Abstract
Activated T cells die when antigen disappears from animals. This death is caused by proteins related to Bcl-2. Two hypotheses have been suggested to explain the actions of the different types of Bcl-2 proteins. One hypothesis suggests that, when T cells prepare to die, Bak and Bax, the proteins that actually kill activated T cells, are released from antiapoptotic proteins such as Bcl-2 and Bcl-xl. Another hypothesis suggests that Bak and Bax are normally free and are triggered to kill cells by release of messenger proteins, such as Bim, from Bcl-2 and Bcl-xl. Here, a form of Bcl-xl, which lacks a long unstructured loop, is used to show that the first hypothesis is not correct. Bcl-xl without its loop protects activated T cells from death, yet Bcl-xl without its loop cannot bind any form of Bak and Bax. Thus, binding of Bcl-xl to Bak or Bax is not involved in T cell life or death. The loop of Bcl-xl is also somewhat involved in Bcl-xl's binding of Bim because Bcl-xl without its loop binds Bim less well than wild-type Bcl-xl. Moreover, the loop may have additional, as yet unknown, functions because it changes its shape when Bcl-xl binds Bim.
The appearance of antigen in animals makes antigen-specific T cells divide and then die (1, 2). It is now well known that the death of these cells is to a large extent governed by members of the Bcl-2 family of proteins (3–6). Overexpression of antiapoptotic members, such as Bcl-2 and Bcl-xl (the Protectors), inhibits activated T cell death (7). Activated T cell death is likewise inhibited by double deficiencies in the Executioner members, Bak and Bax, which are supposed to actually kill the T cells (8, 9). A third group of proteins in this family, the so-called Messengers, also affect T cell death, and deficiencies in one of these proteins (Bim) also protect T cells against death (2, 10).
The fact that members of three different groups of Bcl-2–related proteins affect activated T cell death leads to difficulties in predicting the exact course of events that kills or protects the cells. It is agreed that the Protectors somehow prevent death and the Messengers and Executioners drive death. What is not clear, however, is which proteins must interact with which to control these events. Three models have been suggested. In the first model, the Protectors are supposed to be bound to the Messengers in healthy T cells and the Executioners are in some innocuous form. After activation, as the T cells approach death, it is suggested that some of the Messengers are released from the Protectors and that the freed proteins directly or indirectly activate the killing properties of the Executioners (11–15). In a second model, the Protectors are bound to the Executioners in healthy cells. When the activated T cells approach death, the ratio of these proteins changes such that the Executioners are released from the protective custody of the Protectors and, thus freed, are able to kill the cell (12, 16). Finally it is possible that the Executioners are released to kill the cell via some event that does not directly involve binding to either a Protector or Messenger (17).
The experiments described here test the second of these possibilities by examining whether the Protectors bind the Executioners in healthy cells. We show that the Protector Bcl-2 does not coprecipitate with Bak or Bax even under conditions in which the Executioners are activated by incubation in NP-40. Such coprecipitation experiments could not be interpreted with Bcl-xl because we could not efficiently immunoprecipitate all the protein from normal cells. Thus, in a different approach to the problem, we tested the ability of a tagged variant of Bcl-xl lacking its flexible loop (Bcl-xlΔLoop; references 18–20) to coisolate with Bax or Bak after cosynthesis in insect cells. Normal Bcl-xl coprecipitated with both of these proteins; however, Bcl-xlΔLoop did not. However, like normal Bcl-xl, Bcl-xlΔLoop bound the Messenger protein Bim. In spite of its inability to bind Bak or Bax, Bcl-xlΔLoop protected T cells from death as efficiently as the wild-type protein. Thus, these experiments suggest that, in activated T cells, the Protectors act by binding to Messengers and not by engaging the Executioners directly.
A byproduct of these experiments is a higher resolution structure, by x-ray crystallography, of the structure of Bcl-xl bound to a peptide from Bim. This structure differs from those that have been previously reported (20–22) in that it reveals the configuration of a few more of the residues of the flexible loop (amino acids 22–82) of Bcl-xl. Engagement of Bcl-xl by Bim renders a site in the loop sensitive to proteolytic cleavage and changes the conformation of the loop amino acids that can be resolved. Moreover, Bcl-xlΔLoop binds Bim less well than wild-type Bcl-xl does. Thus, the loop domain of Bcl-xl may play a role in some currently unknown function of Bcl-xl.
RESULTS
Bcl-xl lacking its loop domain protects activated T cells from death
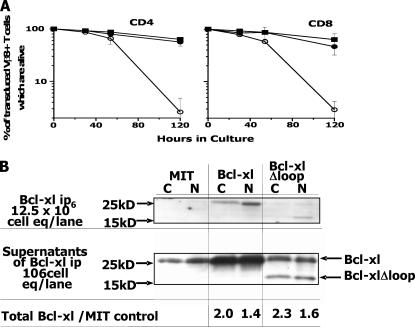
The structure of Bcl-xl has been solved by several groups (20–22). However, the complete structure of the protein is not known. In all reports about Bcl-xl, a portion of the protein stretching from about amino acids 28–82 could not be resolved. To study the contribution of this part of Bcl-xl to the protective activity of the protein, we activated T cells in vivo with the Vβ8-engaging superantigen, staphylococcal enterotoxin B (SEB), isolated the cells 2 d later, and studied the ability of transduced wild-type Bcl-xl or Bcl-xlΔLoop to protect the activated T cells against death. As shown in Fig. 1 A, both constructs of Bcl-xl protected the cells very well against death. Unfortunately, because only ∼5% of the T cells were transduced in this and similar experiments, we could not evaluate directly how much the Bcl-xl transduction had increased levels of Bcl-xl in the T cells. Therefore, to get some estimate of these values, we compared the levels of Bcl-xl in the Phoenix cells that had been transfected either with the control plasmid, expressing only Thy1.1 (MIT), or with plasmids coding for intact Bcl-xl or Bcl-xlΔLoop. Staining showed that between 21 and 25% of the cells were transfected with each plasmid. The cells were lysed and Bcl-xl was immunoprecipitated with an antibody that reacts with both human and mouse Bcl-xl. The immunoprecipitates and supernatants were run on SDS-PAGE and Western blotted for Bcl-xl. Anti–Bcl-xl precipitated Bcl-xl very inefficiently, a consistent finding in our experiments (Fig. 1 B) and one that is common to all anti–Bcl-xl antibodies we have used. Nevertheless, by combining the data from the immunoprecipitates and supernatants we could get an estimate of the increase in Bcl-xl afforded by the Bcl-xl–expressing plasmids.
Figure 1.
