Abstract
Ischemia-reperfusion (I/R) liver injury occurs when blood flow is restored after prolonged ischemia. A short interruption of blood flow (ischemic preconditioning [IP]) induces tolerance to subsequent prolonged ischemia through ill-defined mechanisms. Cardiotrophin (CT)-1, a cytokine of the interleukin-6 family, exerts hepatoprotective effects and activates key survival pathways like JAK/STAT3. Here we show that administration of CT-1 to rats or mice protects against I/R liver injury and that CT-1–deficient mice are exceedingly sensitive to this type of damage. IP markedly reduced transaminase levels and abrogated caspase-3 and c-Jun–NH2-terminal kinase activation after I/R in normal mice but not in CT-1–null mice. Moreover, the protective effect afforded by IP was reduced by previous administration of neutralizing anti–CT-1 antibody. Prominent STAT3 phosphorylation in liver tissue was observed after IP plus I/R in normal mice but not in CT-1–null mice. Oxidative stress, a process involved in IP-induced hepatoprotection, was found to stimulate CT-1 release from isolated hepatocytes. Interestingly, brief ischemia followed by short reperfusion caused mild serum transaminase elevation and strong STAT3 activation in normal and IL-6–deficient mice, but failed to activate STAT3 and provoked marked hypertransaminasemia in CT-1–null animals. In conclusion, CT-1 is an essential endogenous defense of the liver against I/R and is a key mediator of the protective effect induced by IP.
Ischemia-reperfusion (I/R) damage develops when liver blood flow is interrupted, or severely diminished, for a long period of time and then restarted. Ischemia may induce cell death by itself by causing ATP depletion, but mainly primes the cells for the more intense damage that occurs when the liver is reperfused (1). Upon reentry of oxygen, uncoupled dysfunctional mitochondria produce large amounts of oxygen-free radicals, intense oxidative stress, and mitochondrial permeability transition leading to cell death (1). On reperfusion activation of Kupffer cells also occurs, leading to abundant production of reactive oxygen species and proinflammatory cytokines, further enhancing organ damage (1). I/R injury can cause cell death by apoptosis or necrosis (1) depending on the intensity of ATP depletion. I/R liver damage is of great clinical importance because it can cause primary graft nonfunction after liver transplantation and may critically compromise the function of the remaining liver after major hepatic resections (2). The development of new therapeutic approaches to control I/R injury may benefit from better understanding of the defensive mechanisms set into motion in the liver when it is subjected to ischemic insults.
In the liver, and in various tissues, it has been shown that a short period of ischemia protects efficiently against subsequent I/R injury (3). This phenomenon, known as ischemic preconditioning (IP), indicates that a brief ischemic insult triggers a protective biological reaction in the liver which is associated with inhibition of proapoptotic pathways (3, 4). Although several mechanisms have been invoked, there is increasing evidence supporting that a sublethal oxidative stress, as occurs during a short ischemic interval, plays a crucial role in the induction of IP (4). In this regard recent reports have demonstrated that the protective effect granted by IP on subsequent ischemic injury can be mimicked by treatment with H2O2 or an H2O2 analogue (5, 6). However, the downstream effectors of the protective action of reactive oxygen species are still not known.
Cardiotrophin (CT)-1 is member of the IL-6 family of cytokines that binds to a specific receptor that contains gp130 and leukemia inhibitory factor receptor (7). gp130 is common to the receptor complex of other members of IL-6 superfamily and is required for both ligand binding and signal transduction (7). CT-1 is expressed by both parenchymal and nonparenchymal liver cells and exerts potent antiapoptotic effects on hepatocytes (8). In these cells, as in cardiomyocytes and neurons, CT-1 activates cell survival signaling pathways including STAT3, extracellular-regulated kinase (Erk)1/2, and protein kinase B (Akt) (8–10). In the present work we have analyzed the possible role of CT-1 as a natural defense of the liver against I/R injury.
