Abstract
The transplanted liver elicits systemic tolerance, and the underlying mechanism may also account for the persistence of liver infections, such as malaria and viral hepatitis. These phenomena have led to the hypothesis that antigen presentation within the liver is abortive, leading to T cell tolerance or apoptosis. Here we test this hypothesis in an optimized orthotopic liver transplantation model. In direct contradiction to this model, the liver itself induces full CD8+ T cell activation and differentiation. The effects of microchimerism were neutralized by bone marrow transplantation in the liver donor, and the lack of liver-derived antigen-presenting cells was documented by eight-color flow cytometry and by sensitive functional assays. We conclude that local antigen presentation cannot explain liver tolerance. On the contrary, the liver may be an excellent priming site for naive CD8+ T cells.
The liver appears to have a distinctive immunological role, both in terms of intrahepatic responses and in systemic immunity. Hepatotropic pathogens like hepatitis B, hepatitis C, and malaria frequently fail to be cleared by the immune system and become chronic infections (1, 2). In contrast to skin, kidney, or heart transplants, allogeneic liver transplants are accepted by the recipient without immunosuppression in many experimental models. This graft acceptance usually results in donor-specific tolerance, whereas other MHC-disparate tissues are still readily rejected (3–7). In humans, liver transplants require less immunosuppressive therapy and experience fewer and less severe T cell–mediated rejection episodes than other vascularized organ grafts (8). In cer- tain cases, liver transplant recipients can be completely weaned from immunosuppressive therapy without experiencing rejection (9). Tolerance induction has also been observed when antigen is administered via the gastrointestinal tract, a phenomenon known as oral tolerance (10). Evidence that the liver has a crucial role in this process is derived from direct injection of antigen or allogeneic cells into the portal vein, resulting in tolerance (11, 12). Moreover, oral tolerance does not develop if the blood flow from the intestine bypasses the liver as a result of porto-systemic shunting (13). Because the liver is continuously exposed to harmless food antigens and components of the commensal gut bacteria, a tolerogenic predisposition is believed to protect the organ from constant inflammation and consequent bystander damage (14). Despite this bias toward tolerance, there are other situations in which hepatic infections result in a robust immune response, clearance of the pathogen, and functional memory. This is observed in almost all hepatitis A infections and to a variable extent in patients with hepatitis B and C infections (1). In addition, autoimmune hepatitis directed against hepatic antigens indicates that hepatic tolerance can be broken, causing self-destructive inflammation (15).
Because most of these phenomena are in response to intracellular pathogens or antigens, research activities have focused on the modulation of CD8+ T cell responses by hepatic tissue. It has been demonstrated that two cell populations in the liver, hepatocytes and liver sinusoidal endothelial cells (LSECs), can activate naive CD8+ cells. The LSECs are a special type of endothelial cell lining the hepatic sinusoids that, unlike regular endothelial cells, are efficient in the uptake of antigen and its presentation via MHC class I and II. Both in vitro culture experiments and an adoptive transfer model of isolated LSECs indicate that antigen presentation by LSECs can induce tolerance in CD8+ T cells (16). Hepatocytes have also been identified as potential tolerogenic APCs for naive CD8+ lymphocytes. Transgenic expression of allogeneic MHC class I molecules on cultured hepatocytes induced rapid activation of TCR transgenic CD8+ lymphocytes specific for the allo-MHC, followed by their premature death (17). However, adoptive transfer of TCR transgenic lymphocytes caused hepatocyte damage, suggesting that the T cells were fully activated (18). Recent investigations have demonstrated that depending on the promoter used, the transgenic allo-MHC class I antigen was also expressed in lymphatic tissue, which resulted in a productive immune response. This led to the hypothesis that localized antigen presentation by hepatocytes results in tolerance by causing premature T cell death, whereas antigen presentation in lymphatic tissue by professional APCs promotes immunity (19).
Each approach to the analysis of the immune responses to liver antigens carries its own burden of complicating factors. Isolated populations of potential liver APCs are subject to issues of low-level contamination by other cell types, and their isolation from the liver's architecture may change their biological function. Even if such isolated cells are replaced in vivo by adoptive transfer, issues of contamination and anatomical organization remain. Transgenic models of antigen expression circumvent these issues but may be difficult to interpret because of the potential extrahepatic expression of the transgenic antigen. Experiments are further limited by the fact that among the potential liver APC populations, strongly lineage-restricted promoters are available only for the hepatocyte. Finally, the continuous, constitutive expression of a transgenic antigen, either in the liver or at a low level in the thymic medulla, may elicit regulatory T cells with the potential to complicate the analysis of immune responses (20, 21).
To investigate the influence of restricted intrahepatic antigen presentation on all hepatic cell types, we used the novel approach of transplanting the whole liver and exploiting genetic disparities between donor and host to test the APC function of the liver alone. This approach minimizes the role of regulatory cell populations because a peptide antigen may be introduced acutely and presented only by resident cells of the liver grafts. In particular, and in contrast to transgenic models driven by “liver-specific” promoters, our model excludes occult effects of antigen presentation in the thymus. To our surprise, restricted intrahepatic antigen presentation resulted in activation and proliferation of adoptively transferred CD8+ T cells. These T cells did not undergo early apoptosis but completed their differentiation into cytotoxic effector cells.
These experiments demonstrate that antigen presentation in the liver is sufficient to promote the activation and full differentiation of CD8+ cells. To make sense of this result in terms of the evidence for CD8+ T cell tolerance induced by hepatocyte antigen in transgenic models (19), we argue that T cell tolerance in these models is not a direct consequence of restricted intrahepatic antigen presentation, but instead may be due to regulatory T cells that result from the constitutive expression of the antigen. Thus, these models address the basis of self-tolerance while the experiments here address the response to newly expressed liver antigens, such as those encoded by pathogens or neoplasms.
RESULTS
Mouse liver transplantation provides a model of acute hepatic antigen presentation
Several cell types in the liver are known to present antigen to T cells (22, 23). We achieved hepatic presentation of antigen using orthotopic whole organ liver transplantation in mice. Although the surgery is technically challenging, the availability of mutant and congenic strains renders mice the only species in which this kind of analysis is possible. Livers of B6.C-H-2bm8 (bm8) mice were completely removed and replaced by wild-type C57BL/6 livers (for details see Materials and methods). Recipient mice expressed the Kbm8 mutation in the Kb molecule and were therefore unable to present the ovalbumin-derived peptide SIINFEKL, which was introduced as a model antigen throughout the experiments (24). The liver grafts from donor mice expressed the wild-type MHC class I molecule Kb and were competent to present the antigen. Recipient mice were allowed to recover for 4 wk before additional experiments (Fig. 1 B). After successful surgery, mice resumed normal activity by 2 d and regained their initial body weight within 8 d. We excluded any mice with jaundice, which was usually a result of bile duct obstruction. Histologic evaluation of the liver grafts 4 wk after transplantation showed no inflammatory cell infiltration (Fig. 1, C and D) as well as normal liver architecture without significant fibrotic changes or alterations in the intrahepatic leukocyte populations as compared with naive bm8 control animals (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20051775/DC1).
Figure 1.
