Abstract
The proteinase-activated receptors (PARs) are widely recognized for their modulatory properties of inflammation and neurodegeneration. We investigated the role of PAR2 in the pathogenesis of multiple sclerosis (MS) in humans and experimental autoimmune encephalomyelitis (EAE) in mice. PAR2 expression was increased on astrocytes and infiltrating macrophages in human MS and murine EAE central nervous system (CNS) white matter (P < 0.05). Macrophages and astrocytes from PAR2 wild-type (WT) and knockout (KO) mice exhibited differential immune gene expression with PAR2 KO macrophages showing significantly higher interleukin 10 production after lipopolysaccharide stimulation (P < 0.001). PAR2 activation in macrophages resulted in the release of soluble oligodendrocyte cytotoxins (P < 0.01). Myelin oligodendrocyte glycoprotein–induced EAE caused more severe inflammatory gene expression in the CNS of PAR2 WT animals (P < 0.05), together with enhanced T cell proliferation and interferon γ production (P < 0.05), compared with KO littermates. Indeed, PAR2 WT animals showed markedly greater microglial activation and T lymphocyte infiltration accompanied by worsened demyelination and axonal injury in the CNS compared with their PAR2 KO littermates. Enhanced neuropathological changes were associated with a more severe progressive relapsing disease phenotype (P < 0.001) in WT animals. These findings reveal previously unreported pathogenic interactions between CNS PAR2 expression and neuroinflammation with ensuing demyelination and axonal injury.
Proteinase-activated receptors (PARs) are a family of G protein–coupled receptors that are widely expressed on neurons and glial cells in the nervous system (1). PARs are activated through proteolytic cleavage of their extracellular NH2 terminus. The proteolytic cleavage unmasks a “tethered ligand” that binds intramolecularly to the receptor and initiates a signal transduction event (2). Among the four PARs identified to date, PAR1, PAR3, and PAR4 can be activated by thrombin, whereas trypsin and mast cell tryptase can activate PAR2 (2, 3). Signaling through different heterotrimeric G proteins, PARs can affect various cellular functions in the nervous system, including neural cell proliferation, gene transcription, differentiation, and survival (4, 5). The role of PAR2, which is widely distributed throughout the nervous system, has been principally investigated in the peripheral nervous system, where it is known to play major roles in injury, inflammation, neuronal signaling, and nociception (6, 7). PAR2 is also known to be expressed on neurons and astrocytes in rodent and human central nervous systems (CNS), and several studies have implicated it in the pathogenesis of ischemia and neurodegeneration (8–10).
Multiple sclerosis (MS) is a common immune-mediated neurological disorder, which is histopathologically characterized by infiltration of the CNS with inflammatory leukocytes followed by demyelination and axonal loss (11–13). It is generally accepted that an autoimmune response directed against components of myelin is the chief pathogenic event during MS (14). The exact mechanisms leading to the generation of autoimmune responses in MS are not fully known, although components of both the adaptive and innate immune systems are involved (15, 16). Experimental autoimmune encephalomyelitis (EAE) is a widely studied animal model for MS, which recapitulates many of the clinical and neuropathologic aspects of MS (17). EAE can be induced by immunization of genetically susceptible animals with different antigenic components of CNS myelin, including myelin basic protein (MBP), proteolipid protein, or myelin oligodendrocyte glycoprotein (MOG). Immunization leads to the generation of myelin-reactive T cells in the periphery, which then migrate into the CNS and initiate autoimmune inflammation. Although considered largely a T cell–mediated disease, there is increasing evidence for the involvement of other immune cells, including activated macrophages and microglia in both the initiation as well as the effector phases of the immune response associated with the EAE and MS pathogenesis (16, 18). Herein, the expression levels and the cell types expressing PAR2 were investigated in the CNS of MS and control patients. Using EAE as an animal model of MS, we explored the role of PAR2 activation on astrocytes and monocytoid cells. Experiments were performed to evaluate the impact of PAR2-deficient signaling on the generation and responsiveness of myelin-reactive T cells and the severity of MOG-induced EAE.
RESULTS
PAR2 expression is increased in the white matter during MS and EAE
PAR2 has been shown to be widely expressed on neurons and astrocytes in the CNS (19, 20). To investigate the role of PAR2 in the neuroinflammatory process associated with MS, we examined PAR2 transcript levels in CNS white matter from MS and non-MS patients. RT-PCR analysis showed significantly higher PAR2 transcript levels in the white matter of MS compared with non-MS patients (Fig. 1 A). Transcript levels of trypsinogen, a potential PAR2-activating proteinase, were not different between MS and non-MS CNS tissues (Fig. 1 B). Immunohistochemical staining showed that PAR2 immunoreactivity was markedly enhanced in MS (Fig. 1 C) compared with non-MS white matter (Fig. 1 D). In MS CNS tissues, PAR2 immunoreactivity was chiefly detected on infiltrating perivascular cells together with parenchymal glial cells in the areas of active demyelination (Fig. 1 E). Indeed, PAR2 immunoreactivity was colocalized with CD45 leukocyte/macrophage marker in perivascular cells (Fig. 1 E) and with glial fibrillary acidic protein (GFAP) astrocytic marker in parenchymal cells (Fig. 1 F). PAR2 immunoreactivity was present on neurons in the gray matter with no obvious differences between groups (not depicted). Previous studies of blood-derived mononuclear cells have reported that monocytes but not lymphocytes express PAR2, and moreover, the expression levels increase upon differentiation to macrophages (21, 22). We also examined PAR2 expression in PBMCs from healthy human volunteers. Flow cytometric analysis showed PAR2 expression on the monocyte population (Fig. 1 G), whereas expression on lymphocytes was minimal (Fig. 1 H). Treatment of PBMC cultures with phytohemagglutinin for 24 h resulted in a marked up-regulation of PAR2 immunoreactivity on monocytes (Fig. 1 G), but the PAR2 immunoreactivity on the lymphocyte population was unaffected (Fig. 1 H). Thus, these observations indicated that monocytes rather than lymphocytes were the chief cell type expressing PAR2 in both blood and brain, in support of previous studies (21, 22).
Figure 1.
PAR2 is increased in the CNS white matter of MS patients. (A) PAR2 mRNA levels were increased in CNS white matter of MS patients (n = 6) compared with non-MS patients (n = 6), but (B) trypsinogen mRNA levels were not significantly different between the two groups. RFC, relative fold change. Student's t test; *, P < 0.05. (C) PAR2 immunoreactivity was present on perivascular and parenchymal (C, inset) cells in MS white matter, but not in non-MS (D) CNS tissues. Immunolabeling with the anti-CD45 (E) or the anti-GFAP antibodies (F) revealed colocalization of PAR2 on leukocytes and astrocytes in CNS white matter of MS patients in the areas of active demyelination. Original magnification, 400. (G) Flow cytometric analysis of PBMCs from healthy donors disclosed the expression of PAR2 on resting and PHA-stimulated monocytes, whereas (H) expression levels were minimal on resting or PHA-treated lymphocytes. BV, blood vessel.
