Abstract
Epidemiological studies have suggested that the recent increase in the incidence and severity of immunoglobulin (Ig)E-mediated allergic disorders is inversely correlated with Mycobacterium bovis bacillus Calmette Guerin (BCG) vaccination; however, the underlying mechanisms remain uncertain. Here, we demonstrate that natural killer T (NKT) cells in mice and humans play a crucial role in the BCG-induced suppression of IgE responses. BCG-activated murine Vα14 NKT cells, but not conventional CD4 T cells, selectively express high levels of interleukin (IL)-21, which preferentially induces apoptosis in Bɛ cells. Signaling from the IL-21 receptor increases the formation of a complex between Bcl-2 and the proapoptotic molecule Bcl-2–modifying factor, resulting in Bɛ cell apoptosis. Similarly, BCG vaccination induces IL-21 expression by human peripheral blood mononuclear cells (PBMCs) in a partially NKT cell–dependent fashion. BCG-activated PBMCs significantly reduce IgE production by human B cells. These findings provide new insight into the therapeutic effect of BCG in allergic diseases.
The prevalence of IgE-mediated allergic diseases such as asthma, hay fever, and atopic dermatitis has increased dramatically over the past two decades, especially in industrialized countries (1). For example, the incidence of asthma has nearly doubled since 1980 in the United States as well as in Japan (1, 2). However, the precise mechanisms underlying the increased incidence of allergic diseases are not fully understood. One possible explanation has been termed “the hygiene hypothesis,” which proposes that improved hygiene combined with the excessive use of antibiotics in industrial countries has markedly reduced the incidence of infections, particularly in children. This lack of early exposure to infectious agents is associated with accelerated IgE production and an increased incidence of allergic disorders (1–3). Epidemiological studies support this hypothesis (4–6), and bacterial and viral products have been proposed as therapeutic strategies to suppress the development of allergic responses. For example, vaccination with Mycobacterium bovis bacillus Calmette Guerin (BCG) has been reported to suppress IgE production and inhibit the development of allergic diseases in mouse models (7–9) and in humans (10). Furthermore, injection of CpG oligodeoxynucleotides, bacterial DNA surrogates recognized by Toll-like receptor (TLR)9, reduces serum IgE levels in mice (11).
It has been widely accepted that IgE production is totally dependent on Th2 cells, whose functions are reciprocally inhibited by Th1 cells. Mechanistically, therefore, the hygiene hypothesis is based on an imbalance in the Th1/Th2 ratio because bacterial components stimulate Th1 responses that in turn inhibit Th2 responses and IgE production (12). On the other hand, recent findings have indicated that a spectrum of T cells with immunoregulatory properties is involved in the regulation of IgE production and the pathophysiology of allergic diseases (13). For example, CD4+CD25+ regulatory T cells inhibit Th2 responses by producing immunosuppressive cytokines that can directly inhibit B cell activation (14, 15). Furthermore, NKT cells expressing an invariant antigen receptor (Vα14-Jα281 for mice and Vα24-JαQ for humans; reference 16) suppress Th2 and IgE responses via their production of IFN-γ (17).
In addition to these cellular mechanisms, it has also been reported that IL-21 is involved in the suppression of IgE production in both mice and humans (18, 19). IL-21 is a type I cytokine produced by activated CD4+ T cells and has a broad capacity to regulate lymphoid cell functions (20–22). Among these functions, IL-21 directly inhibits antibody production by IgE-bearing B (Bɛ) cells induced by CD40L and IL-4 (18). Conversely, IL-21R–deficient mice exhibit enhanced IgE production (23). IL-21 has been shown to specifically inhibit germ line transcription of the IgE constant region (Cɛ) gene but not of other isotype genes (18). However, there is no direct evidence that this inhibition of germ line transcription is responsible for the suppression of IgE production, as class switch recombination of Ig genes and subsequent antibody secretion are differentially regulated events (24). IL-21 also induces apoptosis in B cells (25, 26), which could partially explain the reduction of IgE production; however, this effect was not shown to be specific for IgE. Hence, the mechanism by which IL-21 specifically inhibits IgE production is not yet fully understood.
Here, we have investigated BCG-mediated IgE suppression and found that NKT cells specifically induced apoptosis in Bɛ cells through the production of IL-21, resulting in a dramatic decrease in IgE production. IL-21 increased the formation of a complex between Bcl-2 and the proapoptotic molecule Bcl-2–modifying factor (Bmf), which is selectively expressed in Bɛ cells and counteracts the antiapoptotic activity of Bcl-2. We have found that similar mechanisms are operative in humans. This is the first report demonstrating that IL-21 produced by Vα14 NKT cells plays an important role in the regulation of IgE responses in both mouse and human immune systems.
RESULTS
Vα14 NKT cell–dependent IgE suppression by BCG treatment
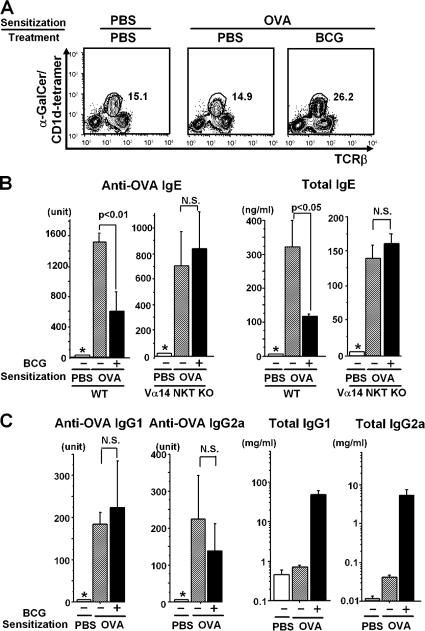
We used an OVA-patched sensitization protocol (27) to determine if BCG activates Vα14 NKT cells. Vα14 NKT cells were detected by α-galactosylceramide (α-GalCer)–loaded CD1d tetramer staining. In control mice treated with PBS or OVA without BCG, ∼15% of the liver mononuclear cells (MNCs) were Vα14 NKT cells (Fig. 1 A, left and middle). However, BCG treatment significantly increased the frequency of Vα14 NKT cells to >25% (Fig. 1 A, right). BCG treatment also increased the absolute number of Vα14 NKT cells because the total number of liver MNCs was also increased by 50–80% (not depicted). Sera were collected from these mice 1 wk after the last sensitization, and IgE levels were evaluated. In WT mice, both total and OVA-specific IgE levels were suppressed by BCG treatment (Fig. 1 B). In mice lacking the Jα18 gene (Vα14 NKT KO), there was no significant BCG-induced suppression of IgE responses, suggesting that suppression requires Vα14 NKT cells.
Figure 1.
