Abstract
Phosphodiesterase-5 (PDE5) inhibitors (sildenafil, tadalafil, and vardenafil) are agents currently in clinical use for nonmalignant conditions. We report the use of PDE5 inhibitors as modulators of the antitumor immune response. In several mouse tumor models, PDE5 inhibition reverses tumor-induced immunosuppressive mechanisms and enables a measurable antitumor immune response to be generated that substantially delays tumor progression. In particular, sildenafil, down-regulates arginase 1 and nitric oxide synthase–2 expression, thereby reducing the suppressive machinery of CD11b+/Gr-1+ myeloid-derived suppressor cells (MDSCs) recruited by growing tumors. By removing these tumor escape mechanisms, sildenafil enhances intratumoral T cell infiltration and activation, reduces tumor outgrowth, and improves the antitumor efficacy of adoptive T cell therapy. Sildenafil also restores in vitro T cell proliferation of peripheral blood mononuclear cells from multiple myeloma and head and neck cancer patients. In light of the recent data that enzymes mediating MDSC-dependent immunosuppression in mice are active also in humans, these findings demonstrate a potentially novel use of PDE5 inhibitors as adjuncts to tumor-specific immune therapy.
Evidence that host immunity plays a critical role in limiting tumor outgrowth in the early stages of tumorigenesis supports the notion of immune surveillance (1, 2). However, to effectively function, endogenous or adoptively transferred tumor-specific T cells must be present in reasonable numbers, maintain their tumor specificity and an activated phenotype, traffic to the tumor site, and kill their targets in situ. Unfortunately, priming tumor-specific T cells and sustaining an immune response that imparts a measurable clinical benefit is limited by the ability of tumors to modify their microenvironment (3). These immunosuppressive mechanisms are also present in transplantable mouse tumors in which stable cell lines are generated after multiple in vivo passages that ultimately select for clones able to avoid immune recognition. As such, these models represent useful tools to identify the cellular and molecular tumor-induced immunosuppressive pathways, as well as discover pharmacological targets and screen immunomodulatory drugs with measurable antitumor activity.
Extensive data exist in mouse models correlating tumor progression with the accumulation of myeloid inhibitory cells such as CD11b+/Gr-1+ myeloid-derived suppressor cells (MDSCs) (4), immature dendritic cells (5), and F4/80+ macrophages (6) that induce local and possibly systemic immunosuppression (7). l-Arginine metabolism is an important pathway used by MDSCs to blunt antitumor immunity (8). In these cells, arginase-1 (ARG1) and nitric oxide synthase–2 (NOS2), the key enzymes in l-arginine catabolism, work either alone or synergistically to suppress T cell function (9). The elimination, functional inhibition, or differentiation of MDSCs in tumor-bearing hosts can restore CD8+ T cell responsiveness (10, 11), thereby implicating their role in tumor-induced immunosuppression.
By increasing the intracellular concentrations of cyclic guanosine monophosphate (cGMP), phosphodiesterase-5 (PDE5) inhibitors such as sildenafil (Viagra), vardenafil (Levitra), and tadalafil (Cialis) have been used therapeutically to treat erectile dysfunction (12), pulmonary hypertension (13), and cardiac hypertrophy (14). More recently, they were shown to induce apoptosis in different human tumors such as colon carcinoma and chronic lymphocyte leukemia (15, 16). In our mouse models, we show that pharmacologic PDE5 blockade down-regulates MDSC suppressive pathways and restores antitumor immunity. Moreover, our in vitro experiments using PBMCs from multiple myeloma (MM) and head and neck cancer patients suggest that the same mechanisms found in mice are also present in humans and demonstrate a possible role for PDE5 inhibitors as an immune adjuvant in the clinical setting.
RESULTS
PDE5 inhibition augments immune-mediated antitumor activity in vivo
When administered in vitro, PDE5 inhibition induces apoptosis in colon carcinoma (15) and chronic lymphocytic leukemia cells (16). To determine whether similar effects could be observed in vivo, we used various transplantable mouse tumors, including CT26WT (a colon carcinoma; Fig. 1 A), the more aggressive variant C26GM (Fig. 1 B), TS/A (a mammary adenocarcinoma; Fig. 1 C), and the MCA203 fibrosarcoma (Fig. 1 D). PDE5 inhibitors were administered starting on the day of tumor challenge. Sildenafil and tadalafil significantly delayed tumor outgrowth by 50 to 70% in immune-competent mice, although all mice ultimately died (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061104/DC1). Similar results were obtained even if sildenafil treatment was started on day 7 after tumor challenge in the CT26WT model (Fig. S2). The fact that no difference in tumor outgrowth was seen between early versus late administration of sildenafil suggests that PDE5 inhibition does not appreciably affect the early phases of tumor uptake but rather influences the later stages of tumor outgrowth. Because the addition of sildenafil to cultured CT26WT cells did not increase their apoptosis or affect their doubling time (unpublished data), we conclude that sildenafil does not have a direct antitumor effect but rather interferes in host–tumor interactions.
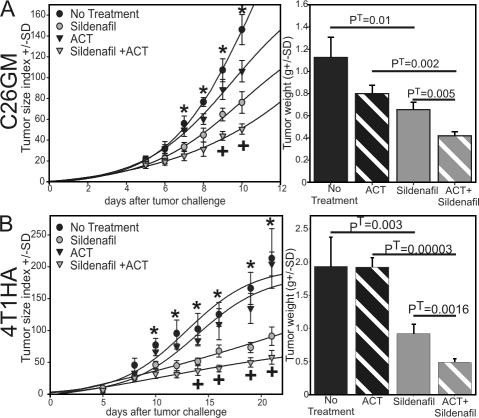
Figure 1.
PDE5 inhibition imparts a measurable immune-mediated antitumor effect. BALB/c or BALB/c Rag-2−/− mice were challenged s.c. with 0.5 × 106 CT26WT (A), C26GM colon carcinoma (B), or TS/A mammary adenocarcinoma (C) cells. (D) C57BL/6 mice were challenged with 0.5 × 106 MCA203 s.c. PDE5 inhibitors were started on day 0. Sildenafil was added to the drinking water or given i.p. daily. Tadalafil was given i.p. daily where indicated in B. Error bar values are shown.
To confirm that the antitumor effect of PDE5 inhibitors was immune mediated, the experiments were repeated in immune-compromised BALB/c-Rag-2−/− mice (Fig. 1, A–C). In these hosts, sildenafil demonstrated no antitumor efficacy. Because these mice lack T and B lymphocytes but have normal or enhanced NK and NKT activity (17, 18), these results strongly suggest that the antitumor activity of PDE5 inhibition in our models is primarily caused by an adaptive immune response with either minimal NK/NKT-mediated activity, direct tumor-induced apoptosis, or tumor angiogenesis inhibition.
