Abstract
Resistance to several prevalent infectious diseases requires both cellular and humoral immune responses. T cell immunity is initiated by mature dendritic cells (DCs) in lymphoid organs, whereas humoral responses to most antigens require further collaboration between primed, antigen-specific helper T cells and naive or memory B cells. To determine whether antigens delivered to DCs in lymphoid organs induce T cell help for antibody responses, we targeted a carrier protein, ovalbumin (OVA), to DCs in the presence of a maturation stimulus and assayed for antibodies to a hapten, (4-hydroxy-3-nitrophenyl) acetyl (NP), after boosting with OVA-NP. A single DC-targeted immunization elicited long-lived T cell helper responses to the carrier protein, leading to large numbers of antibody-secreting cells and high titers of high-affinity antihapten immunoglobulin Gs. Small doses of DC-targeted OVA induced higher titers and a broader spectrum of anti-NP antibody isotypes than large doses of OVA in alum adjuvant. Similar results were obtained when the circumsporozoite protein of Plasmodium yoelii was delivered to DCs. We conclude that antigen targeting to DCs combined with a maturation stimulus produces broad-based and long-lived T cell help for humoral immune responses.
DCs are specialized antigen-presenting cells that are abundant in lymphoid organs and mucosal surfaces (for review see references 1–4). When mature DCs were first compared with other cell types as antigen-presenting cells, they were found to be orders of magnitude more effective in inducing proliferative, cytotoxic, or helper T cell responses in vitro (5–8). Furthermore, small numbers of mature DCs loaded with tumor or pathogen-specific antigens induced protective T cell responses when reinfused into mice or humans (for review see references 9 and 10).
In contrast, when antigens were specifically targeted to immature DCs in vivo, antigen presentation to CD4+ and CD8+ T cells led to profound peripheral T cell tolerance by deletion, anergy, or induction of regulatory T cells (11–14). The same antigens delivered to DCs in conjunction with a stimulus for their differentiation or maturation, such as anti-CD40 antibody (15), elicited strong T cell immune responses (11, 16). We have proposed that this dual function of DCs in tolerance and immunity may be required to prevent anti–self-immune responses. By continually picking up, processing, and presenting antigen in the steady-state, DCs may avert anti–self-responses when a combination of self- and foreign antigens are presented to T cells during infection (17).
In the course of developing a method for antigen delivery to DCs in vivo, we found that proteins delivered to DCs by antibodies to the DEC-205 receptor were at least two orders of magnitude more effective than nontargeted antigens in activating T cell proliferation, production of IFN-γ, and protection against vaccinia virus infection (11, 12, 16). Thus, specific antigen delivery to DCs in combination with a maturation stimulus may be an effective method to produce protein vaccines that induce strong cellular immunity (16, 18). However, cellular immunity is not sufficient for protection against many pathogens, and in these instances, humoral immunity is required for optimal vaccination.
Here, we report on T cell–dependent antibody responses elicited by antigens targeted to DCs in vivo.
RESULTS
Production of fusion mAbs with full-length OVA
Immunization with protein antigens in adjuvant elicits T cell help for antibody formation. The most direct and general method to assess this type of T cell help is to measure antibody responses to haptens coupled to carrier proteins. In this assay, mice primed with the carrier protein are challenged with a conjugate of the same protein with a hapten (19–22). The antihapten antibody responses are dependent on contact between naive hapten-specific B cells and anti-carrier–specific memory T helper cells elicited during the initial immunization (20).
To determine whether antigens targeted to DCs in vivo produce T cell help for antibody responses, we used OVA as the carrier protein and (4-hydroxy-3-nitrophenyl) acetyl (NP) as the hapten. The carrier was delivered to DCs by cloning OVA in frame with the carboxyl terminus of the heavy chain of the αDEC-205 (anti–DEC-OVA) antibody that targets DCs in vivo (11). Constant regions of the original rat αDEC and isotype control antibody (control-OVA) were replaced by that of the mouse IgG1 and modified to avoid Fc-receptor binding (11). The fusion mAbs were produced by transient transfection and binding to DEC-205 was confirmed by flow cytometry (Fig. S1, A and B, available at http://www.jem.org/cgi/content/full/jem.20051639/DC1).