The loop region of Bcl-xl is not required for its antiapoptotic activity in activated T cells. (A) The death of transgenic Vβ8+ T cells was followed in vitro after SEB activation in vivo as described in Materials and methods. In brief, lymph node T cells were removed from Vβ8+ TCR β chain transgenic mice 24 h after SEB injection and transduced with either control MIT retrovirus or MIT expressing either Bcl-xl or Bcl-xlΔloop. The viability of Thy1.1+, Vβ8+ T cells bearing either CD4 or CD8 was assessed by flow cytometry 24 and 48 h after transduction. In this experiment, the rate of T cell death was slowed by incubation of the cells overnight in the cold after spinfection and before culture at 37°C. Similar results were obtained with cells cultured immediately, in two other experiments. Results shown are the means and standard errors of triplicate cultures. (B) Expression of Bcl-xl was compared in Phoenix cells transfected with a control plasmid (MIT) coding for Thy1.1 alone or transfected with plasmids coding for Bcl-xl or Bcl-xlΔloop. Phoenix cells were lysed in CHAPS (C) or NP-40 (N) and Bcl-xl was immunoprecipitated from the lysates with 2H12, anti-human Bcl-xl, or anti-mouse Bcl-xl. Immunoprecipitates were Western blotted as described in materials and Methods. Results show the increase in Bcl-xl in Bcl-xl or Bcl-xlΔloop transfected cells versus MIT transfected cells.
The estimates of Bcl-xl expressed by the transfected genes varied somewhat depending on the detergent used, and was between 40–100% of endogenous levels in Phoenix. Bcl-xl and Bcl-xlΔLoop were expressed equally well (Fig. 1 B).
Bcl-2 does not coprecipitate with Bak or Bax from activated T cells
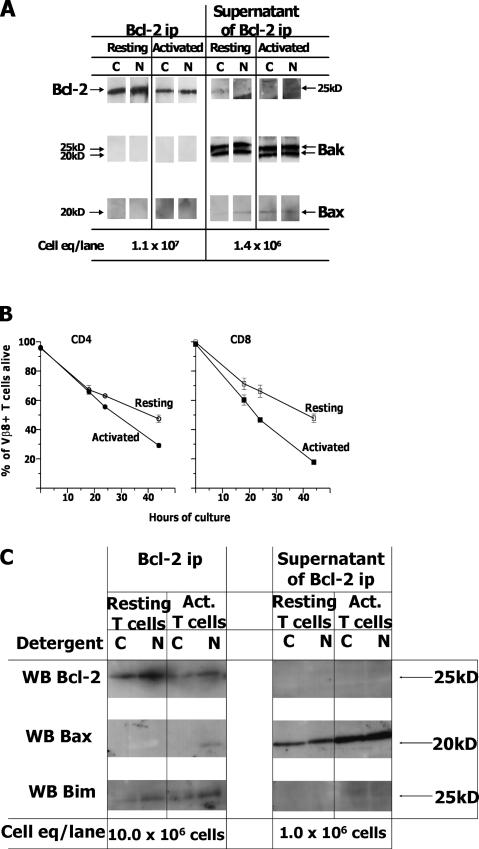
Activated T cells transduced with retroviruses expressing Bcl-2 are protected from death, like the cells transduced with retroviruses expressing Bcl-xl or Bcl-xlΔLoop. To test the idea that Bcl-2 protects activated T cells from death by binding to the Executioners Bak and Bax, Bcl-2 was precipitated from CHAPS or NP-40 lysates of resting and activated T cells, and the precipitates were analyzed by Western blot for coprecipitation of Bak or Bax. Immunoisolates of Bcl-2 contained neither Bak nor Bax (Fig. 2 A). A repeat of this experiment showed that the activated T cells were indeed destined to die more rapidly than the naive cells (Fig. 2 B) and confirmed that Bcl-2 was not bound to Bax in either resting or activated T cells but was bound, as we have reported before, to Bim in both types of cells (reference 23; Fig. 2 C). Similar experiments with immunoprecipitates of Bcl-xl were tried with no consistent sign that Bcl-xl was bound to either of the Executioners in healthy cells (unpublished data). However, under no circumstances could we precipitate all the Bcl-xl from the cells. As illustrated in Fig. 1 B, only a maximum of 30% of the Bcl-xl could be precipitated, even with use of polyclonal antibodies under the best of circumstances. Therefore, we could never be sure that we had isolated the fraction of Bcl-xl that might be associated with Bak or Bax, should such complexes exist. To find out whether or not Bcl-xl bound Bak or Bax in healthy T cells, we therefore turned to a different type of experiment.
Figure 2.
In T cells, Bcl-2 is not bound to Bak or Bax. (A) Live resting and activated T cells were purified and lysed with CHAPS (C) or NP-40 (N) as described in Materials and methods. Lysates were tumbled overnight with beads coupled to anti–Bcl-2 and washed and eluted as described in Materials and methods. Material bound to the beads or supernatants from the beads were run with the indicated cell equivalents and blotted as shown. Results are representative of at least three independent experiments. (B) In a separate experiment the rates of death of resting or activated T cells were measured in culture. Results shown are the means and standard errors of triplicate cultures. (C) The remainder of these cells were lysed in CHAPS (C) or NP-40 (N), lysates were immunoprecipitated with anti-Bcl-2, and the immunoprecipitates and supernatants were Western blotted for Bcl-2, Bax, and Bim.
Unlike wild-type Bcl-xl, Bcl-xlΔL cannot bind a truncated, conformationally altered form of Bax
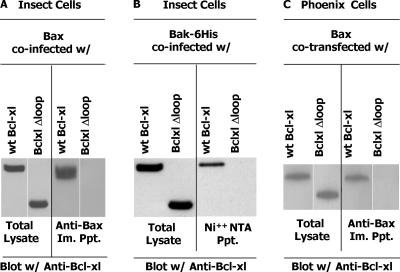
Given our failure to immunoprecipitate Bcl-xl completely from T cells and thus examine its intracellular association with the Executioners, we decided to test the idea that Bak and Bax bind Bcl-xl using insect cells. To perform the test, Bcl-xl or Bcl-xlΔLoop were expressed in insect cells, together with Bax or 6-His–tagged Bak. The insect cells were lysed with NP-40, a detergent, which, like other powerful nonionic detergents, converts innocuous Bax or Bak into their N-terminal–exposing, death dealing forms that have previously been shown to bind Bcl-xl or Bcl-2 (24, 25). Bax was precipitated with the anti-BaxNter monoclonal antibody, 6A7, that reacts with the supposed death-dealing form of Bax (24).