Evidence of an immune-mediated, antitumor effect of PDE5 inhibition is further shown by tetramer analysis on splenocytes obtained from sildenafil-treated, CT26WT tumor- bearing mice, which revealed a higher number of CTLs specific for AH-1 (unpublished data), a CT26 tumor-associated antigen, as compared with their untreated counterparts (19). To confirm immune-mediated antitumor activity in the sildenafil-treated groups, we performed an in vivo cytotoxicity assay. BALB/c mice were injected with PBS (naive), vaccinated with γ-irradiated CT26WT or C26GM, or challenged with CT26WT or C26GM tumors on day 0. On day 12 (CT26WT) or day 5 (C26GM), all mice received carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled splenocytes pulsed with the MHC class I–restricted AH-1 peptide (CFSEhigh), admixed with CFSE-labeled splenocytes pulsed with the irrelevant hemagglutinin (HA)-peptide (CFSElow). In vivo T cell cytotoxicity was determined 40 h later (Fig. 2). These time points were chosen based on the kinetics of tumor outgrowth observed in Fig. 1 A and Fig. 1 B, respectively, when tumor size significantly differed between untreated and sildenafil-treated mice. As expected, an endogenous AH1-specific immune response was observed in the vaccinated mice as compared with their tumor-bearing counterparts. PDE5 inhibition in the vaccine-primed mice failed to augment antigen-specific CD8 responsiveness compared with no treatment. In contrast, tumor-bearing mice treated with sildenafil early after tumor challenge generated antigen-specific immunity that was significantly greater then that observed in their untreated counterparts and similar, or even superior, to that induced by vaccination. Collectively, this is the first indication that PDE5 inhibitors can modulate antitumor immunity. Because the sildenafil-mediated antitumor immune response does not completely eradicate tumors, tumor escape mechanisms may be associated with their outgrowth. To test this hypothesis, the parental CT26WT cell line, as well as the CT26 tumor removed on day 24 from sildenafil-treated mice (either AH-1 pulsed or unpulsed), and BALB/c splenocytes were incubated with either AH-1 peptide-primed (Fig. S1 C) or tumor-primed (Fig. S1 D) effector T cells. Although effector T cells recognized the parental CT26WT line and released IFN-γ in the assay, they failed to recognize the sildenafil-derived tumor. Its recognition, however, was restored by loading the sildenafil-derived tumor with the AH-1 peptide. (Fig. S1, C and D). These results suggest that the immune response in sildenafil-treated mice does not result in complete tumor eradication but rather in the selection of antigen-escape variants.
Figure 2.
Sildenafil enhances antitumor CTL activity in vivo. (A) BALB/c mice were either challenged with CT26WT cells, vaccinated with 106 γ-irradiated CT26WT cells, or injected with PBS on day 0. Sildenafil was added to the drinking water where indicated. On day 12, all groups received 107 AH-1–pulsed CFSEhigh-labeled BALB/c splenocytes admixed with 107 HA-pulsed CFSElow-labeled BALB/c splenocytes. The mice were killed 40 h later. Single cell suspensions from spleens and tumor draining lymph nodes were analyzed by FACS. (B) BALB/c mice were either challenged with C26GM cells, vaccinated with 106 γ-irradiated C26GM cells, or injected with PBS on day 0. Sildenafil was added to the drinking water on day 0. After 5 d, the mice received CFSE-labeled target splenocytes as described in A. Analysis was performed as described in the Materials and methods.
PDE5 inhibition synergizes with adoptive cell therapy (ACT) to delay tumor outgrowth
Considering that sildenafil treatment significantly delayed tumor outgrowth but failed to eradicate it, we sought to determine whether combining sildenafil with tumor-specific CD8+ lymphocytes could enhance the therapeutic efficacy of ACT. 1 d after tumor challenge, C26GM-bearing mice received purified CD8+ T cells derived from mice vaccinated with γ-irradiated C26GM cells. After adoptive transfer of these vaccine-primed CD8+ T cells, the mice were either treated with sildenafil or left untreated. Although adoptive transfer alone demonstrates no statistically meaningful antitumor effect compared with no treatment (Fig. 3 A), PDE5 inhibition significantly reduces tumor outgrowth. However, coupling adoptive immunotherapy with PDE5 inhibition resulted in the greatest antitumor effect.
Figure 3.
Sildenafil improves the efficacy of ACT. BALB/c mice were challenged s.c. with either C26GM (A) or 4T1-HA (B) cells on day 0. Tumor-specific T cells were transferred on day 1. Sildenafil treatment was started on day 1. Tumors were surgically removed and weighed (right) either on day 10 (A) or 21 (B). *, PA < 0.00001 by using one-way ANOVA; +, PT < 0.001 by using a paired t test comparing sildenafil to sildenafil + ACT groups. Error bar values are shown.
To extend this finding to other models, the experiment was repeated using the 4T1 mammary carcinoma genetically modified to express the influenza-derived HA as a model tumor antigen (Fig. 3 B). Mice were challenged on day 0 with 0.5 × 106 4T1-HA cells. On the next day they received 3 × 106 HA-specific naive CD8+ T cells. After adoptive transfer, the mice were either treated with sildenafil or left untreated. As with the C26GM experiments, no therapeutic benefit was observed with ACT alone, whereas sildenafil + ACT imparted the greatest antitumor benefit. Sildenafil treatment alone, instead, substantially delayed tumor progression in immunocompetent BALB/c mice but not in immune-deficient Rag-2−/− mice (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20061104/DC1). These data confirm the immunomodulatory properties of PDE5 inhibitors in augmenting the therapeutic efficacy of ACT and demonstrate a role of sildenafil in modifying the tumor microenvironment rendering it more susceptible to CTL-mediated cytotoxicity.
PDE5 inhibition increases tumor-infiltrating CD8+ T cells
To exert a measurable antitumor effect, tumor-specific T cells must be present in sufficient numbers and capable of trafficking to their targets. A direct correlation exists between the number of tumor-infiltrating lymphocytes (TILs) and a favorable clinical outcome, as demonstrated in patients with metastatic ovarian cancer (20). Furthermore, the functional status of TILs has been correlated with a favorable prognosis in various human malignancies (20–24). Because PDE5 inhibition augments antitumor immunity, we asked whether sildenafil treatment altered both the number and activation state of TILs. Histological examination of CT26WT tumors revealed a greater intratumoral cellular infiltrate in the sildenafil-treated mice compared with the untreated controls (Fig. 4 A). To better evaluate these differences, C26GM-bearing mice received either tumor-primed or no T cells followed by sildenafil treatment or no additional therapy. The tumors were excised 9 d later, and single cell suspensions were obtained. The T cell infiltrate was analyzed by flow cytometry for CD4+ and CD8+ T cells. This approach enabled us to accurately examine the entire tumor mass and reliably quantify the infiltrating lymphocytic population. Although no increase in CD4+ T cells was observed with PDE5 inhibition (Fig. 4 B, inset), sildenafil treatment greatly increased CD8+ intratumoral infiltration with up-regulation of the activation markers CD69 and CD25 (Fig. 4 C). There were no differences in activation markers between the sildenafil-treated group and sildenafil + ACT, whereas a significant increase in intratumoral T cells were observed in the sildenafil + ACT–treated group compared with sildenafil alone (Fig. 4 B and Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20061104/DC1).
Figure 4.
PDE5 inhibition increases intratumoral T cell infiltration and activation of tumor-specific CD8+ T cells. (A) BALB/c mice were challenged with CT26WT cells s.c. on day 0, and 20 mg/kg/d sildenafil was added to the drinking water where indicated. The mice were killed 15 d later, and the tumors were stained with hematoxylin-eosin. (B–D) BALB/c mice were challenged with C26GM on day 0. They were treated with sildenafil, received 20 × 106 C26GM vaccine-primed splenocytes from H2d pIL-2/GFP mice, or were given both treatments as indicated. (B) The tumors were surgically removed 9 d later, and the CD8+ T cell infiltrate was determined by flow cytometry. The percentage of CD8+ T cells was plotted against the tumor size at the time of tumor harvest. The software-provided tool (Sigma plot) was used to fit a three-parameter exponential decay curve (y = 36.13 + 92xe−5.93x). Pearson bivariate correlation, P = 0.0000002. Data are derived from three independent experiments, with each containing five mice per group. (C) Intratumoral single cell suspensions were labeled with anti-CD25 or -CD69 antibodies. Data are expressed as the percentage of positive cells gated on the CD8+ T cell population. (D) IL-2 production in the ACT groups is reported as the percentage of GFP+ CD8+ T cells. Data are derived from two independent experiments. The paired t test p-value is reported. (E) BALB/c mice were challenged with 0.5 × 106 C26GM cells on day 0. They were treated with sildenafil starting on day 0, the anti-CD8+ depleting antibody, or both. The one-way ANOVA p-values (PA) are indicated. Data are reported from one of two similar experiments. Error bar values are shown.
