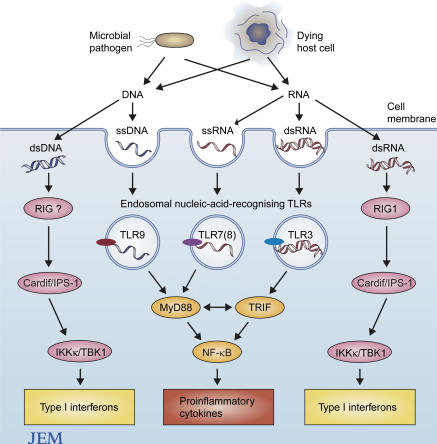
TLR recognition of RNA and DNA
The innate immune system relies on germline-encoded receptors to sense pathogen-derived molecules that are absent in the host. To do this, the innate immune system encodes 11 members of the Toll-like receptor family, each of which senses a distinct set of microbial products. Two major signaling pathways are activated upon ligand-driven TLR dimerization. One pathway requires the adaptor molecule MyD88 and culminates in the production of type I interferons (IFNs) or NF-κB–dependent proinflammatory cytokines including TNF and interleukin (IL)-12. The other pathway requires the adaptor molecule TRIF and primarily culminates in the production of type I IFNs.
In macrophages and dendritic cells (DCs), TLRs that recognize nucleic acids are expressed exclusively in endosomes. These include TLR3, TLR7/8, and TLR9, which sense double-stranded (ds)RNA, single-stranded (ss)RNA, and ssDNA, respectively. Other TLRs, in contrast, reside on the cell membrane. These include TLR2, TLR4, TLR5, and TLR11, which recognize lipopeptides, lipopolysaccharide, flagellin, and propellin, respectively (Fig. 1) (1–3). As TLRs are type I transmembrane proteins, they are likely to sample their specific ligands within either the extracellular space or the endosomal luminal space. For endosomally expressed TLRs, this poses the topological problem of whether and how the appropriate ligands are efficiently translocated from the extracellular space to intracellular endosomes (via receptor-mediated endocytosis or during viral infection), and how this affects the respective TLR specificity. For example, in vitro exposure to ssDNA oligonucleotides containing CpG motifs activates TLR9-positive macrophages and DCs, and this stimulatory activity is lost when cytosine residues in the oligonucleotide are methylated, or when the internal CG dinucleotides are inverted to GC (4). However, if these apparently inactive DNA molecules are formulated within cationic liposomes, which enhance endosomal translocation (5), and perhaps retain the ligand–TLR9 complex in the endosomal compartment (6), these otherwise inactive DNA molecules become immunostimulatory and trigger robust TLR9-dependent cytokine production (5, 7). In support of this finding, binding studies using surface plasmon resonance technology (Biacore) show that TLR9 binds with low affinity to ssDNA molecules that contain phosphodiester (PD) bonds, but lack canonical CpG-motifs (PD-DNA) (8). In this reductionistic system, TLR9 binding is strongly enhanced if the concentration of the respective oligonucleotide is increased (7). A likely interpretation of these results is that high concentrations of endosomal ssPD-DNA, or other nonstimulatory oligonucleotides, can activate TLR9 in a CpG-independent fashion.
Figure 1.
Nucleic acid recognition pathways in innate immune cells. Both pathogen-derived RNA or DNA and host-derived mammalian RNA or DNA are sensed via TLR and TLR-independent recognition pathways. Upon endosomal translocation, viral dsRNA, microbial or mammalian ssRNA and ssDNA are recognized by endosomally expressed TLR3, TLR7, (8) and TLR9, respectively. After ligation of TLR7, TLR8, and TLR9, the adaptor molecule MyD88 is recruited and drives the production of proinflammatory cytokine genes or type 1 interferon genes. TLR3 ligation triggers type 1 interferon genes via the adaptor protein TRIF. Viral dsRNA is also sensed by RIG-1 (retinoic acid inducible gene 1), which was recently shown to recruit Cardif/IPS-1, a new CARD-containing adaptor protein. Cardiff /IPS-1 in turn interacts with Ikkα/β/γ kinases and thus activates IRF3. Mammalian DNA triggers type 1 interferon production by an ill-defined signal pathway. Whether the dsDNA recognition receptor belongs to the RIG family is not yet known.