RESULTS AND DISCUSSION
Treatment with recombinant CT-1 reduces I/R liver injury
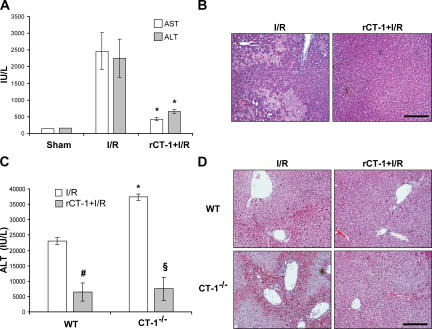
To determine if CT-1 was able to attenuate I/R injury, 400 μg/kg of body weight of recombinant rat CT-1 (rCT-1) was administered to Wistar rats 10 min before clamping the artery of the medium and left liver lobes. Samples were obtained at 6 h of reperfusion after 1 h of ischemia. We found that although untreated rats showed a marked rise of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and exhibited large areas of necrosis in the liver biopsy, those that were pretreated with CT-1 showed little variation of transaminases and no relevant histological changes in the liver parenchyma (Fig. 1, A and B). Subsequent determination of transaminases levels at 12 h of reperfusion showed maintained low values in rats pretreated with rCT-1 but high levels in untreated animals (unpublished data).
Figure 1.
CT-1 defends the liver against I/R damage. (A) AST and ALT levels in the serum of rats after 1 h ischemia and 6 h reperfusion (I/R), sham-operated animals, or rats that were treated with CT-1 (400 μg/kg of weight, i.v.) 10 min before I/R. Values are means ± SD; 8 rats were used per treatment. *, P < 0.01 versus I/R. (B) H&E staining of representative liver tissue sections from rats that received either saline (I/R) or rCT-1 (rCT-1+I/R) before I/R as described previously. (C) ALT levels in the serum of WT and CT-1−/− mice after 75 min of ischemia and 3 h of reperfusion (I/R). Where indicated mice were treated with CT-1 (400 μg/kg of weight, i.v.) 10 min before I/R (rCT-1+I/R). Values are means ± SD; 5 mice were used per treatment. #, P < 0.05 versus untreated WT mice; §, P < 0.01 versus untreated CT-1−/− mice; *, P < 0.05 versus untreated WT mice. (D) H&E staining of representative liver tissue sections from WT and CT-1−/− mice that received either saline (I/R) or rCT-1 (rCT-1+I/R) before I/R as described previously. Bars, 100 μm.
In another set of experiments rCT-1 at the dose of 800 μg/kg of body weight was given at the time of reperfusion after 1 h of ischemia. In these cases serum transaminases at 12 h of reperfusion were higher than in animals that received CT-1 before ischemia, but still there was significant (P < 0.05) protection compared with untreated rats (ALT: 5,726 ± 2,765 and 1,904 ± 478 in untreated and rCT-1–treated animals, respectively). These findings and our previous data showing that CT-1 was able to abrogate concanavalin A–induced hepatitis (8) indicate that this cytokine is able to exert hepatoprotective activity against diverse forms of liver damage.
CT-1 is an essential endogenous defense of the liver against I/R injury
Next, we wished to determine if CT-1 might be involved in the natural biological process that defends the liver against ischemia. To this aim we subjected CT-1–deficient mice to 75-min ischemia of the left and median lobes followed by reperfusion. We observed that the rise of serum transminases and the severity of hemorrhagic necrosis in the liver tissue that was exposed to ischemia were more intense in CT-1–null mice than in WT animals when analyzed at 3 h of reperfusion (Fig. 1, C and D). The higher sensitivity to I/R damage exhibited by CT-1–deficient mice was not caused by some abnormality different from the lack of this cytokine, because these mice were protected against I/R injury by administration of rCT-1 in the same manner as normal animals (Fig. 1, C and D). In rCT-1–treated mice (both WT and CT-1–null animals) serum ALT levels at 6 and 24 h after reperfusion remained significantly (P < 0.05) lower than in untreated animals (unpublished data), indicating that CT-1 treatment effectively prevented tissue injury and did not merely delay it. These data reveal an up to now unrecognized role of CT-1 as a natural defense of the liver against I/R damage.