Requirement of Vα14 NKT cells in BCG-mediated IgE suppression. (A) FACS profiles of liver MNCs. The liver MNCs obtained 1 wk after the last immunization were stained with α-GalCer/CD1d tetramer and anti-TCRβ mAb. Three mice per each group were analyzed and representative data are shown. (B and C) Effects of BCG on antibody responses in WT and Vα14 NKT KO mice. Total and OVA-specific serum IgE (B), IgG1, and IgG2a (C) were assayed by ELISA. Five mice were used in each group. Values are expressed as mean ± SD. The asterisks (*) indicate that the amount of IgE was below the detection level for anti-OVA IgE (<31.2 U/ml), anti-OVA IgG1 (<0.002 U/ml), or anti-OVA IgG2a (<1.25 U/ml). N.S., not significant. All experiments were repeated three times with similar results.
The effect of BCG administration on Th1/Th2 responses
It is well known that the isotype commitment of B cells during Ig class switching is tightly regulated by Th1/Th2 cell cytokines (28) and that Vα14 NKT cells play a regulatory role in T cell differentiation (17, 29, 30). Therefore, we measured serum IgG2a (Th1) and IgG1 (Th2) levels to assess any changes in the Th1/Th2 balance. BCG administration did not significantly alter the levels of OVA-specific IgG1 or IgG2a, although total levels of both isotypes were significantly enhanced (Fig. 1 C).
Innate signaling pathway for BCG-mediated IL-12 production
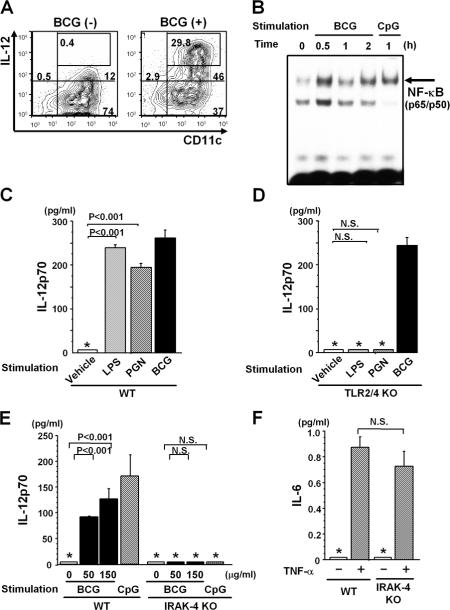
During microbial infection, both CD1d- and IL-12–mediated signals are required for the rapid activation of Vα14 NKT cells (31). Thus, we assessed IL-12 production after BCG treatment. BM-derived DCs (BM-DCs) were stimulated in vitro with 50 μg/ml BCG and examined for IL-12 production by intracellular cytokine staining using an IL-12p40/p70 mAb. Upon BCG stimulation, a large fraction of CD11chigh cells produced IL-12 (Fig. 2 A). NF-κB activation is crucial for IL-12 production, and BCG treatment activated NF-κB to the same extent as treatment with the positive control CpG, as demonstrated by electrophoretic mobility shift assay (Fig. 2 B). These results indicate that BCG directly induces IL-12 production in DCs by activating NF-κB.
Figure 2.
Activation of DCs by BCG. IL-12 production (A) and NF-κB activation (B). (A) Intracellular staining of BM-DCs with anti–IL-12p40/p70 and anti-CD11c mAbs with or without in vitro BCG (50 μg/ml) treatment for 12 h. BCG-treated BM-DCs (10,000 cells) were analyzed by FACS, and the number in each panel indicates the percentage of total cells. (B) NF-κB activation. 2 × 105 BM-DCs were stimulated with or without 50 μg/ml BCG or 1 μM CpG in vitro. NF-κB activity was determined by EMSA. (C and D) No requirement of TLR2 and TLR4 in BCG-mediated IL-12 production. 2 × 105 BM-DCs derived from WT (C) or TLR2/4 double KO (D) mice were stimulated in vitro with or without 10 μg/ml LPS, 10 μg/ml PGN, or 150 μg/ml BCG for 48 h, and IL-12p70 levels were measured by ELISA. (E and F) Requirement of IRAK-4 for IL-12 production. 2 × 105 BM-DCs were assayed for IL-12p70 by ELISA after stimulation with 0, 50, or 150 μg/ml BCG or 1 μM CpG (E), and for IL-6 with 10 ng/ml TNF-α stimulation for 48 h (F). In C–F, values are expressed as mean ± SD of triplicate cultures. The asterisks (*) indicate that the levels were below the detection limits for IL-12p70 (<62.5 pg/ml) and IL-6 (<15.6 pg/ml). N.S., not significant. All experiments were repeated twice with similar results.
It has been reported that mycobacterial cell wall antigens such as peptidoglycan (PGN) or lipoarabinomannan induce proinflammatory gene transcription through TLR2 and TLR4 (32). However, when we compared IL-12p70 production by BCG-stimulated WT and TLR2/TLR4 double KO BM-DCs, there was no difference (Fig. 2 C). As expected, however, the TLR2/4-deficient cells failed to respond to LPS or PGN (Fig. 2 D). These results indicate that receptor(s) other than TLR2 and TLR4 are responsible for the recognition of whole BCG organisms.
To analyze intracellular signaling pathways activated by BCG, we measured IL-12p70 production by BM-DCs from WT and IL-1R–associated kinase (IRAK)-4 KO mice. BM-DCs from IRAK-4 KO mice produced less IL-12p70 than those from WT mice in response to both BCG and CpG (Fig. 2 E), whereas they produced comparable levels of IL-6 in response to TNF-α stimulation (Fig. 2 F). Similarly, BM-DCs from myeloid differentiation factor 88 (MyD88) KO mice produced nearly undetectable IL-12p70 upon BCG stimulation, whereas IL-6 production remained unchanged (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062206/DC1). Therefore, the recognition of BCG organisms is mediated by innate receptors other than TLR2 and TLR4 that signal through both IRAK-4 and MyD88.