Given that the EAE animal model recapitulates many of the neuropathological and clinical features of MS, together with similar underlying pathogenic mechanisms, we next examined PAR2 expression in the CNS of mice with EAE. Interestingly, RT-PCR analysis showed significantly higher levels for PAR2 mRNA in the CNS of animals with EAE compared with healthy control mice (Fig. 2 A). Similar to the MS CNS samples, PAR2 immunoreactivity was detectable in the white matter of EAE animals, where it was localized chiefly on GFAP-immunopositive astrocytes (Fig. 2 C, top) and ionized calcium binding adaptor protein (Iba-1)–immunopositive macrophage/microglia (Fig. 2 C, bottom). Hence, these findings indicated that PAR2 expression was up-regulated on both glial cells and infiltrating leukocytes during MS/EAE-associated neuroinflammation.
Figure 2.
PAR2 expression is up-regulated during EAE on CNS astrocytes and leukocytes. (A) PAR2 mRNA levels were increased in the CNS of mice with EAE (n = 8) as compared with healthy controls (n = 8). (B) Trypsinogen mRNA levels were not significantly different between the two groups of animals. RFC, relative fold change. Student's t test; *, P < 0.05. (C) PAR2 was colocalized with GFAP immunoreactivity on astrocytes (top) and Iba-1 immunoreactivity on macrophages/microglia in the lumbar spinal cord during EAE. Original magnification, 400.
PAR2 deficiency regulates immune gene expression in macrophages and astrocytes
Previous studies have demonstrated important roles for PAR2 in modulating inflammatory processes in the central and peripheral nervous systems (23, 24). As macrophage/microglial activation followed by subsequent demyelination and astrocytosis are predominant neuropathologic features of MS and EAE (25), we investigated the effects of PAR2 expression on the production of inflammatory mediators by these cells. After LPS treatment for 8 h, mRNA levels of proinflammatory or antiinflammatory mediators TNF-α (Fig. 3 A), IL-1β (Fig. 3 B), inducible nitric oxide synthase (iNOS) (Fig. 3 C), and IL-10 (Fig. 3 D) were analyzed in primary astrocytes and macrophages from PAR2 WT and KO mice. Similar levels of TNF-α (Fig. 3 A), IL-1β (Fig. 3 B), and iNOS (Fig. 3 C) mRNA were observed in PAR2 WT and KO macrophages. However, PAR2-deficient macrophages showed significantly higher levels of IL-10 mRNA after LPS treatment (Fig. 3 D). Gene expression analysis also showed significantly higher levels of iNOS mRNA (Fig. 3 G) in PAR2 WT astrocytes after LPS treatment, whereas TNF-α (Fig. 3 E), IL-1β (Fig. 3 F), and IL-10 (Fig. 3 H) levels did not differ between WT and KO astrocytes. Thus, these observations revealed a difference in the expression of immune genes between PAR2 WT and KO astrocytes and macrophages, which depended on the individual cell type.
Figure 3.
PAR2 deficiency regulates immune gene expression in macrophages and astrocytes. PAR2 WT and KO macrophages showed similar levels of TNF-α (A), IL-1β (B), and iNOS (C) expression after stimulation with LPS, but (D) PAR2 KO macrophages show significantly higher levels of IL-10 expression compared with WT cells. mRNA levels of TNF-α (E), IL-1β (F), and IL-10 (H) are not significantly different between PAR2 WT and KO astrocytes, whereas (G) iNOS mRNA levels are significantly higher in WT astrocytes after LPS stimulation. All experiments were performed in triplicate. RFC, relative fold change. Student's t test; ***, P < 0.001.
PAR2 activation mediates oligodendrocyte toxicity
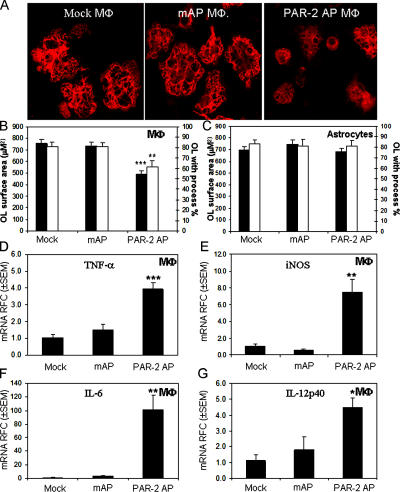
Acute demyelinating lesions in MS are believed to be generated by infiltrating leukocytes and activated microglia that destroy myelin in the presence of autoreactive T cells (26, 27). However, oligodendrocyte injury and apoptosis, even in the absence of a substantial inflammatory reaction, have also been reported to be a principal pathological feature in newly forming lesions (28, 29). We investigated the indirect effects of PAR2 activation on macrophages and astrocytes in terms of oligodendrocyte viability. Supernatants from cultured murine macrophages or astrocytes, treated with the PAR2-activating peptide (PAR2 AP) SLIGRL-NH2 or the mutant inactive peptide (mAP) LSIGRL-NH2, were applied to oligodendrocyte cultures for 24 h, and the surface area of GalC-immunopositive oligodendrocytes as well as the number of cells with processes were quantified. Interestingly, supernatants of PAR2 AP–treated, but not mAP- or mock-treated macrophages, caused a marked reduction in the surface area of exposed oligodendrocytes, leaving a higher number of cells with fewer or no processes (Fig. 4 A). Quantification of the oligodendrocyte area and the number of cells with processes showed significant reductions in cultures treated with the supernatants from PAR2 AP–stimulated macrophages compared with mock- or mAP-treated macrophages (Fig. 4 B). Conversely, supernatants of PAR2 AP–treated astrocytes did not affect oligodendrocyte morphology and surface area (Fig. 4 C). Gene expression studies revealed a significant induction of TNF-α (Fig. 4 D), iNOS (Fig. 4 E), IL-6 (Fig. 4 F), and IL-12p40 (Fig. 4 G) transcript levels in PAR2 AP–treated macrophages compared with mAP- or mock-treated cells. In addition, IL-1β, IFN-inducible protein 10, monocyte chemoattractant protein 1, and macrophage inflammatory protein 1α transcripts were increased significantly in PAR2 AP–treated macrophages, but not in mAP- or mock-treated cells (not depicted). The same effects were not observed for PAR2 AP–treated astrocytes (not depicted). Thus, these findings revealed that direct activation of PAR2 on macrophages caused oligodendrocyte injury associated with inflammatory gene expression, which might contribute to demyelination in MS.
Figure 4.