CT-1 is a key executor of liver protection induced by ischemic preconditioning
The role played by CT-1 in liver defense against I/R prompted us to investigate if this cytokine could be a mediator of the protective biological response induced by IP. We observed that when the left and median liver lobes of normal mice were subjected to a brief period of ischemia and 15 min of reperfusion (IP) followed by 75 min of ischemia and 3 h of reperfusion (I/R injury), the histological liver lesion, the number of apoptotic hepatocyte nuclei (as estimated by the terminal deoxynucleotide transferase–mediated dUDP nick-end labeling [TUNEL] technique), and the rise of serum transaminases were markedly reduced compared with those shown by animals exposed to I/R insult without previous IP (Fig. 2, A–C). It has been reported that I/R damage is associated with phosphorylation of c-Jun–NH2-terminal kinase (JNK), an oxidative stress–responsive kinase activated during IR liver injury (11, 12), and with activation of the proapoptotic caspase 3, a critical executor of I/R liver damage (13, 14). We found that although I/R injury caused activation of caspase 3 and phosphorylation of JNK and c-Jun in liver tissue, these events did not occur when I/R was preceded by IP (Fig. 2 D).
Figure 2.
CT-1 is an indispensable mediator of the hepatoprotective effect induced by ischemic preconditioning. (A) ALT levels in the serum of WT and CT-1−/− mice after 1 h of ischemia and 3 h of reperfusion (I/R), preceded or not by ischemic preconditioning (IP) (10 min of ischemia followed by 15 min of reperfusion). Values are means ± SD; 6 mice were used per treatment. *, P < 0.05 versus WT mice subjected to I/R; #, P < 0.05 versus WT mice without IP. (B) H&E staining of representative liver tissue sections from WT and CT-1−/− mice after I/R preceded or not by ischemic preconditioning (IP). (C) TUNEL staining of representative liver sections from WT and CT-1−/− mice after I/R preceded or not by ischemic preconditioning (IP). (D) Representative Western blot analyses of active caspase 3 p-17, phosphorylated JNK, and phosphorylated c-Jun in liver samples from WT and CT-1−/− mice under basal conditions (C), and after I/R or I/R preceded by IP (IP+I/R). Bars, 100 μm.
In contrast to WT mice, IP lacked protective effect in CT-1–deficient mice. In these animals the rise of serum transaminases, the intensity of the histological liver damage, the abundance of TUNEL-positive hepatocyte nuclei, and the activation of caspase 3, JNK, and c-Jun in hepatic tissue after I/R were similar in all mice independently of whether they had previous exposure to IP or not (Fig. 2, A–D).
STAT3 promotes antiapoptotic effects in many tissues including the liver (15), and it has been shown that the gp130-STAT3 signaling pathway mediates the hepatoprotection induced by gp130 ligands (16). There is also evidence implicating STAT3 activation in the development of heart and brain protection associated with ischemic preconditioning (17, 18), but the mechanisms by which STAT3 is activated in response to IP remain ill understood. In the present work we found activation of STAT3 in hepatic tissue in association with liver protection against I/R injury. Thus, marked STAT3 phosphorylation together with nuclear translocation of STAT3 in hepatocytes were found in normal mice exposed to I/R challenge preceded by IP or rCT-1 administration but not in those subjected to I/R without previous treatment (Fig. 3, A and B). In contrast, IP was unable to induce STAT3 activation in CT-1–null mice in accordance with the absence of protective effect of IP in these animals. However, there was prominent phosphorylation and nuclear translocation of STAT3 in livers from CT-1–null mice after I/R when the animals were pretreated with rCT-1 (Fig. 3, A and B), a therapy that afforded protection against I/R injury.
Figure 3.