BCG-induced IL-21 expression in Vα14 NKT cells
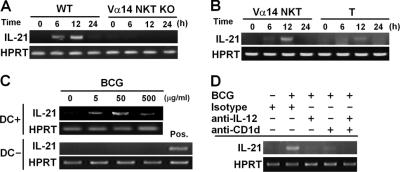
The recently identified IL-21 and its receptor (IL-21R), members of the common γ-chain (γc)-dependent cytokine family, have been shown to regulate IgE production without influencing Th2 cell differentiation (18, 20, 23). Thus, we examined the possibility that IL-21 might be induced by BCG stimulation and might suppress IgE responses in a Vα14 NKT cell–dependent manner. We first measured IL-21 mRNA expression in TCRβ+ liver MNCs by a RT-PCR. IL-21 mRNA was detected in liver TCRβ+ liver MNCs of WT mice within 6 h after BCG injection (Fig. 3 A). In contrast, no IL-21 mRNA was detected in the Vα14 NKT KO mice (Fig. 3 A), suggesting that Vα14 NKT cells are the source of IL-21 in response to BCG. To test this hypothesis, we separated conventional T cells and Vα14 NKT cells and found that IL-21 mRNA was more abundant in the Vα14 NKT cells after BCG injection (Fig. 3 B). Similarly, after stimulation with anti-CD3, IL-21 mRNA levels in Vα14 NKT cells were more than seven times higher than in CD4 T cells, confirming that these cells are the major source of IL-21 in this model (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20062206/DC1).
Figure 3.
IL-21 expression. (A) Vα14 NKT cell–dependent IL-21 production. Liver MNCs were obtained after BCG injection (500 μg/mouse) and examined for IL-21 mRNA expression. (B) Identification of the source of IL-21. Vα14 NKT and conventional T cells were sorted from liver MNCs and examined for IL-21 mRNA expression. (C) Requirement of DCs for BCG-induced IL-21 expression by Vα14 NKT cells. Liver TCRβ+ cells were cultivated in the presence of 50 μg/ml BCG with (top) or without (bottom) BM-DCs for 24 h and analyzed for IL-21 mRNA expression. Liver TCRβ+ cells stimulated with 10 μg/ml anti-CD3 mAb were used as a positive (Pos.) control. (D) Requirement of IL-12– and CD1d-mediated signals for IL-21 mRNA expression upon BCG stimulation. An isotype control, anti–IL-12p40/p70, or anti-CD1d mAb (20 μg/ml) was added to the cultures of liver TCRβ+ cells and BM-DCs as described in C. All experiments were repeated twice with similar results.
Requirement for IL-12 and CD1d in IL-21 expression by Vα14 NKT cells
We next analyzed the role of DCs in BCG-induced IL-21 mRNA expression. Co-culture of Vα14 NKT cells with DCs plus IL-12 strongly induced IL-21 mRNA expression, whereas no IL-21 mRNA was induced in the absence of DCs (Fig. 3 C). Furthermore, IL-21 mRNA expression was inhibited by the addition of anti–IL-12, anti-CD1d, or both into the cultures (Fig. 3 D), indicating that both IL-12 and CD1d are required for IL-21 expression by Vα14 NKT cells.
IL-21–mediated IgE suppression
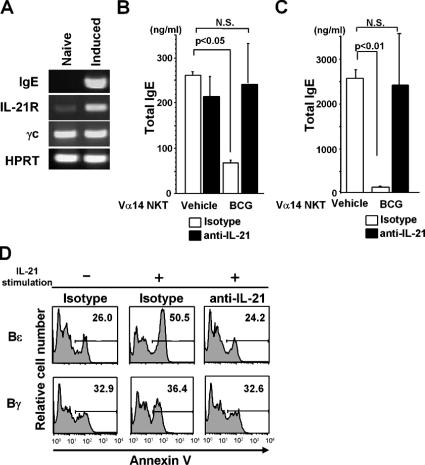
To examine whether BCG-activated Vα14 NKT cells actually suppress IgE production, Bɛ cells were generated from naive CD19+ splenic B cells using the 3-d culture system described by Snapper et al. (33). The starting population of naive B cells expressed negligible IL-21R and contained no Bɛ cells as defined by Cɛ transcripts (Fig. 4 A). However, after 3 d of the culture, the majority of CD19+ B cells became Bɛ cells and expressed IL-21R (Fig. 4 A). We then investigated the effects of BCG treatment on B cells, before and after IgE class switching. The addition of BCG-treated liver MNCs at the onset of the naive B cell cultures significantly suppressed IgE production (∼50%; Fig. 4 B). However, when BCG-activated Vα14 NKT cells were added to the Bɛ cell culture on day 3 and the cells were further cultivated for 5 d, IgE production was even more strongly inhibited (>90% suppression; Fig. 4 C). These results indicate that, even after B cells have undergone Cɛ class switching, BCG-activated Vα14 NKT cells can potently suppress IgE production. The inhibition of IgE production was IL-21 dependent, as an anti–IL-21 mAb completely abrogated the inhibitory effects (Fig. 4, B and C). When the B cells in these cultures were assessed for apoptosis by annexin V staining, there was a significant increase in apoptotic Bɛ cells that was not observed in the Bγ cells (Fig. 4 D, middle). Apoptosis of Bɛ cells was abrogated by the addition of anti–IL-21, a treatment that had no significant effect on Bγ cells (Fig. 4 D, right).
Figure 4.
IL-21–mediated Bɛ cell apoptosis. (A) RT-PCR analysis. Expression of IgE (Cɛ), IL-21R, and γc was investigated in naive B (left) and Bɛ (right) cells. (B) Suppression of IgE production in naive B cell cultures. Naive B cells and Vα14 NKT cells (105 each) were cocultured in the presence of sCD40L and IL-4. (C) Suppression of IgE production in the Bɛ cell culture. 105 Vα14 NKT cells were added to the Bɛ cell (105) cultures. In B and C, 20 μg/ml anti–IL-21 mAb or isotype control mAb was added at the same time as the Vα14 NKT cells. The concentration of total IgE was measured by ELISA in triplicate. Values are expressed as mean ± SD. N.S., not significant. The experiments were repeated three times with similar results. (D) IL-21–mediated Bɛ cell apoptosis. 2 × 105 Bɛ and Bγ cells were generated and then further cultured with or without 30 ng/ml IL-21 for 30 h. Annexin V staining was then performed. The numbers represent percentage of the gated cells. Annexin V+ cells among Bɛ and Bγ cells just before IL-21 treatment was 25.7 and 29.2%, respectively (not depicted). The experiments were repeated three times with similar results.
Bmf-induced Bɛ cell apoptosis
To understand the molecular mechanisms underlying IL-21– induced IgE suppression, we performed DNA microarray analyses to compare gene expression between Bɛ and Bγ cells. The DNA microarray data were deposited in the Center for Information Biology Gene Expression database (CIBEX; http://cibex.nig.ac.jp/) under accession number CBX15. The proapoptotic Bmf gene (34) was dramatically up-regulated in Bɛ cells, a finding that was confirmed by RT-PCR (Fig. 5 A). No significant difference in the expression of IL-21R, Bcl-2, or γc was detected (Fig. 5 A), suggesting that elevated Bmf gene expression in Bɛ, but not in Bγ, cells may account for their differential sensitivity to IL-21–mediated apoptosis.
Figure 5.