To determine whether the immunomodulatory effect of PDE5 inhibition affected T cell activation within the tumor microenvironment, we examined IL-2 production by TILs using a transgenic mouse in which expression of GFP is under an IL-2 promoter (BALB/c–IL-2p/GFP) (25). In this model, T cell stimulation activates the IL-2 promoter and results in expression of the reporter transgene, GFP, which is easily detectable by flow cytometry. C26GM-primed BALB/c–IL-2p/GFP splenocytes were adoptively transferred into tumor-bearing recipients that were either left untreated or treated with sildenafil for 9 d. Single cell suspensions of the tumor-infiltrating CD8+ T cells were analyzed by flow cytometry for GFP expression. Adoptively transferred, vaccine-primed T cells were activated in the tumor microenvironment only in the presence of PDE5 inhibition, whereas in its absence they produced no IL-2 and, hence, were bona fide anergic T cells (Fig. 4 D). To further prove that these effects were dependent on CD8+ T cells, mice were challenged with C26GM and were (a) left untreated, (b) given sildenafil, (c) given an anti-CD8+ depleting antibody, or (d) given both sildenafil and the CD8+ depleting antibody. Sildenafil treatment again demonstrated a statistically significant reduction in tumor outgrowth, an effect completely abrogated by CD8+ depletion (Fig. 4 E). These experiments demonstrate that PDE5 inhibition enhances the tumor-specific T cell response, increases intratumoral T cell infiltration and activation, and underscores the role of CD8+ T cells in sildenafil-mediated antitumor responses.
In vitro sildenafil down-regulates the myeloid suppressor cell suppressive marker IL-4Rα
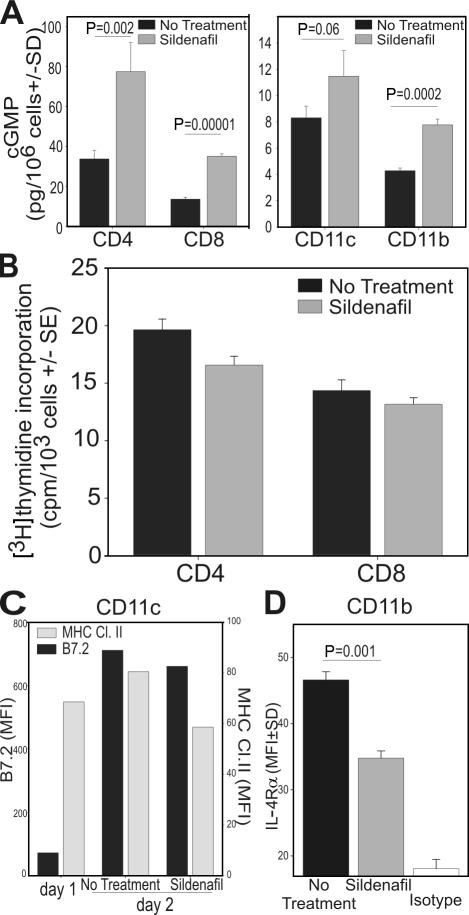
The experiments described thus far demonstrate the ability of PDE5 inhibition to prime/augment antitumor immunity. Yet the mechanisms resulting in T cell activation are unknown. Because PDE5 is expressed in various cells of the immune system (including DCs, macrophages, and T cells [26]), PDE5 inhibitors could putatively target these various populations. Furthermore, numerous factors are present in tumor-bearing hosts that could impair the generation of an effective immune response such as the defective maturation of DCs (7), the accumulation of suppressive MDSCs (4), T cell anergy (27), and/or the accumulation of T reg cells (28). These populations were, therefore, examined separately. Flow cytometric analysis of sildenafil-treated or untreated mice failed to reveal important differences in DC maturation, CD4+Foxp3+ T reg cells, or CD11b+/Gr-1+ MDSC accumulation (unpublished). We evaluated the effect of in vitro PDE5 inhibition on purified CD11c+, CD11b+, CD4+, and CD8+ cells isolated from C26GM tumor-bearing mice. This enabled us to examine purified populations, thereby eliminating the potential for exogenous influences. Although sildenafil treatment increased the intracellular concentration of cGMP in all the populations examined (Fig. 5 A), it had no effect on the proliferation of purified CD4+ and CD8+ T cells stimulated with either ConA (Fig. 5 B) or anti-CD3/CD28 beads (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20061104/DC1), nor on CD11c maturation (Fig. 5 C) as determined by B7.2 or MHC class II up-regulation. It did, however, demonstrate the ability to reverse the suppressive phenotype of MDSCs. In light of recent data identifying IL-4Rα as a functional suppressive marker for MDSCs (29), we examined IL-4Rα expression on CD11b+ cells cultured in the presence or absence of sildenafil. Fig. 5 D shows that sildenafil significantly decreases IL-4Rα expression on MDSCs, suggesting a down-regulation of their suppressive pathways.
Figure 5.
Sildenafil down-regulates IL-4Rα expression on CD11b+ cells. CD4+, CD8+, CD11b+, or CD11c+ cells were magnetically purified from spleens of C26GM-bearing mice challenged 9 d earlier. CD4+ or CD8+ T cells were stimulated with ConA for 3 d, with or without 50 μg/ml sildenafil. (A) Purified cells were cultured separately for 2 d with or without sildenafil, harvested, and used to determine cGMP levels, as described in Materials and methods. (B) T cell proliferation was determined by [3H]thymidine incorporation. (C) CD11c+ cells were analyzed for B7.2 and MHC class II expression. (D) CD11b+ cells were analyzed for IL-4Rα expression by flow cytometry. The t test p-value is reported. Error bar values are shown. MFI, multiplicity of infection.
In vivo PDE5 inhibition down-regulates tumor-associated MDSC suppressive pathways
Although the hallmark feature of MDSCs is immunosuppression, emerging data reveal that the degree of immunosuppression varies among populations of MDSCs isolated from different organs, with intratumoral MDSCs being the most immunosuppressive. Interestingly, these MDSCs express greater levels of NOS2 and ARG1 than their splenic counterparts (5). ARG1 expression is mainly regulated by the STAT-6–IL-4Rα pathway (30). We recently correlated IL-4Rα expression on CD11b+/Gr-1+ with an immunosuppressive phenotype (29), and our in vitro data (Fig. 5) indicate that sildenafil down-regulates IL-4Rα on MDSCs. We then asked whether in vivo PDE5 inhibition reduced ARG1 and NOS2 and down-regulated IL-4Rα in tumor-associated MDSCs. BALB/c mice were challenged with CT26WT, and half were treated with sildenafil. Mice were killed 15 d later, and intratumoral MDSCs were obtained. Sildenafil increased cGMP (Fig. 6 A), reduced IL-4Rα expression (Fig. 6 B), and down-regulated NOS2 and ARG1 expression and reduced their enzymatic activity in the intratumoral MDSCs (Fig. 6, C and D). Considering that ARG1 and NOS2 are key enzymes in MDSC suppressive pathways (8, 31), these findings support the hypothesis that PDE5 inhibition is a novel pharmacologic approach to regulate MDSC-mediated immunosuppressive pathways.
Figure 6.
In vivo PDE5 inhibition down-regulates tumor-associated MDSC suppressive pathways. BALB/c mice were challenged with 0.5 × 106 C26GM cells and treated with sildenafil starting on day 0 or left untreated for 9 d. CD11b+ cells were obtained from the tumors and used to measure (A) intracellular cGMP and (B) IL-4Rα surface expression by flow cytometry. (C) Western blot analysis was performed for NOS2, ARG1, and β-actin expression on purified tumor-associated CD11b+ cells. (D) NO production was determined as the concentration of NO3-NO2 in the supernatant, and arginase activity was determined on cell lysates and normalized for the number of cells. (E) BALB/c mice were challenged with C26GM, treated with sildenafil or the anti–Gr-1 depleting antibody, both treatments, or left untreated. Best fit of the data was obtained by four-parameter sigmoid curves. ANOVA p-values (PA) are reported. Error bar values are shown. MFI, multiplicity of infection.