PAR2 activation on macrophages but not astrocytes mediates oligodendrocyte toxicity and immune gene expression. (A) Supernatants of PAR2 AP–treated but not mAP- or mock-treated macrophages decreased oligodendrocyte process extension. Original magnification, 600. (B) Quantification of oligodendrocyte surface area and the number of cells with processes showed a significant reduction in cells exposed to the supernatants from PAR2 AP–treated macrophages but not (C) astrocytes. (D) Treatment of macrophages with PAR2 AP but not mAP induced TNF-α, (E) iNOS, (F) IL-6, and (G) IL-12p40 transcript expression. RFC, relative fold change. Tukey-Kramer Multiple Comparisons test; **, P < 0.01; ***, P < 0.001. All experiments were performed in triplicate.
PAR2 deficiency reduces neuroinflammation and T cell proliferation during EAE
Given the higher expression of PAR2 in human MS CNS white matter and its contribution to inflammatory gene expression and oligodendrocyte injury, we next investigated the potential role of PAR2 in EAE, as an animal model of MS, using PAR2 WT and KO mice. We examined MOG-induced EAE in 10–12-wk-old PAR2 KO mice compared with their WT littermate controls and corresponding healthy (intact) controls. Neuropathological examination of the CNS of WT animals with EAE showed markedly enhanced immunoreactivity for macrophage/microglial marker, Iba-1, compared with PAR2 KO EAE animals and the nonimmunized intact group (Fig. 5 A, first column). Iba-1–immunopositive cells showed hypertrophy and were more frequently detected in WT and PAR2 KO EAE groups compared with the quiescent morphology observed in the nonimmunized intact group. Quantitative analysis showed a significantly higher number of Iba-1–immunopositive cells in the PAR2 WT EAE group compared with PAR2 KO EAE animals (Fig. 5 B). MBP immunoreactivity showed disrupted integrity of the myelin in both PAR2 WT and KO EAE groups with much more severe demyelination in WT animals (Fig. 5 A, second column), as indicated by quantitative analysis of MBP-immunopositive areas of the white matter (Fig. 5 C). Indeed, myelin loss was closely associated with macrophage infiltration/microglial activation, as indicated by merged MBP–Iba-1 immunoreactivity (Fig. 5 A, third column). CD3+ T cells were also detectable in spinal cords after EAE induction (Fig. 5 A, fourth column), with the WT EAE group showing a significantly higher number of positive cells compared with KO EAE animals (Fig. 5 D). Silver staining followed by counting of the axonal number also showed a significant reduction in the number of axons in the PAR2 WT EAE group compared with the KO EAE and control groups (Fig. 5 E). Of note, PAR2 WT and KO nonimmunized (intact) animals did not differ in terms of neuropathological features and therefore were pooled for these quantitative analyses. Overall, these findings indicated that deficient PAR2 signaling reduced neuroinflammation with greater preservation of myelin and proximate axons in the CNS during MOG-induced EAE.
Figure 5.
PAR2 deficiency diminishes CNS inflammatory cell infiltration, demyelination, and axonal loss during EAE induction. (A) WT EAE animals showed higher number of Iba-1+ cells (first column), associated with worsened demyelination (second and third columns), and a higher number of CD3-immunopositive lymphocytes (fourth column) compared with littermate KO EAE or intact animals. Silver staining displayed more axonal injury/loss in WT EAE animals (fifth column) compared with KO EAE or intact animals. (B) Quantification of macrophage/microglial, (C) myelin, (D) lymphocyte reactivity, and (E) axonal counts showed significantly higher inflammatory cell infiltration together with more severe demyelination and axonal loss in WT EAE animals. All quantitative analyses represented the results of four animals per group. Tukey-Kramer Multiple Comparisons test; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent standard errors.
The generation of myelin-reactive T cells in the peripheral immune system with their subsequent infiltration of the CNS has been considered a key event in EAE and MS pathogenesis (11). To examine the proliferative and cytokine response of T cells in MOG-immunized PAR2 WT and KO animals, we measured [3H]thymidine incorporation and intracellular IFN-γ immunoreactivity by FACS in lymphocytes cocultured with MOG-loaded irradiated splenocytes. Proliferation of T cells measured by [3H]thymidine incorporation was significantly higher in WT animals compared with their PAR2 KO littermates (Fig. 6 A). Intracellular cytokine assays showed a significant increase in IFN-γ reactivity of CD3:CD4 but not CD3:CD8 immunopositive lymphocytes of WT animals compared with PAR-2 KO littermates (Fig. 6, B and C). These observations disclosed that despite the absence of PAR2 on lymphocytes, there was a significant difference in T cell reactivity at the level of proliferative and cytokine responses after MOG immunization of WT and KO animals.
Figure 6.
T cells from PAR2 WT animals show greater proliferation and IFN- γ production. (A) Lymphocytes isolated from lymph nodes of MOG-immunized WT animals (n = 4) show higher [3H]thymidine incorporation after coculture with MOG-loaded irradiated splenocytes compared with lymphocytes from immunized littermate KO animals (n = 4). (B and C) CD3:CD4-immunopositive cells from WT animals show higher levels of IFN-γ production after coculture with MOG-loaded splenocytes compared with the same population in KO animals. Student's t test; *, P < 0.05. Error bars represent standard errors.
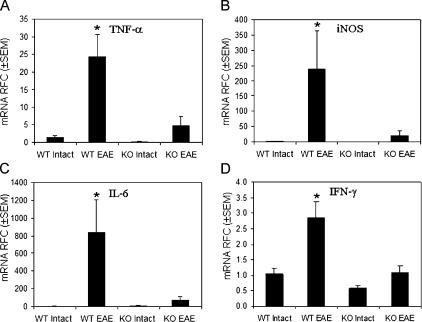
Enhanced host immune responses are apparent within the CNS during MS and EAE (13). Gene expression studies of WT and KO animals showed significantly higher levels of TNF-α (Fig. 7 A), iNOS (Fig. 7 B), IL-6 (Fig. 7 C), and IFN-γ (Fig. 7 D) mRNA in the CNS of WT animals with EAE compared with their PAR-2 KO littermates. TNF-α, iNOS, IL-6, and IFN-γ mRNA was detectable in PAR2 WT and KO animals without EAE (intact) with no differ-ence between the two groups (Fig. 7, A–D). Together with differences in the neuropathological findings and T cell reactivity, these observations emphasized the role of PAR2-deficient signaling in mitigating MOG-induced autoim- mune processes.
Figure 7.
PAR2 expression is associated with greater CNS inflammatory gene expression during EAE induction. mRNA levels of (A) TNF-α, (B) iNOS, (C) IL-6, and (D) IFN-γ were significantly higher in the CNS of PAR2 WT animals with EAE (n = 5) compared with their KO littermates with EAE (n = 5) and intact animals (n = 4) for each genotype. RFC, relative fold change. Tukey-Kramer Multiple Comparisons test; *, P < 0.05.