Experimental design and histologic results of mouse liver transplantation. (A) Mouse liver transplantation with complete replacement of the recipient's liver by a donor graft was used to limit antigen presentation to the liver. Livers from C57BL/6 mice were transplanted into bm8 mice that are unable to present SIINFEKL peptide on MHC class I. (B) The protocol was refined to eliminate extrahepatic antigen presentation by graft-derived passenger leukocytes. Liver donors were irradiated and reconstituted with recipient-type bone marrow 4 wk before transplantation. 4 wk after transplantation, transgenic OT-I cells were adoptively transferred into transplant recipients that presented the specific peptide only within the transplanted livers. Histological sections of naive B6 control animals (C) and bm8 transplant recipients of livers from radiation bone marrow chimeras (D). Hematoxylin and eosin staining at a magnification of 200, and inset at a magnification of 300 (scale bar, 100 μm). The transplanted livers showed no evidence of rejection.
Liver transplantation from bone marrow chimeric donors eliminates antigen presentation by donor-derived migratory leukocytes
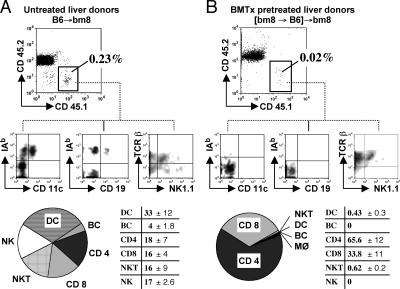
Despite extensive perfusion of the liver grafts with saline solution before transplantation, bone marrow–derived cells of organ donors have been found in transplant recipients, a phenomenon termed microchimerism (25, 26). Therefore, we next investigated the extent and phenotype of microchimeric donor-derived cells in liver transplant recipients. Liver grafts from congenic B6.SJL mice (CD45.1 background) were transplanted into bm8 animals (CD45.2 background). 4 wk after liver transplantation, spleens and peripheral lymph nodes of transplant recipients were harvested and analyzed by eight-color flow cytometry. Based on their expression of the CD45.1 congenic marker, 0.1–0.3% of all leukocytes in spleens (Fig. 2 A) and 0.05–0.2% of peripheral lymph node cells (not depicted) were of donor origin. Among these donor leukocytes, 30–50% were professional APCs expressing dendritic cell or B cell lineage markers. These made up 0.03–0.14% of total leukocytes in the spleen and <0.1% in the peripheral lymph node compartment. Remaining lymphocytes were CD4+ and CD8+ T lymphocytes, NK cells, and NKT cells (Fig. 2 A). To minimize the possibility of extrahepatic antigen presentation by donor-derived passenger leukocytes, we created bone marrow chimeras, in which B6.SJL (H-2b, CD45.1) mice were reconstituted with bone marrow of the liver transplant recipient strain bm8 (Fig. 1 B). These bone marrow chimeras were allowed to reconstitute for 4 wk, and then were used as donors in liver transplantation experiments. 4 wk after liver transplantation, the percentage of CD45.1+ cells had decreased ∼10-fold in spleens to 0.02–0.04% (Fig. 2 B). In the lymph nodes, donor-specific cell numbers were reduced by half to 0.03–0.05% (not depicted). More significantly, donor-derived H-2b passenger leukocytes in these animals consisted almost entirely of T lymphocytes and NKT cells, whereas donor-derived dendritic cells were only detectable as a few individual events on a dot plot (too few to quantitate, <0.01%), and B cells of donor origin were no longer detectable (Fig. 2 B).
Figure 2.
Microchimerism in transplant recipients of liver grafts from untreated B6 and [bm8→B6] radiation bone marrow chimeras. (A) bm8 (CD45.2) recipients of untreated B6.SJL (CD45.1) livers were killed 4 wk after liver transplantation. The frequency and phenotype of donor-derived passenger leukocytes in spleens and peripheral lymph nodes was assessed by eight-color flow cytometry based on the congenic marker CD45.1. (B) Microchimerism in recipients of livers from radiation bone marrow chimeras, in which the bone marrow–derived cells were replaced by bone marrow of the recipient mouse strain (bm8) 4 wk before liver transplantation. Data are representative of two independent experiments with three mice per group. Bottom: average percentage (±SEM) of donor-derived passenger leukocytes as a fraction of the total number of donor-derived CD45.1+ cells (six animals per group). The radiation reduced microchimerism by 90% and abolished the transfer of professional APCs (dendritic cells and B cells).
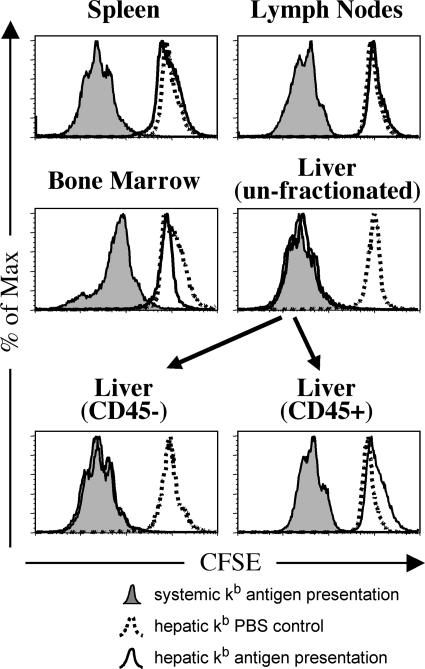
To explicitly test whether the presentation of SIINFEKL antigen could be mediated by these very rare donor-derived APCs, we used an in vitro proliferation assay in which the ability of extrahepatic tissues to induce proliferation of transgenic OT-I T cells was evaluated. 4 wk after transplantation, bm8 animals grafted with a [bm8→B6] bone marrow chimeric liver were injected on three consecutive days with SIINFEKL peptide. Leukocytes were isolated from spleens, peripheral and mesenteric lymph nodes, and bone marrow. Livers were harvested, and a whole organ cell suspension containing hepatocytes, endothelial cells, and nonparenchymal leukocytes was prepared. Cell suspensions from all organs were cocultured for 3 d with CFSE-labeled transgenic OT-I cells, which were on a CD90.1 (Thy-1.1) background. Their antigen-specific proliferation was assessed by flow cytometry based on the dilution of CFSE in CD90.1+ T cells. As shown in Fig. 3, extensive proliferation occurred in all analyzed organs in control transplant recipients, in which a B6 donor was irradiated and reconstituted with B6 bone marrow, and the liver was transplanted into a B6 recipient ([B6→B6]→B6). However, in bm8 recipients of B6 liver grafts that had been irradiated and reconstituted with bm8 bone marrow ([bm8→B6]→bm8), proliferation of OT-I cells was observed only in coculture with CD45− or unfractionated cells from the liver grafts. This was not due to limiting antigen because the results were consistent even when SIINFEKL peptide was added to the cultures in a concentration of 1 μM. Therefore, we concluded that the transplantation of a liver from a [bm8→B6] donor resulted in restricted intrahepatic antigen presentation in recipient bm8 animals. In contrast, antigen presentation was systemic in control animals in which all components (bone marrow and parenchyma of the graft as well as the recipient) were of B6 origin.