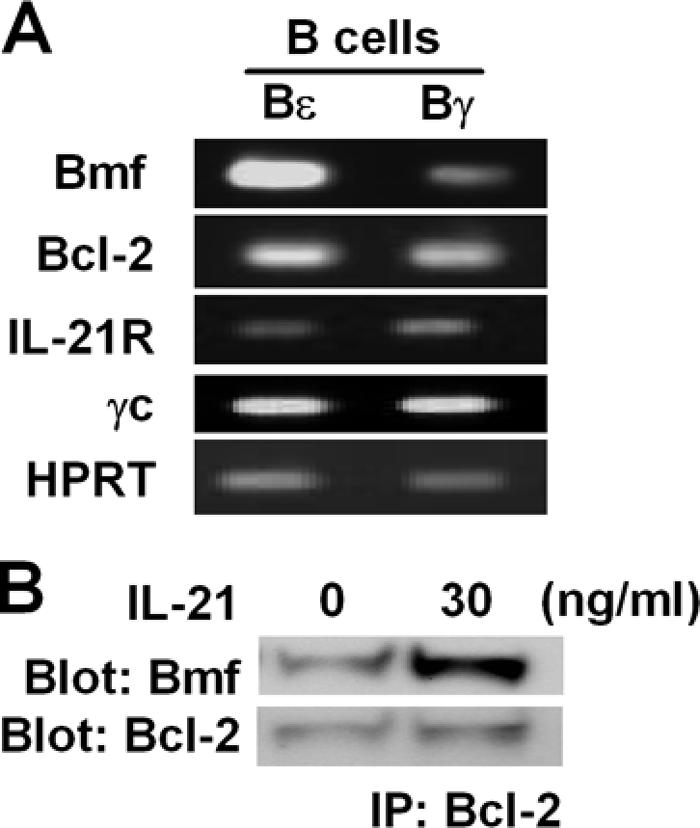
Bmf-mediated Bɛ cell apoptosis. (A) RT-PCR. RNA from Bɛ and Bγ cells was analyzed for its expression of the indicated genes by RT-PCR. Note that no significant differences in Bcl-2 and IL-21R expression between Bɛ and Bγ cells were observed. (B) Western blotting. Bɛ cells were stimulated with IL-21 at 37°C for 30 min, and their cell lysates (6 × 106) were subjected to immunoprecipitation with anti–Bcl-2 mAb and immunoblotting with anti-Bmf antibody (top) or anti–Bcl-2 mAb (bottom). All experiments were repeated three times with similar results.
To investigate whether the Bmf expressed in Bɛ cells is functional in its proapoptotic activity, Bmf cDNA was isolated from Bɛ cells and used to prepare several mutants of enhanced GFP–fused Bmf. These mutations included an A69P mutation in the dynein light chain 2 binding motif and an L138A mutation in the BH3 domain. These Bmf mutants were transfected into Baf3 cells. Upon IL-3 deprivation, mock transfectants underwent apoptosis. Transfection with WT Bmf or Bmf-A69P to Baf3 cells also significantly augmented apoptosis (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20062206/DC1). However, reduced apoptosis was seen in Baf3 cells transfected with BH3 mutants, such as Bmf-L138A or Bmf-A69P/L138A (Fig. S3), indicating that Bmf in Bɛ cells is functional and the BH3 domain of the protein is important for mediating its proapoptotic activity.
Based on the understanding of proapoptotic activity of Bmf expressed in Bɛ cells, we investigated the formation of Bmf–Bcl-2 complexes in Bɛ cells after activation with IL-21. Bmf in Bɛ cells faintly binds to Bcl-2 in unstimulated cells (Fig. 5 B, left). However, when Bɛ cells were stimulated with IL-21, the formation of Bmf–Bcl-2 complexes was significantly augmented (Fig. 5 B, right).
BCG-mediated IL-21 induction in human Vα24 NKT cells
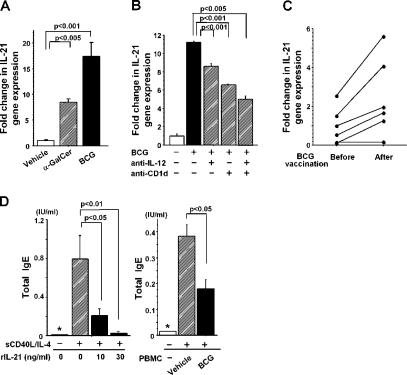
To determine how widespread our findings are, we investigated whether IL-21 and Vα24 NKT cells are required for the BCG-mediated suppression of human IgE responses. When human PBMCs were stimulated with α-GalCer or BCG, a significant up-regulation of IL-21 mRNA was detected by quantitative PCR (Fig. 6 A). The BCG-induced up-regulation of IL-21 mRNA was effectively suppressed by blocking with antibodies against CD1d, IL-12p40/p70, or both (Fig. 6 B), indicating that the CD1d-restricted NKT cell–dependent suppression of IgE responses observed in mice also operates in the human immune system. IL-21 mRNA expression by anti-CD1d and anti–IL-12 treatment was significantly reduced but was not as effective as in the mouse Vα14 NKT cell system (Fig. 3 D), perhaps suggesting a significant contribution of human conventional CD4+ T cells (Fig. S2 B).
Figure 6.
IL-21 mRNA expression and IL-21–induced IgE suppression in humans. (A) IL-21 mRNA expression in PBMCs. 106 human PBMCs were stimulated with 100 ng/ml α-GalCer or 50 μg/ml BCG and examined for IL-21 expression by quantitative real-time PCR with Taqman probes. The data are representative of five donors. (B) IL-12 and CD1d are required for IL-21 expression. 106 PBMCs were stimulated in vitro with 50 μg/ml BCG in the presence of 10 μg/ml anti-CD1d and/or anti–IL-12p40/p70 mAb. Representative data from five donors are shown. (C) IL-21 mRNA expression in PBMCs. Healthy volunteers were inoculated intradermally with BCG (two drops of 26.7 mg/ml of BCG emulsion per person). In A–C, the data for IL-21 expression were normalized to 18S ribosomal RNA expression, and relative expression levels are shown. Statistical analysis was performed using a matched pairs t test in C. (D) Suppression of IgE production. Left, suppression of IgE production by IL-21. 2 × 105 human B cells were cultured with sCD40L and IL-4 in the presence of human IL-21 for 14 d. Right, suppression of IgE production by BCG-activated human PBMCs. 105 Bɛ cells were cocultured with 105 PBMCs, sCD40L, and IL-4 in the presence of 50 μg/ml BCG for 14 d. Total IgE was measured by ELISA. Values are expressed as mean ± SD of triplicate cultures. The asterisks (*) indicate that the IgE levels are below the detection limit for total IgE (<0.014 IU/ml). Data shown are representative of three donors. Results were expressed as a fold difference in human IL-21 gene expression relative to a control sample (vehicle) after being normalized with 18S ribosomal RNA expressions in each sample.
