Abstract
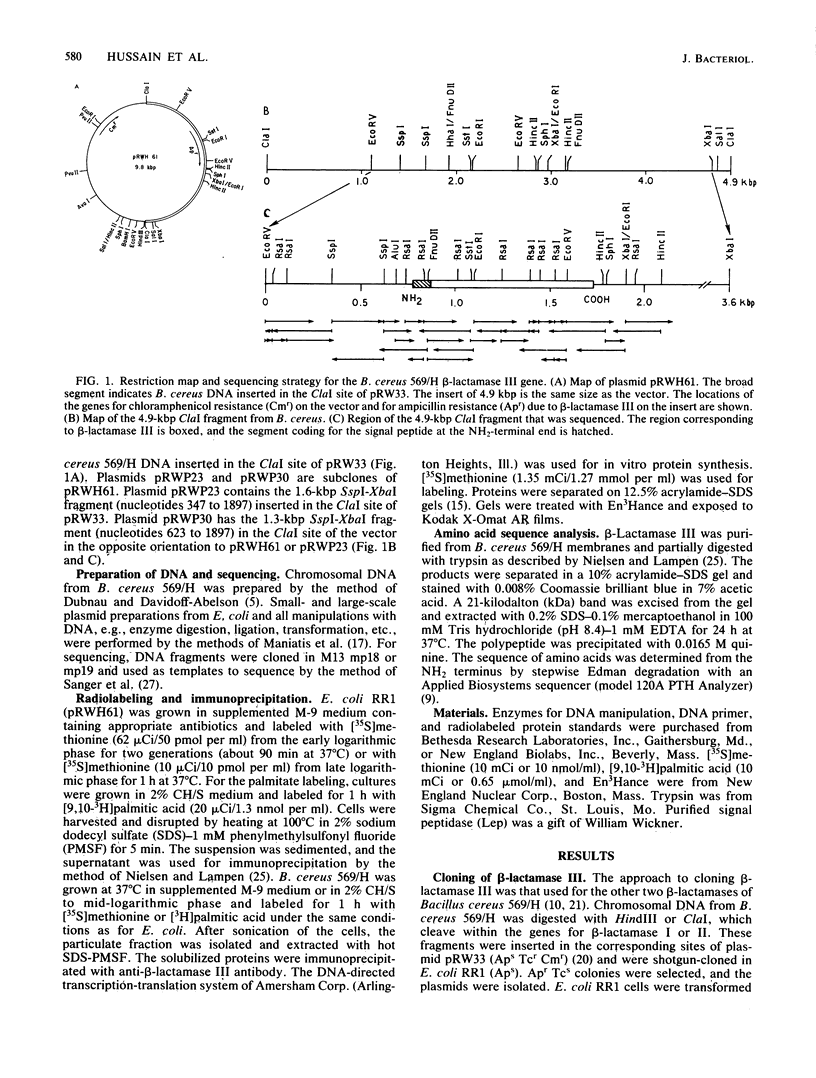
It has not been clear whether the membrane-bound beta-lactamase III of Bacillus cereus 569 is a separate enzyme or a modified form of the secreted beta-lactamase I. The membrane enzyme is an acyl-glyceride thioether-linked lipoprotein (J. B. K. Nielsen and J. O. Lampen, Biochemistry 22:4652-4656, 1983) and thus is probably a separate entity. We cloned the beta-lactamase III gene (blaZ) on a 4.9-kilobase-pair ClaI fragment from mutant strain 569/H (constitutive for high-level production of beta-lactamases I, II, and III), and the nucleotide sequence was determined. The structural gene was flanked by typical promoter, transcription termination, and translation initiation sequences. Expression of the cloned gene in Escherichia coli was low in exponential-phase cultures and increased only as the cultures reached the stationary phase. The deduced amino acid sequence indicates a pre-beta-lactamase III of 316 amino acid residues (35,021 daltons), with a 29-residue signal peptide and a mature lipoprotein form of approximately 32,500 daltons. The 12 NH2-terminal residues of a 21-kilodalton tryptic peptide from the B. cereus membrane enzyme were in agreement with the sequence deduced from the cloned gene. The amino acid sequence was highly homologous to the class A beta-lactamases, especially that of Bacillus licheniformis 749. beta-Lactamase III is a distinct class A enzyme and the product of a separate gene (blaZ).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Connolly A. K., Waley S. G. Characterization of the membrane beta-lactamase in Bacillus cereus 569/H/9. Biochemistry. 1983 Sep 27;22(20):4647–4651. doi: 10.1021/bi00289a006. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Giam C. Z., Chai T., Hayashi S., Wu H. C. Prolipoprotein modification and processing in Escherichia coli. A unique secondary structure in prolipoprotein signal sequence for the recognition by glyceryl transferase. Eur J Biochem. 1984 Jun 1;141(2):331–337. doi: 10.1111/j.1432-1033.1984.tb08196.x. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Chang S. Y., Chang S., Wu H. C. Modification and processing of Bacillus licheniformis prepenicillinase in Escherichia coli. Fate of mutant penicillinase lacking lipoprotein modification site. J Biol Chem. 1984 Aug 25;259(16):10448–10454. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hussain M., Carlino A., Madonna M. J., Lampen J. O. Cloning and sequencing of the metallothioprotein beta-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol. 1985 Oct;164(1):223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J Biol Chem. 1980 Apr 25;255(8):3707–3712. [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem. 1982 May 10;257(9):5177–5182. [PubMed] [Google Scholar]