Strong T cell responses to a single dose of anti–DEC-OVA plus maturation stimulus
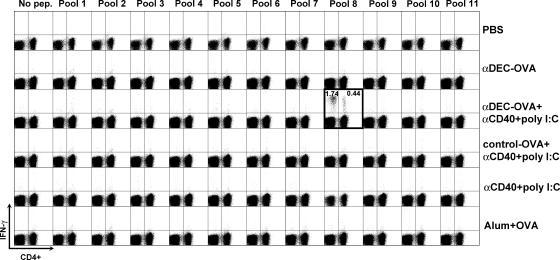
Naive C57BL/6 mice were immunized with 10 μg anti–DEC-OVA or control-OVA (corresponding to ∼3.5 μg OVA) in the presence or absence of a DC maturation stimulus (anti-CD40 plus poly I:C) or with 100 μg of alum-precipitated LPS-free OVA (alum plus OVA) because the latter is considered to be effective in producing T cell help for antibody responses. 14 d later, T cells isolated from immunized mice were pulsed with peptide pools from a library of overlapping OVA 12–17-mer peptides (Table S1, available at http://www.jem.org/cgi/content/full/jem.20051639/DC1), and IFN-γ secretion was measured by flow cytometry. We detected IFN-γ secretion by CD4+ and CD8+ T cells in response to OVA peptides in the group immunized with anti–DEC-OVA plus maturation stimulus, but not in any of the other groups, including mice primed with alum plus OVA (Fig. 1, boxed plot). When we broke down the pools into individual peptides, the CD8+ T cells recognized peptides containing the known H-2Kb binding sequence SIINFEKL and two other mimetope peptides, whereas the CD4+ T cell response was restricted to a previously undefined helper peptide, EKLTEWTSSNVMEER (Fig. S1 C). We conclude that immunization with ∼3.5 μg OVA in the form of anti–DEC-OVA fusion protein induces CD4+ and CD8+ T cell responses, whereas the immunization with alum-precipitated OVA does not.
Figure 1.
Immunization with anti–DEC-OVA fusion mAb primes OVA-specific T cells. IFN-γ production by CD4+ and CD8+ T cells is shown as dot plots of gated CD3+ T cells stained for CD4 and intracellular IFN-γ. Immunization conditions are displayed on the right, and the peptide pool numbers are shown on top. Numbers represent the percent of IFN-γ+ CD3+ CD4− (left panels) or IFN-γ+ CD3+ CD4+ (right panels) T cells.
Broad and long-lived humoral immune responses with DC-targeted T cell help
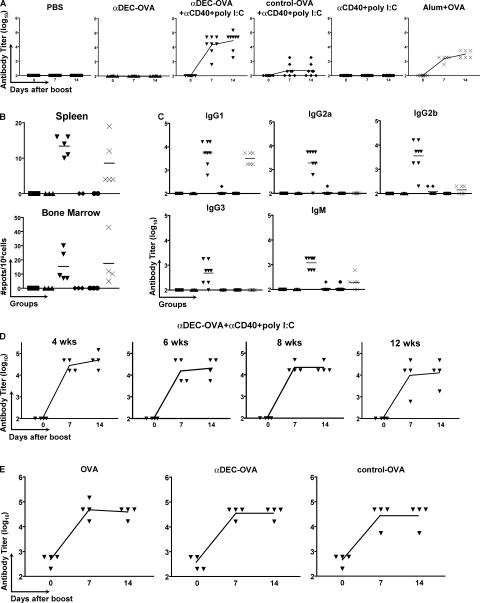
To examine humoral immune responses, C57BL/6 mice were primed with 3 μg of the fusion antibodies ( corresponding to ∼1.0 μg OVA) and boosted with 1 μg OVA-NP11 in PBS 4 wk after the primary immunization. Mice were bled before and 7 and 14 d after the boost, and IgG antibody responses were measured by ELISA using NP2-BSA for antibody capture because high-affinity antibodies bind to proteins derivatized with small numbers of NP groups, whereas low-affinity antibodies do not (23). High titers of high-affinity anti-NP antibodies were found in mice primed with anti–DEC-OVA plus maturation stimulus, but not with anti–DEC-OVA or maturation stimulus alone (Fig. 2 A). Immunization with 100 μg of alum-precipitated OVA (LPS-free) or control-OVA plus maturation stimulus was one and two orders of magnitude less effective, respectively, than immunization with anti–DEC-OVA and maturation stimulus (Fig. 2 A). The number of IgG-producing cells in the spleen and bone marrow was measured by ELISPOT. We found NP-specific antibody-producing cells in the spleen and bone marrow (Fig. 2 B) of mice immunized with anti–DEC-OVA plus maturation stimulus and with alum plus OVA. Collectively, these results lead us to conclude that immunization by antigen targeting to DCs is effective in eliciting T cell help for humoral responses.
Figure 2.
Immunization with anti–DEC-OVA fusion mAb primes helper T cells for antibody responses. (A) Anti-NP IgG antibody titers before and 7 or 14 d after an i.p. boost injection with 1 μg OVA-NP11 in PBS. Immunization conditions are displayed at the top. Symbols represent individual mice, and the curves represent the mean value of each group. (B) Antibody-secreting cells in the spleen or bone marrow measured by ELISPOT on NP2-BSA 14 d after an i.p. boost with 1 μg OVA-NP11 in PBS. Symbols indicate individual mice. Initial immunization conditions were as follows: PBS (▪), anti–DEC-OVA (▴), anti–DEC-OVA plus anti-CD40 plus poly I:C (▾), control-OVA plus anti-CD40 plus poly I:C (♦), anti-CD40 plus poly I:C (•), and alum plus OVA (×). (C) Anti-NP antibody titers for individual IgG isotypes and IgM 14 d after an i.p. boost with 1 μg OVA-NP11 in PBS. The horizontal lines represent the mean value of each group. Symbols indicate individual mice and initial immunization conditions as described in B. (D) Anti-NP IgG antibody responses measured by ELISA in mice immunized with anti–DEC-OVA plus anti-CD40 plus poly I:C 4, 6, 8, or 12 wk before i.p. boosting with 1 μg OVA-NP11 in PBS. Antibody titers were measured as described in A. (E) Anti-OVA IgG antibody titers in mice immunized with anti–DEC-OVA plus anti-CD40 plus poly I:C and then boosted 6 wk later with 1 μg of either OVA, anti–DEC-OVA, or control-OVA. Antibody titers were measured as described in A.