PAR2 deficiency is neuroprotective during EAE
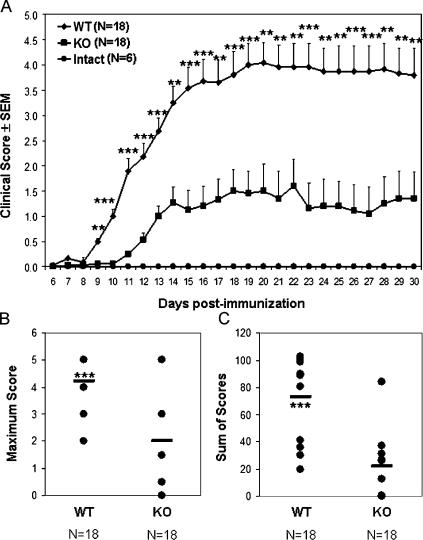
Consistent with the differences in inflammatory gene expression, T cell response, and neuropathologic findings in EAE induced in PAR2 WT and KO animals, we observed a significant difference in the neurobehavioral phenotype severity between PAR2 WT and KO animals after the induction of EAE. Displaying a progressive relapsing disease course (Fig. 8 A), PAR2 WT animals showed a significantly earlier disease onset together with significantly more severe neurological disability during the course of the disease. In addition, maximal disease score (Fig. 8 B) and cumulative neurological disability (Fig. 8 C) were significantly higher for WT EAE animals compared with the KO EAE group. Hence, our findings indicated that PAR2 expression contributed to the severity of neuroinflammation and neurological disability during MOG-induced EAE.
Figure 8.
PAR2 signaling exacerbates EAE severity. (A) Neurobehavioral analysis during EAE showed a later onset accompanied by less severe EAE disease in PAR2 KO animals compared with their WT littermates. (B) Likewise, the maximum disease score during the disease course and (C) cumulative neurological disability was significantly lower in PAR2 KO animals compared with WT littermate controls during EAE. Student's t test; **, P < 0.01; ***, P < 0.001. Error bars represent standard errors.
DISCUSSION
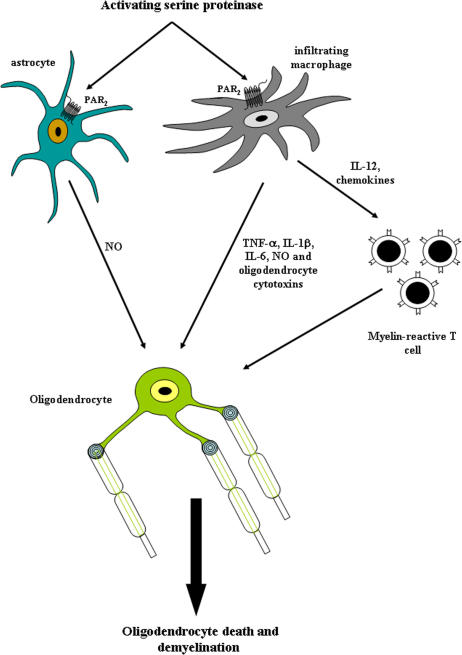
This study highlights previously unrecognized interactions between PAR2 expression and induction of neuroinflammation during MS/EAE, thereby disclosing new therapeutic opportunities for CNS autoimmune demyelinating disorders. We have demonstrated that PAR2 expression is enhanced in the CNS white matter during MS and EAE in which it is chiefly expressed on perivascular macrophages and astrocytes (Fig. 9). PAR2 WT and deficient macrophages and astrocytes exhibited differential inflammatory gene expression, with PAR2 activation on macrophages leading to the induction of proinflammatory cytokines and chemokines adversely affecting oligodendrocyte viability (Fig. 9). Indeed, absent PAR2 signaling suppressed EAE disease severity, which was associated with decreased T cell reactivity and neuroinflammation.
Figure 9.
PAR2's neuroinflammatory actions in the CNS during MS/EAE. Activation of PAR2 on macrophages and astrocytes results in the release of proinflammatory (NO, TNF-α, IL-1β, and IL-6) and oligodendrocytotoxic molecules together with chemokines that contribute to the recruitment of myelin-reactive T lymphocytes into the CNS during MS/EAE.
It is widely recognized that autoreactive T cells infiltrating the white matter during MS initiate demyelination through the release of toxins together with recruiting and activating monocytoid cells (16). However, an alternative view holds that oligodendrocyte death, perhaps due to apoptosis, together with microglial activation in the absence of marked lymphocyte infiltration might herald newly forming demyelinating lesions (29). Indeed, several studies indicate that innate immune mechanisms are potent determinants of MS and EAE disease onset and severity (30–33). Extensive oligodendrocyte death likely overwhelms normal mechanisms of dead cell clearance, prompting a T cell–mediated inflammatory response that arises from the release of myelin antigens (29). In this study, we observed less T cell reactivity and infiltration after EAE induction in PAR2-deficient mice, which may partly explain the milder disease severity in this group of animals compared with their WT littermates. Nonetheless, we also found that PAR2 activation on macrophages led to the production of soluble cytotoxic factors, which diminished oligodendrocyte viability. These two observations could be considered reciprocal mechanisms by which PAR2 expression affects the underlying pathogenic processes in EAE, likely reflecting the interaction of the adaptive and innate immune systems. However, it is also plausible that PAR2 expression and activation on monocytoid cells (unlike astrocytes) contributes directly to oligodendrocyte injury (34), which could prime a subsequent myelin-specific T cell response, as mentioned above.
A recent study has reported a neuroprotective role for neuronal PAR2 in experimental stroke (35). Moreover, our group showed a neuroprotective role for neuronal PAR2 expression and activation in the context of HIV-induced dementia, a neurodegenerative disorder caused by the HIV infection of brain (24). Herein, up-regulation and activation of PAR2 on monocytoid cells contributed to the neuroinflammatory/degenerative process in EAE. Of interest, PAR2 expression is up-regulated on monocytes upon differentiation to macrophages (22), and its activation leads to the production of proinflammatory cytokines IL-1β, IL-6, and IL-8 (21). A concomitant localized increase in tryptic serine proteinases, which act as potential activators of PAR2, may accentuate PAR2-mediated effects in the context of MS/EAE neuroinflammation. Indeed, there is also a report describing the role of PAR2 in dendritic cell development (36), indicating that dendritic cells do not spontaneously develop from the bone marrow cells of PAR2-deficient mice after IL-4/GM-CSF treatment, despite differentiating to mature dendritic cells in the presence of inflammatory mediators (36). In our hands, treatment of bone marrow cells from WT and PAR2 KO animals with IL-4/GM-CSF led to the generation of morphologically indistinguishable dendritic cells, which matured normally after LPS treatment (not depicted). Nonetheless, given the administration of adjuvants upon MOG immunization with the resulting immune activation, it is unlikely that differences in antigen presentation exert major effects on the ensuing autoimmune processes in the present studies.