Figure 3.
Organ-specific detection of antigen presentation by ex vivo T cell proliferation assay. Antigen presentation in spleens, peripheral and mesenteric lymph nodes, bone marrow, and livers (unfractionated and fractionated into CD45+ and CD45− cells) was assessed independently using an in vitro T cell proliferation assay 4 wk after transplantation. bm8 recipients of liver transplants from [bm8→B6] bone marrow chimeras (hepatic kb expression, open graphs) were compared with B6 recipients of liver grafts from [B6→B6] bone marrow chimeras (systemic kb expression, filled graphs). 4 wk after transplantation, transplant recipients were injected with SIINFEKL peptide, and then cell suspensions from spleens, peripheral and mesenteric lymph nodes, bone marrow, and livers were cocultured with CFSE-stained OT-I cells (CD90.1 background). Organ-specific antigen presentation was determined by dilution of CFSE in OT-I cells. The histograms show OT-I T cells based on their expression of CD90.1. These data demonstrate that the capacity to present the SIINFEKL peptide in the hepatic kb expression group was exclusive to the CD45− and unfractionated intrahepatic cells of the transplanted livers. Data are representative of three independent experiments with two mice per group, and fractionation of liver cells based on CD45 expression was repeated twice with two independently analyzed animals in each group.
Intrahepatic presentation of antigen leads to proliferation of naive CD8+ T cells
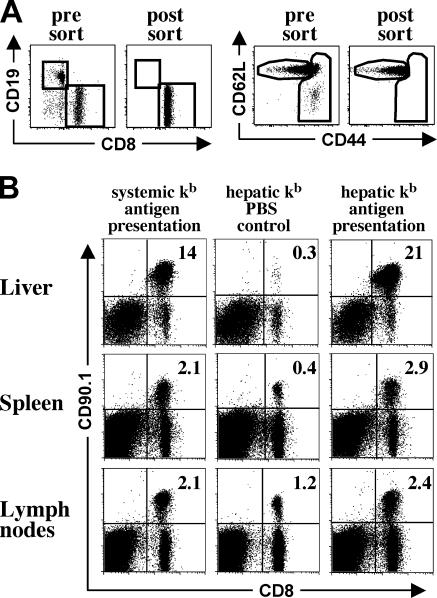
We tested whether the restricted presentation of antigen within the liver in our model would be sufficient for the activation of naive CD8+ T cells. We adoptively transferred TCR transgenic OT-I cells into transplant recipients. To ensure a naive phenotype, transgenic OT-I cells were FACS sorted selecting for the CD44low CD62Lhigh cell population (Fig. 4 A). After SIINFEKL injection for three consecutive days, control transplant recipients with systemic Kb antigen presentation showed expansion of antigen-specific OTI cells in livers, spleens, and peripheral lymph nodes (Fig. 4 B). In animals with restricted hepatic Kb antigen presentation, OT-I expansion was similar or even exceeded the expansion seen in animals with systemic Kb antigen presentation. Expansion did not occur in the absence of specific antigen (Fig. 4 B, hepatic Kb PBS control).
Figure 4.
In vivo expansion of naive OT-I T cells after hepatic and systemic antigen presentation. Naive OT-I cells for adoptive transfer were obtained by magnetic bead depletion, followed by FACS sorting for transgenic T cells with a naive phenotype. (A) Purity and activation status of adoptively transferred OT-I T cells before and after flow cytometric cell sorting. (B) Expansion of adoptively transferred OT-I T cells detected in livers, spleens, and peripheral lymph nodes of transplant recipients. For restricted hepatic kb antigen presentation, livers from [bm8→B6] bone marrow chimeras were transplanted into bm8 recipients. Systemic kb antigen presentation was achieved by transplanting livers from [B6→B6] bone marrow chimeras into B6 recipients. 4 wk after transplantation, 5 × 106 naive OT-I cells (CD90.1 background) were adoptively transferred and the animals received three i.p. injections of either SIINFEKL peptide or PBS (hepatic kb PBS control). 4 d after the initial antigen contact, transplant recipients were killed and the number of OT-I cells was assessed by flow cytometry. Data are representative of three independent experiments with three mice per group, and sorted and unsorted OT-I cells were used for adoptive transfer without significant differences in the extent of prolif-eration. This shows that antigen presentation in the transplanted liver can cause extensive clonal expansion.
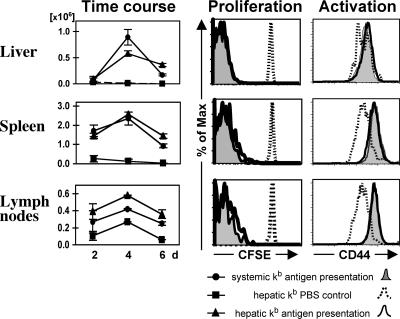
To evaluate whether the similar percentages of OT-I cells in the liver and lymphatic organs of both groups were a result of equally expanding populations or simply a reflection of a disproportionate cell distribution, we transferred CFSE-stained OT-I cells and evaluated proliferation and cell division by dilution of CFSE. By day 4 after the first antigen encounter, cytoplasmic CFSE staining in both groups was completely diluted, indicating more than six cell divisions (Fig. 5). There was also no detectable difference in CFSE dilution between both groups earlier on day 2 (not depicted). In the absence of antigen, no CFSE dilution was observed (Fig. 5). During the early phase of the immune response, OT-I T cells in the hepatic Kb antigen presentation group were found in the spleens and lymph nodes of these animals, indicating that they recirculated after intrahepatic antigen encounter. The proliferative response peaked on day 4 after either systemic or hepatic antigen presentation, followed by a contraction phase. However, OT-I cell numbers decreased more gradually in animals with restricted hepatic Kb presentation as compared with the control group with systemic Kb presentation (Fig. 5).
Figure 5.
Kinetics and activation status of intrahepatically activated OT-I T cells. Left column: Absolute cell numbers of adoptively transferred OT-I T cells 2, 4, and 6 d after initial SIINFEKL injection. Transplant recipients with hepatic or systemic capability to present antigen received 5 × 106 OT-I T cells and were injected with SIINFEKL or PBS (hepatic kb PBS control) on days 0, 1, and 2. OT-I cell numbers from livers, spleens, and peripheral lymph nodes were calculated based on flow cy-tometry data and organ cell counts. Right column: Proliferation and activation status of OT-T cells in animals with hepatic and systemic kb antigen presentation demonstrated by dilution of CFSE staining and expression of CD44 on day 4 after initial antigen or PBS injection. The data show that CD8+ T cell activation in the liver causes massive proliferation and seeding of the lymphoid organs. The data are representative of three independent experiments with three mice per group, and error bars indicate SEM.
Activation of TCR transgenic OT-I cells was similar in both groups, based on the surface expression of activation markers. Up-regulation of CD44 expression on OT-1 T cells was observed in the livers, spleens, and lymph nodes of mice with either systemic or exclusively hepatic antigen presentation (Fig. 5). Similarly, there was equivalent and synchronous down-regulation of CD62L on the OT-1 T cells in both groups of liver-transplanted hosts (not depicted).