To further characterize the antibody response, we determined the subclass specificity of the anti-NP Ig response. Immunization with anti–DEC-OVA plus maturation stimulus elicited effective T cell help for B cells and antibodies of all IgG subclasses as well as IgM (Fig. 2 C). In contrast, NP responses in mice primed with alum plus OVA were mainly restricted to the IgG1 subclass (Fig. 2 C). As an additional control, we immunized groups of mice with anti–DEC-OVA plus poly I:C. The administration of poly I:C in conjunction with anti–DEC-OVA elicited very low antibody responses (unpublished data). We conclude that immunization by DC targeting produces T cell help that induces a broad spectrum of antibody isotypes.
To determine whether antigen targeting to DCs leads to long-lasting helper T cell responses, we primed mice with anti–DEC-OVA plus maturation stimulus and boosted them with 1 μg OVA-NP11 4, 6, 8, or 12 wk after primary immunization. High titers of anti-NP antibodies were elicited by the boost 12 wk after the primary immunization, the longest point we tested, indicating the persistence of helper T cells long after a single immunization with anti–DEC-OVA plus maturation stimulus (Fig. 2 D). Thus, antigen targeting to DCs leads to efficient long-lived helper T cell responses for antibody formation.
To determine whether anti–DEC-OVA would be an effective secondary immunogen we compared it with OVA. Mice primed with anti–DEC-OVA plus maturation stimulus were boosted with 1 μg OVA, anti–DEC-OVA, or control-OVA in PBS, and anti-OVA responses were measured by ELISA (Fig. 2 E). We found no difference in the antibody titers among the various groups, suggesting that boosting with anti–DEC-OVA fusion antibody is effective in eliciting T-dependent antibody responses.
T cell responses to a malaria protein after DEC-205–mediated delivery
To determine whether antigen targeting to DCs by αDEC-205 elicits antibodies to potential vaccine target antigens, we measured immune responses to the circumsporozoite protein (CSP) of Plasmodium yoelii, which causes murine malaria. We chose CSP because it is the major immunogen on the surface of malaria sporozoites, and it has demonstrated efficacy in murine and human vaccine trials (24–27).
αDEC-CS and control-CS fusion antibodies were produced and tested for binding to DEC-205 as described for anti–DEC-OVA above (Fig. S2, A and B, available at http://www.jem.org/cgi/content/full/jem.20051639/DC1). In addition, we verified antigen processing and presentation in vivo using transgenic CD8+ T cells specific for the previously characterized CSP CD8 epitope (SYVPSAEQI; reference 28). Anti-CSP transgenic CD8+ T cells transferred into BALB/c mice proliferated extensively in response to immunization with 2 μg αDEC-CS (corresponding to ∼0.7 μg CSP) and only minimally to control-CS antibody, suggesting that DCs targeted with αDEC-CS efficiently process and present CSP to CD8+ T cells in vivo (Fig. S2 C).
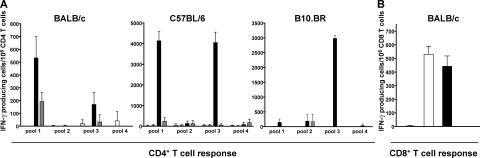
To assess the efficacy of T cell immunization by αDEC-CS plus a maturation stimulus, we compared it with irradiated sporozoites in different strains of naive mice. Mice were immunized i.p. with a single dose of αDEC-CS plus maturation stimulus or i.v. with a single dose of 7.5 × 104 irradiated sporozoites. CD4+ T cell responses were assayed by ELISPOT by measuring IFN-γ secretion in response to pools of overlapping CSP 12–16-mer peptides comprising the N- and COOH-terminal domains of CSP (Fig. 3 A and Table S2, available at http://www.jem.org/cgi/content/full/jem.20051639/DC1). One injection of αDEC-CS plus a maturation stimulus elicited strong CD4+ T cell responses to at least two separate epitopes in naive BALB/c, C57BL/6, and B10.BR mice (i.e., three different MHC haplotypes: H-2d, H-2b, and H-2k), but irradiated sporozoites and other controls did not (Fig. 3 A). In BALB/c mice, the response was directed against the previously described peptides KIYNRNIVNRLLGDA (29) and SQLTEEWSQCSVTCG (30), whereas in C57BL/6 mice, CD4+ T cells recognized peptides KIYNRNIVNRLLGDA and AQNKLNQPVVADENV. CD8+ T cell responses were measured in BALB/c mice by assaying IFN-γ secretion in response to a previously described peptide epitope (SYVPSAEQI; Fig. 3 B; reference 31). We found that immunization with αDEC-CS induced similar numbers of IFN-γ–producing CD8+ T cells as generated by irradiated sporozoites (Fig. 3 B). Thus, when compared with a single dose of irradiated sporozoites, one injection with αDEC-CS plus maturation stimulus is as effective in eliciting CSP-specific CD8+ T cells, and even better in eliciting CD4+ T cells.