Gr-1+ cells are known to facilitate tumor outgrowth. As such, it is conceivable that strategies seeking to eliminate this population may have a measurable antitumor effect. In certain tumor models, the Gr-1 depleting antibody inhibited tumor outgrowth even in the absence of T cells, although the antitumor effect was more pronounced in immune competent mice (32, 33). In light of these results and to verify that MDSCs are the target of sildenafil-mediated antitumor activity, we examined the effect of antibody-mediated MDSC depletion in combination with PDE5 inhibition in vivo. BALB/c mice were challenged on day 0 with C26GM tumor and were (a) left untreated; (b) injected with 100 μg of anti–Gr-1 antibody i.p. on days 0, 3, and 6; (c) treated with sildenafil; or (d) treated with a combination of the two treatments. As shown in Fig. 6 E, Gr-1 depletion delayed tumor outgrowth similarly to PDE5 inhibition, whereas no synergistic effect was seen with the combination. Collectively, these data demonstrate the immunosuppressive nature of Gr-1+ cells and the ability of PDE5 inhibition to reduce their suppressive phenotype in vivo.
PDE5 inhibition abrogates MDSC suppressive activity
Freshly isolated MDSCs suppress the in vitro proliferation of activated lymphocytes. Interestingly, the suppressive mechanisms appear to be strain specific. In the Th1 cell prone strain C57BL/6, it is mediated by NOS2 through NO production (34), whereas, in the mixed Th1/Th2 cell BALB/c strain, suppression requires peroxynitrite formation via ARG1 and NOS2 coexpression (8) or l-arginine depletion secondary to ARG1 overexpression (35). Reductions of both ARG1 and NOS2 expression via PDE5 inhibition should affect both suppressive pathways, resulting in less MDSC-mediated immunosuppression and, therefore, enhanced antigen-specific T cell proliferation. To test this hypothesis, tumor-derived CD11b+ MDSCs were isolated from C26GM-bearing BALB/c mice. MDSC suppressive activity was determined by admixing MDSCs with CFSE-labeled HA-specific CD8+ (clone 4) or CD4+ (6.5) T cells pulsed with their relevant peptide in the presence or absence of sildenafil (Fig. 7, A and B). Although the addition of tumor-derived MDSCs significantly impaired antigen-specific T cell proliferation as demonstrated by the low percentage of CFSElow clonotypic T cells, sildenafil almost completely restored both CD4+ and CD8+ responsiveness of these antigen-specific T cells. The absence of sildenafil-mediated enhancement in T cell function in the groups lacking CD11b+ cells underscores the targeted role of sildenafil on the MDSC population. Because in a Th1 cell–prone environment MDSC suppression is only NOS2 dependent (34), we examined the role of PDE5 in MDSCs in a C57BL/6 background where NOS2−/− mice are also available. CD11b+ MDSCs were isolated from either C57BL/6-NOS2+/+ or B16GM-bearing C57BL/6-NOS2−/− B16GM melanoma-bearing mice. A suppression assay was performed by stimulating OVA-specific CD4+ T cells with the relevant peptide in the presence or absence of MDSCs obtained from either NOS2+/+ or NOS2−/− tumor-bearing mice (Fig. 7 C). Although the addition of C57BL/6-NOS2+/+ MDSCs induced considerable T cell suppression, no suppression was observed with MDSCs from NOS2−/− mice. Furthermore, although PDE5 inhibition reversed MDSC suppression in NOS2+/+ mice, sildenafil failed to augment T cell responsiveness in the NOS2−/−-derived MDSC suppression assay. These results confirm the role of NOS2 in MDSC-mediated T cell suppression (Fig. 7 C) and underscore the ability of PDE5 inhibition to reverse the two major suppressive pathways in MDSCs (ARG1 and NOS2).
Figure 7.
PDE5 inhibition reverts MDSC suppressive pathways. Splenic CD11b+ cells from C26GM tumor-bearing mice were isolated and added to CFSE-labeled splenocytes containing either (A) naive HA-specific CD8+ (CL4) cells or (B) naive HA-specific CD4+ (6.5) cells. The cultures were stimulated for 4 d with the relevant peptide in the presence or absence of 50 μg/ml sildenafil. Proliferation was evaluated as CFSE dilution by FACS analysis. (C) Splenic CD11b+ cells were magnetically purified from B16GM tumor-bearing C57BL/6-NOS+/+ or C57BL/6-NOS−/− mice and added to CFSE-labeled splenocytes containing naive OVA-specific CD4+ T cells. The cultures were stimulated for 4 d with the relevant peptide in the presence or absence of 50 μg/ml sildenafil. Proliferation was evaluated as CFSE dilution by flow cytometry. Data derived from one of two independent experiments with similar results are reported. Error bar values are shown.
PDE5 inhibition restores T cell proliferation in MM and head and neck cancer patients
Having demonstrated that PDE5 inhibition can impair MDSC suppressive mechanisms in BALB/c and C57BL/6 tumor-bearing mice, we next sought to determine whether similar results could be obtained in humans. Head and neck cancers are known to be highly immunosuppressive. Their high levels of GM-CSF production are likely the major mediator of immune suppression observed in these patients and are probably responsible for the intratumoral infiltration by MDSCs (36). In fact, peripheral blood lymphocytes (PBLs) from these patients are functionally impaired in their ability to be activated and to proliferate upon stimulation (37). Similar results were also seen in prostate cancer (38) and in nonsmall cell cancer (35). Although this anergic state in solid tumors may be attributable to the ARG1- and/or NOS-dependent suppressive activity of MDSCs, MDSC-mediated immunosuppression has not been previously reported in hematological malignancies. PBMCs from MM patients were stimulated with anti-CD3/CD28 antibody-coated beads in the presence of N-(omega)-hydroxy-nor-l-arginine (NorNOHA; an ARG1-specific inhibitor), N G-monomethyl-l-arginine (l-NMMA; an NOS2 inhibitor), both inhibitors, or neither. As shown in Fig. 8 A (top), T cell expansion was considerably enhanced in the presence of both NorNOHA and l-NMMA, whereas the single inhibitors failed to increase T cell proliferation over the baseline. Interestingly, sildenafil yielded results equivalent to the combination of NorNOHA and l-NMMA. These results suggest involvement of both ARG1 and NOS2 in MDSC-mediated immunosuppression in myeloma and confirm the in vitro results demonstrating the ability of PDE5 inhibitors to affect both pathways. Recent data from our lab identified human MDSCs as ARG+, CD14+ cells (unpublished data). We therefore examined T cell expansion in CD14+-depleted PBMCs under the same conditions (Fig. 8 A, bottom). Although CD14+ depletion alone increased CD3+ T cell expansion fourfold, pharmacologic inhibitors failed to further enhance proliferation, suggesting that CD14+ cells are the mediators of ARG1- and NOS2-mediated immunosuppression in MM. As seen with purified mouse T cells (Fig. 5 B), sildenafil also failed to enhance the CD3+ T cell proliferation of CD14-depleted PBMCs from cancer patients.
Figure 8.
PDE5 inhibition restores proliferation of head and neck and myeloma lymphocytes. (A) Unfractionated or CD14-depleted PBMCs from MM patients were stimulated with anti-CD3/CD28 antibody–coated beads in the presence of NorNOHA, l-NMMA, both NorNOHA and l-NMMA, sildenafil, or no inhibitor. The CD3+ T cell expansion was measured 5 d later by flow cytometry. (B) Ficoll-purified PBMCs from healthy donors (n = 4), head and neck cancer patients (H &N; n = 7), or MM patients (n = 7) were stimulated as described in A in the presence or absence of sildenafil. CD4+ and CD8+ T cell expansion was measured by flow cytometry 5 d later. Data are reported as fold change. t test p-values are reported. Horizontal lines represent the median, the 10th and 90th percentile.