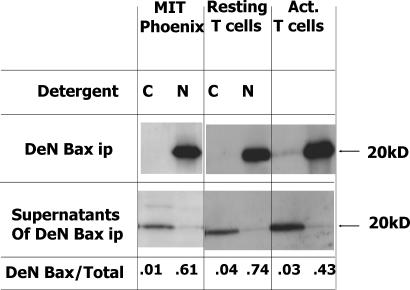
Immunoisolation of Bax from the NP-40 lysates readily coprecipitated Bcl-xl. Bcl-xlΔLoop, however, did not coisolate with Bax. Likewise, Bak coprecipitated with wild-type Bcl-xl but not with Bcl-xlΔLoop (Fig. 3). To check that this was not some artifact caused by the environment inside insect cells, we repeated the experiment with transfections in Phoenix cells (Fig. 3 C). Again, Bax coisolated with Bcl-xl but not with Bcl-xlΔLoop. As expected, Bax did not coprecipitate with Bcl-xl if the Phoenix cells were lysed in CHAPS rather than NP-40 (unpublished data). This result was reflected in the degree of conformational change of Bax by NP-40, with virtually none of the cellular Bax reacting with the anti-conformationally changed Bax antibody 6A7 after Phoenix of T cell lysis in CHAPS and up to 70% of the Bax reacting with 6A7 after lysis in NP-40 (Fig. 4).
Figure 3.
The loop region of Bcl-xl is essential for interaction with Bax and Bak. The interactions of Bax with Bcl-xl and Bcl-xlΔloop were studied in baculovirus-infected High Five insect cells as described in Materials and methods. In brief, insect cells were coinfected with baculovirus expressing Bax and a second virus expressing either Bcl-xl or Bcl-xlΔloop. (A) After 4 d, whole cell lysates (105) or anti-Bax (mAb 6A7) precipitates from the lysates of 5 × 105 infected cells were analyzed by SDS-PAGE and blotted with anti–Bcl-xl antibody. (B) Same as A except baculovirus-expressing 6-His–tagged Bak instead of Bax was used and the precipitation was performed with the Ni-NTA beads. (C) Phoenix cells were cotransfected with Bax and a full-length version of either Bcl-xl or Bcl-xlΔloop as described in Materials and methods. Total cell lysates and anti-Bax immunoprecipitates were analyzed by SDS-PAGE and blotting with anti-Bax as in A. Care was taken throughout to ensure equal cell equivalents were loaded in each lane.
Figure 4.
Lysis in NP-40 changes the conformation of Bax. Phoenix cells were transfected with a control plasmid (MIT) expressing Thy1.1. These cells, and resting or activated T cells, were lysed in CHAPS or NP-40. The lysates were immunoprecipitated with the anti–N-terminal Bax antibody 6A7. Bax immunoprecipitated by 6A7 is here termed DeN. Immunoprecipitates and their supernatants were Western blotted for Bax. Shown are the results of immunoprecipitates of 12.5 × 106 Phoenix cells or 107 resting or activated T cells and the supernatants of 106 of each of these cells. Also indicated are the ratios of Bax obtained in the immunoprecipitates versus all Bax in the cells.
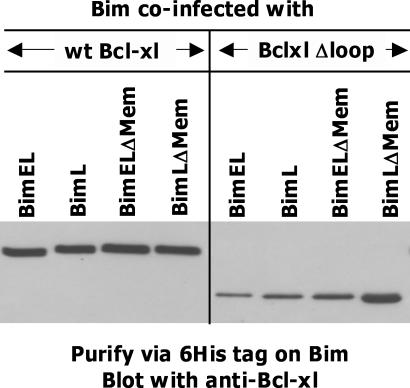
To demonstrate that the Bcl-xlΔLoop was functional in insect cells, we coexpressed, in the cells, Bcl-xl or Bcl-xlΔLoop together with Bim, a protein to which Bcl-xl binds well. Both forms of Bcl-xl were associated with Bim, although less Bcl-xlΔLoop bound to Bim than Bcl-xl did, especially in the case of BimEL (Fig. 5).
Figure 5.
Role of the loop region of Bcl-xl in interactions with Bim. The interactions of various versions of Bim with Bcl-xl and Bcl-xlΔloop were studied in baculovirus-infected High Five insect cells as described in Materials and methods. In brief, insect cells were coinfected with baculovirus expressing one of the versions of 6-His–tagged Bim (BimEL, BimL, BimELΔTM, and BimLΔTM) and a second virus expressing either Bcl-xl or Bcl-xlΔloop. After 4 d, Ni-NTA precipitates from lysates of equal numbers of the infected cells (5 × 105) were analyzed by SDS-PAGE and blotted with anti–Bcl-xl antibody. Care was taken throughout to ensure that equal cell equivalents were loaded in each lane.
Thus, Bcl-xlΔLoop does not bind Bak or Bax, even when the latter proteins are conformationally changed to their supposed death-dealing form. Nevertheless, Bcl-xlΔLoop protects activated T cells from death, suggesting that interaction of Bcl-xl with conformationally altered Executioners is not important to the protective effects of Bcl-xl.
The flexible loop of Bcl-xl is affected by binding of Bcl-xl to Bim
These experiments led us to be curious about the functions of Bcl-xl's flexible loop. Somehow this loop affects the ability of Bcl-xl to bind the conformationally changed forms of the Executioners, even though this interaction is not important in the ability of Bcl-xl to protect T cells from death. Also, the loop modestly affects the ability of Bcl-xl to bind intact Bim, even though the major site of this interaction is thought to be via the engagement of Bim's Bcl-2 homology 3 (BH3) region by the BH3 binding groove of Bcl-xl, a site on Bcl-xl that does not include Bcl-xl's flexible loop.
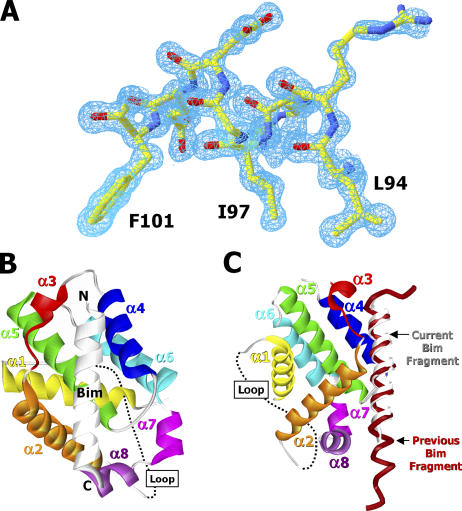
To address the function of Bcl-xl's loop, we made another attempt to resolve its structure. To this end, hanging drops containing Bcl-xl and Bim were set up, with each protein lacking only its transmembrane region. Crystals appeared. Their characteristics, shown in Table I, revealed some differences between these crystals and those we have previously reported between Bcl-xl and a fragment of Bim (22). Most notably, the new crystals diffracted to a 1.2-Å resolution, allowing a much higher quality electron density map and analysis of the complex (Fig. 6 A).