Figure 3.
Immunization with αDEC-CS primes helper and CD8+ T cells. (A) IFN-γ production by CD4+ (A) and CD8+ (B) T cells measured by ELISPOT assay 14 d after immunization. Plots show numbers of spots per 106 T cells from mice immunized i.p. with 10 μg αDEC-CS plus anti-CD40 plus poly I:C (black bars) or PBS (striped bars), or anti-CD40 plus poly I:C (gray bars) or i.v. with 7.5 × 104 irradiated sporozoites (white bars). Mouse strains are indicated at the top.
Strong anti-CSP antibody responses after priming with DEC-205–targeted CSP
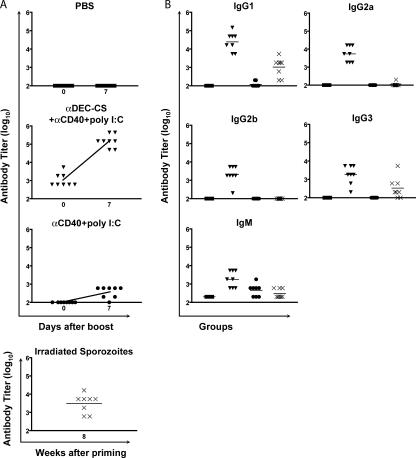
P. yoelii CSP has a central domain composed of two tandem repeats corresponding to major and minor immunodominant serological epitopes (QGPGAP and QQPP, respectively; reference 32). To examine humoral responses against CSP, we immunized BALB/c mice with αDEC-CS plus maturation stimulus or with maturation stimulus alone and boosted them with αDEC-CS after 8 wk. As a positive control, we also immunized mice with a single dose of irradiated sporozoites. Mice were bled before and 7 d after the boosting, and the IgG antibody titers were measured by ELISA against the major B cell epitope (29) and by indirect immunofluorescence assay on P. yoelii parasites. We found that mice primed with 10 μg αDEC-CS plus maturation stimulus and boosted with 10 μg αDEC-CS produced high antibody titers when compared with controls (Fig. 4 A and Table I). As shown above for anti–DEC-OVA, αDEC-CS immunization elicited antibodies of all IgG isotypes (Fig. 4 B). We conclude that immunization by targeting antigens to DCs by DEC-205 together with a maturation stimulus produces a broad spectrum of T cell help for humoral immune responses to P. yoelii CSP.
Figure 4.
Immunization with DEC-CS primes helper cells for antibody responses. (A) Anti-QGPGAP IgG antibody titers before and 7 d after an i.p. boost injection with 10 μg αDEC-CS in PBS. Immunization conditions are displayed at the top of each panel. Symbols represent individual mice, and the curves represent the mean value of each group. (B) Anti-QGPGAP antibody titers for individual IgG isotypes and IgM 7 d after an i.p. boost with 10 μg αDEC-CS in PBS. The horizontal lines represent the mean value of each group. Initial immunization conditions were as follows: PBS (▪), αDEC-CS plus anti-CD40 plus poly I:C (▾), and anti-CD40 plus poly I:C (•). As control, one group of mice was immunized with 7.5 × 104 irradiated sporozoites (×) and bled 8 wk later at the same time as the other groups. Symbols represent individual mice.
Table I.
Immunofluorescence titers in mice immunized with αDEC-CS antibody
| Primea | Boostb | Reciprocal IFA titerc |
|---|---|---|
| PBS | αDEC-CS | <600 |
| αDEC-CS+anti-CD40+poly I:C | αDEC-CS | 106,920 (±53,237) |
| anti-CD40+poly I:C | αDEC-CS | 900 (±600) |
| Irradiated sporozoites | — | 4,500 (±1,800) |
Groups of four mice were primed with 10 μg αDEC-CS plus maturation stimulus, maturation stimulus alone, 7.5 × 104 irradiated sporozoites, or PBS.
Mice were boosted with 10 μg αDEC-CS or nothing.
Immunofluorescence assay was performed 7 d after boost using serial dilutions of serum from individual mice. Results are depicted as the mean of reciprocal titers ±SD.