CT-1–deficient mice fail to activate STAT-3 in liver cells after ischemic preconditioning. (A) Representative Western blot analyses of STAT3 phosphorylation (tyr 705) and STAT3 protein levels in the liver of WT and CT-1−/− mice under basal conditions (C), and after I/R or I/R preceded by IP (IP+I/R) or by CT-1 treatment (rCT-1+I/R). (B) Immunohistochemical detection of phosphorylated STAT3 in representative liver sections from WT and CT-1−/− mice after I/R, I/R preceded by IP, or in mice treated with CT-1 (400 μg/kg of weight, i.v.) 10 min before I/R (rCT-1+I/R). Bar, 100 μm.
Although it has been reported that rCT-1 may defend cardiac cells against hypoxic damage (19) and neurones against oxidative injury (20), there was no information as to whether endogenous CT-1 participates in the biological protective response elicited by IP. Our results in CT-1–deficient mice reveal that CT-1 is a critical mediator of STAT3 activation and nuclear translocation in animals exposed to IP and that CT-1 is an essential component of the hepatoprotective reaction set into motion by IP.
Administration of neutralizing antibodies to CT-1 blunt the protective effect of IP
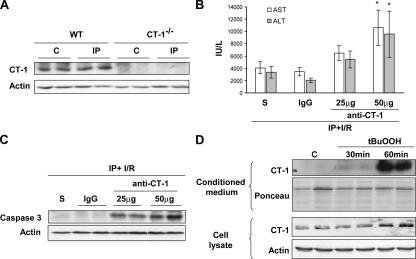
Once we found that CT-1 was a critical component of the defensive mechanism promoted by IP, we wished to determine whether the IP could affect the expression of CT-1 in liver tissue. We observed that the hepatic levels of CT-1 protein did not change after 10 min of ischemia and 15 min of reperfusion (Fig. 4 A), suggesting that IP has no manifest effect on CT-1 synthesis. We reasoned that IP might provoke the release of preformed cytokine to the extracellular milieu to induce local autocrine and paracrine effects. To evaluate this possibility we performed an experiment consisting of the administration of neutralizing anti–CT-1 antibodies to mice subjected to IP followed by I/R. In agreement with our previous observation showing that IP was not effective in CT-1−/− mice (Fig. 2, A–D), neutralization of CT-1 in WT mice significantly (P < 0.05) blunted the protective effect of IP on I/R liver injury. This was indicated by the rise in serum AST and ALT levels, and by the activation of caspase 3 in the liver of mice treated with anti–CT-1 antibody compared with controls (IgG) (Fig. 4, B and C). Anti–CT-1 antibody administration to sham-operated mice had no effect on serum transaminases levels (unpublished data). These observations further confirmed the hepatoprotective role of CT-1, and also suggested that CT-1 must be released from intracellular stores to the extracellular milieu to mediate the hepatoprotective effects of IP. We were unable to detect by ELISA circulating CT-1 either in basal condition or after IP (unpublished data). It seems possible that CT-1 may act mainly paracrinally during IP.
Figure 4.
The protective effects of IP are blunted by anti–CT-1–neutralizing antibodies. Release of CT-1 from isolated hepatocytes upon induction of oxidative stress. (A) CT-1 is not up-regulated in IP. Representative Western blot analysis of CT-1 protein levels in the liver of WT and CT-1−/− mice under basal conditions (C) or after ischemic preconditioning (IP). Actin levels are shown as loading control. (B) Neutralizing antibody to CT-1 impairs the protective effect granted by IP on I/R liver injury. ALT and AST levels in the serum of mice that received saline (S), 50 μg of preimmune IgG (IgG), or increasing doses of CT-1–neutralizing antibody (anti–CT-1) 15 min before IP, and subsequently underwent I/R. *, P < 0.05 versus mice that received preimmune IgG. (C) Representative Western blot analyses of active caspase 3 p-17 in liver samples from mice treated as described in B. Actin levels are shown as loading control. (D) Representative Western blot analyses of CT-1 protein levels in the conditioned culture medium and cell lysates obtained from control mouse hepatocytes (C) or hepatocytes treated with tBuOOH (500 μM) for different periods of time. Ponceau S stain of Western blot membranes and actin levels are shown as loading controls.