- Hussain M., Lampen J. O. Accumulation of glyceride-modified pre-penicillinase of Bacillus licheniformis in Escherichia coli treated with globomycin. FEBS Lett. 1983 Jun 27;157(1):31–36. doi: 10.1016/0014-5793(83)81110-7. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Dideberg O., Charlier P., Wery J. P., Libert M., Moews P. C., Knox J. R., Duez C., Fraipont C., Joris B. On the origin of bacterial resistance to penicillin: comparison of a beta-lactamase and a penicillin target. Science. 1986 Mar 21;231(4744):1429–1431. doi: 10.1126/science.3082007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mézes P. S., Blacher R. W., Lampen J. O. Processing of Bacillus cereus 569/H beta-lactamase I in Escherichia coli and Bacillus subtilis. J Biol Chem. 1985 Jan 25;260(2):1218–1223. [PubMed] [Google Scholar]
- Mézes P. S., Wang W., Yeh E. C., Lampen J. O. Construction of penP delta 1, Bacillus licheniformis 749/C beta-lactamase lacking site for lipoprotein modification. Expression in Escherichia coli and Bacillus subtilis. J Biol Chem. 1983 Sep 25;258(18):11211–11218. [PubMed] [Google Scholar]
- Mézes P. S., Yang Y. Q., Hussain M., Lampen J. O. Bacillus cereus 569/H beta-lactamase I: cloning in Escherichia coli and signal sequence determination. FEBS Lett. 1983 Sep 19;161(2):195–200. doi: 10.1016/0014-5793(83)81006-0. [DOI] [PubMed] [Google Scholar]
- Neugebauer K., Sprengel R., Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a Gram-positive bacterium. Nucleic Acids Res. 1981 Jun 11;9(11):2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Beta-lactamase III of Bacillus cereus 569: membrane lipoprotein and secreted protein. Biochemistry. 1983 Sep 27;22(20):4652–4656. doi: 10.1021/bi00289a007. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Membrane-bound penicillinases in Gram-positive bacteria. J Biol Chem. 1982 Apr 25;257(8):4490–4495. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloma A., Gross M. Molecular cloning and nucleotide sequence of the type I beta-lactamase gene from Bacillus cereus. Nucleic Acids Res. 1983 Jul 25;11(14):4997–5004. doi: 10.1093/nar/11.14.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wolfe P. B., Wu H. C. Prolipoprotein signal peptidase in Escherichia coli is distinct from the M13 procoat protein signal peptidase. J Biol Chem. 1982 Sep 10;257(17):9922–9925. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yamagata H., Mizushima S. The major outer membrane lipoprotein and new lipoproteins share a common signal peptidase that exists in the cytoplasmic membrane of Escherichia coli. FEBS Lett. 1984 Jan 23;166(1):179–182. doi: 10.1016/0014-5793(84)80068-x. [DOI] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]