T cells (Fig. 2 D) and eosinophils (Fig. 3 D) and the reemergence of AHR (Fig. 4). This pattern appears repeatable for at least a third cycle, and possibly beyond.
Collectively, these data provide “Koch's postulates” equivalent evidence that immunological and physiological homeostasis in the airway mucosa of sensitized animals is maintained in the face of chronic local antigen exposure via establishment of a state of dynamic equilibrium between T reg cells and specific Th cells resulting in functional silencing of the Th cells, and prevention of recurrence of repeated cycles of T cell activation in the airway mucosa and associated induction of AHR. Of note, we have demonstrated that the maintenance of this stable state is dependent on continuing allergen exposure, as T reg cells activity wanes after temporary removal of the antigen stimulus, rendering the animals again susceptible to another Th cell activation cycle once allergen exposure is reinstituted. Such a scenario provides a plausible explanation for the intermittent pattern of asthma exacerbations typical of human atopic asthmatics.
It is additionally noteworthy that during this T reg cell–controlled steady-state, the expression levels of GATA-3 within the tracheal Th cell population remain high, consistent with the maintenance (or possibly expansion) of Th2-polarised memory cells driven by the repeated allergen exposures. This suggests that translocation of aerosol-derived allergen to draining lymph nodes and/or central lymphoid organs where Th memory cells expansion is regulated may continue independently of events occurring at the challenge site. The maintenance of OVA-specific Th2 memory cells during this process infers that these aeroallergen-derived signals emanating from the airway mucosa of chronically exposed animals continue to be immunogenic as opposed to tolerogenic, which superficially appears to conflict with reports implicating Foxp3+ CD4+ CD25+ T reg cells in the generation of immunological tolerance to inhaled antigen (27, 28). Our laboratory was the first to describe this respiratory tract tolerance process in relation to control of IgE responses to inhaled allergen (29), and demonstrated contributions from a variety of T cell populations with regulatory properties (30–32). However, a hallmark feature of this tolerance process is that it can only be induced in immunologically naive animals—once CD4+ Th cell priming has been instituted, recirculating Th memory cells are resistant to deletion via these forms of T cell regulation and ongoing aerosol exposure instead slowly expands this memory population (33). Our present findings are consistent with this conclusion but additionally emphasize that T reg cell activity plays a key role in prevention of tissue damage via reactivation of effector–Th memory cells at challenge sites in the respiratory tract.
The findings reported here have important theoretical implications in relation to design of immunotherapeutic strategies for long-term control of atopic asthma, as they emphasize the importance of both the clonal expansion of allergen-specific Th cells within the central lymphoid compartment, and their reactivation within the airway mucosa, as independently regulated processes and by inference as separate drug targets.
MATERIALS AND METHODS
Animals and allergen exposures.
PVG rats were bred and maintained specific pathogen free using techniques approved by our institutional Animal Ethics Committee. Randomly selected animals of both sexes aged 8–13 wk were used. Presensitization of animals to OVA was achieved by i.p. immunization with 100 μg OVA in an alum mucaine suspension 14 d before use in challenge experiments. Generation of OVA-primed inguinal LN T cells for in vitro use was by s.c. injection with 100 μg OVA in Complete Freunds adjuvant. OVA aerosol challenge (using preselected LPS-low OVA [Sigma-Aldrich]) was performed over a 60-min period (15, 33).
Media and reagents.
Tissue culture medium and isolation reagents for preparation of airway mucosal and lymphoid tissue cells were as described (15, 33). All mAbs and immunostaining reagents (BD Biosciences) used for flow cytometry have been previously described (15, 33). For intracellular staining of Foxp3, an anti–mouse/rat Foxp3-FLR staining kit (eBioscience) was used as described by the manufacturer. Immunophenotypic analysis was performed using a FACSCalibur flow cytometer (BD Immunocytometry Systems) and analyzed using Flowjo software (version 4.6.1, Tree Star Inc.).
Cell preparation and culture.