CD8+ T cells acquire full effector functions when primed in the liver
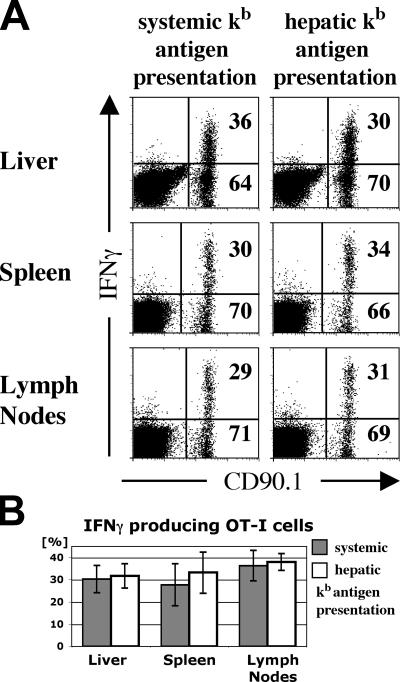
Because naive CD8+ T cells responded to intrahepatic antigen presentation by activation and proliferation, we were interested in the functional potential of these cells. OT-I cells from liver grafts, spleens, and lymph nodes were isolated on days 2 and 4 after antigen encounter, and their ability to produce IFN-γ was tested by intracellular cytokine staining after a 6-h restimulation period. Restricted hepatic antigen presentation had no negative effect on IFN-γ production, which was the same as that observed in mice with systemic presentation (Fig. 6 A). In the absence of antigen restimulation (media without added SIINFEKL peptide), no significant cytokine production was detectable at the end of the culture period. OT-I cells that were primed within liver grafts readily produced IFN-γ upon restimulation, as early as 2 d after antigen contact. During the course of the immune response, IFN-γ–producing OT-I cells initially recirculated into peripheral lymphatic organs and later accumulated in the livers of both groups, resulting in a higher percentage compared with spleens and lymph nodes on day 4 of the immune response (Fig. 4 B). Despite this higher percentage in the liver, the fraction of OT-I cells that responded to peptide restimulation by IFN-γ production was not significantly different between the liver and the lymphatic organs (Fig. 6 B; liver, P = 0.77; spleen, P = 0.52; lymph nodes, P = 0.71). This indicates that the liver environment does not negatively influence the ability of CD8+ T cells to produce IFN-γ. In PBS-injected transplant recipients, no cytokine production was observed 6 h after restimulation. Also, the percentage of cytokine-producing OT-I cells as well as the total OT-I cell number in transplanted animals was similar to that observed in nontransplanted B6 mice after OT-I transfer and peptide injection. This indicated that the transplantation technique did not affect the quality of the immune response as determined by cytokine production (not depicted).
Figure 6.
IFN-γ production of OT-I T cells after restimulation with cognate antigen. (A) Liver transplant recipients with restricted intrahepatic or systemic antigen presentation were killed on day 4 after initial antigen injection. Cell isolates from livers, spleens, and lymph nodes were restimulated with SIINFEKL peptide for 6 h and their IFN-γ production was assessed by intracellular cytokine staining. (B) Average percentage of IFN-γ producers among isolated OT-I T cells. These experiments show that priming in liver produces fully functional CD8+ T cells. Data are representative of three independent experiments with three mice per group, and the bars in B represent the mean ± SEM.
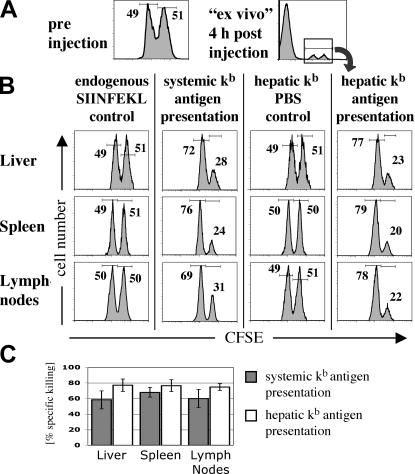
The hallmark of CD8+ T cell effector function is cytotoxic activity. Therefore, we investigated the cytotoxic potential of intrahepatically activated CD8+ T cells using an in vivo cytotoxicity assay. Wild-type B6 splenocytes were pulsed with the SIINFEKL peptide and stained with a high CFSE concentration. These specific target cells were mixed in a 1:1 ratio with nonspecific target cells, which were pulsed with the influenza PA peptide and stained with a lower CFSE concentration (Fig. 7 A). On days 5 and 6 of the immune response, transplant recipients were injected with the 1:1 mixture of specific and nonspecific targets. 4 h later, livers, spleens, and lymph nodes were harvested and analyzed for CFSE+ cells (Fig. 7 A). Cytotoxicity was defined by the reduction of SIINFEKL-pulsed specific targets (CFSEhigh) compared with nonspecific target cells (CFSElow) as shown in Fig. 7, B and C. Antigen-specific disappearance of SIINFEKL-pulsed splenocytes provided evidence of antigen-specific cytotoxicity and was observed in both groups of liver-transplanted mice. There was no clear difference in the priming of cytotoxic function between systemic and hepatic CD8+ T cell activation (liver, P = 0.2; spleen, P = 0.5; lymph nodes, P = 0.21). In control animals in which OT-I cells had not been activated by their specific peptide (hepatic Kb PBS control), the ratio of specific versus nonspecific target cells remained unchanged from the preinjection ratio. Cytotoxicity was not mediated by an endogenous SIINFEKL-specific cell population; transplant recipients that had not received OT-I cells but were injected with the SIINFEKL peptide for 3 d showed no disappearance of specific target cells (Fig. 7 B). Compared with systemic priming, hepatic Kb presentation resulted in slightly greater specific killing, especially in livers and lymph nodes. However, this increased killing was more likely due to higher OT-I cell numbers during the late phase of the immune response rather than a difference in cytotoxicity on a single cell level (Fig. 5).
Figure 7.
In vivo CTL challenge with SIINFEKL-pulsed target cells. (A) OT-I cells were adoptively transferred into transplant recipients with restricted hepatic or systemic kb antigen presentation and primed by SIINFEKL injection. 5–6 d after initial antigen encounter, mice were tested for cytotoxic T cells by injection of SIINFEKL-pulsed target cells. Splenocytes from naive B6 animals, stained in a 2-μM CFSE solution (CFSEhigh) and pulsed in a 1-μM SIINFEKL solution, were used as specific target cells. Nonspecific control targets were stained in a 0.2-μM CFSE solution (CFSElow) and pulsed with the influenza PA peptide. Specific and nonspecific target cells were mixed in a 1:1 ratio, and a total of 2 × 107 cells was injected i.v. into transplant recipients (A, preinjection). 4 h after the injection of target cells, transplant recipients were killed and cell suspensions from livers, spleens, and peripheral lymph nodes were analyzed for CFSE+ cells by flow cytometry (A, right). (B) Cytotoxicity was determined by the increased ratio of specific versus nonspecific target cells (see Results and Materials and methods for details). Cytotoxic activity of endogenous SIINFEKL-specific cells was excluded in transplant recipients by three injections of cognate antigen in the absence of previous adoptive OT-I transfer (endogenous SIINFEKL control). (C) Percentage of specific cytotoxicity in transplant recipients. These experiments show that antigen presented in the transplanted liver can prime CTLs. Data are representative of three independent experiments with three mice per group, and the bars in C represent the average ± SEM.