Table I.
Data collection and refinement statistics
| Data collection | |
|---|---|
| Data set | Bcl-xL–Bim |
| Space group | P21 |
| Unit cell dimensions (Å) | a = 36.52 b = 46.21 c = 51.18 |
| Unit cell angles (°) | β = 100.25 |
| Number of molecules in AU | 1 |
| Resolution limits (Å) | 20.0−1.20 (1.28−1.20)a |
| Unique reflections | 49,728 (6,983) |
| Completeness (%) | 92.1 (77.4) |
| Average redundancy | 3.1 (2.7) |
| Average I/σ | 9.2 (2.2) |
| Rmerge(%)b | 7.4 (47.8) |
| Refinement | |
| Data set | Bcl-xL–Bim |
| Resolution (Å) | 20.0−1.20 |
| I/σ cut off | 1.0 |
| Total reflections | 48,364 (6,410) |
| Reflections used for Rfree | 2,460 (317) |
| Rworking (%)c | 20.8 (25.3) |
| Rfree (%) | 21.2 (27.6) |
| Average B factors (Å2) | 16.0 |
| Ramachandran data (% of residuesd in:) | |
| Favored regions | 95.9 |
| Allowed regions | 4.1 |
| Generously allowed regions | 0.0 |
| Disallowed regions | 0.0 |
| Rmsd | |
| Bonds (Å) | 0.004 |
| Angles (°) | 1.01 |
| B factor main chain (Å2) | 0.89 |
| B factor side chain (Å2) | 1.40 |
| Cross-validated coordinate error (Å) | 0.4 |
All data (outer shell).
Rmerge = ∑ (‖I – 〈I〉‖)/∑ (I).
Rworking/free = ∑‖Fo‖ – ‖Fc‖/∑‖Fo‖.
Excluding glycine and proline.
Figure 6.
The overall structure of Bcl-xl–Bim. (A) The 2Fo-Fc electronic density (1.4σ cutoff) of the BH3 region of the Bim peptide. Three conserved BH3 hydrophobic amino acids, L94, I97, and F101, are labeled. (B) A ribbon representation of the complex of Bcl-xl and the Bim fragment. The eight α helices of Bcl-xl are labeled and colored sequentially: yellow, orange, red, blue, green, cyan, magenta, and purple. (C) A superimposition (Swiss PDB Viewer [reference 47]) of the current and previous (available from GenBank/EMBL/DDBJ under accession no. 1PQ1) Bcl-xl–Bim complexes.
As before, the crystals contained Bcl-xl bound to a peptide of Bim. The Bim protein had, once again, been degraded by proteolysis during the time the crystals took to form (Fig. 6 B). In general, the conformation of Bcl-xl in this new complex was very similar to that previously reported (20–22) with the exception that the Bim peptide was somewhat shorter at both ends than that which we observed in our previous structure (Fig. 6 C). This allowed for a change in the crystal packing, accounting for the changes in crystal parameters between this and our previous paper (22). Nevertheless, the Bim peptide was bound to Bcl-xl very much as before with hydrophobic and hydrophilic interactions allowing a very stable complex and similar changes in the structure of Bcl-xl by comparison with its unengaged fold.
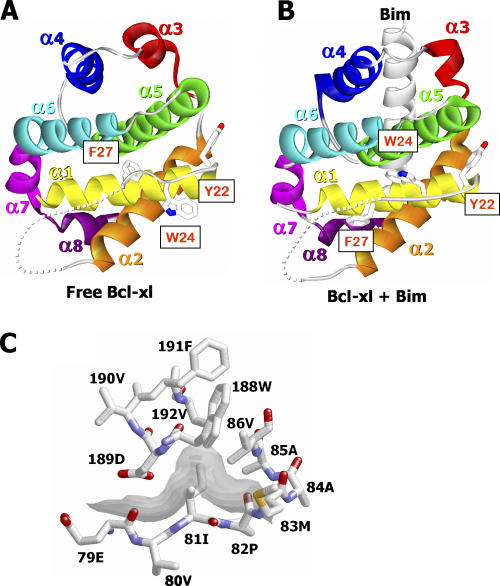
Most of the loop region between Bcl-xl's α1 and α2 helices remained unresolved in this new structure. The higher resolution of these crystals allowed us to see some of the amino acids at the beginning and end of the loop that had not previously been visible in complexes between Bcl-xl and BH3 peptides, but that were, to some extent, seen in the structure of Bcl-xl alone (20–22). Thus our new structure showed that the conformation of the N-terminal end of the Bcl-xl loop domain is changed by binding to Bim (Fig. 7, A and B). In free Bcl-xl, a hydrophobic pocket formed between Bcl-xl's α1 and α5 helices is occupied by Phe27, whereas in the Bcl-xl–Bim BH3 complex, the pocket is occupied by Trp24. Likewise, in free Bcl-xl the stretch of amino acids 22–28 has an extended helix-like conformation, whereas this region is completely linearized in the Bcl-xl–Bim BH3 complex. At the other end of the loop, clear density and low temperature factors can be seen for amino acids 79–82 and, in the complex, these amino acids are interacting stably with the body of Bcl-xl (Fig. 7 C). The easy identification of these amino acids in the complex, but not in free Bcl-xl, suggests that they have changed position in the complexed versus free Bcl-xl and are now attached more intimately to the rest of the protein.
Figure 7.
Details of the Bcl-xl loop region. The beginning of the loop region of free Bcl-xl (available from GenBank/EMBL/DDBJ under accession no. 1PQ1; A) and the Bcl-xl–Bim complex (B) are shown as ribbon representations colored as in Fig. 1. Wire frame representations of Tyr22, Trp24, and Phe27 are also shown, with CPK coloring. This figure was created with WebLab Viewer Pro. (C) Wire frame representations of the amino acids of Bcl-xl, which contact the end of the loop domain (amino acids 79–82) are shown (CPK coloring). The contact surface of amino acids 79–82 with the rest of the molecule is also shown shaded from light gray (closer) to dark gray (distant). This figure was created with Protein Explorer (reference 48).