Oxidative stress induces the release of CT-1 from hepatocytes to extracellular milieu
It has been shown that a sublethal oxidative stress is a key event that mediates the cytoprotective effect of IP in the liver (5, 6). Sublethal concentrations of oxygen-free radicals, likely produced by Kupffer cells (6), are thought to trigger protective mechanisms on subsequent periods of ischemia; however, the identity of such mechanisms remains elusive (5). Therefore, we analyzed whether isolated hepatocytes from normal mice could release CT-1 upon exposure to a prooxidant such as the H2O2 analogue tert-butyl-hydroperoxide (tBuOOH), previously shown to mimic the effect of IP in mice (5). Western blot analysis of the supernatant of cultured hepatocytes at 30 and 60 min of incubation showed absence of CT-1 in the medium of nonstimulated cells, whereas a strong signal was observed at 60 min of incubation with tBuOOH (Fig. 4 D). A slight increase in the intracellular levels of CT-1 protein was observed after 60 min of treatment with tBuOOH (Fig. 4 D). This could be interpreted as a compensatory response to replenish intracellular stores of CT-1 after oxidative stress–stimulated release of this cytokine. From these observations it is conceivable that the oxidative stress generated during IP is responsible for the release of CT-1 to extracellular milieu. Our present data shed light on the mechanism by which oxidative stress promotes IP-induced hepatoprotection by showing that this event leads to CT-1 release, and that this cytokine is essential for the cytoprotective effect to occur because it is absent in CT-1–null mice.
CT-1 but not IL-6 mediates the liver defense against brief ischemia
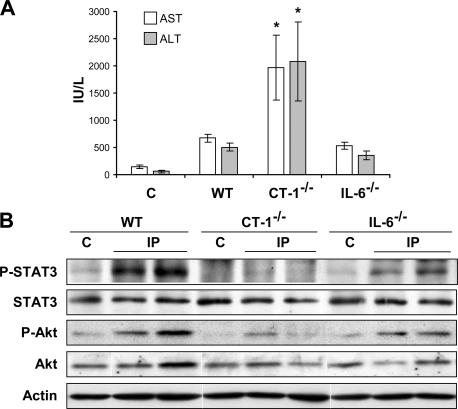
The phenomenon of IP indicates that normal livers tolerate a short period of ischemia (and reperfusion) well by setting into motion protective mechanisms that adapt the phenotype of the tissue not only to resist this brief I/R insult but also to acquire tolerance to subsequent I/R of longer duration. The inability of CT-1–null mice to elicit protective IP suggests that CT-1 might also be essential to defend the liver against ischemia of short duration. Because IL-6 has been suggested to play a role in the modulation of I/R liver damage, and treatment with recombinant IL-6 substantially protects from ischemic liver injury (21), we decided to compare the relative role of CT-1 and IL-6 in the defense of the liver against brief I/R. To this aim we exposed the left and median lobes of the liver of WT mice, CT-1–null mice, and IL-6–null mice to 10 min of ischemia followed by 15 min of reperfusion, and at the end of this time we analyzed serum AST and ALT values and the activation of survival factors STAT3 and Akt in hepatic tissue. We observed that in both WT and IL-6–null mice AST and ALT levels showed little change with respect to control values. This resistance to short I/R exposure was associated with STAT3 and Akt activation in the two groups of animals, although with less intensity in IL-6–deficient mice (Fig. 5, A and B). In sharp contrast, in CT-1–null mice AST and ALT were considerably elevated and there was no sign of STAT3 activation and only a faint signal of phosphorylated Akt (Fig. 5, A and B). This finding reveals that CT-1, rather than IL-6, is a critical factor in the defense of the liver against ischemia of short duration.