DISCUSSION
Several aspects of hepatic biology demonstrate that immune responses tend to be skewed toward immunological tolerance in the liver. Several mechanisms have been identified that are orchestrated to cause this phenomenon. Hepatic dendritic cells with an immature phenotype have been shown to migrate to lymphatic organs and exert tolerogenic functions (27). Activated CD8+ lymphocytes are effectively trapped in the liver and eliminated from the circulating blood by induction of apoptosis (28, 29). Although these mechanisms may contribute to the regulation of systemic immune responses, the initiation of immune responses in the liver itself may also result in immunological tolerance, as seen in oral tolerance and other models. Recent studies comparing mouse models with transgenic intrahepatic plus or minus extrahepatic antigen expression have concluded that although initial presentation of antigen in lymphatic organs results in immunogenic responses, antigen presentation in the liver promotes tolerance induction of specific CD8+ T cells (19).
In this work, this hypothesis was tested using a novel approach. Antigen was acutely introduced and its presentation was restricted to resident cells of a previously transplanted liver graft. Surprisingly, local intrahepatic antigen presentation in our model was sufficient for the full activation and differentiation of naive CD8+ T cells without the need for initial priming in lymphatic organs.
It is generally agreed that CD4+ T cell responses are dependent on antigen presentation and costimulatory signals of mature dendritic cells within regional lymph nodes, and that priming does not occur outside lymphatic organs (30). In the case of initial priming of CD8+ lymphocytes, the absolute need for costimulation is less well established. Full activation and differentiation was observed in CD28-deficient mice that were infected with lymphocytic choriomeningitis virus (31), and the T cell–mediated destruction of pancreatic β cells was unaffected in CD28-deficient nonobese diabetic mice (32). In vitro, full activation and differentiation of transgenic CD8+ T cells has been induced by peptide-loaded tetramers, despite the lack of costimulation (33). From these studies it would be predicted that the special costimulatory properties of dendritic cells are optional for CD8+ T cells. The current work, in which we show that priming and full activation of naive CD8+ T cells can occur independent of lymphatic organs, is consistent with this view of CD8+ T cell priming. The complete, acute activation and differentiation of naive CD8+ T cells was demonstrated by specific cytotoxicity until day 6 after initial peptide activation. This argues against a defective initial activation of naive CD8 T+ cells as the exclusive reason of liver-mediated CD8+ T cell tolerance. Because this experimental model depends on the intrahepatic expression of an MHC class I restriction element, and the antigen is a class I–restricted peptide, the model excludes the participation of CD4+ T helper cells, which have been shown to be essential for the long-term survival of memory cells. Without help, memory CD8+ T cell survival is impaired; therefore, we do not draw the conclusion that the fully activated CD8+ T cells would be likely to differentiate into memory cells.
In the context of the liver transplantation model, it is appropriate to consider whether the observed CD8+ T cell activation could be occurring in neo-lymphatic tissue, induced in the liver grafts by chronic inflammation, due to low-grade rejection. Liver transplants in mice are generally accepted by the recipient without the need for immunosuppression and result in donor-specific tolerance (34). In our model, stable graft acceptance and tolerance induction toward the B6 liver parenchyma was observed by 4 wk after liver transplantation. Adoptive transfer of B6 lymphocytes, either in the form of OT-I cells in the proliferation experiments or as B6 splenocytes in the in vivo cytotoxicity assays, were accepted without rejection, consistent with the induction of full transplantation tolerance in the first 4 wk after transplantation of the chimeric liver graft. In contrast to this, adoptive transfer of B6 cells into either nontransplanted bm8 mice as well as bm8 recipients of [bm8→bm8] bone marrow chimeric livers resulted in rejection and complete disappearance of these cells within the first 2 d after transfer (not depicted). However, there was no histological evidence of lymphocyte infiltration or chronic rejection detectable in the liver transplants 4 wk after transplantation (Fig. 1 and Fig. S1). Other potential causes of liver inflammation, such as infection with pathogens (in particular mouse hepatitis virus), were not observed in our mouse colonies for the entire time of the experiments.
Transplantation of whole organs is accompanied by the transfer of passenger leukocytes from the organ donor. These donor-derived cells migrate out of the transplant into the recipient and relocate to lymphoid and nonlymphoid organs, a phenomenon known as microchimerism (35, 36). Consequently, in our model, passenger leukocytes in transplant recipients were a potential source of antigen presentation outside the liver graft. To address this concern, we transplanted the livers from radiation bone marrow chimeras, in which the bone marrow–derived cells were replaced by bone marrow of the recipient mouse strain 4 wk before liver transplantation (Fig. 1 B). This resulted in the complete disappearance of the donor-derived APCs from the recipient's spleens, peripheral lymph nodes, and liver grafts as detected by flow cytometry (Fig. 2 and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20051775/DC1). To confirm the restriction of antigen presentation to the liver grafts in a sensitive functional assay, we separated hepatic antigen presentation from lymphatic antigen presentation in an ex vivo T cell proliferation experiment. Constituent cells from livers, peripheral and mesenteric lymph nodes, spleens, and bone marrow were evaluated independently for their ability to activate antigen-specific T cells and promote their proliferation. Indeed, this demonstrated that by using bone marrow chimeras as liver donors, microchimerism of effective APCs was eliminated and antigen presentation was restricted to non-bone marrow–derived cells of the liver itself (Fig. 3).
However, the immune response in vivo was not restricted to the transplanted organ. Instead, OT-I T cells recirculated systemically and were found in lymphatic tissues of the recipient early in the immune response. At various time points during the immune response, OT-I cells isolated from spleens, lymph nodes, and livers showed a similar activated phenotype and proliferation status, and resembled each other in their ability to produce IFN-γ upon restimulation. This suggests constant recirculation between the antigen expressing liver and the lymphatic tissues during the ongoing immune response. The actual site of expansion was not addressed by our experiments, and it is quite conceivable that mitotic cell divisions and/or T cell maturation of OT-I cells took place in lymphatic tissues as well as in the liver. However, the activation and proliferation of CD8+ T cells in our model was clearly initiated by antigen presentation in the liver. Surprisingly, there was no significant difference in the magnitude of T cell expansion when antigen was presented systemically or restricted to the liver. Our data indicate that the capacity of the liver to present antigen is sufficient to promote activation and proliferation of a large number of T cells. Several physiologic aspects of the liver contribute to this. The hepatic environment, unlike that of other parenchymal organs, promotes the interaction of naive lymphocytes with potential tissue APCs. The liver is perfused by 25% of the cardiac output, which results in constant exposure of circulating T cells with presented antigen. A unique combination of narrow hepatic sinuses with a fenestrated endothelium and a lack of basement membrane, together with a low velocity blood flow, allow perpetual contact of circulating lymphocytes with potential APCs in the sinusoids and the subendothelial space of Disse (12, 14). In addition, exposure to endotoxins and other bacterial products derived from the intestine provide a constant source of “endogenous” immunological stimulation that results in up-regulation of adhesion molecules on Kupffer cells and LSECs (37).