High frequencies of anti-CSP hybridomas after priming with DEC-205–targeted CSP
As a measure of the number of activated B cells in mice immunized with αDEC-CS, we produced hybridomas after immunization and boosting with 10 μg αDEC-CS antibody. For comparison, we also generated hybridomas after immunization and boosting with 7.5 × 104 irradiated sporozoites. Hybridomas were screened by ELISA for reactivity against the major (QGPGAP) or minor (QQPP) immunodominant B cell epitopes. We found that 16.4% of all wells screened from the αDEC-CS immunization were reactive to the major or minor epitopes. In contrast, only 0.8% of all wells screened from the irradiated sporozoite immunization were positive, and these were all specific for the major immunodominant repeat (Table II). Finally, the hybridoma antibody isotypes obtained by αDEC-CS immunization were representative of the broad range of isotypes found in serum by ELISA (Table III). We conclude that immunization with αDEC-CS in the presence of maturation stimulus produces effective T cell help for a broad spectrum of anti-CSP antibodies.
Table II.
Number of mAbs generated by immunization with irradiated sporozoites or αDEC-CS plus maturation stimulus
| Immunization method | Number of screened clonesa | Number of positive clones (% of the total screened) |
Positive clones (%)b | |
|---|---|---|---|---|
| Major repeat | Minor repeat | |||
| Irradiated sporozoitesc | 1,277 | 10 (0.8) | 100 | 0 |
| αDEC-CS+anti-CD40+polyI:Cd | 736 | 121 (16.4) | 55.4 | 44.6 |
Growing clones were screened for antibody secretion.
Growing clones were screened for reactivity against sequences representing the major repeat (QGPGAP)2 or the minor repeat (QQPP)2 of CSP.
Prime and boost were performed i.v. with 7.5 × 104 irradiated sporozoites in the interval of a month. The fusion was performed 3 d after the boost dose.
Prime i.p. with 10 μg αDEC-CS plus anti-CD40 plus poly I:C. 21 wk later, the boost was performed i.p. with 10 μg αDEC-CS. The fusion was performed 3 d after the boost dose.
Table III.
mAb subclasses generated by the immunization with irradiated sporozoites or with αDEC-CS plus maturation stimulus
| Immunization methoda | Positive clones against the major repeat (%)b | Positive clones against the minor repeat (%)b | ||||||
|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | IgG1 | IgG2a | IgG2b | IgG3 | |
| Irradiated sporozoites | 70 | 10 | 0 | 20 | 0 | 0 | 0 | 0 |
| αDEC-CS+anti-CD40+poly I:C | 56.7 | 9 | 7.5 | 26.9 | 25.9 | 25.9 | 40.7 | 7.4 |
Mice were immunized as described in Table II.
All positive clones were rescreened against the major and minor repeats of CSP using anti–mouse subclass-specific secondary antibodies.
DISCUSSION
T cell–dependent antibody responses are initiated at the interface of the T and B cell follicle where antigen-specific T and B cells interact (33, 34). The requirements for this interaction include capture and display of processed antigen by the B cell to a helper T cell, costimulation by cell surface molecules such as CD40-CD40L, and secretion of cytokines by activated T cells (35). The initial T–B cell interaction leads to germinal center formation, somatic hypermutation, and isotype switching. The isotype of the switched antibodies produced in this reaction is determined mainly by the cytokine secreted by the helper T cells. For example, the helper type 1 cytokine IFN-γ is associated with production of IgG2a, whereas the helper type 2 cytokine IL-4 supports switching to IgG1 (for review see reference 36). Therefore, the isotypes of the T cell–dependent antibodies produced during an immune response are a reflection of T cell polarization. Immunization by DC targeting induces helper T type 1 IFN-γ–producing CD4+ T cells and CD8+ T cells, as well as protection against vaccinia infection (16). Our experiments establish that immunization by DC targeting also leads to long-lived T cell help for a broad range of different antibody isotypes and therefore a combination of helper T type 1 and 2 responses.
Vaccination against many pathogens, including HIV and malaria, requires both cellular and humoral responses. In the case of HIV, mAbs to the conserved epitopes on proteins gp120 and gp41 (37–39) are sufficient for viral neutralization, and in malaria, mAbs to CSP are protective in mice and monkeys (40–42). However, it has been difficult to obtain effective immunization to protein antigens in humans or mice using conventional approaches. We would like to suggest that direct antigen targeting to DCs in vivo in combination with a maturation stimulus may be a safe and effective means to produce long-lived, broad-based cellular and humoral immune responses to specific candidate vaccine proteins.
MATERIALS AND METHODS
Construction and production of the hybrid antibodies.
The entire cDNA encoding chicken OVA was cloned in frame with the carboxyl terminus of the heavy chain of mouse αDEC-205 or isotype control antibodies (11). Likewise, an 870-bp sequence comprising amino acids 57–346 from the CSP of P. yoelii (17X NL strain; reference 32) was cloned into the antibodies to produce αDEC-CS or isotype control. Hybrid antibodies were produced by transient transfection in 293T cells using calcium phosphate as described previously (11), and the fusion antibodies were purified on Protein G columns (GE Healthcare). These were assayed for binding to stable DEC-205 transfectants, which were provided by C.G. Park (The Rockefeller University, New York, NY).