Figure 5.
CT-1, and not IL-6, is the critical liver defense against a short period of ischemia. (A) AST and ALT levels in the serum of WT, CT-1−/−, and IL-6−/− mice after 10 min liver ischemia followed by 15 min of reperfusion. Values are means ± SD; 5 mice were used per treatment. (B) Representative Western blot analyses of STAT3 phosphorylation (tyr 705), STAT3 protein levels, Akt phosphorylation, and Akt protein levels in the liver of WT, CT-1−/−, and IL-6−/− mice under basal conditions (C) and after 10 min of liver ischemia and 15 min of reperfusion. Actin levels are shown as loading control.
The role of CT-1 as a natural defense against I/R liver injury and the ability of rCT-1 to protect against this form of hepatocellular damage point to potential therapeutic applications of rCT-1. New effective therapies are urgently needed for patients undergoing large hepatic resections because avoidance of I/R damage may have an important impact on postoperative morbidity and mortality by improving the function of the remaining small liver. The attractiveness of rCT-1 as a potential drug in liver surgery is enhanced by the striking increase in the number of hepatic surgical interventions during the last years owing to the frequent practice of major hepatic resections for primary or metastatic liver cancer and the increasing application of living donor liver transplantation.
MATERIAL AND METHODS
Animals.
We followed University of Navarra guidelines for the use of laboratory animals. Male Wistar rats (250–275 g) were from Harlan. C57/BL6 CT-1–null mice (CT-1−/−) (22) and WT mice were a gift from Dr. M. Selzner (Zurich University Hospital, Zurich, Switzerland). C57/BL6 IL-6–null mice (IL-6−/−) were from The Jackson Laboratory.
Surgical procedure.
Rats anesthetized with isoflurane (Abbott) were subjected to segmental hepatic ischemia followed by reperfusion (23). They were killed after 1 h of ischemia and 6 or 12 of reperfusion (I/R), and serum and liver biopsies were harvested. Sham animals were manipulated identically but without vascular clamping.
A similar I/R procedure was performed in CT-1−/– and WT male mice 8–10 wk old (5). The left and median lobes were occluded for 75 min, and after 3 h of reperfusion mice were killed for blood and tissue sampling. Where indicated IP consisting of 10 min of ischemia followed by 15 min of reperfusion was performed before I/R. Also where indicated, goat preimmune IgG (Sigma-Aldrich) or CT-1–neutralizing antibodies (R&D Systems) were administered i.v. to WT mice 15 min before IP+I/R. WT, CT-1−/–, and IL-6−/– mice were subjected to brief ischemia of 10 min and killed after 15 min of reperfusion.
Liver samples were snap frozen in liquid nitrogen or formalin fixed and paraffin embedded for histological studies. Serum was used for AST and ALT aminotransferases analysis.
Rats were given 400 μg/kg body weight of rCT-1 (8) i.v. 10 min before ischemia or 800 μg/kg body weight just after declamping. In mice 400 μg/kg body weight of rCT-1 was injected 10 min before ischemia. These doses were selected based on dose–response studies performed in mice and were extrapolated to rat experiments. In these experiments, a clear protective effect was already observed at 200 μg/kg body weight of rCT-1, and protection was maximal at 400 μg/kg body weight. The rCT-1 that was used for these studies contained 0.14 pg/μg protein of LPS (Limulus amoebocyte lysate assay; Cambrex).
Histological analysis.
Hematoxylin and eosin (H&E) staining and TUNEL assay (Roche Applied Science) were performed on paraffin-embedded liver sections as described (8, 24). Immunohistochemistry was performed on paraffin-embedded liver sections using a polyclonal anti–P-STAT3 (tyr 705) antibody (Cell Signaling) (25). The EnVision kit (Dako) was used for detection.
Western blot.
Western blot was performed (24) using antibodies specific for caspase 3, P-STAT3 (tyr 705), STAT3, P-Akt, Akt (Cell Signaling), P-JNK, P–c-Jun (Santa Cruz Biotechnology, Inc), actin (Sigma-Aldrich), and CT-1 (R&D Systems).