Although many studies have indicated proinflammatory roles for PAR2 in respiratory, gastrointestinal, musculoskeletal, skin, and peripheral nervous systems (23, 37–42), PAR2 agonists have also been shown to be beneficial in some mouse models of mucosal inflammation (43, 44). These studies underscore the different roles played by PAR2 based on the individual cell type and specific animal model or disease context. Elucidation of mechanisms leading to altered receptor expression and differential downstream effects, together with identifying specific activating proteinases, will permit the development of new therapeutic strategies for modulating detrimental effects of inflammation in the nervous system.
MATERIALS AND METHODS
Human brain tissue samples.
CNS tissue (frontal lobe white matter) was collected at autopsy with consent from each experimental group (MS, n = 6; non-MS, n = 6) and stored at −80°C as described previously (45–47). Brain samples from MS and non-MS patients were obtained from the Laboratory for Neurological Infection and Immunity Brain Bank, University of Alberta. Normal-appearing frontal white matter was homogenized, lysed in TRIzol (Invitrogen), and used in real-time PCR analyses as described previously (48). The MS patient group (female/male, 4:2) was classified as relapsing-remitting (n = 1), primary progressive (n = 2), and secondary progressive (n = 3; mean age 57 ± 10 yr) with estimated disability status scores of 7–10 at death. The non-MS group (female/male, 3:3) was classified as anoxic encephalopathy (n = 1), HIV-1 infection (encephalitis, n = 1; toxoplasmosis encephalitis, n = 1), Alzheimer's disease (n = 2), and sepsis (n = 1; mean age 61 ± 12 yr). Human CNS sections from non-MS and MS patients with acute demyelinating lesions were provided by A. Clark (University of Calgary, Calgary, Canada).
Cell cultures.
Mouse primary astrocyte cultures were established from CNS tissue of 2-d-old PAR2 WT and KO mice as described previously (49). Cells were cultured in MEM containing 10% FBS, 1 mM sodium pyruvate, and 2 mM l-glutamine. Mouse bone marrow–derived macrophages were isolated from the pelvic and femoral bone marrow of adult PAR2 WT and KO mice (50) as described previously (47, 51). Bone marrow cells were cultured in DMEM containing 10% FBS, 10% L929 cell–conditioned medium as a source of M-CSF, and 2 mM l-glutamine (Invitrogen). Cells were incubated in 10% CO2 for 5 d before additional treatments. Macrophages or astrocytes were treated with 100 ng/ml LPS for 8 h before TRIzol lysis and RNA extraction. For PAR2 activation experiments, cells were treated with 100 μM PAR2 AP (SLIGRL-NH2) or mAP (LSIGRL-NH2) for 4 h (Peptide Synthesis Facility, University of Calgary). Cells were then washed twice and fresh AIM-V serum-free medium (Invitrogen) was added. Media was harvested 24 h later and stored at −80°C for subsequent oligodendrocyte toxicity experiments. Oligodendrocytes were isolated from adult Sprague-Dawley rat brains and plated in polyornithine-coated chamber slides (Nunc) as described previously (52). Oligodendrocytes were maintained in MEM containing 5% FBS, 0.1% dextrose, 2 mM l-glutamine, and 1 mM sodium pyruvate for 5 d. Oligodendrocytes were then treated with the supernatants of PAR2 AP–treated macrophages or astrocytes for 24 h before fixation and immunostaining. Human PBMCs from healthy individuals were isolated by density separation over Ficoll-hypaque and seeded in 24-well plates in RPMI containing 10% FBS. Cells were treated with 5 μg/ml phytohemagglutinin for 24 h before staining and flow cytometric analysis.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded sections of human brain tissue (frontal lobe) were deparaffinized and rehydrated using decreasing concentrations of ethanol. Antigen retrieval was performed by boiling the slides in 0.01 M trisodium citrate buffer, pH 6, for 10 min followed by incubation with 0.3% hydrogen peroxide to block endogenous peroxidases. Sections were then preincubated with 10% normal goat serum, 0.2% Triton X-100 overnight at 4°C to block nonspecific binding. To detect PAR2 immunoreactivity, slides were incubated overnight at 4°C with a rabbit polyclonal antibody (B5) raised against rat PAR2 (1:500; reference 53) (GPN SKG RSL IGR LDT 46P YGG C; 1/500) diluted in 5% normal goat serum, 0.2% Triton X-100 (47). A secondary biotinylated goat anti–rabbit antibody followed by avidin–biotin–peroxidase complexes (Vector Laboratories) and 3,3′-diaminobenzidine tetrachloride (Vector Laboratories) were used for single labeling. For double labeling with anti-CD45 (1:100; Zymed Laboratories) and anti-GFAP (1:50; BD Biosciences) mouse monoclonal antibodies, an alkaline phosphatase–conjugated goat anti–mouse antibody (Jackson ImmunoResearch Laboratories) followed by NBT/BCIP substrate (Vector Laboratories) were used.
Immunofluorescence and confocal laser scanning microscopy.
Rabbit polyclonal anti-PAR2 (B5) antibody (53) together with mouse monoclonal anti-GFAP (1:50; BD Biosciences) and goat polyclonal anti-PAR2 antibody (1:50; Santa Cruz Biotechnology, Inc.) together with rabbit polyclonal antibody against Iba-1 (1:500; Wako) followed by appropriate secondary antibodies were used to detect PAR2 immunoreactivity and localization in lumbar spinal cords of mice (47). Antibodies against Iba-1 (1:500; Wako), CD3 (1:100; Santa Cruz Biotechnology, Inc.), and MBP (1:1,000; Sternberger Monoclonals) followed by appropriate secondary antibodies were used to detect macrophage/microglial, T lymphocyte, and myelin immunoreactivity. Slides were examined on an Olympus Fluoview (FV300) confocal laser scanning microscope. For quantification, Iba-1 or CD3-immunopositive cells were counted using a regular fluorescent microscope (Axioskop2; Carl Zeiss MicroImaging, Inc.) in ventral, lateral, and dorsal columns of the spinal cord white matter. Quantitative analysis of MBP reactivity was performed using Scion Image software (Scion Corporation). Cultured oligodendrocytes derived from adult rat brains were stained with O1 anti-galactocerebroside monoclonal antibody (provided by V.W. Yong, University of Calgary, Calgary, Canada) that recognizes mature oligodendrocytes, followed by a Cy3-conjugated goat anti–mouse antibody. Slides were scanned by laser scanning confocal microscope, and Scion Image software was used to measure the surface area of O1-immunopositive oligodendrocytes (52).
Real-time RT-PCR.