T cell activation in this special environment has been shown previously. However, the final result of hepatic T cell activation was often premature activation-induced cell death, defective activation and tolerance induction in CD8+ T cells, or the formation of a regulatory cell type in CD4+ T cells (17, 38). These observations led to the hypothesis that immunological tolerance might be established in the liver by local antigen presentation and implicated a special role in this process for two intrahepatic cell types: the LSECs and the hepatocytes (38, 39).
Antigen presentation by isolated LSECs skews CD4+ T cell activation toward a regulatory phenotype with the expression of IL-4 and IL-10 (40). Priming of CD8+ T cells by LSECs resulted in incomplete activation with loss of IFN-γ and IL-2 cytokine production and a lack of cytotoxic activity. However, the exclusive role of LSECs in T cell priming has recently been questioned on the basis of experiments using LSECs that were isolated based on surface marker expression rather than counterflow elutriation. The problem is to determine whether low-level contamination of LSEC preparation by other APCs might have complicated the interpretation of previous results (41). Whether LSECs are finally agreed to be self-sufficient APCs does not impact directly on our present work, but would change the probability that they, rather than hepatocytes, are the important local intrahepatic APC.
Incomplete activation of CD8+ T cells with a lack of cytotoxicity and premature T cell death within the first few days after T cell priming has also been reported when transgenic antigen was expressed on hepatocytes (acting as nonprofessional APCs). However, continuous expression of a transgenic antigen is known to induce regulatory T cells, creating an alternative mechanism of tolerance. Similarly, the promiscuous gene expression of tissue-specific self-antigens in medullary thymic epithelial cells results in systemic central tolerance and formation of tolerogenic regulatory T cells (42–44). Therefore, modulation of CD8+ T cell responses by transgenic hepatic antigen could simulate mechanisms of self-tolerance and the control of autoimmunity rather than immune failure to an exogenous pathogen. In this work, antigen expression was introduced acutely by peptide injection, which would not be expected to induce thymic, self-specific regulatory T cell populations.
Our findings support a model in which a productive immune response is initially provoked by acute presentation of antigen within the liver, as seen in hepatic infections. In cases of antigen persistence, this immune response is modulated by regulatory systems over time, which might ultimately result in tolerance. This is reflected by the initial immune response in viral hepatitis infections preceding the chronic stage (45) or the immediate phase after liver transplantation with the occurrence of alloreactive lymphocytes before tolerance is established (7). The mechanisms through which such tolerance is established are still poorly understood. However, in this work we have tested and rigorously excluded the possibility that local, intrahepatic presentation of antigen to CD8+ T cells results in abortive interaction, maturation failure, and premature death. The reality is quite different. The interaction of CD8+ T cells with the liver results in the proliferation and maturation of CTLs with full effector function.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6J and B6.SJL-Ptprca (B6.CD45.1) mice were purchased from The Jackson Laboratory. The bm8 mice were provided by L.R. Pease (Mayo Clinic, Rochester, MN). A colony of OT-I transgenic mice was maintained on a CD90.1 (Thy1.1) background. All mice were bred and housed in a specific pathogen-free environment and used between 6 to 10 wk of age. Experiments were performed in accordance with regulatory guidelines and standards set by the University Committee on Animal Resources of the University of Rochester.
Mouse liver transplantation.
Orthotopic mouse liver transplantation, initially reported by Qian et al. (46), was performed according to a technique described by Steger et al. (47) in the non-rearterialized version. The donor liver was obtained by dissection of the surrounding hepatic ligaments; the right adrenal vein, pyloric vein, and proper hepatic artery were ligated and divided. For continuous bile flow the gallbladder was ligated and removed. A polyethylene stent tube (inner diameter, 0.28 mm; SIMS Portex) was inserted into the lumen of the common bile duct and secured with 8–0 silk (Pearsalls). The infrahepatic inferior vena cava (IVC) and portal vein were clamped, and the organ was perfused with 10 ml of 4°C normal saline through the portal vein. The liver was removed and stored in 4°C PBS solution until transplantation. The transplantation procedure was performed under inhalation anesthesia with isoflurane. After clamping of the infrahepatic and suprahepatic IVC and the portal vein, the recipient's liver was completely removed and the donor organ was placed orthotopically into the abdominal cavity. The suprahepatic and the infrahepatic IVC were anastomosed with continuous running sutures using 10–0 nylon (Ethicon), and the portal vein was reconnected by cuff anastomosis. Reconstruction of the bile flow was achieved by inserting the graft's stent tube into the recipient's bile duct and securing it with three single 10–0 nylon sutures.
Radiation bone marrow chimera.
6–8-wk-old recipient mice were irradiated with a dose of 10 Gy (1,000 rad) using an RS2000 X-ray irradiator (RadSource Technologies). Depletion of mature T lymphocytes from donor bone marrow cells was achieved by incubation with anti-CD4 mAb (clone RL172.4) and anti-CD8 mAb (clone 3-16.8) and subsequent lysis using Guinea pig complement (Invitrogen). Recipient mice were injected with 107 cells via the tail vein and were allowed to reconstitute for 28 d until additional experiments.
Cell isolation procedures.
For single cell suspensions, peripheral lymph nodes and spleens were mechanically homogenized between frosted glass slides. Liver leukocytes were isolated as described previously (48). In brief, livers were perfused with PBS, mashed through a cell strainer, and incubated for 40 min at 37°C in RPMI digestion buffer containing 0.05% collagenase IV and 0.002% DNase I (Sigma-Aldrich). The leukocyte population was obtained by density gradient centrifugation using a 22% Opti-prep (Axis-Shield) solution.
Flow cytometry and statistical analysis.
Cell solutions were stained for 20 min at 4°C with mAbs specific for TCR-β, CD90.1, CD44, I-Ab, CD4, CD19, and IFN-γ (all from BD Biosciences); F4/80, CD8, and CD11b (all from Caltag Laboratories); and CD45.1, CD45.2, CD62L, and CD11c (all from eBioscience). For intracellular cytokine staining, the Cytofix/Cytoperm kit (BD Biosciences) was used according to the manufacturer's instructions after restimulation in the presence or absence of 1 μM SIINFEKL peptide for 6 h. Ungated cell samples of the described organs were acquired using a BD LSR II flow cytometer (BD Biosciences) and analyzed by FlowJo software (Tree Star) based on a lymphocyte size gate.
Adoptive transfer of OT-I cells and in vivo activation.
Transgenic CD8+ cells from spleens and lymph nodes were enriched by magnetic depletion of B cells, CD4+ T cells, dendritic cells, and NK cells using primary antibody (clone 212.Al specific for MHC class II molecules, clone GK1.5 specific for CD4, clone 2.4.G2 specific for FcRs, and clone HB.191 specific for NK1.1), followed by magnetic beads coated with secondary antibodies (QIAGEN). The purity of enriched CD8+ OT-I cells was >93% (±4%). To obtain a naive OT-I population, cells were sorted for a CD44low CD62Lhigh phenotype using a FACSAria cell sorting system (BD Biosciences). The purity of CD8+ OT-I cells with a naive phenotype was 99% after sorting. 5 × 106 OT-I cells were injected i.v. into transplant recipients and activated by daily i.p. injections of 25 nmol SIINFEKL peptide (New England Peptide) for 3 d starting 12 h after injection of OT-I cells.