BAL cells were obtained by standard methods and stained using Diff Quik (Lab Aids Pty Ltd). For airway mucosal cell preparation, isolated trachea were flushed with PBS, cut finely transversely, and minced with scissors. Tissue digestion and subsequent preparation of mononuclear cells, and selective depletion of macrophages and B cells was performed using methodology described in detail in forerunner publications (15, 33). DCs were finally isolated by immunostaining the remaining cells with FITC-conjugated anti–rat MHC class II (OX 6 FITC; BD Biosciences), which were then sorted using a Coulter EPICS Elite ESP, routinely to > 94% purity on the basis of morphology and staining characteristics. Contaminating cells were mainly CD3+ T cells or cytokeratin-positive cells, both <3%. Isolation of CD4+CD25+ cell populations was achieved by first enriching for CD25+-expressing cells using an autoMACS separator system based on positive selection (posseld) of labeled cells as per manufacturer's instructions (Miltenyi Biotech). CD25+-enriched cells were immunostained with PE-conjugated anti–rat CD4 (OX35PE; BD Bioscience), and CD4+CD25+ cells were sorted to >96% purity. For isolation of CD4+CD25− cells, the first enrichment step using the autoMACS separator system was for CD4+ cells. CD4-enriched cells were immunostained with biotinylated anti–rat CD25 (OX39bio; BD Biosciences) followed by streptavidin CyC (BD Biosciences), and CD4+CD25− cells were sorted using a Coulter EPICS Elite ESP, routinely to >93%.
Determination of APC in vitro activity.
Presentation of OVA by purified DCs used standard techniques described previously (15, 33). In brief, DCs were cultured together with CD4+-enriched inguinal LN preparations from OVA-immunized animals. CD4+ LN T cells were cultured at 1 × 105 per well in RPMI-5% FCS plus DC added at ratios indicated and OVA added at 50 μg/ml final concentration (if required) in a total volume of 200 μl for 48 h, and then pulsed with 3H-thymidine for a further 18 h. Cell proliferation was determined as mean counts per minute (CPM).
In vitro suppression assay.
OVA-primed LN cells were cultured at 105 per well in RPMI-5% FCS, with CD4+CD25+ cells added at ratios specified. OVA was added at 50 μg/ml final concentration, and the cultures incubated for 48 h, then pulsed with 3H-thymidine for a further 18 h. Cell proliferation was determined as mean CPM.
DC–T cell coculture.
In DC–T cell coculture experiments, tracheal digests prepared from animals exposed multiple times to OVA aerosols were depleted of various cell phenotypes using standard Dynabead depletion protocols as per manufacturer's instructions. CD4+ T cells from draining lymph nodes were prepared as described here and in reference 15.
Adoptive transfer experiments.
In adoptive transfer experiments, 1 × 106 CD4+CD25+ or CD4+CD25− cells sorted from the DLN after multiple OVA aerosols were injected i.v. into sensitized animals. The next day (18 h after injection) recipients were exposed to OVA aerosol. Responsiveness to inhaled MCh was measured at 24 h after aerosol. Because of limitations in attainable numbers of CD4+CD25+ cells for the adoptive transfers, on any given day recipients were limited to two per experiment. Each experiment also included AHR measurements made on naive controls and sensitized/exposed to OVA aerosol to enable direct comparisons to be made between all groups.
Lung function and MCh hyperresponsiveness.
The techniques used were as described previously (15, 34). In brief, respiratory impedance (Zrs) was measured by forced oscillation between 0.5 and 20 Hz using a computer-controlled piston ventilator. The constant–phase model was fitted to give estimates of airway and tissue mechanics (unpublished data). After baseline lung function, MCh challenge was performed by delivering aerosols (2 min) of saline (control) and MCh (0.1, 0.3, 1.0, 3.0, 10.0, 30.0 mg/ml) during tidal ventilation. Five measurements of Zrs were made after each dose and peak responses reported.
RT-PCR.
Total RNA was isolated from cells using RNeasy Mini kit and QIAshredder (Qiagen) and DNase treated using Turbo DNA-free kits (Ambion). Reverse transcription was performed using Omniscript RT kit (Qiagen). Total cDNA was then used as template in real-time PCR using ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) for 40 cycles of 15 s at 95°C and 1 min at 60°C. Primer pairs for PCR were designed in house. The PCR reaction contained Quantitect SYBR Green Master Mix (Qiagen) and 300 nM of each primer. Copy numbers of the amplicons were determined using standard curves generated with 10-fold serial dilutions of the respective standards and normalized against the reference Eef1α-1 gene.
Statistics.