We next sought to determine whether we could restore T cell proliferation of PBMCs isolated from head and neck and MM patients stimulated with anti-CD3/CD28 antibody-coated beads in the presence or absence of sildenafil. The expansion of CD4+ and CD8+ T cells obtained from MM or head and neck patients was significantly less than that observed using healthy donors (Fig. 8 B). In contrast, in the presence of sildenafil, PBMCs from MM and head and neck patients expanded similarly to sildenafil-treated PBMCs from healthy donors (Fig.8 B). Interestingly, qualitative differences in T cell expansion emerged between the two malignancies. In head and neck patients, CD8+ T cell proliferation is restored with PDE5 inhibition while CD4+ lymphocyte expansion in the same condition is only partially augmented. In contrast, CD4+ T cell proliferation is restored and minimal effects are seen on CD8+ lymphocyte expansion with PDE5 blockade in myeloma (Fig. 8 B).
In summary, these data confirm that PDE5 inhibition can augment immune responsiveness through its effect on an accessory CD14+ population. Moreover, they suggest that the same immunosuppressive mechanisms found in mice are conserved in human malignancies and that PDE5 can be a useful therapeutic approach to enhance tumor-specific immunotherapy.
DISCUSSION
This is the first demonstration that PDE5 blockade exerts an indirect, immune-mediated, antineoplastic effect through inhibition of MDSC-mediated immunosuppressive mechanisms. These findings could establish a new role for PDE5 inhibition as a viable and effective immunological adjunct in the treatment of various malignancies, thereby adding to the growing list of therapeutic applications of these agents. In this paper we show that PDE5 inhibition can significantly overcome the immunosuppressive mechanisms present in a tumor-bearing host to generate immune responses that are similar to (if not greater than) those observed in a non– tumor-bearing, vaccine-primed host (Fig. 2). However, although a CTL response can be elicited, PDE5 blockade is incapable of complete tumor eradication (Fig. S1). Several hypotheses may account for these findings. For example, chronic sildenafil blockade may up-regulate PDE5 expression, making it difficult to achieve complete and long-term pharmacologic inhibition (39). Alternatively, the sildenafil-mediated immune response against the self tumor-associated antigen can predominantly involve low affinity CD8+ T cells incapable of complete tumor cell killing. Finally, the tumor-specific immune response may ultimately result in immune editing of the tumor that escapes immune recognition (Fig. S1, C and D).
PDE5 blockade not only increases intratumoral CD8+ infiltration and activation but also enhances their tumoricidal activity (Fig. 2). The ability to favorably alter the intratumoral microenvironment, thereby permitting tumor-specific T cells to directly interact with their targets, is critical for maximal antitumor immunity. Effective immunotherapy requires tumor-specific CTLs to infiltrate the tumor and kill their target in situ. Although no clinical advantage was derived by the presence of tumor-specific CTLs in the peripheral blood in melanoma patients (40), their frequency in the tumor correlated with a favorable prognosis (41). These clinical observations seem to be confirmed in our model where sildenafil treatment (a) increased the CD8+ T cell tumor infiltration (Fig. 4 B) and (b) increased the percentage of activated T cells (Fig. 4, C and D). Moreover, the number of tumor-infiltrating T cells directly correlated with a measurable antitumor effect (Fig. 3).
Although sildenafil can increase cGMP in T cells, DCs, and CD11b+ cells (Fig. 5), the following data indicate that Gr-1+/CD11b+ MDSCs are its primary cellular target. Gr-1 depletion does not augment sildenafil-mediated antitumor activity (Fig. 6 E), and sildenafil down-regulates MDSC suppressive pathways in vivo (Fig. 6, B–D). Moreover, sildenafil reverses MDSC suppression in vitro (Fig. 7). MDSCs and/or tumor-associated macrophages have been shown to induce apoptosis or anergy in CD8+ and CD4+ T cells through NOS2- and/or ARG1-dependent mechanisms (34). In fact, NO production anergizes Th1 cells through inhibition of IL-2 signaling (34). Alternatively, in a mixed Th1/Th2 cell environment where ARG-induced pathways also mediate immunosuppression, MDSCs produce NO and super-oxide radicals to generate peroxynitrites that induce apoptosis of activated CD8+ T cells (9). A greater understanding of the role of MDSCs in tumor-induced immune dysfunction (7, 42) will establish the scientific rationale for a targeted pharmacologic approach to disrupt these suppressive mechanisms and may serve as an adjunct to immunotherapy. We previously showed that nitroaspirin could abrogate the inhibitory activity of MDSCs by enhancing the preventive and therapeutic efficacy of antitumor vaccines (43). However, despite its use as a vaccine adjuvant, nitroaspirin demonstrated no antitumor efficacy when used alone. In contrast, down-modulation of both ARG1 and NOS2 in MDSCs (Fig. 6) with PDE5 inhibitors effectively abrogates MDSC-mediated immune suppression, resulting in a measurable antitumor response (Fig. 1, Fig. 3, and Fig. 4). We have recently shown that to effectively exert their suppressive function, MDSCs must (a) be activated by IFN-γ production from antigen-stimulated T cells, (b) release their own IFN-γ, and (c) be responsive to IL-13 (29). Cooperation between these two cytokines leads to the activation of ARG1 and NOS2 enzymes. Sildenafil neither alters IFN-γ production from activated lymphocytes (not depicted) nor changes IL-13 and IFN-γ production from MDSCs (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20061104/DC1). Rather, PDE5 inhibition down-regulates IL-4Rα expression on MDSCs (Fig. 5 and Fig. 6), likely impairing their responsiveness to IL-13.
MDSCs can promote tumor growth not only by preventing tumoricidal CTL activity but also by supporting tumor angiogenesis. The Gr-1+/CD11b+ cells, in fact, produce high levels of the matrix metalloprotease 9, which regulates the bioavailability of vascular endothelial growth factor. The selective deletion of the matrix metalloprotease 9 gene in these cells abolishes their ability to promote tumor growth and inhibits tumor formation (44). Although PDE5 inhibition could also affect these proangiogenic mechanisms, the lack of antitumor activity in sildenafil-treated Rag-2−/− mice (Fig. 1) and the dependence on CD8+ T cells (Fig. 4) would imply otherwise.
Although we have shown that PDE inhibitors reduce NOS2 and ARG1, the full mechanisms underlying these effects remain to be elucidated. One putative mechanism involves the impact of these inhibitors on mRNA stability. cGMP destabilizes NOS2 mRNA by reducing the ubiquitous mRNA binding protein, human antigen R (45). As such, destabilization of NOS2 mRNA via PDE5 inhibition would abrogate NO-mediated immunosuppression more effectively than would competitive inhibition of NO itself. However, because ARG1 mRNA does not possess adenylate/ uridylate-rich elements and has not been described to be stabilized by human antigen R, other mechanisms are likely involved in PDE5-mediated down-regulation of ARG1. One possibility is that high levels of cGMP induced by PDE5 blockade reduce the cytosolic Ca2+ concentration (46), leading to a reduction of the calcium-dependent protein kinase C activity (47) that in turn prevents up-regulation of IL-4Rα (48). The link between IL-4Rα and ARG1 in MDSCs is supported by recent data (29) demonstrating a direct correlation between ARG1 expression and IL-4Rα expression. ACT of tumor-primed CD8 T cells completely eradicated a preestablished C26GM tumor in the LysMCreIL-4Rα−/flox mice in which IL-4Rα expression has been deleted in neutrophils and macrophages, whereas no effect was seen in the control littermates (29). Our findings support these data by demonstrating that PDE5 blockade down-regulates IL-4Rα expression on tumor-infiltrating MDSCs (Fig. 6) and synergizes with the adoptive transfer of tumor-primed CD8+ T cells (Fig. 3 and Fig. 4). This effect appears to specifically target MDSCs, because IL-4Rα expression on isolated CD11b+ cells from tumor-bearing mice is significantly reduced when co-cultured in the presence of sildenafil (Fig. 5). Collectively, these findings underscore the critical role of the IL-4Rα–ARG1 pathway in MDSCs, as well as the use of PDE5 inhibitors as therapeutically effective drugs to overcome tumor-induced immunosuppression.