TLR7 and TLR8 have recently been shown to recognize viral or synthetic RNA molecules that are rich in GU or U sequences, provided the RNA is formulated within cationic lipids (9; 10). However, as TLR7 reportedly also recognizes small interfering RNA molecules (11), which do not have a high GU content, there must be mechanisms in place that prevent host-derived ssRNA molecules from causing abnormal activation of innate immune cells via TLR7 or TLR8. Rapid degradation of endogenous RNA by RNases and the topology of endosomal TLR expression are two likely security mechanisms. However, the most important security mechanism may be the fact that most host-derived ssRNA molecules contain a high frequency of modified nucleotides—such as 5-methylcytidine or pseudouridine—that render them nonstimulatory (12). Of note, such modifications also affect TLR3 signaling in response to viral dsRNA molecules, which normally triggers type I IFN production via the adaptor molecule TRIF. Whereas the presence of pseudouridine—which stabilizes RNA duplex formation—does not affect TLR3 recognition, the presence of N6-methyladenosine—which destabilizes RNA duplexes—dampens TLR3 recognition (12). Overall, these results imply that selective posttranscriptional nucleoside modifications suppress the TLR3-, TLR7- and TLR8-mediated immunostimulatory effects of host-derived RNA.
TLRs and anti-DNA (and RNA) autoantibodies
Systemic lupus erythematosus (SLE) is a prototypical human autoimmune disease in which increased serum levels of type I IFNs correlate with disease activity and severity. The sera of SLE patients contain immune complexes (ICs) made up of either host DNA and anti-DNA IgG molecules, or host RNA and anti-RNA IgG molecules. These anti-DNA (and RNA) ICs result from the loss of tolerance to a restricted set of nuclear self-antigens. In vitro, these anti-DNA ICs activate rheumatoid factor–positive (RF+) B cells via sequential engagement of B cell receptor (BCR) and TLR9. In this system, the RF+ BCR first recognizes the isotype of the autoantibody, and this recognition triggers the subsequent translocation of the ICs into TLR-expressing endosomes (13). Similarly, anti-DNA ICs that are translocated to endosomes via FcγRIII-mediated (mouse) or FcγRIIa-mediated (human) endocytosis activate the TLR9-dependent production of cytokines, including type I IFNs (14, 15). Interestingly, lupus-prone mice that lack TLR9 fail to generate anti-dsDNA antibodies, yet the production of auto-antibodies to Smith antigens is not affected (16), suggesting that not all autoantibodies depend on TLR ligation for their generation.
Mammalian DNA can also stimulate cytokine production when formulated with cationic lipids. In this form, mammalian DNA has been shown to stimulate murine plasmacytoid (pDCs)—also termed natural interferon-producing cells—which respond by producing high concentrations of type I IFNs in a partly TLR9–dependent fashion (5). Together these data imply that host (self) DNA can activate immune cells via TLR9, particularly under conditions of enhanced endosomal translocation. A similar conclusion can now be extended to self-RNA. ICs containing U1 small nuclear ribonucleotide proteins, which are found in a subset of SLE patients, activate the TLR7-dependent production of type I IFNs and other proinflammatory cytokines by murine pDCs (17–20). Thus, in SLE, both self-RNA and self-DNA can act as ligands for TLR7 and TLR9, respectively, provided they do not contain certain posttranscriptional nucleoside modifications. Perhaps enforced endosomal translocation of host-DNA or host-RNA is the key to unraveling their TLR-dependent immunostimulatory potential.