Cultured cells, human brain (frontal lobe white matter), or lumbar spinal cords from mice were homogenized in TRIzol (Invitrogen) according to the manufacturer's guidelines. Total RNA was isolated and dissolved in diethylpyrocarbonate-treated water, and 1 μg RNA was used for the synthesis of complementary DNA and subsequent PCR as described previously (48). Primer sequences were as follows: human GAPDH, 5′ primer: 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′,3′primer: 5′-CGGAGTCAACGGATTTGGTCG-3′; human PAR2, 5′ primer: 5′-CTGGCCATTGGGGTCTTTCTGTTC-3′, 3′ primer: 5′-GGCCCTCTTCCTTTTCTTCTCTGA-3′; human trypsinogen, 5′ primer: 5′-TCAGCGAACAGTGGGTGGTATCAG-3′, 3′ primer: 5′-GAGGGGCGGTGGGCAGAG-3′; mouse PAR2, 5′ primer: 5′-TGGCCATTGGAGTCTTCCTGTT-3′, 3′ primer: 5′-TAGCCCTCTGCCTTTTCTTCTC-3′; and mouse trypsinogen, 5′ primer: 5′-ATCTCTGGCTGGGGCAACACTC-3′, 3′ primer: 5′-CTAGGAAGCCAGCACAGACCA T-3′. Mouse trypsinogen primers were designed based on the mouse mesotrypsin (mouse serine protease 3, Prss3) sequence. The other mouse oligonucleotide primer sequences have been reported previously (54). Semiquantitative analysis was performed by monitoring in real time the increase of fluorescence of the SYBR green dye on an i-Cycler (Bio-Rad Laboratories) as reported previously (48). All data were normalized against the GAPDH mRNA levels and expressed as fold increases relative to controls ± SE.
Induction and assessment of EAE.
10–12-wk-old female PAR2 homozygous KO mice (50) and littermate homozygous WT controls were used for EAE induction. Age-matched female mice were injected subcutaneously with 50 μg MOG (MOG35–55 peptide; prepared by the Peptide Synthesis Facility, University of Calgary) emulsified in 100 μl of complete Freund's adjuvant (Difco Laboratories; reference 47). Animals received intraperitoneal injections of pertussis toxin (0.3 μg; List Biological Laboratories) at the same time as MOG immunization and 48 h later. Vehicle-treated animals were only injected with complete Freund adjuvant and pertussis toxin. Animals were assessed daily for EAE severity for 30 d using a 0–5 rating scale as reported previously (55). Animals were killed by cardiac puncture under ketamine/xylazine anesthesia. Spinal cords were removed and fixed in 4% neutral buffered formalin before paraffin embedding or lysed in TRIzol for RNA extraction. All experiments were approved by the University of Calgary Animal Care Committee.
Histological analysis.
Formalin-fixed spinal cords of EAE or vehicle-treated animals were embedded in paraffin before sectioning (56). 4-μm sections from lumbar spinal cords were stained with Bielschowsky's silver impregnation method (33). Five randomly chosen fields in each animal's white matter were scanned and photographed using a microscope (Axioskop2; Carl Zeiss MicroImaging, Inc.) and the Spot imaging system (Diagnostic Instruments). The number of silver-positive axons was quantified in square millimeters using Scion Image software (Scion Corporation).
T cell proliferation and flow cytometric analysis.
Splenocytes isolated from the spleens of nonimmunized animals by density separation over Ficoll-hypaque were γ irradiated, suspended at a density of 2 × 106 cells/ml, and incubated with 40 μg/ml MOG35–55 peptide for 30 min (57). Splenocytes incubated with vehicle were used as control. Draining lymph nodes were isolated from MOG-immunized PAR2 WT and KO animals 7 d after immunization. Lymph nodes were homogenized in PBS, and lymphocytes isolated from dissociated lymph nodes were washed and suspended at a density of 2 × 106 cells/ml. Splenocytes and lymphocytes were plated 1:1 in 96-well U-bottom microtiter plates. Cells were incubated at 37°C for 48 h before adding 1 μCi [3H]thymidine (GE Healthcare) to each well. Cells were harvested after 24 h and counted on a liquid scintillation counter. Intracellular cytokine assay was performed using a cytofix/cytoperm kit according to the manufacturer's guidelines (BD Biosciences). In brief, Golgistop protein transport inhibitor was added to splenocyte/lymphocyte cocultures 48 h after plating. Cells were harvested after 12 h and immunostained using PerCP- labeled anti–mouse CD3 (1:50), FITC-labeled anti–mouse CD4 (1:50), and PE-labeled anti–IFN-γ (1:50) monoclonal antibodies. All monoclonal antibodies were purchased from BD Biosciences. For PAR2 immunodetection, cultured PBMCs were stained with B5 rabbit anti-PAR2 antibody followed by Alexa 488–conjugated goat anti–rabbit secondary antibody. FACS analysis was performed on a FACSCalibur apparatus using CELLQuest software (Becton Dickinson).
Acknowledgments
This work was supported by the Multiple Sclerosis Society of Canada (MSSC) and Canadian Institutes for Health Research (CIHR) operating grants (N. Vergnolle, M.D. Hollenberg, and C. Power) and a CIHR Proteinase and Inflammation Network Group grant. S. Tsutsui is an MSSC Fellow. N. Vergnolle is an Alberta Heritage Foundation for Medical Research Scholar/CIHR New Investigator. C. Power holds a Canada Research Chair (Tier 1) in Neurological Infection and Immunity.
The authors have no conflicting financial interests.
Abbreviations used: CNS, central nervous system(s); EAE, experimental autoimmune encep-halomyelitis; GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor protein; iNOS, inducible nitric oxide syn-thase; mAP, mutant inactive peptide; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; PAR, proteinase-activated receptor; PAR2 AP, PAR2-activating peptide.