In vitro studies.
Mouse primary hepatocytes were isolated and cultured as described (25). After adhesion, hepatocytes were treated for 30 or 60 min with 500 μM of tBuOOH (Sigma-Aldrich). Cells were lysed for Western blot analyses as previously described (24). Conditioned medium was harvested and concentrated before Western blot analysis. Cell viability was not affected by tBuOOH treatment because no differences were observed in lactate dehydrogenase activity between the supernatant of control and treated hepatocytes, as assessed by the CytoTox-ONE assay from Promega.
Statistical analysis.
Statistical methods used were as described previously (26). Data are means ± SD; a p value of < 0.05 was considered significant.
Acknowledgments
The technical help of Eva Petri and Sonia Gárate is acknowledged.
This work was supported by grant SAF-2005-03513 (J. Prieto) and by the agreement UTE project CIMA.
The authors have no conflicting financial interests.
M.A. Avila and J. Prieto are senior authors on this paper.
References
- 1.Jaeschke, H., and J.J. Leamsters. 2003. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 125:1246–1257. [DOI] [PubMed] [Google Scholar]
- 2.Teoh, N.C., and G.C. Farrell. 2003. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J. Gastroenterol. Hepatol. 18:891–902. [DOI] [PubMed] [Google Scholar]
- 3.Selzner, N., H. Rudiger, R. Graf, and P.A. Clavien. 2003. Protective strategies against ischemic injury of the liver. Gastroenterology. 125:917–936. [DOI] [PubMed] [Google Scholar]
- 4.Carini, R., and E. Albano. 2003. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 125:1480–1491. [DOI] [PubMed] [Google Scholar]
- 5.Rudiger, H.A., R. Graf, and P.A. Clavien. 2003. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J. Hepatol. 39:972–977. [DOI] [PubMed] [Google Scholar]
- 6.Tejima, K., A. Mashairo, I. Hitoshi, T. Tomoaki, M. Yanase, Y. Inoue, K. Nagashima, T. Nishikawa, N. Watanabe, M. Omata, and K. Fujiwara. 2004. Ischemic preconditioning protects hepatocytes via reactive oxygen species derives from Kupffer cells in rats. Gastroenterology. 127:1488–1496. [DOI] [PubMed] [Google Scholar]
- 7.Pennica, D., W.I. Wood, and K.R. Chien. 1996. Cardiotrophin-1: a multifunctional cytokine that signals via LIF receptor-gp130 dependent pathways. Cytokine Growth Factor Rev. 7:81–91. [DOI] [PubMed] [Google Scholar]
- 8.Bustos, M., N. Beraza, J.J. Lasarte, E. Baixeras, P. Alzuguren, T. Bordet, and J. Prieto. 2003. Protection against liver damage by cardiotrophin-1: a hepatocyte survival factor up-regulated in the regenerating liver in rats. Gastroenterology. 125:192–201. [DOI] [PubMed] [Google Scholar]
- 9.Lopez, N., J. Diez, and M.A. Fortuno. 2005. Characterization of the protective effects of cardiotrophin-1 against non-ischemic death stimuli in adult cardiomyocytes. Cytokine. 30:282–292. [DOI] [PubMed] [Google Scholar]
- 10.Dolcet, X., R.M. Soler, T.W. Gould, J. Egea, R.W. Oppenheim, and J.X. Comella. 2001. Cytokines promote motoneuron survival through the Janus kinase-dependent activation of the phosphatidylinositol 3-kinase pathway. Mol. Cell. Neurosci. 18:619–631. [DOI] [PubMed] [Google Scholar]
- 11.Schwabe, R.F., and D.A. Brenner. 2006. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G583–G589. [DOI] [PubMed] [Google Scholar]