The experiments were performed using single animals (AHR studies, BAL studies, and IgE) or pooled tissue from 5–20 animals that were repeated two to five times as indicated. Unless indicated, unpaired Student's t test was performed. Data is shown as mean ± SE or SD as indicated.
Acknowledgments
This work was funded by the National Health & Medical Research Council of Australia.
The authors have no conflicting financial interests.
Abbreviations used: AHR, airways hyperresponsiveness; AMDC, airway mucosal dendritic cell; CPM, counts per minute; DLN, draining lymph node; LPR, late phase reaction; MCh, methacholine; T reg cell, T regulatory cell.
P.A. Stumbles's present address is Division of Health Sciences, Murdoch University, South Street, Murdoch, Western Australia, 6150.
References
- 1.Busse, W.W., and R.F. Lemanske. 2001. Asthma. N. Engl. J. Med. 344:350–362. [DOI] [PubMed] [Google Scholar]
- 2.Haselden, B.M., A.B. Kay, and M. Larche. 1999. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J. Exp. Med. 189:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platts-Mills, T.A.E., R.B. Sporik, G.W. Ward, P.W. Heymann, and M.D. Chapman. 1995. Dose–response relationships between asthma and exposure to indoor allergens. In Progress in Allergy and Clinical Immunology. S.G.O. Johansson, editor Hogrefe & Huber, Seattle, WA. 90–96.
- 4.Woolcock, A.J., J.K. Peat, and L.M. Trevillion. 1995. Is the increase in asthma prevalence linked to increase in allergen load? Allergy. 50:935–940. [DOI] [PubMed] [Google Scholar]
- 5.Hawrylowicz, C.M., and A. O'Garra. 2005. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5:271–283. [DOI] [PubMed] [Google Scholar]
- 6.Akdis, M., J. Verhagen, A. Taylor, F. Karamloo, C. Karagiannidis, R. Crameri, S. Thunberg, G. Deniz, R. Valenta, H. Fiebig, et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karagiannidis, C., M. Akdis, P. Holopainen, N.J. Woolley, G. Hense, B. Ruckert, P.-Y. Mantel, G. Menz, C.A. Akdis, K. Blaser, and C.B. Schmidt-Weber. 2004. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 114:1425–1433. [DOI] [PubMed] [Google Scholar]
- 8.Lewkowich, I.P., N.S. Herman, K.W. Schleifer, M.P. Dance, B.L. Chen, K.M. Dienger, A.A. Sproles, J.S. Shah, J. Kohl, Y. Belkaid, and M. Wills-Karp. 2005. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 202:1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbari, O., G.J. Freeman, E.H. Meyer, E.A. Greenfield, T.T. Chang, A.H. Sharpe, G. Berry, R.H. DeKruyff, and D.T. Umetsu. 2002. Antigen-specific regulatory T cells develop via ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 10.Stock, P., O. Akbari, G. Berry, G.J. Freeman, R.H. DeKruyff, and D.T. Umetsu. 2004. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat. Immunol. 5:1149–1156. [DOI] [PubMed] [Google Scholar]
- 11.de Heer, H.J., H. Hammad, T. Soullie, D. Hijdra, N. Vos, M.A.M. Willart, H.C. Hoogsteden, and B.N. Lambrecht. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearley, J., J.E. Barker, D.S. Robinson, and C.M. Lloyd. 2005. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 202:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson, M.S., M.D. Taylor, A. Balic, C.A.M. Finney, J.R. Lamb, and R.M. Maizels. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuany-Amorim, C., E. Sawicka, C. Manlius, A. Le Moine, L.R. Brunet, D.M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625–629. [DOI] [PubMed] [Google Scholar]
- 15.Huh, J.C., D.H. Strickland, F.L. Jahnsen, D.J. Turner, J.A. Thomas, S. Napoli, I. Tobagus, P.A. Stumbles, P.D. Sly, and P.G. Holt. 2003. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T-cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J. Exp. Med. 198:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Garnier, C., L. Filgueira, M.E. Wikström, M. Smith, J.A. Thomas, D.H. Strickland, P.G. Holt, and P.A. Stumbles. 2005. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 175:1609–1618. [DOI] [PubMed] [Google Scholar]