Although most of the work to date on MDSCs has focused on mouse Gr-1+/CD11b+ cells, emerging data confirm the presence of these cells in human malignancies (7, 42, 49). In this paper we show that a nonlymphoid CD14+ population mediates the hypo-responsiveness in PBLs from MM patients. Although the low proliferative capacity may be caused by intrinsic T cell defects, an additional explanation for T cell unresponsiveness is the presence of a nonlymphoid suppressor population whose function is abrogated by PDE5 inhibition. Evidence supporting this is the ability of sildenafil to augment the proliferative index of lymphocytes obtained from unfractionated PBMCs but not from purified CD4+ or CD8+ T cells (Fig. 8 and Fig. S6). The ability of sildenafil to restore proliferation of PBLs from both head and neck and myeloma patients suggests that the mechanisms found in mice also play a major role in cancer-mediated immunosuppression in two very different human malignancies.
Although drugs such l-NMMA, Nor-NOHA, NO-aspirin, or Vitamin D3 (4) have all been used in vitro and in mouse models to alter MDSC suppressive mechanisms, they have either not been extensively tested in humans or have been found to be extremely toxic, as is the case with l-NMMA (50). In demonstrating the ability to use clinically available PDE5 inhibitors to overcome the MDSC-mediated immunosuppressive pathways, these observations open the opportunity for the rapid translation of our preclinical findings into the clinic and give new hope for the development of more effective immune-based treatments for a broad range of human malignancies.
MATERIALS AND METHODS
Cell lines.
The following cell lines were used: CT26, a carcinogen-induced, undifferentiated colon carcinoma obtained from BALB/c mice (51); TS/A, a mouse mammary adenocarcinoma derived from BALB/c mice (52); MCA203, a C57BL/6-derived fibrosarcoma (53); and B16-GM, a C57BL/6 melanoma cell line genetically modified to secrete GM-CSF (54). The 4T1-HA cell line was obtained by lentiviral transduction of 4T1 mammary carcinoma and was provided by K. Whartenby (Johns Hopkins University, Baltimore, MD). These cell lines were grown in DMEM or RPMI 1640 (Invitrogen) with 10% FBS (Invitrogen). The C26GM cell line derived from the C26 colon carcinoma was genetically modified to produce GM-CSF (8) and was grown in the presence of 800 μg/ml G418.
Drugs and cytokines.
Sildenafil (Pfizer) was dissolved in the drinking water (20 mg/kg/24 h), given i.p. daily where indicated (20 mg/kg/24 h), or added to the cell cultures (50 mg/ml). 2 mg/kg/24 h tadalafil (Lilly ICOS) was administered i.p. In vivo treatments started on day 0 unless otherwise indicated.
NorNOHA and l-NMMA (Calbiochem) were used at 500 μM in vitro.
Mice and in vivo experiments.
4–6-wk-old BALB/c and C57BL/6 mice were purchased from Harlan. C57BL/6-NOS2−/− mice (strain B6;129P2-Nos2tm1Lau) and control mice (strain B6;129PF2/J-100903) were purchased from the Jackson Laboratory. BALB/c-Rag-2−/− (17), clone 4 mice transgenic for the H-2Kd–restricted TCR recognizing the influenza virus, HA peptide (HAp512–520) TCR-transgenic (6.5) mice recognizing the HAp110–120 presented by I-Ed, and OT-II TCR-transgenic mice recognizing OVAp329–337 presented by I-Ab were all bred in the Johns Hopkins University animal facility. BALB/c–pIL-2/GFP mice were a gift of C.T. Weaver (University of Alabama, Tuscaloosa, AL) (25). All mouse experiments were in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. 0.5 × 106 tumor cells were injected s.c. in the inguinal area. Tumor measurements were performed with a caliper by measuring the largest diameter and its perpendicular length. The tumor size index is the average of the product of these diameters measured independently by two operators. Gr-1 depletion was performed by i.p. injection of 100 μg of anti–Gr-1 depleting antibody (clone RB6.8C5-18) per mouse on days 0, 3, and 6. CD8 depletion used 200 μg of the anti-CD8 depleting antibody (clone 2.43) on days 0, 2, 4, and 6. All of the experiments were performed at least twice with five mice per group unless otherwise indicated in the figures.
In vivo CTL assay.
BALB/c splenocytes were stained either with 5 μM CFSE (Invitrogen) for 10 min at 37°C and pulsed with the relevant AH-1 MHC class I peptide (corresponding to amino acids 423–431 of gp70, SPSYVYHQF; CFSEhigh cells) or with 0.5 μM CFSE and pulsed with an irrelevant peptide (HA) as a control (CFSElow cells). 107 splenocytes (per population) were transferred i.v. to each host. The draining inguinal lymph nodes and spleens were harvested 40 h later and analyzed by FACS. Percent lysis was calculated as 1 − CFSEhigh/CFSElow normalized by the same ratio in naive BALB/c mice (%lysis = 100 × {1 − [(CFSEhigh exp/CFSElow exp)/(CFSEhigh BALB/c/CFSElow BALB/c)]}).
Vaccination and ACT.
The vaccine consisted of 106 γ-irradiated CT26 or C26GM cells injected s.c. For ACT experiments, mice were vaccinated on days −14 and −7 before ACT in all four limbs with 106 γ-irradiated C26GM. The CD8+ T cells were negatively purified using the CD8+ isolation kit (Miltenyi Biotec), and 3 × 106 CD8+ T cells were transferred per mouse. In the 4T1-HA experiments, HA-specific CD8+ naive T cells were isolated from spleens and lymph nodes of pCL4-TCR mice.
Flow cytometry.
Single cell suspensions from spleens or tumors were stained with PE-conjugated anti–mouse CD8 (CD8-PE; BD Biosciences), allophycocyanin-conjugated anti–mouse CD4 (CD4-allophycocyanin; BD Biosciences), FITC-conjugated anti–mouse CD11c, PE-conjugated anti–mouse B7.2, cychrome (Cy)-conjugated anti–mouse MHC class II, or with allophycocyanin-conjugated anti–mouse CD11b (BD Biosciences) and PE-conjugated anti–mouse Gr-1 (CD8-PE). IL-4Rα expression was determined on purified CD11b+ cells with PE-conjugated anti–mouse CD124 (BD Biosciences). Isotype-matched antibodies were used as controls, and live cells were gated based on 7-amino-actinomycin D, annexin V staining. Samples were run on a flow cytometer (FACSCalibur; BD Biosciences), and the data were analyzed using FCSexpress software (v 2.0; De Novo Software).
Cell purification.
CD11b+ purification was performed with mouse CD11b MicroBeads (Miltenyi Biotec). The positive and negative fractions were sorted with the LS columns according to the manufacturer's instructions. CD11c+ cells were positively selected from splenocytes of tumor-bearing mice using mouse CD11c MicroBeads (Miltenyi Biotec). CD4+ or CD8+ T cells were negatively selected, using the CD4+ or CD8+ T cell isolation kit (Miltenyi Biotec), from CD11b-depleted splenocytes. CD14 depletion of human PBMCs was obtained by adding PE-labeled, anti–human CD14 antibody (BD Biosciences). The CD14-negative population was collected using a cell sorter (FACSVantage SE; BD Biosciences).
Suppressive assay.
2 × 105 purified splenic or intratumoral CD11b+ cells were added to 106 clonotypic CFSE-labeled splenocytes. They were then peptide pulsed and cultured for 3 d in 96-well flat-bottom plates. Sildenafil was added where indicated in the figures.
Proliferation assay.