- 12.Tsung, A., R. Sahai, H. Tanaka, A. Nakao, M.P. Fink, M.T. Lotze, H. Yang, J. Li, K.J. Tracey, D.A. Geller, and T.R. Billiar. 2005. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 201:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller, T.H., K. Kienle, A. Beham, E.K. Geissler, K.W. Jauch, and M. Rentsch. 2004. Caspase 3 inhibition improves survival and reduces early graft after ischemia and reperfusion in rat liver transplantation. Transplantation. 78:1267–1273. [DOI] [PubMed] [Google Scholar]
- 14.Contreras, J.L., M. Vilatoba, C. Eckstein, G. Bilbao, J. Anthony Thompson, and D.E. Eckhoff. 2004. caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver inmice. Surgery. 136:390–400. [DOI] [PubMed] [Google Scholar]
- 15.Haga, S., K. Terui, H.Q. Zhang, S. Enosawa, W. Ogawa, H. Inoue, T. Okuyama, K. Takeda, S. Akira, T. Ogino, et al. 2003. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J. Clin. Invest. 112:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, C., T. Wustfeld, U. Assmus, T. Roskams, S. Rose-John, M. Muller, M.P. Manns, M. Esrnst, and C. Trautwein. 2005. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J. Clin. Invest. 115:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori, R., N. Maulik, H. Otani, L. Zhu, G. Cordis, R.M. Engelman, M.A. Siddiqui, and D.K. Das. 2001. Role of STAT3 in ischemic preconditioning. J. Mol. Cell. Cardiol. 33:1929–1936. [DOI] [PubMed] [Google Scholar]
- 18.Smith, R.M., N. Suleman, L. Lacerda, L.H. Opie, S. Akira, K.R. Chien, and M.N. Sack. 2004. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc. Res. 63:611–616. [DOI] [PubMed] [Google Scholar]
- 19.Brar, B.K., A. Stephanou, Z. Liao, R.M. O'Leary, D. Pennica, D.M. Yellon, and D.S. Latchman. 2001. Cardiotrophin-1 can protect cardiac myocytes from injury when added both prior to simulated ischemia and at reoxygenation. Cardiovasc. Res. 51:265–274. [DOI] [PubMed] [Google Scholar]
- 20.Wen, T.C., M.R. Rogido, J.E. Moore, T. Genetta, H. Peng, and A. Sola. 2005. Cardiotrophin-1 protects cortical neuronal cells against free radical-induced injuries in vitro. Neurosci. Lett. 387:38–42. [DOI] [PubMed] [Google Scholar]
- 21.Camargo, C.A., J.F. Madden, W. Gao, R.S. Selvan, and P.A. Clavien. 1997. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 26:1513–1520. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim, R.W., S. Wiese, D. Prevette, M. Armanini, S. Wang, L.J. Houenou, B. Holtzmann, R. Grotz, D. Pennica, and M. Sendtner. 2001. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J. Neurosci. 21:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peralta, C., G. Hotter, D. Closa, N. Prats, C. Xaus, E. Gelpi, and J. Rosello-Catafau. 1999. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 29:126–132. [DOI] [PubMed] [Google Scholar]
- 24.Berasain, C., E.R. Garcia-Trevijano, J. Castillo, E. Erroba, M. Santamaria, D.C. Lee, J. Prieto, and M.A. Avila. 2005. Novel role of amphiregulin in protection from liver injury. J. Biol. Chem. 280:19012–19020. [DOI] [PubMed] [Google Scholar]
- 25.Larrea, E., R. Aldabe, E. Molano, C.M. Fernandez-Rodriguez, A. Ametzazurra, M.P. Civeira, and J. Prieto. 2005. Altered expression and activation of STATs (signal transduction and activator of transcription) in HCV infection: in vivo and in vitro studies. Gut. 55:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berasain, C., E.R. Garcia-Trevijano, J. Castillo, E. Erroba, D.C. Lee, J. Prieto, and M.A. Avila. 2005. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 128:424–432. [DOI] [PubMed] [Google Scholar]