To evaluate in vivo responses, we inoculated BCG into healthy volunteers and examined IL-21 mRNA levels in PBMCs 1 wk later. There was a significant up-regulation of IL-21 mRNA levels in five out of six individuals (Fig. 6 C), and, furthermore, IL-21 suppressed IgE production by human Bɛ cells (Fig. 6 D, left). As expected, the addition of BCG-stimulated, but not control, PBMCs significantly inhibited IgE production (Fig. 6 D, right).
DISCUSSION
It is widely accepted that the mechanistic basis of the hygiene hypothesis for suppression of IgE responses is an increase in the Th1/Th2 ratio (12). However, in reality, the Th1 response exacerbates allergic reactions, as human asthma is associated with the production of IFN-γ, a cytokine that appears to contribute to the pathogenesis of the disease (35). Furthermore, the adoptive transfer of allergen-specific Th1 cells causes severe airway inflammation (36). Thus, a shift in the Th1/Th2 ratio alone cannot explain all of the immunological findings observed in allergic diseases (1). Furthermore, there are several studies suggesting that BCG vaccination has little or no effect on the development and prevalence of allergic diseases (37, 38). Therefore, it is necessary to better understand the precise mechanism of IgE suppression in BCG-treated animals or humans.
In this study, neither a Th1/Th2 imbalance nor an involvement of regulatory T cells was observed in response to BCG treatment (Fig. 1). Instead, we demonstrated that IL-21–induced Bɛ cell apoptosis is the mechanism responsible for BCG-mediated suppression of IgE production (Figs. 1, 3, and 5). Because the human IL-21 responses to BCG vaccination were heterogeneous (Fig. 6 C), it seems likely that the magnitude of the response in each individual could cause different degrees of BCG-induced IgE suppression and might be prognostic.
Previous studies have indicated that IL-21 is preferentially expressed by activated CD4+ T cells (20), the results that are partially in agreement with the present data, as half of peripheral Vα14 NKT cells are CD4+ (39, 40). Interestingly, upon anti-CD3 mAb stimulation, Vα14 NKT cells, but not conventional T cells, preferentially expressed IL-21 (Fig. S2 A), similar to the results with BCG (Fig. 3 B). Therefore, the major IL-21 producers in response to BCG in mice are Vα14 NKT cells.
It has been proposed that, for full activation of Vα14 NKT cells to produce IFN-γ, two signals are required: one CD1d-dependent and the other TLR-mediated IL-12–dependent signals (31). In agreement with this, IL-21 expression by BCG-activated Vα14 NKT cells was significantly inhibited by blocking with antibodies to IL-12 and/or CD1d (Fig. 3 D). Therefore, it is likely that Vα14 NKT cells recognize endogenous antigens presented by CD1d molecules but require IL-12 signals to produce IL-21. Nevertheless, it is still possible that glycolipid BCG components such as phosphatidylinositol mannoside may directly stimulate Vα14 NKT cells to produce IL-21 in a CD1d-dependent manner (41, 42).
In terms of the receptors on DCs that are required for BCG recognition and signal transduction, we showed in this study that BCG-induced IL-12 production is IRAK-4 and MyD88 dependent (Fig. 2 E and Fig. S1). These results in mice are consistent with a recent report indicating that BCG cannot induce IL-12 or IFN-γ production by PBMCs from IRAK-4–deficient patients (43). In addition, it has been reported that BCG enhances NF-κB–dependent gene transcription through the activation of phosphatidylinositol 3 kinase and c-Jun N-terminal kinase cascades (44). The activated NF-κB is then liberated for nuclear translocation and transactivates a variety of immune response genes, including IL-12.
In contrast to a previous report that implicated TLR2 and TLR4 in the recognition of mycobacterial antigens (32), we could not identify any involvement of these receptors in IL-12 production by BCG-stimulated BM-DCs (Fig. 2, C and D). In agreement with our findings, it has recently been reported that TLR2/4 double KO mice infected with live BCG have normal adaptive immune responses and survived as long as WT mice (45). As whole BCG contains multiple components including mycobacterial glycolipids, proteins, and DNA, several receptors that use IRAK-4 and MyD88 as the signal transducer appear to be involved in the complex recognition of BCG.
In IL-21R–deficient mice, the level of circulating IgE is high, whereas that of IgG1 is low (23, 46). Similarly, in human B cells, IL-21 inhibits IgE production and stimulates IgG4 (analogous to mouse IgG1) production (19). These results suggest that IL-21 differentially regulates IgE and IgG1 (IgG4 in humans) class switching. In fact, Suto et al. (18) reported that IL-21 specifically suppresses IgE production by inhibiting germ line Cɛ transcripts. Our present findings do not exclude this possibility. IL-21 has also been reported to induce apoptosis in resting and activated B cells by reducing the expression levels of apoptosis-related genes (25, 26). However, in this report, we have shown that IL-21 selectively induces apoptosis in Bɛ, but not Bγ, cells (Fig. 4 D). Thus, our findings that BCG-activated IL-21–expressing Vα14 NKT cells suppressed IgE production even after class switching (Fig. 4 C) suggests that the role of IL-21 on Bɛ cells is to control cell growth and viability, rather than to regulate the differentiation and maturation of these cells.
We found that expression of a proapoptotic gene, Bmf, was significantly higher in Bɛ cells than in Bγ cells (Fig. 5 A). Under physiological conditions, Bmf, which is a BH3 domain–only Bcl-2 family member that inhibits Bcl-2 function and accelerates apoptosis, binds to myosin V motors via the dynein light chain 2 domain of Bmf (34). In response to certain cellular damage signals, Bmf is supposed to be released from the myosin V motors and trigger apoptosis (34). Because Bmf from Bɛ cells induced apoptosis and a mutation in the BH3 domain of Bmf failed to induce apoptosis (Fig. S3), we confirmed that Bmf expressed in Bɛ cells is functional, and that the BH3 domain is important for the binding to Bcl-2 and is essential for its proapoptotic activity. In fact, the binding of Bmf with Bcl-2 was up-regulated by IL-21R signaling (Fig. 5 C). Therefore, BCG-mediated Bɛ cell apoptosis is due to the augmented formation of Bmf–Bcl-2 complexes generated by IL-21R signaling in Bɛ cells.