References
- 1.Ossovskaya, V.S., and N.W. Bunnett. 2004. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 84:579–621. [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg, M.D., and S.J. Compton. 2002. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol. Rev. 54:203–217. [DOI] [PubMed] [Google Scholar]
- 3.Steinhoff, M., J. Buddenkotte, V. Shpacovitch, A. Rattenholl, C. Moormann, N. Vergnolle, T.A. Luger, and M.D. Hollenberg. 2005. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr. Rev. 26:1–43. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane, S.R., M.J. Seatter, T. Kanke, G.D. Hunter, and R. Plevin. 2001. Proteinase-activated receptors. Pharmacol. Rev. 53:245–282. [PubMed] [Google Scholar]
- 5.Noorbakhsh, F., N. Vergnolle, M.D. Hollenberg, and C. Power. 2003. Proteinase-activated receptors in the nervous system. Nat. Rev. Neurosci. 4:981–990. [DOI] [PubMed] [Google Scholar]
- 6.Cottrell, G.S., S. Amadesi, F. Schmidlin, and N. Bunnett. 2003. Protease-activated receptor 2: activation, signalling and function. Biochem. Soc. Trans. 31:1191–1197. [DOI] [PubMed] [Google Scholar]
- 7.Vergnolle, N., M. Ferazzini, M.R. D'Andrea, J. Buddenkotte, and M. Steinhoff. 2003. Proteinase-activated receptors: novel signals for peripheral nerves. Trends Neurosci. 26:496–500. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Swintosky, V.L., C.T. Cheo-Isaacs, M.R. D'Andrea, R.J. Santulli, A.L. Darrow, and P. Andrade-Gordon. 1997. Protease-activated receptor-2 (PAR-2) is present in the rat hippocampus and is associated with neurodegeneration. J. Neurochem. 69:1890–1896. [DOI] [PubMed] [Google Scholar]
- 9.Olejar, T., R. Matej, M. Zadinova, and P. Pouckova. 2002. Proteinase-activated receptor-2 expression on cerebral neurones after radiation damage: immunohistochemical observation in Wistar rats. Int. J. Tissue React. 24:81–88. [PubMed] [Google Scholar]
- 10.Rohatgi, T., P. Henrich-Noack, F. Sedehizade, M. Goertler, C.W. Wallesch, K.G. Reymann, and G. Reiser. 2004. Transient focal ischemia in rat brain differentially regulates mRNA expression of protease-activated receptors 1 to 4. J. Neurosci. Res. 75:273–279. [DOI] [PubMed] [Google Scholar]
- 11.Hemmer, B., J.J. Archelos, and H.P. Hartung. 2002. New concepts in the immunopathogenesis of multiple sclerosis. Nat. Rev. Neurosci. 3:291–301. [DOI] [PubMed] [Google Scholar]
- 12.Steinman, L., R. Martin, C. Bernard, P. Conlon, and J.R. Oksenberg. 2002. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 25:491–505. [DOI] [PubMed] [Google Scholar]
- 13.Prat, A., and J. Antel. 2005. Pathogenesis of multiple sclerosis. Curr. Opin. Neurol. 18:225–230. [DOI] [PubMed] [Google Scholar]
- 14.Hafler, D.A., J.M. Slavik, D.E. Anderson, K.C. O'Connor, P. De Jager, and C. Baecher-Allan. 2005. Multiple sclerosis. Immunol. Rev. 204:208–231. [DOI] [PubMed] [Google Scholar]
- 15.Ristori, G., C. Montesperelli, A. Perna, S. Cannoni, L. Battistini, G. Borsellino, P. Riccio, G. Pesole, A. Chersi, C. Pozzilli, et al. 2000. Global immune disregulation in multiple sclerosis: from the adaptive response to the innate immunity. J. Neuroimmunol. 107:216–219. [DOI] [PubMed] [Google Scholar]
- 16.Sospedra, M., and R. Martin. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23:683–747. [DOI] [PubMed] [Google Scholar]
- 17.Owens, T., and S. Sriram. 1995. The immunology of multiple sclerosis and its animal model, experimental allergic encephalomyelitis. Neurol. Clin. 13:51–73. [PubMed] [Google Scholar]
- 18.Behi, M.E., S. Dubucquoi, D. Lefranc, H. Zephir, J. De Seze, P. Vermersch, and L. Prin. 2005. New insights into cell responses involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol. Lett. 96:11–26. [DOI] [PubMed] [Google Scholar]
- 19.Ubl, J.J., C. Vohringer, and G. Reiser. 1998. Co-existence of two types of [Ca2+]i-inducing protease-activated receptors (PAR-1 and PAR-2) in rat astrocytes and C6 glioma cells. Neuroscience. 86:597–609. [DOI] [PubMed] [Google Scholar]
- 20.D'Andrea, M.R., C.K. Derian, D. Leturcq, S.M. Baker, A. Brunmark, P. Ling, A.L. Darrow, R.J. Santulli, L.F. Brass, and P. Andrade-Gordon. 1998. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J. Histochem. Cytochem. 46:157–164. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, U., C. Lawson, M. Dabare, D. Syndercombe-Court, A.C. Newland, G.L. Howells, and M.G. Macey. 2005. Human peripheral blood monocytes express protease receptor-2 and respond to receptor activation by production of IL-6, IL-8, and IL-1{beta}. J. Leukoc. Biol. 78:967–975. [DOI] [PubMed] [Google Scholar]
- 22.Colognato, R., J.R. Slupsky, M. Jendrach, L. Burysek, T. Syrovets, and T. Simmet. 2003. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 102:2645–2652. [DOI] [PubMed] [Google Scholar]
- 23.Steinhoff, M., N. Vergnolle, S.H. Young, M. Tognetto, S. Amadesi, H.S. Ennes, M. Trevisani, M.D. Hollenberg, J.L. Wallace, G.H. Caughey, et al. 2000. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 6:151–158. [DOI] [PubMed] [Google Scholar]
- 24.Noorbakhsh, F., N. Vergnolle, J.C. McArthur, C. Silva, M. Vodjgani, P. Andrade-Gordon, M.D. Hollenberg, and C. Power. 2005. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J. Immunol. 174:7320–7329. [DOI] [PubMed] [Google Scholar]
- 25.Bruck, W., and C. Stadelmann. 2005. The spectrum of multiple sclerosis: new lessons from pathology. Curr. Opin. Neurol. 18:221–224. [DOI] [PubMed] [Google Scholar]
- 26.Bar-Or, A., E.M. Oliveira, D.E. Anderson, and D.A. Hafler. 1999. Molecular pathogenesis of multiple sclerosis. J. Neuroimmunol. 100:252–259. [DOI] [PubMed] [Google Scholar]
- 27.Markovic-Plese, S., and H.F. McFarland. 2001. Immunopathogenesis of the multiple sclerosis lesion. Curr. Neurol. Neurosci. Rep. 1:257–262. [DOI] [PubMed] [Google Scholar]
- 28.Barnett, M.H., and J.W. Prineas. 2004. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55:458–468. [DOI] [PubMed] [Google Scholar]
- 29.Matute, C., and F. Perez-Cerda. 2005. Multiple sclerosis: novel perspectives on newly forming lesions. Trends Neurosci. 28:173–175. [DOI] [PubMed] [Google Scholar]
- 30.Hendriks, J.J., C.E. Teunissen, H.E. de Vries, and C.D. Dijkstra. 2005. Macrophages and neurodegeneration. Brain Res. Brain Res. Rev. 48:185–195. [DOI] [PubMed] [Google Scholar]