In vitro proliferation assay.
Transplant recipients were injected i.p. for three consecutive days with 25 nmol SIINFEKL peptide. Cells from spleens and lymph nodes (cervical, axillary, brachial, inguinal, mesenteric, and celiac lymph nodes) were isolated by mechanical disruption. Bone marrow cells were obtained from femora and tibiae. Livers were perfused with a 37°C PBS solution containing 5% collagenase IV for 8–10 min, disrupted, and shaken to liberate intrahepatic cells into the media. To obtain an unbiased representation of all types of intrahepatic cells, the cell suspensions were simply washed, filtered to remove aggregates, and used in the assay without any further separation steps. To further differentiate APCs in the assay we fractionated such liver cells based on their CD45 expression by fluorescence- activated cell sorting using a FACSAria system (BD Biosciences). 3 × 105 cells from spleens, lymph nodes, and bone marrow, as well as 2 × 104 unseparated liver cells and 3 × 105 fractionated liver cells were resuspended in culture media (DMEM/F12, 10% FBS, insulin-transferrin-selenium/ITS, and penicillin-streptomycin) and cocultured with 3 × 105 CFSE-stained OT-I cells. Cultures were maintained for 96 h before flow cytometric analysis.
In vivo cytotoxicity assay.
Splenocytes from C57BL/6J mice were separated from the RBCs by density gradient centrifugation (Lympholyte-M; Cedarlane Laboratories), divided into two equal portions, and stained in a 2-or 0.2-μM CFSE solution (Invitrogen), respectively. Cell suspensions were pulsed for 1 h with either a 1-μM solution of SIINFEKL peptide as a specific target cell population (2 μM CFSE-stained cells) or a 1-μM solution of PA peptide as a control population (0.2 μM CFSE-stained cells). Specific target cells and control population were counted and mixed in a 1:1 ratio, and a total of 2 × 107 cells was injected i.v. into transplant recipients. After 5 h, spleens, lymph nodes, and livers were harvested from recipient mice and analyzed by flow cytometry to determine the ratio of specific target cells versus control cells. Specific killing was calculated by the following formula: 100 − ([(percentage of SIINFEKL pulsed in immunized/percentage of PA pulsed in immunized)/(percentage of SIINFEKL pulsed in uninmmunized/percentage of PA pulsed in uninmmunized)] × 100).
Statistical analysis.
The statistical significance of the differences between groups of mice was tested using Student's t test. A value of P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 depicts hepatic micro architecture and intrahepatic cell populations in transplanted and naive animals. Fig. S2 shows the fraction and subpopulations of radioresistant intrahepatic kb+ wild-type leukocytes. Figs. S1 and S2 are available at http://www.jem.org/cgi/content/full/jem.20051775/DC1.
Supplemental Material
Acknowledgments
The authors would like to thank Nathan Laniewski for expert assistance with the cell sorting, Robert Pierce, Noelle Polakos, and Linda Johnstone for histopathology, and Jim Miller for critical reading of the manuscript.
This work was supported by National Institutes of Health grant numbers AI063353 and AI054517 (to I.N. Crispe). I. Klein was supported by the German Research Foundation grant number KL1403/2-1 and by funds from the Federal Ministry of Education and Research supplied to the Interdisciplinary Centre for Clinical Research of the University of Wuerzburg (research project grant no. 01 KS 9603).
The authors have no conflicting financial interests.
Abbreviations used: bm8, B6.C-H-2bm8 mouse strain; IVC, inferior vena cava; LSEC, liver sinusoidal endothelial cell.
References
- 1.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229. [DOI] [PubMed] [Google Scholar]
- 2.Good, M.F. 1995. Development of immunity to malaria may not be an entirely active process. Parasite Immunol. 17:55–59. [DOI] [PubMed] [Google Scholar]
- 3.Calne, R., and H. Davies. 1994. Organ graft tolerance: the liver effect. Lancet. 343:67–68. [DOI] [PubMed] [Google Scholar]
- 4.Gassel, H.J., I.V. Hutchinson, R. Engemann, and P.J. Morris. 1992. The role of T suppressor cells in the maintenance of spontaneously accepted orthotopic rat liver allografts. Transplantation. 54:1048–1053. [DOI] [PubMed] [Google Scholar]
- 5.Farges, O., P.J. Morris, and M.J. Dallman. 1994. Spontaneous acceptance of liver allografts in the rat. Analysis of the immune response. Transplantation. 57:171–177. [DOI] [PubMed] [Google Scholar]
- 6.Dahmen, U., S. Qian, A.S. Rao, A.J. Demetris, F. Fu, H. Sun, L. Gao, J.J. Fung, and T.E. Starzl. 1994. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 58:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian, S., L. Lu, F. Fu, Y. Li, W. Li, T.E. Starzl, J.J. Fung, and A.W. Thomson. 1997. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J. Immunol. 158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 8.Calne, R.Y. 2000. Immunological tolerance–the liver effect. Immunol. Rev. 174:280–282. [DOI] [PubMed] [Google Scholar]
- 9.Mazariegos, G.V., J. Reyes, I.R. Marino, A.J. Demetris, B. Flynn, W. Irish, J. McMichael, J.J. Fung, and T.E. Starzl. 1997. Weaning of immunosuppression in liver transplant recipients. Transplantation. 63:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowat, A.M., L.A. Parker, H. Beacock-Sharp, O.R. Millington, and F. Chirdo. 2004. Oral tolerance: overview and historical perspectives. Ann. N Y Acad. Sci. 1029:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Gorczynski, R.M., Z. Chen, H. Zeng, and X.M. Fu. 1998. A role for persisting antigen, antigen presentation, and ICAM-1 in increased renal graft survival after oral or portal vein donor-specific immunization. Transplantation. 66:339–349. [DOI] [PubMed] [Google Scholar]
- 12.Knolle, P.A., and G. Gerken. 2000. Local control of the immune response in the liver. Immunol. Rev. 174:21–34. [DOI] [PubMed] [Google Scholar]
- 13.Yang, R., Q. Liu, J.L. Grosfeld, and M.D. Pescovitz. 1994. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J. Pediatr. Surg. 29:1145–1148. [DOI] [PubMed] [Google Scholar]
- 14.Crispe, I.N. 2003. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3:51–62. [DOI] [PubMed] [Google Scholar]
- 15.Czaja, A.J. 2001. Understanding the pathogenesis of autoimmune hepatitis. Am. J. Gastroenterol. 96:1224–1231. [DOI] [PubMed] [Google Scholar]