Finally, we defined the mechanism of BCG-induced IL-21–dependent suppression of IgE production in humans (Fig. 6). In a broader context, these findings may explain the mechanisms underlying the BCG-mediated suppression of allergic diseases and the epidemiological data indicating a reduction in the morbidity of allergic diseases in patients who have been infected with Mycobacterium tuberculosis. Interestingly, IL-21–mediated B cell responses in C57BL/6 mice differ from those in BALB/c mice (26), suggesting that there is a genetic polymorphism with respect to the outcome of IL-21 signaling in B cells. In fact, a recent report indicated that polymorphisms in the IL-21R gene locus differentially affect serum IgE levels in humans (47). In this study, consistent with our data, the levels of IL-21 expression induced by BCG stimulation varied among the individuals examined (Fig. 6 C). These results suggest that the response to BCG in humans is dependent, at least in part, on genetic background. The specific genes responsible for the heterogeneity in BCG-mediated IL-21 production have not been identified. However, this observation may be applied to the development of diagnostic or therapeutic strategies in which the levels of IL-21 expression are used to evaluate the efficacy of BCG treatment, or in determining the potential benefit of therapy using bacterial products such as CpG for allergic diseases.
MATERIALS AND METHODS
Mice.
7–10-wk-old female BALB/c mice were purchased from Japan CREA Inc. Vα14 NKT-deficient (Vα14 NKT KO) mice on a BALB/c background (48), IRAK-4 KO (49), TLR2 KO, TLR4 KO, and MyD88 KO mice (50, 51) have been described. TLR2 and TLR4 double KO mice were generated by breeding. Mice were kept under specific pathogen-free conditions, maintained on an OVA-free diet, and treated in accordance with the guidelines for animal care at RIKEN Research Center for Allergy and Immunology.
Allergic sensitization and BCG.
Allergic epicutaneous sensitization was performed as described previously (27). In brief, a 1-cm2 sterile patch infused with 100 μl of PBS solution with or without 100 μg OVA (grade V; Sigma-Aldrich) was placed on the shaved back of mice and fixed in place with a bio-occlusive dressing and an elastic bandage. Patches were left on for 48 h and removed. The sensitization course was repeated at the same skin site every week for 4 wk. For BCG vaccination, mice were given a weekly i.p. injection of BCG (500 μg/mouse) or PBS at the time of OVA sensitization. The attenuated BCG (strain Tokyo) was purchased from the Japan BCG Laboratory.
Flow cytometry.
Cells were stained with antibodies after adding 2.4G2 (BD Biosciences) for Fc blocking. The following antibodies were used: FITC–anti-CD19 (1D3), FITC–anti-IgE (R35-72), APC–anti-IgG1 (X59), FITC–anti-TCRβ (H57-597), APC–anti–IL-12p40/70 (C15.6), and PE–anti-CD11c (HL3; BD Biosciences). PE-conjugated α-GalCer–loaded CD1d tetramer (α-GalCer/CD1d tetramer) was prepared as described previously (52). For intracellular staining, BM-DCs were fixed and permeabilized with BD Cytofix and Cytoperm kits after staining with PE–anti-CD11c. They were then stained with APC–anti–IL-12p40/70. FACS analysis of at least 10,000 cells and cell sorting were performed with a FACSCalibur (BD Biosciences) with FlowJo software (TreeStar) or with a MoFlo cell sorter (DakoCytomation).
Cell preparations and cultures.
2 × 106 BM-DCs obtained by culturing BM for 6 d with 10 ng/ml GM-CSF were further cultured in the presence or absence of BCG, CpG, LPS (Invivogen), PGN from Escherichia coli (Invivogen), or 10 μg/ml anti-CD3 mAb (2C11; BD Biosciences) for 48 h at 37°C. For blocking experiments, mAb against CD1d or IL-12p40/p70 (clones 1B1 and C17.8, respectively; BD Biosciences), or an isotype control was added at a concentration of 20 μg/ml after 2.4G2 treatment. TCRβ+ cells or Vα14 NKT cells with a purity of >98% were obtained from liver MNCs (52) using an Auto MACS (Miltenyi Biotec) after staining with FITC–anti-TCRβ and sorting with anti-FITC magnetic beads (Miltenyi Biotec). Vα14 NKT cells were then isolated from TCRβ+ cells by MoFlo using PE–α-GalCer/CD1d tetramer. Conventional T or CD4+ T cells were isolated from an α-GalCer/CD1d tetramer− fraction of TCRβ+ liver MNCs. Bɛ and Bγ cells generated from splenic CD19+ cells in the presence of 10 μg/ml sCD40L (ALX-850-075; Qbiogene) and 20 ng/ml of recombinant IL-4 (PeproTech) for 3 d (33) were cultured for 30 h for the apoptosis assay or for an additional 5 d to investigate IgE responses.
ELISA.
Cytokines (IL-12p70 and IL-6) and Ig subclasses (IgG1, IgG2a, and IgE) were measured by ELISA using kits or sets of antibodies (BD Biosciences) according to the manufacturer's protocol. Specific antibodies were also measured as described previously (7).
RT-PCR.
Total RNA was extracted by RNAeasy (QIAGEN), and cDNA was synthesized with random primers after DNase treatment. The following RT-PCR primer sets were used for mouse genes: IL-21, 5′-CCCTTGTCTGTCTGGTAGTCATC-3′ and 5′-ATCACAGGAAGGGCATTTAGC-3′; IgE (Cɛ), 5′-AGGAACCCTCAGCTCTACCC-3′ and 5′-GCCAGCTGACAGAGACATCA-3′; mIL-21R, 5′-TGTCAATGTGACGGACCAGT-3′ and 5′-CAGCATAGGGGTCTCTGAGG-3′; γc, 5′-GTCGACAGAGCAAGCACCATGTTGAAACTA-3′ and 5′-GGATCCTGGGATCACAAGATTCTGTAGGTT-3′; Bmf, 5′-CAGACCCTCAGTCCAGCTTC-3′ and 5′-CGTATGAAGCCGATGGAACT-3′; Bcl-2, 5′-GGTGGTGGAGGAACTCTTCA-3′ and 5′-CATGCTGGGGCCATATAGTT-3′; and HPRT, 5′-AGCGTCGTGATTAGCGATG-3′ and 5′-CTTTTATGTCCCCCGTTGAC-3′. The numbers of PCR cycles were as follows: 30 for HPRT; 35 for IgE, γc, and IL-21R; 40 for IL-21 and Bmf; and 45 for Bcl-2. The amounts of cDNA were standardized by quantification of the housekeeping gene HPRT using primers for mouse samples. The human IL-21 mRNA levels were quantified by real-time quantitative PCR on the ABI Prism 7000 sequence detection system (Applied Biosystems) by using TaqMan assay kits and TaqMan Gene Expression Assays (primers and TaqMan probes).
Electrophoretic mobility shift assay.