- 31.Olson, J.K., J. Ludovic Croxford, and S.D. Miller. 2004. Innate and adaptive immune requirements for induction of autoimmune demyelinating disease by molecular mimicry. Mol. Immunol. 40:1103–1108. [DOI] [PubMed] [Google Scholar]
- 32.Jack, C., F. Ruffini, A. Bar-Or, and J.P. Antel. 2005. Microglia and multiple sclerosis. J. Neurosci. Res. 81:363–373. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsui, S., F. Noorbakhsh, A. Sullivan, A.J. Henderson, K. Warren, K. Toney-Earley, S.E. Waltz, and C. Power. 2005. RON-regulated innate immunity is protective in an animal model of multiple sclerosis. Ann. Neurol. 57:883–895. [DOI] [PubMed] [Google Scholar]
- 34.Buntinx, M., P. Stinissen, P. Steels, M. Ameloot, and J. Raus. 2002. Immune-mediated oligodendrocyte injury in multiple sclerosis: molecular mechanisms and therapeutic interventions. Crit. Rev. Immunol. 22:391–424. [PubMed] [Google Scholar]
- 35.Jin, G., T. Hayashi, J. Kawagoe, T. Takizawa, T. Nagata, I. Nagano, M. Syoji, and K. Abe. 2005. Deficiency of PAR-2 gene increases acute focal ischemic brain injury. J. Cereb. Blood Flow Metab. 25:302–313. [DOI] [PubMed] [Google Scholar]
- 36.Fields, R.C., J.G. Schoenecker, J.P. Hart, M.R. Hoffman, S.V. Pizzo, and J.H. Lawson. 2003. Protease-activated receptor-2 signaling triggers dendritic cell development. Am. J. Pathol. 162:1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrell, W.R., J.C. Lockhart, E.B. Kelso, L. Dunning, R. Plevin, S.E. Meek, A.J. Smith, G.D. Hunter, J.S. McLean, F. McGarry, et al. 2003. Essential role for proteinase-activated receptor-2 in arthritis. J. Clin. Invest. 111:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cenac, N., A.M. Coelho, C. Nguyen, S. Compton, P. Andrade-Gordon, W.K. MacNaughton, J.L. Wallace, M.D. Hollenberg, N.W. Bunnett, R. Garcia-Villar, et al. 2002. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor- 2. Am. J. Pathol. 161:1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebeling, C., P. Forsythe, J. Ng, J.R. Gordon, M. Hollenberg, and H. Vliagoftis. 2005. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J. Allergy Clin. Immunol. 115:623–630. [DOI] [PubMed] [Google Scholar]
- 40.Seeliger, S., C.K. Derian, N. Vergnolle, N.W. Bunnett, R. Nawroth, M. Schmelz, P.Y. Von Der Weid, J. Buddenkotte, C. Sunderkotter, D. Metze, et al. 2003. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 17:1871–1885. [DOI] [PubMed] [Google Scholar]
- 41.Coughlin, S.R., and E. Camerer. 2003. PARticipation in inflammation. J. Clin. Invest. 111:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su, X., E. Camerer, J.R. Hamilton, S.R. Coughlin, and M.A. Matthay. 2005. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J. Immunol. 175:2598–2605. [DOI] [PubMed] [Google Scholar]
- 43.Cocks, T.M., B. Fong, J.M. Chow, G.P. Anderson, A.G. Frauman, R.G. Goldie, P.J. Henry, M.J. Carr, J.R. Hamilton, and J.D. Moffatt. 1999. A protective role for protease-activated receptors in the airways. Nature. 398:156–160. [DOI] [PubMed] [Google Scholar]
- 44.Fiorucci, S., A. Mencarelli, B. Palazzetti, E. Distrutti, N. Vergnolle, M.D. Hollenberg, J.L. Wallace, A. Morelli, and G. Cirino. 2001. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc. Natl. Acad. Sci. USA. 98:13936–13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston, J.B., C. Silva, J. Holden, K.G. Warren, A.W. Clark, and C. Power. 2001. Monocyte activation and differentiation augment human endogenous retrovirus expression: implications for inflammatory brain diseases. Ann. Neurol. 50:434–442. [DOI] [PubMed] [Google Scholar]
- 46.Antony, J.M., G. van Marle, W. Opii, D.A. Butterfield, F. Mallet, V.W. Yong, J.L. Wallace, R.M. Deacon, K. Warren, and C. Power. 2004. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 7:1088–1095. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsui, S., J. Schnermann, F. Noorbakhsh, S. Henry, V.W. Yong, B.W. Winston, K. Warren, and C. Power. 2004. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 24:1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power, C., S. Henry, M.R. Del Bigio, P.H. Larsen, D. Corbett, Y. Imai, V.W. Yong, and J. Peeling. 2003. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann. Neurol. 53:731–742. [DOI] [PubMed] [Google Scholar]
- 49.Corley, S.M., U. Ladiwala, A. Besson, and V.W. Yong. 2001. Astrocytes attenuate oligodendrocyte death in vitro through an alpha(6) integrin-laminin-dependent mechanism. Glia. 36:281–294. [DOI] [PubMed] [Google Scholar]
- 50.Damiano, B.P., W.M. Cheung, R.J. Santulli, W.P. Fung-Leung, K. Ngo, R.D. Ye, A.L. Darrow, C.K. Derian, L. de Garavilla, and P. Andrade-Gordon. 1999. Cardiovascular responses mediated by protease- activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR- 1. J. Pharmacol. Exp. Ther. 288:671–678. [PubMed] [Google Scholar]
- 51.Riches, D.W., and G.A. Underwood. 1991. Expression of interferon-beta during the triggering phase of macrophage cytocidal activation. Evidence for an autocrine/paracrine role in the regulation of this state. J. Biol. Chem. 266:24785–24792. [PubMed] [Google Scholar]
- 52.Oh, L.Y., P.H. Larsen, C.A. Krekoski, D.R. Edwards, F. Donovan, Z. Werb, and V.W. Yong. 1999. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J. Neurosci. 19:8464–8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Ani, B., M. Saifeddine, A. Kawabata, B. Renaux, S. Mokashi, and M.D. Hollenberg. 1999. Proteinase-activated receptor 2 (PAR(2)): development of a ligand-binding assay correlating with activation of PAR(2) by PAR(1)- and PAR(2)-derived peptide ligands. J. Pharmacol. Exp. Ther. 290:753–760. [PubMed] [Google Scholar]
- 54.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 11:305–312. [DOI] [PubMed] [Google Scholar]
- 55.Liu, J., M.W. Marino, G. Wong, D. Grail, A. Dunn, J. Bettadapura, A.J. Slavin, L. Old, and C.C. Bernard. 1998. TNF is a potent anti-inflam- matory cytokine in autoimmune-mediated demyelination. Nat. Med. 4:78–83. [DOI] [PubMed] [Google Scholar]
- 56.Brundula, V., N.B. Rewcastle, L.M. Metz, C.C. Bernard, and V.W. Yong. 2002. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 125:1297–1308. [DOI] [PubMed] [Google Scholar]
- 57.Youssef, S., O. Stuve, J.C. Patarroyo, P.J. Ruiz, J.L. Radosevich, E.M. Hur, M. Bravo, D.J. Mitchell, R.A. Sobel, L. Steinman, and S.S. Zamvil. 2002. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 420:78–84. [DOI] [PubMed] [Google Scholar]