- 17.Huang, C.-T., C.J. Workman, D. Flies, X. Pan, A.L. Marson, G. Zhou, E.L. Hipkiss, S. Ravi, J. Kowalski, H.I. Levitsky, et al. 2004. Role of LAG-3 in regulatory T cells. Immunity. 21:503–513. [DOI] [PubMed] [Google Scholar]
- 18.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte- associated antigen 4 plays an essential role in the function of CD25(+) CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt, P.G., M.A. Schon-Hegrad, M.J. Phillips, and P.G. McMenamin. 1989. Ia-positive dendritic cells form a tightly meshed network within the human airway epithelium. Clin. Exp. Allergy. 19:597–601. [DOI] [PubMed] [Google Scholar]
- 20.Schon-Hegrad, M.A., J. Oliver, P.G. McMenamin, and P.G. Holt. 1991. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J. Exp. Med. 173:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stumbles, P.A., J.A. Thomas, C.L. Pimm, P.T. Lee, T.J. Venaille, S. Proksch, and P.G. Holt. 1998. Resting respiratory tract dendritic cells preferentially stimulate Th2 responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 188:2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Rijt, L.S., and B.N. Lambrecht. 2005. Dendritic cells in asthma: a function beyond sensitization. Clin. Exp. Allergy. 35:1125–1134. [DOI] [PubMed] [Google Scholar]
- 23.Cederbom, L., H. Hall, and F. Ivars. 2000. CD4+ CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 30:1538–1543. [DOI] [PubMed] [Google Scholar]
- 24.Tadokoro, C.E., G. Shakhar, S. Shen, Y. Ding, A.C. Lino, A. Maraver, J.J. Lafaille, and M.L. Dustin. 2006. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 203:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannie, M.D., J.G. Dawkins, M.R. Walker, B.A. Clayson, and D.M. Patel. 2004. MHC class II biosynthesis by activated rat CD4+ T cells: development of repression in vitro and modulation by APC-derived signals. Cell. Immunol. 230:33–43. [DOI] [PubMed] [Google Scholar]
- 26.Tang, Q., J.Y. Adams, A.J. Tooley, M. Bi, B.T. Fife, P. Serra, P. Santamaria, R.M. Locksley, M.F. Krummel, and J.A. Bluestone. 2005. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 7:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostroukhova, M., C. Sequin-Devaux, T.B. Oriss, B. Dixon-McCarthy, L. Yang, B.T. Ameredes, T.E. Corcoran, and A. Ray. 2004. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Invest. 114:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umetsu, D.T., O. Akbari, and R.H. DeKruyff. 2003. Regulatory T cells control the development of allergic disease and asthma. J. Allergy Clin. Immunol. 112:480–487. [PubMed] [Google Scholar]
- 29.Holt, P.G., J.E. Batty, and K.J. Turner. 1981. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology. 42:409–417. [PMC free article] [PubMed] [Google Scholar]
- 30.McMenamin, C., and P.G. Holt. 1993. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I–restricted CD8+ T cell–mediated but MHC class II–restricted CD4+ T cell–dependent immune deviation resulting in selective suppression of IgE production. J. Exp. Med. 178:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMenamin, C., C. Pimm, M. McKersey, and P.G. Holt. 1994. Regulation of CD4+-TH-2-dependent IgE responses to inhaled antigen in mice by antigen-specific g/d T-cells. Science. 265:1869–1871. [DOI] [PubMed] [Google Scholar]
- 32.van Halteren, A., M. van der Cammen, D. Cooper, H. Savelkoul, G. Kraal, and P. Holt. 1997. Regulation of antigen-specific IgE, IgG1 and mast cell responses to ingested allergen by mucosal tolerance induction. J. Immunol. 159:3009–3015. [PubMed] [Google Scholar]
- 33.McWilliam, A.S., S. Napoli, A.M. Marsh, F.L. Pemper, D.J. Nelson, C.L. Pimm, P.A. Stumbles, T.N.C. Wells, and P.G. Holt. 1996. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J. Exp. Med. 184:2429–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petak, F., Z. Hantos, A. Adamicza, T. Asztalos, and P.D. Sly. 1997. Methacholine-induced bronchoconstriction in rats: effects of intravenous vs. aerosol delivery. J. Appl. Physiol. 82:1479–1487. [DOI] [PubMed] [Google Scholar]