Engagement of Bim makes Bcl-xl's loop susceptible to proteolysis
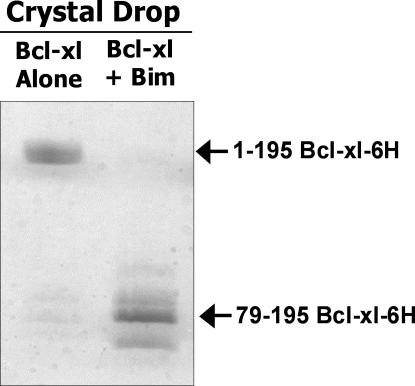
Even at the high resolution of our new crystal, electron density for the loop faded gradually after Phe27, indicating that the rest of the loop is relatively unstructured. At the C-terminal end of the loop, however, electron density reappeared abruptly at Glu79, suggesting that in the crystal Bcl-xl may have been cleaved at this point (Fig. 7). To test this, we ran the protein in the crystal on SDS-PAGE and found fragments that were consistent with proteolysis of Bcl-xl (Fig. 8). Such fragments were not seen in the preparation of Bcl-xl before it was set up to crystallize, nor in crystallization drops set up at the same time as those analyzed in Fig. 8, but containing Bcl-xl without Bim. Because all the proteins were purified similarly from insect cell supernatants, and thus had the same chance of contamination with insect cell proteases, we concluded that engagement of Bcl-xl by Bim may have rendered the flexible loop of Bcl-xl susceptible to proteolysis.
Figure 8.
Cleavage of the Bcl-xl loop region during crystallization. The contents of hanging drops containing crystals of either Bcl-xl alone (left) or Bcl-xl and Bim (right) were subjected to SDS gel analysis. The samples were prepared from crystallization trays after 11 mo of incubation at 4°C.
DISCUSSION
In spite of many years' work, we still don't know exactly how the Executioners Bak and Bax are triggered to kill activated cells. The work described here suggests that in T cells triggering does not occur because of release of Bak and Bax from the embrace of Protectors such as Bcl-2 and Bcl-xl. We can see no evidence that Bcl-2 binds the innocuous forms of Bak or Bax in T cells. Bcl-xl in both its intact and truncated (Bcl-xlΔLoop) form protects T cells from death. Nevertheless, Bcl-xlΔLoop does not bind the Executioners, even after they have been converted by incubation in NP-40 to their cell-killing forms (24). One could argue that this latter experiment was done only in insect or Phoenix cells and not in the environment of interest, i.e., in activated T cells, where conditions may be different. Although this is possible, we think it unlikely, especially because in the insect cells, for example, both Bcl-xl and Bcl-xlΔLoop are well able to bind a verified partner (Bim) for these proteins.
It could be that Bcl-xlΔLoop does not function in the same way as normal Bcl-xl. For example, both proteins may bind Bim, as shown here, but denatured Bax and Bak may be able to displace Bim from Bcl-xl but not from Bcl-xlΔLoop. If this were correct, one might expect Bcl-xlΔLoop to be a better Protector than Bcl-xl. However, that doesn't seem to be the case. Activated T cells appear to be equally well protected by the two proteins (Fig. 1), suggesting that the two proteins act similarly.
The involvement of Bcl-xl's flexible loop in the binding of the protein to Bax after cell solubilization in NP-40 has been studied previously (19) in the paper that first investigated the functions of the loop. Chang et al. (19) reported that Bcl-xl lacking its loop continued to bind Bax, a result that is exactly the opposite to that reported here. There are several differences in methodology and cell type that could account for the discrepancy. One explanation stems from the fact that in the previous paper, the band that coprecipitated with Bcl-xlΔLoop was identified as Bax on the basis of its molecular weight, not by Western blot with a specific antibody. Bax and Bim have approximately the same molecular weight, and the band thought to have been Bax could have been some other protein of similar molecular weight, e.g., Bim, a protein which is known to bind Bcl-xl (15, 23).
The function of Bcl-xl's flexible loop, and the comparable structure on Bcl-2, has long been a mystery. Several papers have shown that the ability of these proteins to protect cells from death is more or less unaffected by absence of their loops (18, 26, 27), which is a result we confirm here. Indeed, sometimes the absence of the loop improves the antiapoptotic activities of the proteins (19, 28). This may be because serines on the loops can be phosphorylated, and such phosphorylation inhibits the protective and increases the death-dealing properties of the proteins (28–30).
It has also been reported that asparagines in the flexible loop of Bcl-xl can be deamidated in tumor cells in response to DNA damage (31, 32) and that deamidation renders Bcl-xl less able to block the proapoptotic activity of BH3-only messenger proteins. This result suggests a relationship between Bcl-xl's engagement of a Messenger protein such as Bim and the structure of Bcl-xl's loop. As such, the result is reminiscent of our finding that although Bim engages Bcl-xl very well via binding of its BH3 region to the BH3 binding groove of Bcl-xl, somehow engagement of Bim's BH3 affects the structure of Bcl-xl's flexible loop.
Finally, caspase-dependent cleavage of the flexible loop of Bcl-xl, a phenomenon that converts Bcl-xl into a proapoptotic protein (33, 34), has been described. Interestingly, one of the cleavage sites reported is only two amino acids away from Glu79, the amino acid at the N terminus of one of the Bcl-xl fragments we found in our crystal structures. Perhaps the protein is particularly sensitive to proteolysis in this region, particularly after engagement of Bim.
In summary, the data presented here suggest that the Executioners are not kept in check by binding to antiapoptotic proteins. How then are the Executioners triggered? Most likely, in activated T cells, this process begins when, as antigen disappears from animal, levels of Bcl-2 in the activated T cells fall without a concomitant fall in levels of Bim (35). In healthy T cells, most of the Bim in the cell is bound to Bcl-2 or Bcl-xl (23). We predict that when Bcl-2 levels fall in T cells, some Bim is released. The free Bim then directly or indirectly causes Executioner activation.
At first thought, this hypothesis is opposed by our finding that Bcl-xlΔLoop protects activated T cells as efficiently as wild-type Bcl-xl does, and yet the former protein binds Bim less well than the latter. These apparently contradictory results may be reconciled by the observation that Bcl-xlΔLoop binds BimL quite well and, in fact, binds BimL lacking a transmembrane region as efficiently as Bcl-xl does (Fig. 5). BimL may be a more effective mediator of T cell death than BimEL because the latter can be ubiquitinylated and degraded after phosphorylation of a serine residue that is absent in BimL (36, 37). It is also possible that Bim's transmembrane region is not available to interfere with the binding of Bcl-xlΔLoop when T cells are being protected from death.
We do not know how the freed Bim acts to mediate cell death because, in the same way that we cannot see association between Protectors and Executioners, we cannot measure any association between Bim and Bak or Bax. Perhaps Bim acts, as has been suggested in the past, via “hit and run” in which a very quick, low level interaction between Bim and the Executioners catalyzes a catastrophic change in the conformation of a large amount of the Executioner proteins (13).
MATERIALS AND METHODS
Measurement of the antiapoptotic activity of Bcl-xl and Bcl-xlΔLoop.