Mice.
6–8-wk-old adult female BALB/c, B10.BR, and C57BL/6 mice were purchased from Taconic Farms or Charles River Laboratories and used according to institutional guidelines. SYVPSAEQI-specific T cell receptor transgenic mice were described previously (28). Protocols were approved by the Institutional Animal Care and Use Committee at The Rockefeller University.
Peptide libraries.
Peptide libraries for OVA and CSP were synthesized in collaboration with the Proteomics Resource Center, The Rockefeller University. Details on how the libraries were prepared are available in the online supplemental material (see below).
Immunizations.
Mice were primed once i.p. with 3 or 10 μg anti–DEC-OVA (as indicated in Results) or 10 μg αDEC-CS in the presence of 50 μg anti-CD40 antibody (clone 1C10) and 50 μg poly I:C (Invivogen) or the same doses of αCD40 and poly I:C alone or with 100 μg of LPS-free OVA (Seikagaku Corp.) precipitated in alum (Pierce Chemical Co.) or 7.5 × 104 irradiated sporozoites (γ-source, 20 krad). Antibodies were elicited by boosting the mice once i.p. with either 1 μg OVA-NP11, anti–DEC-OVA, control-OVA, or 10 μg of the αDEC-CS in PBS.
Intracellular cytokine staining.
Intracellular IFN-γ production by primed CD4+ and CD8+ T cells was evaluated using bulk splenocytes incubated with 2 μM of the OVA peptide pools or medium alone in the presence of 2 μg/ml of costimulatory αCD28 antibody (clone 37.51). Cells were cultured for 6 h in the presence of brefeldin A (BD Biosciences), incubated with αCD16/CD32 antibody to block Fcγ receptors, and stained with anti–mouse CD4 (clone GK 1.5), CD8 (clone 53-6.7), and CD3 (clone 145-2C11) antibodies for 15 min at 4°C. After fixation with Cytofix/Cytoperm Plus (BD Biosciences), cells were stained for intracellular IFN-γ (XMG1.2) for 15 min at room temperature. All mAbs were purchased from BD Biosciences. Data was collected using FACSCalibur and analyzed using FlowJo software (Tree Star).
ELISA and B cell ELISPOT.
For the detection of NP- or CSP-specific antibodies, high-binding ELISA plates (Costar) were coated overnight with 5 μg/ml NP2-BSA (Biosearch Technologies) or (QGPGAP)2 peptide in PBS. Plates were then washed three times with PBS-Tween 20 0.02% and blocked with PBS-BSA 1% for 1 h at room temperature. Serial dilutions of the sera in PBS-BSA 0.25% were incubated for 2 h at room temperature and visualized with goat anti–mouse IgG Fc-specific antibody conjugated to horseradish peroxidase (1:2,000; Jackson ImmunoResearch Laboratories) or anti–mouse subclass-specific antibodies (1:500; SouthernBiotech), followed by colorimetric assay using 1-Step ABTS. OD405 was measured using a VERSAmax microplate reader (Molecular Devices). Titers represent the highest dilution of serum showing an OD405 >0.1. The results are presented as the log10 antibody titer of each individual mouse. For the anti-NP antibody-producing B cell assay, ELISPOT plates (MAIPS; Millipore) were coated as described above and blocked with RPMI 5% FBS (Invitrogen) for 1 h at 37°C and 5% CO2. Serial dilutions of splenocyte suspensions were prepared in RPMI 5% and incubated in the coated wells for 6 h at 37°C in 5% CO2. Anti-NP antibody-secreting cells were visualized by incubation with goat anti–mouse IgG Fc-specific antibody conjugated to horseradish peroxidase (1:2,000; Jackson ImmunoResearch Laboratories), followed by substrate from the AEC kit (Vector Laboratories). Spots were counted in an ELISPOT reader (Autoimmun Diagnostika GmbH).
IFN-γ ELISPOT.
ELISPOT plates (MAHAS; Millipore) were coated with 10 μg of the anti–mouse IFN-γ mAb (clone R4-6A2; BD Biosciences) overnight at room temperature, and plates were blocked by incubation with PBS-BSA 1% for at least 2 h at 37°C. Spleen CD8+ T cells were purified by negative selection using αCD19 and αCD11c antibody beads, followed by positive selection on αCD8 beads (Miltenyi Biotec). The flow through was CD4+ T cell enriched. Purified T cells were then cultured for 36–40 h at 37°C with 5% CO2 in the presence of 2 μM peptide, and CD11c+ DCs purified by positive selection. Plates were developed with anti–IFN-γ biotinylated antibody (BD Biosciences), and spots visualized with avidin–horseradish peroxidase (Vector Laboratories), followed by DAB as substrate (Invitrogen). Spots were counted in an ELISPOT reader (Autoimmun Diagnostika GmbH).
mAbs.