- 16.Limmer, A., J. Ohl, C. Kurts, H.G. Ljunggren, Y. Reiss, M. Groettrup, F. Momburg, B. Arnold, and P.A. Knolle. 2000. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6:1348–1354. [DOI] [PubMed] [Google Scholar]
- 17.Bertolino, P., M.C. Trescol-Biemont, J. Thomas, B. Fazekas de St Groth, M. Pihlgren, J. Marvel, and C. Rabourdin-Combe. 1999. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 11:1225–1238. [DOI] [PubMed] [Google Scholar]
- 18.Bertolino, P., D.G. Bowen, G.W. McCaughan, and B. Fazekas de St Groth. 2001. Antigen-specific primary activation of CD8+ T cells within the liver. J. Immunol. 166:5430–5438. [DOI] [PubMed] [Google Scholar]
- 19.Bowen, D.G., M. Zen, L. Holz, T. Davis, G.W. McCaughan, and P. Bertolino. 2004. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Invest. 114:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. [DOI] [PubMed] [Google Scholar]
- 21.Su, M.A., and M.S. Anderson. 2004. Aire: an update. Curr. Opin. Immunol. 16:746–752. [DOI] [PubMed] [Google Scholar]
- 22.Lohse, A.W., P.A. Knolle, K. Bilo, A. Uhrig, C. Waldmann, M. Ibe, E. Schmitt, G. Gerken, and K.H. Meyer Zum Buschenfelde. 1996. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 110:1175–1181. [DOI] [PubMed] [Google Scholar]
- 23.Bertolino, P., M.C. Trescol-Biemont, and C. Rabourdin-Combe. 1998. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur. J. Immunol. 28:221–236. [DOI] [PubMed] [Google Scholar]
- 24.Nikolic-Zugic, J., and F.R. Carbone. 1990. The effect of mutations in the MHC class I peptide binding groove on the cytotoxic T lymphocyte recognition of the Kb-restricted ovalbumin determinant. Eur. J. Immunol. 20:2431–2437. [DOI] [PubMed] [Google Scholar]
- 25.Bettens, F., J.M. Tiercy, N. Campanile, E. Giostra, P. Majno, L. Rubbia, E. Roosnek, G. Mentha, and J. Villard. 2005. Microchimerism after liver transplantation: absence of rejection without abrogation of anti-donor cytotoxic T-lymphocyte-mediated alloreactivity. Liver Transpl. 11:290–297. [DOI] [PubMed] [Google Scholar]
- 26.Rao, A.S., A.W. Thomson, R. Shapiro, and T.E. Starzl. 1994. Chimerism after whole organ transplantation: its relationship to graft rejection and tolerance induction. Curr. Opin. Nephrol. Hypertens. 3:589–595. [DOI] [PubMed] [Google Scholar]
- 27.Lau, A.H., and A.W. Thomson. 2003. Dendritic cells and immune regulation in the liver. Gut. 52:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehal, W.Z., A.E. Juedes, and I.N. Crispe. 1999. Selective retention of activated CD8+ T cells by the normal liver. J. Immunol. 163:3202–3210. [PubMed] [Google Scholar]
- 29.Mehal, W.Z., F. Azzaroli, and I.N. Crispe. 2001. Antigen presentation by liver cells controls intrahepatic T cell trapping, whereas bone marrow-derived cells preferentially promote intrahepatic T cell apoptosis. J. Immunol. 167:667–673. [DOI] [PubMed] [Google Scholar]
- 30.Itano, A.A., and M.K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733–739. [DOI] [PubMed] [Google Scholar]
- 31.Shahinian, A., K. Pfeffer, K.P. Lee, T.M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P.S. Ohashi, C.B. Thompson, and T.W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 261:609–612. [DOI] [PubMed] [Google Scholar]
- 32.Lenschow, D.J., K.C. Herold, L. Rhee, B. Patel, A. Koons, H.Y. Qin, E. Fuchs, B. Singh, C.B. Thompson, and J.A. Bluestone. 1996. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 5:285–293. [DOI] [PubMed] [Google Scholar]
- 33.Wang, B., R. Maile, R. Greenwood, E.J. Collins, and J.A. Frelinger. 2000. Naive CD8+ T cells do not require costimulation for proliferation and differentiation into cytotoxic effector cells. J. Immunol. 164:1216–1222. [DOI] [PubMed] [Google Scholar]
- 34.Qian, S., A.J. Demetris, N. Murase, A.S. Rao, J.J. Fung, and T.E. Starzl. 1994. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 19:916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl, T.E. 2004. Chimerism and tolerance in transplantation. Proc. Natl. Acad. Sci. USA. 101:14607–14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood, K.J. 2003. Passenger leukocytes and microchimerism: what role in tolerance induction? Transplantation. 75:17S–20S. [DOI] [PubMed] [Google Scholar]
- 37.van Oosten, M., E. van de Bilt, H.E. de Vries, T.J. van Berkel, and J. Kuiper. 1995. Vascular adhesion molecule-1 and intercellular adhesion molecule-1 expression on rat liver cells after lipopolysaccharide administration in vivo. Hepatology. 22:1538–1546. [DOI] [PubMed] [Google Scholar]
- 38.Knolle, P.A., and A. Limmer. 2001. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 22:432–437. [DOI] [PubMed] [Google Scholar]
- 39.Bertolino, P., G.W. McCaughan, and D.G. Bowen. 2002. Role of primary intrahepatic T-cell activation in the ‘liver tolerance effect’. Immunol. Cell Biol. 80:84–92. [DOI] [PubMed] [Google Scholar]
- 40.Knolle, P.A., E. Schmitt, S. Jin, T. Germann, R. Duchmann, S. Hegenbarth, G. Gerken, and A.W. Lohse. 1999. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 116:1428–1440. [DOI] [PubMed] [Google Scholar]
- 41.Katz, S.C., V.G. Pillarisetty, J.I. Bleier, A.B. Shah, and R.P. DeMatteo. 2004. Liver sinusoidal endothelial cells are insufficient to activate T cells. J. Immunol. 173:230–235. [DOI] [PubMed] [Google Scholar]
- 42.Jolicoeur, C., D. Hanahan, and K.M. Smith. 1994. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc. Natl. Acad. Sci. USA. 91:6707–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 44.von Boehmer, H., I. Aifantis, F. Gounari, O. Azogui, L. Haughn, I. Apostolou, E. Jaeckel, F. Grassi, and L. Klein. 2003. Thymic selection revisited: how essential is it? Immunol. Rev. 191:62–78. [DOI] [PubMed] [Google Scholar]
- 45.Racanelli, V., and B. Rehermann. 2003. Hepatitis C virus infection: when silence is deception. Trends Immunol. 24:456–464. [DOI] [PubMed] [Google Scholar]
- 46.Qian, S.G., J.J. Fung, A.V. Demetris, S.T. Ildstad, and T.E. Starzl. 1991. Orthotopic liver transplantation in the mouse. Transplantation. 52:562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steger, U., B. Sawitzki, A.M. Gassel, H.J. Gassel, and K.J. Wood. 2003. Impact of hepatic rearterialization on reperfusion injury and outcome after mouse liver transplantation. Transplantation. 76:327–332. [DOI] [PubMed] [Google Scholar]
- 48.John, B., and I.N. Crispe. 2004. Passive and active mechanisms trap activated CD8+ T cells in the liver. J. Immunol. 172:5222–5229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.