Constructs of Bcl-xl and Bcl-xlΔLoop (Bcl-xl lacking amino acids 45–84; reference 21) were cloned into a plasmid version of the Thy1.1 expressing retroviral construct MIT (38) and cotransfected by standard methods with pCL-Eco (a plasmid vector expressing the structural proteins of Maloney murine leukemia virus; a gift of I. Verma (Salk Institute, La Jolla, CA [reference 39]) into the packaging cell line Phoenix-Eco. Activated T cells were purified from the lymph nodes of mice expressing a Vβ8+ transgene, VβDO (23), which had been treated 2–3 d before with 100 μg of mouse SEB, which is a Vβ8-specific superantigen that activates all the T cells in VβDO mice. The activated T cells were transduced with retrovirus by centrifugation at 1000 g for 2 h, followed by washing and incubation at 37°C in Dulbecco's enriched culture medium. In some experiments, death of the cells was slowed by maintaining them in the cold overnight after the spinfection and before culture at 37°C. At various times thereafter, the cells were stained with antibodies against Vβ8, Thy-1.1, and either CD4 or CD8 and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). The viability of Thy-1.1+/Vβ8+ cells bearing either CD4 or CD8 was determined by FSC/SSC gating (38, 40).
Use of mice for these experiments has been approved by the National Jewish Medical and Research Center Animal Care and Use Committee.
Analysis, by immunoprecipitation, of association between Bcl-2 and Bak or Bax in T cells.
T cells were purified from VβDO mice, which were untreated or had been treated 2 d before with 100 μg SEB to yield resting and activated cells, respectively. The cells were lysed with solutions containing protease inhibitors and 2% CHAPS or 0.5% NP-40 and spun at 14,000 rpm in a microfuge for 15 min to remove nuclei and unlysed cells. The lysates were incubated overnight with beads coupled to hamster anti–Bcl-2, 3F11 (41). The beads were then extensively washed. Bound proteins were released by boiling in SDS loading buffer, run on 10–20% gradient gels (Bio-Rad Laboratories), and Western blotted with mouse anti–human and mouse Bcl-2 (Zymed Corp.), rabbit anti-Bak NT (Upstate Biotechnology), or rabbit anti-Bax N-20 (Santa Cruz Biotechnology, Inc.). Secondary antibodies were horseradish peroxidase–coupled donkey anti–mouse IgG or donkey anti–rabbit IgG (The Jackson Laboratory). In experiments in which we failed to satisfactorily immunoprecipitate, Bcl-xl lysates were tumbled overnight with excess beads bearing two different hamster anti–Bcl-xl antibodies: 2H12 (24) or Ham151-297.5 (unpublished data) or rabbit polyclonal anti–Bcl-xl (Transduction Laboratories). Bcl-xl was Western blotted with rabbit anti–Bcl-xl (Transduction Laboratories) followed by donkey anti–rabbit IgG (The Jackson Laboratory).
Analysis by immunoprecipitation of the association of Bcl-xL or Bcl-xLΔloop with Bak or Bax.
Baculoviruses encoding Bak, Bax, 6-His–tagged Bcl-xl lacking its transmembrane region (Bcl-xlΔMem), or lacking both its transmembrane region and its flexible loop (amino acids 45–84 [Bcl-xlΔLoopΔMem]) were constructed as previously described (22, 42). High Five insect cells (Invitrogen) were coinfected with viruses expressing either Bak or Bax and with viruses expressing one of the forms of Bcl-xl. 4 d later the cells were lysed in Ni-NTA binding buffer by sonication. 0.5% NP-40 was added to the lysis buffer to increase the likelihood that Bcl-xl would bind Bak or Bax (24). After centrifugation (13,000 g for 60 min), supernatants were incubated with beads bearing an antibody to activated Bax (6A7 [reference 23]) or Ni-NTA beads for 1 h in 4°C. After thorough washing using the binding buffer, complexes were isolated by boiling in SDS loading buffer, run on SDS-PAGE, and Western blotted as described in the previous paragraph.
To study the interaction of Bcl-xl and Bax in Phoenix cells, Bax was cloned into the MIT plasmid (38). Phoenix cells were cotransfected with the Bax plasmid and the MIT plasmid encoding either Bcl-xl or Bcl-xlΔLoop as described in Measurement of the antiapoptotic activity…, except that the pCL-Eco helper plasmid was omitted. Transfected cells were incubated at 37°C. After 24 h, their supernatants were replaced with new Dulbecco's enriched culture medium. After another 24 h, cells were harvested and the cell pellets were lysed with 0.5% NP-40 in PBS buffer (50 mM NaH2PO4-Na2HPO4 and 150 mM NaCl, pH 7.5). The lysates were cleared by centrifugation at 10,000 g for 30 min. Bax was immunoprecipitated from the lysate with the 6A7 monoclonal antibody (24) and subjected to SDS-PAGE. Any Bcl-xl associated with the immunoprecipitated Bax was detected by blotting the gel with anti-Bcl-xl antibody.
Coprecipitation of Bim with Bcl-xL or Bcl-xLΔloop.
Baculoviruses encoding BimEL–6-His, BimL–6-His, BimELΔMem–6-His, and BimLΔMem–6-His were constructed and used to coinfect insect cells with viruses expressing Bcl-xlΔLoop or Bcl-xlΔLoopΔMem. 4d later, complexes were isolated from the insect cells. Equal cell equivalents of every sample were loaded, separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with an anti–Bcl-xl antibody (Santa Cruz Biotechnology, Inc.).
Preparation of the complex of Bcl-xl and Bim.
The cDNA fragment encoding amino acids 1–196 of mouse Bcl-xl was cloned under the control of the polyhedron promoter into a previously described baculovirus transfer plasmid (22, 42). Active virus was made by cotransfection into SF9 insect cells (Invitrogen) of the plasmid and BacVector 3000 baculovirus DNA (Novagen) by the calcium phosphate coprecipitation method. A high tittered virus stock was made by further propagation in SF9 cells. Virus producing BimL with an additional C-terminal 6-His tag was made by the same method (22). The C-terminal membrane interaction domain of BimL was truncated to increase the solubility of the protein (BimLΔMem).