BALB/c mice were immunized either i.p. with 10 μg αDEC-CS plus maturation stimulus or i.v. with 7.5 × 104 irradiated sporozoites. The mouse that received the αDEC-CS plus maturation stimulus was boosted 21 wk later i.p. with 10 μg αDEC-CS. The mouse immunized with irradiated sporozoites was boosted 4 wk later i.v. with another dose of 7.5 × 104 irradiated sporozoites. 3 d after boost, splenocytes were fused with an appropriate myeloma partner in the Monoclonal Antibody Core Facility, Memorial Sloan-Kettering Cancer Center. Screening was performed by ELISA using the peptides (QGPGAP)2 or (QQPP)2 and the same conditions described above.
Online supplemental material.
Fig. S1, A and B, shows the purification and binding of the antibodies anti–DEC-OVA and control-OVA to CHO cells stably transfected with DEC-205, and C shows IFN-γ CD8+ and CD4+ T cell responses for individual peptides contained in pool 8. Fig. S2, A and B, shows the purification and binding of the antibodies αDEC-CS and control-CS to CHO cells stably transfected with DEC-205, and C shows transgenic CD8+ T cell proliferation in mice injected with αDEC-CS or control-CS. Tables S1 and 2 show the peptide libraries generated for OVA and CSP, respectively. Details on the peptide libraries generation, adoptive transfer, and analysis of TCR transgenic T cell proliferation and immunofluorescence on P. yoelii sporozoites can be found in supplemental Materials and methods. Figs. S1 and S2 and Tables S1–S2 are available at http://www.jem.org/cgi/content/full/jem.20051639/DC1.
Supplemental Material
Acknowledgments
The authors would like to thank J. Overholser and Dr. F. Weis-Garcia for their help with the generation of the mAbs, Dr. V. Nussenzweig and Dr. H. Wardemann for critical review of the manuscript, T. Schwickert for help with alum immunizations, and Dr. K. Liu for valuable advice.
This work was supported by the National Institutes of Health (NIH) grant AI57158 to R.M. Steinman and M.C. Nussenzweig and is funded in part by a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative. S.B. Boscardin is the recipient of a PEW Foundation Latin American Fellowship, and J.C.R. Hafalla was the recipient of an American Liver Foundation Fellowship. M.C. Nussenzweig is a Howard Hughes Medical Institute Investigator. R.M. Steinman and M.C. Nussenzweig are consultants to Celldex, which is developing human DEC-205–based vaccines.
All other authors do not have a conflict of interest.
Abbreviations used: CSP, circumsporozoite protein; NP, (4-hydroxy-3-nitrophenyl) acetyl.
References
- 1.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 2.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell. 106:263–266. [DOI] [PubMed] [Google Scholar]
- 3.Liu, Y.J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 106:259–262. [DOI] [PubMed] [Google Scholar]
- 4.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621–667. [DOI] [PubMed] [Google Scholar]
- 5.Steinman, R.M., and M.D. Witmer. 1978. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl. Acad. Sci. USA. 75:5132–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussenzweig, M.C., R.M. Steinman, B. Gutchinov, and Z.A. Cohn. 1980. Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J. Exp. Med. 152:1070–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Voorhis, W.C., J. Valinsky, E. Hoffman, J. Luban, L.S. Hair, and R.M. Steinman. 1983. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J. Exp. Med. 158:174–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inaba, K., and R.M. Steinman. 1985. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 229:475–479. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau, J., and A.K. Palucka. 2005. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 5:296–306. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran, B. 2004. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 199:227–250. [DOI] [PubMed] [Google Scholar]
- 11.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifaz, L., D. Bonnyay, K. Mahnke, M. Rivera, M.C. Nussenzweig, and R.M. Steinman. 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196:1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M.C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. In press. [DOI] [PubMed]
- 14.Hawiger, D., R.F. Masilamani, E. Bettelli, V.K. Kuchroo, and M.C. Nussenzweig. 2004. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 20:695–705. [DOI] [PubMed] [Google Scholar]
- 15.van Kooten, C., and J. Banchereau. 1997. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 9:330–337. [DOI] [PubMed] [Google Scholar]