TLR-independent recognition of RNA and DNA
Although viral dsRNA is known to be sensed by TLR3, DCs or fibroblasts that lack TLR3 still produce type I IFNs after intracellular introduction of dsRNA molecules. This TLR3-independent induction does not require TRIF, but depends on the kinase TBK1 (TANK-binding kinase-1) and the transcription factor IRF-3 (IFN regulatory factor-3) (21). In this system, the RNA is recognized by the cytoplasmic helicase domain of the helicase protein retinoic acid–induced gene 1 (RIG-1). The downstream signaling events that result from this recognition require the NH2-terminal caspase recruitment domain (CARD) of RIG-1, which binds to the adaptor molecule Cardif (also called IPS-1) (22, 23). Another candidate for the sensing of cytoplasmic dsRNA molecules is Mda5 (melanoma differentiation-associated gene 5, also termed Helicard). Unexpectedly, RIG-1, but not the TLRs, plays an essential role in the antiviral responses of many cell types. In contrast, the TLR system is indispensable for type I IFN production by pDCs. Thus, RIG-1 and the TLRs appear to exert their antiviral responses in a cell type–specific manner (24).
Mammalian DNA can also be sensed in a TLR-independent manner. For example, the FcγR-mediated translocation of ICs from SLE patients into endosomes triggers type I IFN secretion, and this response is only partially independent of TLR9 and MyD88 (14). In addition, the production of type I IFNs in response to mammalian DNA formulated in cationic lipids is also partially TLR independent (5). Finally, cells lacking TLR9 or MyD88 produce type I IFNs in response to herpes simplex virus infection, suggesting that this response is at least partly TLR (MyD88) independent (25). Altogether, these findings corroborate recent data showing that transfection of mammalian dsDNA induces TLR-independent antiviral responses (26).
In a recent issue, Okabe et al. provided perhaps the clearest evidence to date of TLR-independent recognition of host DNA (27). The group analyzed mice lacking the enzyme DNase II and noted that macrophages in the embryonic livers of these mice accumulate undigested DNA. As a result, these embryos died in feto because of excessive type I IFN production (28). Backcrossing the DNase-deficient mice onto mouse strains lacking TLR3, TLR9, TRIF, or MyD88 did not rescue lethality. However, backcrossing the DNase-deficient mice to a strain that lacked the type I IFN receptor did rescue the mice (27), demonstrating that the IFN-triggered death of the mice did not require DNA-specific TLR signaling pathways. Altogether these data clearly imply that a cytosolic DNA receptor exists—possibly a member of the RIG family (26)—that is able to sense endogenous DNA that escapes lysosomal degradation.
Why do we need sensing mechanisms for endogenous DNA?
In mammals, DNA is actively degraded during various developmental processes, including programmed cell death (apoptosis), embryogenesis, and erythropoesis. Under these homeostatic conditions, apoptotic cells are engulfed by macrophages and self-DNA is degraded in lysosomes by DNase II, thus hindering an inflammatory response to endogenous (“sterile”) DNA. One may speculate on conditions under which it might be useful for the organism to sense endogenous DNA via TLR-dependent and TLR-independent pathways. For example, it might be an advantage to sense large quantities of self-DNA that are released during massive tissue apoptosis to promote cytokine-driven tissue repair under otherwise sterile conditions. Clearly much work remains to be done to understand the precise biological role of TLR-dependent and -independent recognition of self-DNA under both homeostatic and autoimmune conditions.