To prepare the complex of Bcl-xl and Bim, High Five insect cells were coinfected with the two viruses and cultured for 24 h at 27°C. The infected cells were then moved to 19°C and harvested by centrifugation (1,500 g for 10 min) 4 d later. The cells were lysed by sonication in Ni-NTA binding buffer (50 mM Na2HPO4, 300 mM NaCl, and 5 mM imidazole, pH 8.0). Lysates were cleared by high-speed centrifugation (100,000 g for 60 min), and proteins were purified from the supernatant using Ni-NTA columns (QIAGEN). The Bcl-xl–Bim complex was eluted from the column with 500 mM imidazole and purified by Superdex 200 size exclusion column (GE Healthcare) in PBSA buffer (50 mM NaH2PO4-Na2HPO4, 150 mM NaCl, and 5 mM NaN3, pH 7.5). The complex was further purified using ion exchange chromatography on a Mono-Q column (GE Healthcare) and a Tris-NaCl gradient (25 mM Tris, 100–1,000 mM NaCl, pH 8.2). The complex was concentrated to 15 mg/ml in Tris-NaCl buffer (10 mM Tris and 100 mM NaCl, pH 8.2) for crystallization.
Crystallization and data collection.
Crystallization trays were set up using the hanging drop vapor diffusion method. Equal amounts of protein solution and mother liquor (25% PEG4000, 0.1 M NaAc, pH 4.6, and 0.2 M (NH4)2SO4) were mixed in the hanging drops. After ∼11 mo at 4°C, crystals appeared. The crystals were flash-frozen in liquid nitrogen after brief transfer to a solution containing the mother liquor supplemented with 20% glycerol. A 1.2-Å high-resolution diffraction dataset was collected under cryogenic conditions at beam line SBC 19BM at Advanced Photon Source at Argonne National Laboratory. The data were processed with HKL2000 package (43). The data processing results are summarized in Table I.
Structure determination and refinement.
The structure of the Bcl-xl–Bim complex was determined by molecular replacement. The mouse Bcl-xl crystal structure (available from GenBank/EMBL/DDBJ under accession no. 1PQ0) was used to determine the initial phases of Bcl-xl–Bim. AMoRe (44, 45) was used to find the initial position of the molecules. The CNS suite (46) was used for further refinement. At the beginning of refinement, the α2 and α3 helices around the BH3 binding groove of Bcl-xl were omitted because of large discrepancies in the initial electronic density map. After a few cycles of refinement, α2 and α3 were rebuilt based on 2Fo-Fc and Fo-Fc electronic density maps. The Bim peptide in the binding groove was built later, taking advantage of a previous structure of Bcl-xl bound to a Bim peptide (available from GenBank/EMBL/DDBJ under accession no. 1PQ1). Finally, we built as much of the details of the Bcl-xl loop region as we could, ending at Phe27 and beginning again at Glu79. There was still some continuous electronic density after Phe27, but this was not strong enough to allow us to build in additional residues. In contrast, the electronic density diminished abruptly preceding Glu79. Hydrogen atoms were built in and refined in the last cycles. The structure was refined to 1.2 Å and 252 water molecules were added to the final structure. The results of the refinement are summarized in Table I. All figures were constructed with WebLab Viewer Pro (Accelrys Inc.).
Atomic coordinates of the Bcl-xL–Bim complex reported here have been deposited in the Protein Data Bank (accession no. 1TD8).
Acknowledgments
This work was supported by U.S. Public Health Service grants AI-17134, AI-18785, and AI-22295 and by CA-046934 via its support of the Cell Culture Core of the Cancer Center at the University of Colorado Health Sciences Center. The Argonne National Laboratory is supported by the U.S. Department of Energy, Office of Biological and Environmental Research, under contract W-31-109-ENG-38.
The authors have no conflicting financial interests.
Abbreviations used: BH3, Bcl-2 homology 3; SEB, staphylococcal enterotoxin B.
X. Liu's present address is Dept. of Biological Science, Purdue University, West Lafayette, IN 47907.
Y. Zhu's present address is Dept. of Microbiology and Immunology, University of California, San Francisco, San Francisco, CA 94143.
References
- 1.Pape, K.A., A. Khoruts, A. Mondino, and M.K. Jenkins. 1997. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 159:591–598. [PubMed] [Google Scholar]
- 2.Hildeman, D.A., Y. Zhu, T.C. Mitchell, P. Bouillet, A. Strasser, J. Kappler, and P. Marrack. 2002. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 16:759–767. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell, T.J., N. Deane, F.M. Platt, G. Nunez, U. Jaeger, J.P. McKearn, and S.J. Korsmeyer. 1989. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 57:79–88. [DOI] [PubMed] [Google Scholar]
- 4.Sentman, C.L., J.R. Shutter, D. Hockenbery, O. Kanagawa, and S.J. Korsmeyer. 1991. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 67:879–888. [DOI] [PubMed] [Google Scholar]
- 5.Strasser, A., A.W. Harris, and S. Cory. 1991. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 67:889–899. [DOI] [PubMed] [Google Scholar]
- 6.Vaux, D.L., S. Cory, and J.M. Adams. 1988. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 335:440–442. [DOI] [PubMed] [Google Scholar]
- 7.Petschner, F., C. Zimmerman, A. Strasser, D. Grillot, G. Nunez, and H. Pircher. 1998. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur. J. Immunol. 28:560–569. [DOI] [PubMed] [Google Scholar]
- 8.Rathmell, J.C., T. Lindsten, W.X. Zong, R.M. Cinalli, and C.B. Thompson. 2002. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 3:932–939. [DOI] [PubMed] [Google Scholar]
- 9.Zhu, Y., X. Liu, D. Hildeman, F.W. Peyerl, J. White, E. Kushnir, J. Kappler, and P. Marrack. 2006. Bax does not have to adopt its final form to drive T cell death. J. Exp. Med. 203:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouillet, P., D. Metcalf, D.C. Huang, D.M. Tarlinton, T.W. Kay, F. Kontgen, J.M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 11.Boyd, J.M., G.J. Gallo, B. Elangovan, A.B. Houghton, S. Malstrom, B.J. Avery, R.G. Ebb, T. Subramanian, T. Chittenden, R.J. Lutz, et al. 1995. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 11:1921–1928. [PubMed] [Google Scholar]
- 12.Zha, H., C. Aime-Sempe, T. Sato, and J.C. Reed. 1996. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271:7440–7444. [DOI] [PubMed] [Google Scholar]
- 13.Wei, M.C., T. Lindsten, V.K. Mootha, S. Weiler, A. Gross, M. Ashiya, C.B. Thompson, and S.J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi, H., and H.G. Wang. 2002. Bcl-XL protects BimEL-induced Bax conformational change and cytochrome C release independent of interacting with Bax or BimEL. J. Biol. Chem. 277:41604–41612. [DOI] [PubMed] [Google Scholar]