- 16.Bonifaz, L.C., D.P. Bonnyay, A. Charalambous, D.I. Darguste, S. Fujii, H. Soares, M.K. Brimnes, B. Moltedo, T.M. Moran, and R.M. Steinman. 2004. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman, R.M., and M.C. Nussenzweig. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 99:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman, R.M., and M. Pope. 2002. Exploiting dendritic cells to improve vaccine efficacy. J. Clin. Invest. 109:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul, W.E., G.W. Siskind, and B. Benacerraf. 1966. Studies on the effect of the carrier molecule on antihapten antibody synthesis. II. Carrier specificity of anti-2,4-dinitrophenyl-poly-l-lysine antibodies. J. Exp. Med. 123:689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajewsky, K., V. Schirrmacher, S. Nase, and N.K. Jerne. 1969. The requirement of more than one antigenic determinant for immunogenicity. J. Exp. Med. 129:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz, D.H., W.E. Paul, E.A. Goidl, and B. Benacerraf. 1970. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J. Exp. Med. 132:261–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchison, N.A. 1971. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur. J. Immunol. 1:10–17. [DOI] [PubMed] [Google Scholar]
- 23.Reth, M., G.J. Hammerling, and K. Rajewsky. 1978. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur. J. Immunol. 8:393–400. [DOI] [PubMed] [Google Scholar]
- 24.Zavala, F., J.P. Tam, P.J. Barr, P.J. Romero, V. Ley, R.S. Nussenzweig, and V. Nussenzweig. 1987. Synthetic peptide vaccine confers protection against murine malaria. J. Exp. Med. 166:1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues, M., S. Li, K. Murata, D. Rodriguez, J.R. Rodriguez, I. Bacik, J.R. Bennink, J.W. Yewdell, A. Garcia-Sastre, R.S. Nussenzweig, et al. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 153:4636–4648. [PubMed] [Google Scholar]
- 26.Stoute, J.A., M. Slaoui, D.G. Heppner, P. Momin, K.E. Kester, P. Desmons, B.T. Wellde, N. Garcon, U. Krzych, and M. Marchand. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 336:86–91. [DOI] [PubMed] [Google Scholar]
- 27.Nardin, E.H., G.A. Oliveira, J.M. Calvo-Calle, K. Wetzel, C. Maier, A.J. Birkett, P. Sarpotdar, M.L. Corado, G.B. Thornton, and A. Schmidt. 2004. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect. Immun. 72:6519–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sano, G., J.C. Hafalla, A. Morrot, R. Abe, J.J. Lafaille, and F. Zavala. 2001. Swift development of protective effector functions in naive CD8+ T cells against malaria liver stages. J. Exp. Med. 194:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grillot, D., M. Michel, I. Muller, C. Tougne, L. Renia, D. Mazier, G. Corradin, P.H. Lambert, J.A. Louis, and G. Del Guidice. 1990. Immune responses to defined epitopes of the circumsporozoite protein of the murine malaria parasite, Plasmodium yoelii. Eur. J. Immunol. 20:1215–1222. [DOI] [PubMed] [Google Scholar]
- 30.Takita-Sonoda, Y., M. Tsuji, K. Kamboj, R.S. Nussenzweig, P. Clavijo, and F. Zavala. 1996. Plasmodium yoelii: peptide immunization induces protective CD4+ T cells against a previously unrecognized cryptic epitope of the circumsporozoite protein. Exp. Parasitol. 84:223–230. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues, M.M., A.S. Cordey, G. Arreaza, G. Corradin, P. Romero, J.L. Maryanski, R.S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:579–585. [DOI] [PubMed] [Google Scholar]
- 32.Lal, A.A., V.F. de la Cruz, J.A. Welsh, Y. Charoenvit, W.L. Maloy, and T.F. McCutchan. 1987. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii. A rodent model for examining antimalarial sporozoite vaccines. J. Biol. Chem. 262:2937–2940. [PubMed] [Google Scholar]
- 33.Liu, Y.J., J. Zhang, P.J. Lane, E.Y. Chan, and I.C. MacLennan. 1991. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21:2951–2962. [DOI] [PubMed] [Google Scholar]
- 34.Jacob, J., C. Miller, and G. Kelsoe. 1992. In situ studies of the antigen-driven somatic hypermutation of immunoglobulin genes. Immunol. Cell Biol. 70:145–152. [DOI] [PubMed] [Google Scholar]
- 35.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 36.Stavnezer, J. 2000. Molecular processes that regulate class switching. Curr. Top. Microbiol. Immunol. 245:127–168. [DOI] [PubMed] [Google Scholar]
- 37.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 10:1651–1658. [DOI] [PubMed] [Google Scholar]
- 38.Trkola, A., A.B. Pomales, H. Yuan, B. Korber, P.J. Maddon, G.P. Allaway, H. Katinger, C.F. Barbas III, D.R. Burton, D.D. Ho, et al. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 17:1757–1765. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida, N., R.S. Nussenzweig, P. Potocnjak, V. Nussenzweig, and M. Aikawa. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 207:71–73. [DOI] [PubMed] [Google Scholar]
- 41.Charoenvit, Y., W.E. Collins, T.R. Jones, P. Millet, L. Yuan, G.H. Campbell, R.L. Beaun, J.R. Broderson, and S.L. Hoffman. 1991. Inability of malaria vaccine to induce antibodies to a protective epitope within its sequence. Science. 251:668–671. [DOI] [PubMed] [Google Scholar]
- 42.Charoenvit, Y., S. Mellouk, C. Cole, R. Bechara, M.F. Leef, M. Sedegah, L.F. Yuan, F.A. Robey, R.L. Beaun, and S.L. Hoffman. 1991. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J. Immunol. 146:1020–1025. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.