2 × 106 BM-DCs were stimulated with 50 μg/ml BCG or 1.0 μM CpG-B for the indicated periods. Nuclear extracts were prepared and used for Gel Shift Assay Systems (Promega) as described previously (50).
Bɛ cell–derived Bmf and its mutants.
cDNAs encoding bmf were amplified from Bɛ cells by PCR using primers 5′-CCGAATTCGGATGGAGCCACCTCAGTGTGT-3′ and 5′-GCGGCCGCCTGCATTCCTGGTGATCCAT-3′ (EcoRI and NotI sites for cloning are underlined). The amplified products were cloned using the pGEM-T Easy Vector System (Promega). Mutant cDNAs were generated by PCR using point-mutated primer pairs.
Immunoprecipitation and Western blotting.
Interaction of Bmf with Bcl-2 in Bɛ cells was detected by immunoprecipitation with anti–Bcl-2 mAb (clone 7; BD Transduction Laboratories) and subsequent immunoblotting with anti-Bmf rabbit antibody (Cell Signaling). The protein levels were visualized by ECL (GE Healthcare) using horseradish peroxidase–conjugated Protein A/G (Pierce Chemical Co.).
Human studies.
All human specimens were obtained under informed consent. The protocol for the human research project has been approved by the Ethics Committee of Chiba University and RIKEN, and conformed to the provisions of the Declaration of Helsinki in 1995. 108 PBMCs from healthy volunteers were prepared by Ficoll-Paque density gradient centrifugation and used for the cultures. Human recombinant IL-21 was purchased from BIOSOURCE Inc. Human total IgE was measured with a sensitive immune assay (GE Healthcare).
Statistical analysis.
Statistical analyses were performed using the Student's t test or matched pairs t test. P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 provides data demonstrating that MyD88 signaling in DCs is required for BCG-induced activation. Fig. S2 contains data demonstrating IL-21 mRNA expression by NKT cells, CD4+ T cells, and CD8+ T cells of murine and human origin. Fig. S3 provides the data indicating proapoptotic activity of Bɛ cell–derived Bmf and functional domain analysis using mutant Bmf in Baf3 cells. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20062206/DC1.
Supplemental Material
Acknowledgments
The authors thank Prof. Peter Burrows for critical reading and Ms. Norie Takeuchi for secretarial assistance.
This work was partly supported by a grant from The Ministry of Education, Culture, Sports, Science and Technology (RR2002).
The authors have no conflicting financial interests.
Abbreviations used: α-GalCer, α-galactosylceramide; BCG, Mycobacterium bovis bacillus Calmette Guerin; BM-DC, BM-derived DC; Bmf, Bcl-2–modifying factor; γc, common γ-chain; IRAK, IL-1R– associated kinase; MNC, mononuclear cell; MyD88, myeloid differentiation factor 88; PGN, peptidoglycan; TLR, Toll-like receptor.
References
- 1.Umetsu, D.T., J.J. Mcintire, O. Akbari, C. Macaubas, and R.H. Dekruyff. 2002. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 3:715–720. [DOI] [PubMed] [Google Scholar]
- 2.Cookson, W.O., and M.F. Moffatt. 1997. Asthma: an epidemic in the absence of infection? Science. 275:41–42. [DOI] [PubMed] [Google Scholar]
- 3.Von Mutius, E. 2000. The environmental predictors of allergic disease. J. Allergy Clin. Immunol. 105:9–19. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen, S.O., P. Aaby, A.J. Hall, D.J. Barker, C.B. Heyes, A.W. Shiell, and A. Goudiaby. 1996. Measles and atopy in Guinea-Bissau. Lancet. 347:1792–1796. [DOI] [PubMed] [Google Scholar]
- 5.Shirakawa, T., T. Enomoto, S. Shimazu, and J.M. Hopkin. 1997. The inverse association between tuberculin responses and atopic disorder. Science. 275:77–79. [DOI] [PubMed] [Google Scholar]
- 6.Adams, J.F., E.H. Scholvinck, R.P. Gie, P.C. Potter, N. Beyers, and A.D. Beyers. 1999. Decline in total serum IgE after treatment for tuberculosis. Lancet. 353:2030–2033. [DOI] [PubMed] [Google Scholar]
- 7.Herz, U., K. Gerhold, C. Gruber, A. Braun, U. Wahn, H. Renz, and K. Paul. 1998. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J. Allergy Clin. Immunol. 102:867–874. [DOI] [PubMed] [Google Scholar]
- 8.Wang, C.C., and G.A. Rook. 1998. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 93:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang, X., S. Wang, Y. Fan, and L. Zhu. 1999. Systemic mycobacterial infection inhibits antigen-specific immunoglobulin E production, bronchial mucus production and eosinophilic inflammation induced by allergen. Immunology. 98:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallo, G.P., M. Elia, D. Giordano, C. Baldi, and R. Cammarota. 2002. Decrease of specific and total IgE levels in allergic patients after BCG vaccination: preliminary report. Arch. Otolaryngol. Head Neck Surg. 128:1058–1060. [DOI] [PubMed] [Google Scholar]
- 11.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 12.Renz, H., and U. Herz. 2002. The bidirectional capacity of bacterial antigens to modulate allergy and asthma. Eur. Respir. J. 19:158–171. [DOI] [PubMed] [Google Scholar]
- 13.Akbari, O., P. Stock, R.H. Dekruyff, and D.T. Umetsu. 2003. Role of regulatory T cells in allergy and asthma. Curr. Opin. Immunol. 15:627–633. [DOI] [PubMed] [Google Scholar]
- 14.Stassen, M., H. Jonuleit, C. Muller, M. Klein, C. Richter, T. Bopp, S. Schmitt, and E. Schmitt. 2004. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J. Immunol. 173:267–274. [DOI] [PubMed] [Google Scholar]
- 15.Robinson, D.S., M. Larche, and S.R. Durham. 2004. Tregs and allergic disease. J. Clin. Invest. 114:1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483–513. [DOI] [PubMed] [Google Scholar]
- 17.Cui, J., N. Watanabe, T. Kawano, M. Yamashita, T. Kamata, C. Shimizu, M. Kimura, E. Shimizu, J. Koike, H. Koseki, et al. 1999. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 190:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suto, A., H. Nakajima, K. Hirose, K. Suzuki, S. Kagami, Y. Seto, A. Hoshimoto, Y. Saito, D.C. Foster, and I. Iwamoto. 2002. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 100:4565–4573. [DOI] [PubMed] [Google Scholar]
- 19.Wood, N., K. Bourque, D.D. Donaldson, M. Collins, D. Vercelli, S.J. Goldman, and M.T. Kasaian. 2004. IL-21 effects on human IgE production in response to IL-4 or IL-13. Cell. Immunol. 231:133–145. [DOI] [PubMed] [Google Scholar]