- 15.Morishima, N., K. Nakanishi, K. Tsuchiya, T. Shibata, and E. Seiwa. 2004. Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J. Biol. Chem. 279:50375–50381. [DOI] [PubMed] [Google Scholar]
- 16.Wang, K., A. Gross, G. Waksman, and S.J. Korsmeyer. 1998. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol. 18:6083–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chipuk, J.E., and D.R. Green. 2004. Cytoplasmic p53: bax and forward. Cell Cycle. 3:429–431. [PubMed] [Google Scholar]
- 18.Burri, S.H., C.N. Kim, G. Fang, B.S. Chang, C. Perkins, W. Harris, L.W. Davis, C.B. Thompson, and K.N. Bhalla. 1999. ‘Loop’ domain deletional mutant of Bcl-xL is as effective as p29Bcl-xL in inhibiting radiation-induced cytosolic accumulation of cytochrome c (cyt c), caspase-3 activity, and apoptosis. Int J Radiat Oncol Biol Phys. 43:423–430. [DOI] [PubMed] [Google Scholar]
- 19.Chang, B.S., A.J. Minn, S.W. Muchmore, S.W. Fesik, and C.B. Thompson. 1997. Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2. EMBO J. 16:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muchmore, S.W., M. Sattler, H. Liang, R.P. Meadows, J.E. Harlan, H.S. Yoon, D. Nettesheim, B.S. Chang, C.B. Thompson, S.L. Wong, et al. 1996. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 381:335–341. [DOI] [PubMed] [Google Scholar]
- 21.Petros, A.M., D.G. Nettesheim, Y. Wang, E.T. Olejniczak, R.P. Meadows, J. Mack, K. Swift, E.D. Matayoshi, H. Zhang, C.B. Thompson, and S.W. Fesik. 2000. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 9:2528–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, X., S. Dai, Y. Zhu, P. Marrack, and J.W. Kappler. 2003. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity. 19:341–352. [DOI] [PubMed] [Google Scholar]
- 23.Zhu, Y., B.J. Swanson, M. Wang, D.A. Hildeman, B.C. Schaefer, X. Liu, H. Suzuki, K. Mihara, J. Kappler, and P. Marrack. 2004. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc. Natl. Acad. Sci. USA. 101:7681–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu, Y.T., and R.J. Youle. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272:13829–13834. [DOI] [PubMed] [Google Scholar]
- 25.Ruffolo, S.C., and G.C. Shore. 2003. BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J. Biol. Chem. 278:25039–25045. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa, B., Jr., T.M. Sauerwald, G.A. Oyler, J.M. Hardwick, and M.J. Betenbaugh. 2003. A comparison of the properties of a Bcl-xL variant to the wild-type anti-apoptosis inhibitor in mammalian cell cultures. Metab. Eng. 5:230–245. [DOI] [PubMed] [Google Scholar]
- 27.Zhou, H., Q. Hou, Y. Chai, and Y.T. Hsu. 2005. Distinct domains of Bcl-XL are involved in Bax and Bad antagonism and in apoptosis inhibition. Exp. Cell Res. 309:316–328. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava, R.K., Q.S. Mi, J.M. Hardwick, and D.L. Longo. 1999. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc. Natl. Acad. Sci. USA. 96:3775–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassik, M.C., L. Scorrano, S.A. Oakes, T. Pozzan, and S.J. Korsmeyer. 2004. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 23:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto, K., H. Ichijo, and S.J. Korsmeyer. 1999. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol. Cell. Biol. 19:8469–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deverman, B.E., B.L. Cook, S.R. Manson, R.A. Niederhoff, E.M. Langer, I. Rosova, L.A. Kulans, X. Fu, J.S. Weinberg, J.W. Heinecke, et al. 2002. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 111:51–62. [DOI] [PubMed] [Google Scholar]
- 32.Li, C., and C.B. Thompson. 2002. Cancer. DNA damage, deamidation, and death. Science. 298:1346–1347. [DOI] [PubMed] [Google Scholar]
- 33.Clem, R.J., E.H. Cheng, C.L. Karp, D.G. Kirsch, K. Ueno, A. Takahashi, M.B. Kastan, D.E. Griffin, W.C. Earnshaw, M.A. Veliuona, and J.M. Hardwick. 1998. Modulation of cell death by Bcl-XL through caspase interaction. Proc. Natl. Acad. Sci. USA. 95:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita, N., A. Nagahashi, K. Nagashima, S. Rokudai, and T. Tsuruo. 1998. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene. 17:1295–1304. [DOI] [PubMed] [Google Scholar]
- 35.Hildeman, D.A., T. Mitchell, B. Aronow, S. Wojciechowski, J. Kappler, and P. Marrack. 2003. Control of Bcl-2 expression by reactive oxygen species. Proc. Natl. Acad. Sci. USA. 100:15035–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ley, R., K. Balmanno, K. Hadfield, C. Weston, and S.J. Cook. 2003. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278:18811–18816. [DOI] [PubMed] [Google Scholar]
- 37.Qi, X.J., G.M. Wildey, and P.H. Howe. 2006. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J. Biol. Chem. 281:813–823. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell, T.C., D. Hildeman, R.M. Kedl, T.K. Teague, B.C. Schaefer, J. White, Y. Zhu, J. Kappler, and P. Marrack. 2001. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat. Immunol. 2:397–402. [DOI] [PubMed] [Google Scholar]
- 39.Naviaux, R.K., E. Costanzi, M. Haas, and I.M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veis, D.J., C.L. Sentman, E.A. Bach, and S.J. Korsmeyer. 1993. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J. Immunol. 151:2546–2554. [PubMed] [Google Scholar]
- 42.Kozono, H., J. White, J. Clements, P. Marrack, and J. Kappler. 1994. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 369:151–154. [DOI] [PubMed] [Google Scholar]
- 43.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. In Macromolecular Crystallography Part A. C.W. Carter Jr. and R.M. Sweet, editors. Academic Press, New York. 307–326. [DOI] [PubMed]
- 44.Collaborative Computational Project, Number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50:760–763. [DOI] [PubMed] [Google Scholar]
- 45.Navaza, J. 2001. Implementation of molecular replacement in AMoRe. Acta Crystallogr. D Biol. Crystallogr. 57:1367–1372. [DOI] [PubMed] [Google Scholar]
- 46.Brunger, A.T., P.D. Adams, G.M. Clore, W.L. DeLano, P. Gros, R.W. Grosse-Kunstleve, J.S. Jiang, J. Kuszewski, M. Nilges, N.S. Pannu, et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54:905–921. [DOI] [PubMed] [Google Scholar]
- 47.Guex, N., and M.C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 18:2714–2723. [DOI] [PubMed] [Google Scholar]
- 48.Martz, E. 2002. Protein Explorer: easy yet powerful macromolecular visualization. Trends Biochem. Sci. 27:107–109. [DOI] [PubMed] [Google Scholar]