PBLs were obtained from MM or head and neck cancer patients after obtaining informed consent using an Institutional Review Board–approved protocol. Ficolled PBMCs were stimulated with anti-CD3/28 antibody-coated Dynal beads (3:1 bead/T cell ratio) for 5 d in a 96-well round-bottom plate and analyzed by flow cytometry. Sildenafil was added where indicated in the figures. Results are reported as fold change (number of activated cells/number of unactivated cells).
cGMP.
cGMP was measured on purified CD11b+, CD11c+, CD4+, or CD8+ cells using the Cyclic GMP EIA kit (Cayman Chemical). Data analysis was performed with the workbooks available at http://www.caymanchem.com/neptune/servlet/neptune/template/analysis%2CEIA.vm/a/z. Data are expressed as mean ± SE of quadruplicate wells.
Western blot.
Lysates from purified cells were denaturated at 95°C for 10 min and subjected to SDS-PAGE, and proteins were transferred overnight to polyvinylidene difluoride membranes. The membranes were incubated with a rabbit polyclonal anti-NOS2 antibody (Santa Cruz Biotechnology, Inc.), mouse anti-ARG1 antibody (a gift from Augusto C. Ochoa, Louisiana State University, New Orleans, LA), or a polyclonal rabbit anti-actin antibody (Sigma-Aldrich). Proteins were detected using the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Chemical Co.) according to the manufacturer's instructions.
NO measurement.
NO was measured using a nitrate/nitrite assay kit (Cayman Chemical) according to the manufacturer's instructions. Results were normalized to 106 cells. Data are from triplicate wells.
Arginase assay.
The arginase assay was performed as previously described (8) on purified intratumoral MDSCs.
Statistical analysis.
Bivariate Pearson and analysis of variance (ANOVA) analyses were performed using SPSS (v 7.0). All experiments were repeated at least twice, and all p-values were two sided (t test) or one sided (ANOVA).
Online supplemental material.
Fig. S1 shows that PDE-5 inhibitors reduce tumor growth but that persistent tumors show evidence of immune editing. In Fig. S2, the antitumor effect of sildenafil is maintained even with delayed treatment. Fig. S3 shows that sildenafil increases CD8+ TILs. Fig. S4 depicts sildenafil as it fails to induce an antitumor effect in 4T1-HA–bearing Rag-2−/− mice. In Fig. S5, sildenafil fails to augment proliferation of purified T cells. Fig. S6 shows that sildenafil does not alter IL-13 or IFN-γ production from CD11b+ cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061104/DC1.
Supplemental Material
Acknowledgments
We would like to thank Drs. Drew Pardoll, Elizabeth Jaffee, Katie Whartenby, Eduardo Sotomayor, Alessia Zoso, and Stephanie Mgebroff for their critical reading of the manuscript and Dr. Michael Lee for his assistance with the Western blot.
This work has been supported by grants from the MIUR-PRIN (project no. 2005069853), Cariverona, Bando 2004 “Integrazione tra tecnologia e sviluppo di settore - Bando per progetti di ricerca a indirizzo biomedico,” and the Italian Association for Cancer Research.
The authors declare no conflicting financial interests.
Abbreviations used: ACT, adoptive cell therapy; ANOVA, analysis of variance; ARG1, arginase-1; CFSE, carboxyfluorescein diacetate succinimidyl ester; cGMP, cyclic guanosine monophosphate; HA, hemagglutinin; l-NMMA, N G-monomethyl-l-arginine; MDSC, myeloid-derived suppressor cell; MM, multiple myeloma; NorNOHA, N-(omega)-hydroxy-nor-l-arginine; NOS2, nitric oxide synthase–2; PBL, peripheral blood lymphocyte; PDE5, phosphodiesterase-5; TIL, tumor-infiltrating lymphocyte.
References
- 1.Shankaran, V., H. Ikeda, A.T. Bruce, J.M. White, P.E. Swanson, L.J. Old, and R.D. Schreiber. 2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 410:1107–1111. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan, D.H., V. Shankaran, A.S. Dighe, E. Stockert, M. Aguet, L.J. Old, and R.D. Schreiber. 1998. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 95:7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou, W. 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 5:263–274. [DOI] [PubMed] [Google Scholar]
- 4.Serafini, P., I. Borrello, and V. Bronte. 2005. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 16:53–65. [DOI] [PubMed] [Google Scholar]
- 5.Kusmartsev, S., and D.I. Gabrilovich. 2005. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 174:4880–4891. [DOI] [PubMed] [Google Scholar]
- 6.Lin, H.H., D.E. Faunce, M. Stacey, A. Terajewicz, T. Nakamura, J. Zhang-Hoover, M. Kerley, M.L. Mucenski, S. Gordon, and J. Stein-Streilein. 2005. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 201:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich, D. 2004. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 4:941–952. [DOI] [PubMed] [Google Scholar]
- 8.Bronte, V., P. Serafini, C. De Santo, I. Marigo, V. Tosello, A. Mazzoni, D.M. Segal, C. Staib, M. Lowel, G. Sutter, et al. 2003. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170:270–278. [DOI] [PubMed] [Google Scholar]
- 9.Bronte, V., P. Serafini, A. Mazzoni, D.M. Segal, and P. Zanovello. 2003. l-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 24:302–306. [DOI] [PubMed] [Google Scholar]
- 10.Terabe, M., S. Matsui, J.M. Park, M. Mamura, N. Noben-Trauth, D.D. Donaldson, W. Chen, S.M. Wahl, S. Ledbetter, B. Pratt, et al. 2003. Transforming growth factor–β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte–mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 198:1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte, V., M. Wang, W.W. Overwijk, D.R. Surman, F. Pericle, S.A. Rosenberg, and N.P. Restifo. 1998. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol. 161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 12.Setter, S.M., J.L. Iltz, J.E. Fincham, R.K. Campbell, and D.E. Baker. 2005. Phosphodiesterase 5 inhibitors for erectile dysfunction. Ann. Pharmacother. 39:1286–1295. [DOI] [PubMed] [Google Scholar]
- 13.Lee, A.J., T.B. Chiao, and M.P. Tsang. 2005. Sildenafil for pulmonary hypertension. Ann. Pharmacother. 39:869–884. [DOI] [PubMed] [Google Scholar]
- 14.Takimoto, E., H.C. Champion, M. Li, D. Belardi, S. Ren, E.R. Rodriguez, D. Bedja, K.L. Gabrielson, Y. Wang, and D.A. Kass. 2005. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 11:214–222. [DOI] [PubMed] [Google Scholar]
- 15.Liu, L., H. Li, T. Underwood, M. Lloyd, M. David, G. Sperl, R. Pamukcu, and W.J. Thompson. 2001. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J. Pharmacol. Exp. Ther. 299:583–592. [PubMed] [Google Scholar]
- 16.Sarfati, M., V. Mateo, S. Baudet, M. Rubio, C. Fernandez, F. Davi, J.L. Binet, J. Delic, and H. Merle-Beral. 2003. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 101:265–269. [DOI] [PubMed] [Google Scholar]
- 17.Shinkai, Y., G. Rathbun, K.P. Lam, E.M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A.M. Stall, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 68:855–867. [DOI] [PubMed] [Google Scholar]
- 18.Suzue, K., E.L. Reinherz, and S. Koyasu. 2001. Critical role of NK but not NKT cells in acute rejection of parental bone marrow cells in F1 hybrid mice. Eur. J. Immunol. 31:3147–3152. [DOI] [PubMed] [Google Scholar]