- 20.Parrish-Novak, J., S.R. Dillon, A. Nelson, A. Hammond, C. Sprecher, J.A. Gross, J. Johnston, K. Madden, W. Xu, J. West, et al. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 408:57–63. [DOI] [PubMed] [Google Scholar]
- 21.Parrish-Novak, J., D.C. Foster, R.D. Holly, and C.H. Clegg. 2002. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J. Leukoc. Biol. 72:856–863. [PubMed] [Google Scholar]
- 22.Habib, T., A. Nelson, and K. Kaushansky. 2003. IL-21: a novel IL-2-family lymphokine that modulates B, T, and natural killer cell responses. J. Allergy Clin. Immunol. 112:1033–1045. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki, K., R. Spolski, C.G. Feng, C.F. Qi, J. Cheng, A. Sher, H.C. Morse III, C. Liu, P.L. Schwartzberg, and W.J. Leonard. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science. 298:1630–1634. [DOI] [PubMed] [Google Scholar]
- 24.Purkerson, J.M., and P.C. Isakson. 1994. Independent regulation of DNA recombination and immunoglobulin (Ig) secretion during isotype switching to IgG1 and IgE. J. Exp. Med. 179:1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta, D.S., A.L. Wurster, M.J. Whitters, D.A. Young, M. Collins, and M.J. Grusby. 2003. IL-21 induces the apoptosis of resting and activated primary B cells. J. Immunol. 170:4111–4118. [DOI] [PubMed] [Google Scholar]
- 26.Jin, H., R. Carrio, A. Yu, and T.R. Malek. 2004. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 173:657–665. [DOI] [PubMed] [Google Scholar]
- 27.Nelde, A., M. Teufel, C. Hahn, A. Duschl, W. Sebald, E.B. Brocker, and S.M. Grunewald. 2001. The impact of the route and frequency of antigen exposure on the IgE response in allergy. Int. Arch. Allergy Immunol. 124:461–469. [DOI] [PubMed] [Google Scholar]
- 28.Gould, H.J., B.J. Sutton, A.J. Beavil, R.L. Beavil, N. Mccloskey, H.A. Coker, D. Fear, and L. Smurthwaite. 2003. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 21:579–628. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa, Y., K. Takeda, H. Yagita, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166:6012–6018. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto, T., A. Bendelac, C. Watson, J. Hu-Li, and W.E. Paul. 1995. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 270:1845–1847. [DOI] [PubMed] [Google Scholar]
- 31.Brigl, M., L. Bry, S.C. Kent, J.E. Gumperz, and M.B. Brenner. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4:1230–1237. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect. Immun. 68:6883–6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snapper, C.M., P. Zelazowski, F.R. Rosas, M.R. Kehry, M. Tian, D. Baltimore, and W.C. Sha. 1996. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 156:183–191. [PubMed] [Google Scholar]
- 34.Puthalakath, H., A. Villunger, L.A. O'Reilly, J.G. Beaumont, L. Coultas, R.E. Cheney, D.C. Huang, and A. Strasser. 2001. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 293:1829–1832. [DOI] [PubMed] [Google Scholar]
- 35.Corrigan, C.J., and A.B. Kay. 1990. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am. Rev. Respir. Dis. 141:970–977. [DOI] [PubMed] [Google Scholar]
- 36.Hansen, G., G. Berry, R.H. Dekruyff, and D.T. Umetsu. 1999. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arikan, C., N.N. Bahceciler, G. Deniz, M. Akdis, T. Akkoc, C.A. Akdis, and I.B. Barlan. 2004. Bacillus Calmette-Guerin-induced interleukin-12 did not additionally improve clinical and immunologic parameters in asthmatic children treated with sublingual immunotherapy. Clin. Exp. Allergy. 34:398–405. [DOI] [PubMed] [Google Scholar]
- 38.Vargas, M.H., D.A. Bernal-Alcantara, M.A. Vaca, F. Franco-Marina, and R. Lascurain. 2004. Effect of BCG vaccination in asthmatic schoolchildren. Pediatr. Allergy Immunol. 15:415–420. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apostolou, I., Y. Takahama, C. Belmant, T. Kawano, M. Huerre, G. Marchal, J. Cui, M. Taniguchi, H. Nakauchi, J.J. Fournie, et al. 1999. Murine natural killer T(NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl. Acad. Sci. USA. 96:5141–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer, K., E. Scotet, M. Niemeyer, H. Koebernick, J. Zerrahn, S. Maillet, R. Hurwitz, M. Kursar, M. Bonneville, S.H. Kaufmann, and U.E. Schaible. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA. 101:10685–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feinberg, J., C. Fieschi, R. Doffinger, M. Feinberg, T. Leclerc, S. Boisson-Dupuis, C. Picard, J. Bustamante, A. Chapgier, O. Filipe-Santos, et al. 2004. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur. J. Immunol. 34:3276–3284. [DOI] [PubMed] [Google Scholar]
- 44.Darieva, Z., E.B. Lasunskaia, M.N. Campos, T.L. Kipnis, and W.D. Da Silva. 2004. Activation of phosphatidylinositol 3-kinase and c-Jun- N-terminal kinase cascades enhances NF-kappaB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding. J. Leukoc. Biol. 75:689–697. [DOI] [PubMed] [Google Scholar]
- 45.Nicolle, D., C. Fremond, X. Pichon, A. Bouchot, I. Maillet, B. Ryffel, and V.J. Quesniaux. 2004. Long-term control of Mycobacterium bovis BCG infection in the absence of Toll-like receptors (TLRs): investigation of TLR2-, TLR6-, or TLR2-TLR4-deficient mice. Infect. Immun. 72:6994–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasaian, M.T., M.J. Whitters, L.L. Carter, L.D. Lowe, J.M. Jussif, B. Deng, K.A. Johnson, J.S. Witek, M. Senices, R.F. Konz, et al. 2002. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 16:559–569. [DOI] [PubMed] [Google Scholar]
- 47.Hecker, M., A. Bohnert, I.R. Konig, G. Bein, and H. Hackstein. 2003. Novel genetic variation of human interleukin-21 receptor is associated with elevated IgE levels in females. Genes Immun. 4:228–233. [DOI] [PubMed] [Google Scholar]
- 48.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 278:1623–1626. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, N., S. Suzuki, G.S. Duncan, D.G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, et al. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 416:750–756. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 51.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 52.Harada, M., K. Seino, H. Wakao, S. Sakata, Y. Ishizuka, T. Ito, S. Kojo, T. Nakayama, and M. Taniguchi. 2004. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int. Immunol. 16:241–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.