References
- 1.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 3.Wagner, H. 2004. The immunobiology of the TLR9 subfamily. Trends Immunol. 25:381–386. [DOI] [PubMed] [Google Scholar]
- 4.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda, K., P. Yu, C.J. Kirschning, B. Schlatter, F. Schmitz, A. Heit, S. Bauer, H. Hochrein, and H. Wagner. 2005. Endo- somal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J. Immunol. 174:6129–6136. [DOI] [PubMed] [Google Scholar]
- 6.Honda, K., Y. Ohba, H. Yanai, H. Negishi, T. Mizutani, A. Takaoka, C. Taya, and T. Taniguchi. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 434:1035–1040. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda, K., M. Rutz, B. Schlatter, J. Metzger, B.P. Luppa, F. Schmitz, T. Haas, A. Heit, S. Bauer, and H. Wagner. 2005. CpG-motif independent activation of TLR9 upon endosomal translocation of “natural” phosphodiester DNA. Eur. J. Immunol. In press. [DOI] [PubMed]
- 8.Rutz, M., J. Metzger, T. Gellert, P. Luppa, G.B. Lipford, H. Wagner, and S. Bauer. 2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 34:2541–2550. [DOI] [PubMed] [Google Scholar]
- 9.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 10.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 11.Hornung, V., M. Guenthner-Biller, C. Bourquin, A. Ablasser, M. Schlee,S. Uematsu, A. Noronha, M. Manoharan, S. Akira, A. de Fougerolles, et al. 2005. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR 7. Nat. Med. 11:263–270. [DOI] [PubMed] [Google Scholar]
- 12.Kariko, K., M. Buckstein, H. Ni, and D. Weissman. 2005. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolu tionary origin of RNA. Immunity. 23:165–175. [DOI] [PubMed] [Google Scholar]
- 13.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]
- 14.Boule, M.W., C. Broughton, F. Mackay, S. Akira, A. Marshak-Rothstein, and I.R. Rifkin. 2004. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 199:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Means, T.K., E. Latz, F. Hayashi, M.R. Murali, D.T. Golenbock, and A.D. Luster. 2005. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR 9. J. Clin. Invest. 115:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen, S.R., M. Kashgarian, L. Alexopoulou, R.A. Flavell, S. Akira, and M.J. Shlomchik. 2005. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 202:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savarese, E., O. Chae, S. Trowitzsch, G. Weber, B. Kastner, S. Akira, H. Wagner, R. Schmid, S. Bauer, and A. Krug. 2005. U1 small nuclear ribonucleoprotein immune complexes induce type I Interferon in plasmacytoid dendritic cells via TLR 7. Blood. 10.1182/blood-20005-07-2650. [DOI] [PubMed]
- 18.Barrat, F.J., T. Meeker, J. Gregorio, J.H. Chan, S. Uematsu, S. Akira, B. Chang, O. Duramad, and R.L. Coffman. 2005. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau, C.M., C. Broughton, A.S. Tabor, S. Akira, R.A. Flavell, M.J. Mamula, S.R. Christensen, M.J. Shlomchik, G.A. Viglianti, I.R. Rifkin, and A. Marshak-Rothstein. 2005. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollmer, J., S. Tluk, C. Schmitz, S. Hamm, M. Jurk, A. Forsbach, S. Akira, K.M. Kelly, W.H. Reeves, S. Bauer, and A.M. Krieg. 2005. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmi, H., O. Takeuchi, S. Sato, M. Yamamoto, T. Kaisho, H. Sanjo, T. Kawai, K. Hoshino, K. Takeda, and S. Akira. 2004. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737. [DOI] [PubMed] [Google Scholar]
- 23.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 437:1167–1172. [DOI] [PubMed] [Google Scholar]
- 24.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 23:19–28. [DOI] [PubMed] [Google Scholar]
- 25.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA. 101:11416–11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii, K.J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, et al. 2005. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40–48. [DOI] [PubMed] [Google Scholar]
- 27.Okabe, Y., K. Kawane, S. Akira, T. Taniguchi, and S. Nagata. 2005. Toll-like receptor-independent gene induction progam activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 202:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata, S. 2005. DNA degradation in development and programmed cell death. Annu. Rev. Immunol. 23:853–875. [DOI] [PubMed] [Google Scholar]