- 19.Huang, A.Y., P.H. Gulden, A.S. Woods, M.C. Thomas, C.D. Tong, W. Wang, V.H. Engelhard, G. Pasternack, R. Cotter, D. Hunt, et al. 1996. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc. Natl. Acad. Sci. USA. 93:9730–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, L., J.R. Conejo-Garcia, D. Katsaros, P.A. Gimotty, M. Massobrio, G. Regnani, A. Makrigiannakis, H. Gray, K. Schlienger, M.N. Liebman, et al. 2003. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348:203–213. [DOI] [PubMed] [Google Scholar]
- 21.Mihm, M.C., Jr., C.G. Clemente, and N. Cascinelli. 1996. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab. Invest. 74:43–47. [PubMed] [Google Scholar]
- 22.Naito, Y., K. Saito, K. Shiiba, A. Ohuchi, K. Saigenji, H. Nagura, and H. Ohtani. 1998. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58:3491–3494. [PubMed] [Google Scholar]
- 23.Schumacher, K., W. Haensch, C. Roefzaad, and P.M. Schlag. 2001. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 61:3932–3936. [PubMed] [Google Scholar]
- 24.Vesalainen, S., P. Lipponen, M. Talja, and K. Syrjanen. 1994. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur. J. Cancer. 30A:1797–1803. [DOI] [PubMed] [Google Scholar]
- 25.Saparov, A., F.H. Wagner, R. Zheng, J.R. Oliver, H. Maeda, R.D. Hockett, and C.T. Weaver. 1999. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 11:271–280. [DOI] [PubMed] [Google Scholar]
- 26.Essayan, D.M. 2001. Cyclic nucleotide phosphodiesterases. J. Allergy Clin. Immunol. 108:671–680. [DOI] [PubMed] [Google Scholar]
- 27.Staveley-O'Carroll, K., E. Sotomayor, J. Montgomery, I. Borrello, L. Hwang, S. Fein, D. Pardoll, and H. Levitsky. 1998. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl. Acad. Sci. USA. 95:1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, R.F. 2006. Immune suppression by tumor-specific CD4+ regulatory T-cells in cancer. Semin. Cancer Biol. 16:73–79. [DOI] [PubMed] [Google Scholar]
- 29.Gallina, G., L. Dolcetti, P. Serafini, C.D. Santo, I. Marigo, M.P. Colombo, G. Basso, F. Brombacher, I. Borrello, P. Zanovello, et al. 2006. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8 T cells. J. Clin. Invest. 116:2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauleau, A.L., R. Rutschman, R. Lang, A. Pernis, S.S. Watowich, and P.J. Murray. 2004. Enhancer-mediated control of macrophage-specific arginase I expression. J. Immunol. 172:7565–7573. [DOI] [PubMed] [Google Scholar]
- 31.Kusmartsev, S., Y. Nefedova, D. Yoder, and D.I. Gabrilovich. 2004. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172:989–999. [DOI] [PubMed] [Google Scholar]
- 32.Pekarek, L.A., B.A. Starr, A.Y. Toledano, and H. Schreiber. 1995. Inhibition of tumor growth by elimination of granulocytes. J. Exp. Med. 181:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seung, L.P., D.A. Rowley, P. Dubey, and H. Schreiber. 1995. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc. Natl. Acad. Sci. USA. 92:6254–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzoni, A., V. Bronte, A. Visintin, J.H. Spitzer, E. Apolloni, P. Serafini, P. Zanovello, and D.M. Segal. 2002. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168:689–695. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez, P.C., D.G. Quiceno, J. Zabaleta, B. Ortiz, A.H. Zea, M.B. Piazuelo, A. Delgado, P. Correa, J. Brayer, E.M. Sotomayor, et al. 2004. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64:5839–5849. [DOI] [PubMed] [Google Scholar]
- 36.Pak, A.S., M.A. Wright, J.P. Matthews, S.L. Collins, G.J. Petruzzelli, and M.R.I. Young. 1995. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 1:95–103. [PubMed] [Google Scholar]
- 37.Young, M.R., M.A. Wright, and R. Pandit. 1997. Myeloid differentiation treatment to diminish the presence of immune-suppressive CD34+ cells within human head and neck squamous cell carcinomas. J. Immunol. 159:990–996. [PubMed] [Google Scholar]
- 38.Bronte, V., T. Kasic, G. Gri, K. Gallana, G. Borsellino, I. Marigo, L. Battistini, M. Iafrate, T. Prayer-Galetti, F. Pagano, and A. Viola. 2005. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin, C.S., S. Chow, A. Lau, R. Tu, and T.F. Lue. 2001. Regulation of human PDE5A2 intronic promoter by cAMP and cGMP: identification of a critical Sp1-binding site. Biochem. Biophys. Res. Commun. 280:693–699. [DOI] [PubMed] [Google Scholar]
- 40.van Oijen, M., A. Bins, S. Elias, J. Sein, P. Weder, G. de Gast, H. Mallo, M. Gallee, H. Van Tinteren, T. Schumacher, and J. Haanen. 2004. On the role of melanoma-specific CD8+ T-cell immunity in disease progression of advanced-stage melanoma patients. Clin. Cancer Res. 10:4754–4760. [DOI] [PubMed] [Google Scholar]
- 41.Haanen, J.B., A. Baars, R. Gomez, P. Weder, M. Smits, T.D. de Gruijl, B.M. von Blomberg, E. Bloemena, R.J. Scheper, S.M. van Ham, et al. 2006. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol. Immunother. 55:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baniyash, M. 2004. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat. Rev. Immunol. 4:675–687. [DOI] [PubMed] [Google Scholar]
- 43.De Santo, C., P. Serafini, I. Marigo, L. Dolcetti, M. Bolla, P. Del Soldato, C. Melani, C. Guiducci, M.P. Colombo, M. Iezzi, et al. 2005. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl. Acad. Sci. USA. 102:4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, L., L.M. DeBusk, K. Fukuda, B. Fingleton, B. Green-Jarvis, Y. Shyr, L.M. Matrisian, D.P. Carbone, and P.C. Lin. 2004. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 6:409–421. [DOI] [PubMed] [Google Scholar]
- 45.Pilz, R.B., and D.E. Casteel. 2003. Regulation of gene expression by cyclic GMP. Circ. Res. 93:1034–1046. [DOI] [PubMed] [Google Scholar]
- 46.Rotella, D.P. 2002. Phosphodiesterase 5 inhibitors: current status and potential applications. Nat. Rev. Drug Discov. 1:674–682. [DOI] [PubMed] [Google Scholar]
- 47.Webb, B.L., S.J. Hirst, and M.A. Giembycz. 2000. Protein kinase C isoenzymes: a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br. J. Pharmacol. 130:1433–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vellenga, E., W. Dokter, and R.M. Halie. 1993. Interleukin-4 and its receptor; modulating effects on immature and mature hematopoietic cells. Leukemia. 7:1131–1141. [PubMed] [Google Scholar]
- 49.Zea, A.H., P.C. Rodriguez, M.B. Atkins, C. Hernandez, S. Signoretti, J. Zabaleta, D. McDermott, D. Quiceno, A. Youmans, A. O'Neill, et al. 2005. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65:3044–3048. [DOI] [PubMed] [Google Scholar]
- 50.Freeman, B.D., R.L. Danner, S.M. Banks, and C. Natanson. 2001. Safeguarding patients in clinical trials with high mortality rates. Am. J. Respir. Crit. Care Med. 164:190–192. [DOI] [PubMed] [Google Scholar]
- 51.Griswold, D.P., and T.H. Corbett. 1975. A colon tumor model for anticancer agent evaluation. Cancer. 36:2441–2444. [DOI] [PubMed] [Google Scholar]
- 52.Nanni, P., C. de Giovanni, P.L. Lollini, G. Nicoletti, and G. Prodi. 1983. TS/A: a new metastasizing cell line from a BALB/c spontaneous mammary adenocarcinoma. Clin. Exp. Metastasis. 1:373–380. [DOI] [PubMed] [Google Scholar]
- 53.Spiess, P.J., J.C. Yang, and S.A. Rosenberg. 1987. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J. Natl. Cancer Inst. 79:1067–1075. [PubMed] [Google Scholar]
- 54.Dranoff, G., E. Jaffee, A. Lazenby, P. Golumbek, H. Levitsky, K. Brose, V. Jackson, H. Hamada, D. Pardoll, and R.C. Mulligan. 1993. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 90:3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.