Abstract
Current human immunodeficiency virus (HIV) vaccine approaches emphasize prime boost strategies comprising multiple doses of DNA vaccine and recombinant viral vectors. We are developing a protein-based approach that directly harnesses principles for generating T cell immunity. Vaccine is delivered to maturing dendritic cells in lymphoid tissue by engineering protein antigen into an antibody to DEC-205, a receptor for antigen presentation. Here we characterize the CD4+ T cell immune response to HIV gag and compare efficacy with other vaccine strategies in a single dose. DEC-205–targeted HIV gag p24 or p41 induces stronger CD4+ T cell immunity relative to high doses of gag protein, HIV gag plasmid DNA, or recombinant adenovirus-gag. High frequencies of interferon (IFN)-γ– and interleukin 2–producing CD4+ T cells are elicited, including double cytokine-producing cells. In addition, the response is broad because the primed mice respond to an array of peptides in different major histocompatibility complex haplotypes. Long-lived T cell memory is observed. After subcutaneous vaccination, CD4+ and IFN-γ–dependent protection develops to a challenge with recombinant vaccinia-gag virus at a mucosal surface, the airway. We suggest that a DEC-targeted vaccine, in part because of an unusually strong and protective CD4+ T cell response, will improve vaccine efficacy as a stand-alone approach or with other modalities.
Vaccine development for major global infectious diseases will likely require strategies that induce strong T cell–mediated immunity, which is implicated in resistance to infections like HIV/AIDS, malaria, tuberculosis, and human papilloma and Epstein Barr viruses (1–5). One critical element of T cell–mediated immunity is the CD4+ helper T cell. These T cells are able to produce high levels of IFN-γ, exert cytolytic activity on MHC class II–bearing targets, and help other elements of the immune response, such as antibody formation and CD8+ cytolytic killer cells including memory (6). HIV-infected patients who have a better clinical course and are long-term nonprogressors tend to have stronger CD4+ T cell responses to the virus (7, 8), and HIV-specific CD4+ T cells are able to promote the function of HIV-specific CD8+ T cells in vitro (9). It is therefore important to identify and harness principles of immune function that would improve CD4+ T cell immunity to HIV vaccines (10, 11).
Prior studies have used tissue culture systems, as well as adoptive transfer of DCs into animals and people, to show that these cells induce strong T cell–mediated immunity (for review see references 12–17). For example, isolated DCs are able to initiate CD4+ helper T cell responses in culture (18) and after reinfusion into mice (19). When human (20) or mouse (21) DCs are loaded with antigen ex vivo and reinfused, the DCs expand antigen-specific helper cells that primarily produce IFN-γ and not IL-4; i.e., a Th1 type of CD4+ T cell that is thought to be valuable in host defense against viral infection (2, 3).
We have been developing a different approach to study the function of DCs directly in lymphoid tissues in situ and to harness the immunizing capacities of DCs in vaccine design. The approach is to deliver antigens within antibodies that selectively deliver vaccine proteins to DCs in lymphoid tissues. Our first experiments have targeted DEC-205/CD205, an endocytic receptor (22, 23) that was originally termed the NLDC-145 antigen and is expressed at high levels on DCs (24), particularly a subset of DCs, in lymphoid tissues (25). Although DEC-205 is expressed at high levels on several epithelia, and at low levels on many leukocytes (26, 27), the injected antibody primarily binds to DCs in the T cell areas (28). When antigens are incorporated into the anti–DEC-205 mAb, there is efficient antigen presentation on both MHC class I and II products; i.e., low doses of the targeted antigen relative to nontargeted antigen are required to present antigen in vivo (28–31). It is important to extend the concept of directed delivery of antigen to DCs in situ to more clinically relevant antigens, to additional immune readouts, and to comparisons with other vaccine modalities.
In our prior studies of antigen presentation by DCs in situ, we have chemically coupled the protein OVA to the anti-DEC antibody, or we have engineered the cDNA of the heavy chain of the antibody to express sequences for antigenic peptides in frame at its carboxy terminus. We regard the latter engineering method to be preferable in that fusion antibodies can be expressed that reliably contain a single copy of the antigen on every heavy chain. For immunization to take place after injection of antigen within anti–DEC-205 mAb, we also observed that it is necessary to overcome the normal capacity of the DEC-205+ DCs in situ to induce peripheral tolerance. This can be achieved by administering agonistic anti-CD40 mAb as a stimulus for the maturation of DCs in vivo (28–30). Using OVA as an antigen, we have shown that the combination of DC targeting and a maturation stimulus improves CD4+ and CD8+ T cell responses in naive mice, as assessed with single MHC class I and II binding peptides (30).
Here we have engineered the p24 and p41 proteins from HIV gag into the heavy chain of anti–DEC-205 and studied the immune responses to the fusion mAb along with a maturation stimulus. We find that DC targeting with an anti-DEC-205–HIV gag fusion mAb vaccine induces CD4+ T cell immunity of a quantity and quality that has not been seen before with safe vaccines. The CD4+ T cell response to a single dose of vaccine, as assessed by IFN-γ and IL-2 production, is much greater than that observed with plasmid gag DNA and recombinant adenoviral gag vaccination, although these other approaches produce equal or more CD8+ IFN-γ–producing T cells. The immune response to anti–DEC-HIV gag comprises several potentially valuable features, including a broad response to many peptides in different MHC backgrounds as well as CD4-dependent protection at mucosal surfaces.
RESULTS
DEC-205 targeting of HIV gag p24 enhances antigen presentation in vivo
To harness the antigen processing (32) and immunizing functions of DCs (16, 17) within intact lymphoid organs, we cloned HIV gag p24 protein in frame with the carboxyl terminus of the heavy chain of an mAb to mouse DEC-205, an endocytic receptor for antigen presentation (22, 23). We also engineered the heavy chain of a control mAb that does not react with DCs. In prior studies, sequences for peptides were fused to the heavy chain (28, 31), but here we introduced gag protein (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20052005/DC1). The fusion mAbs were successfully expressed and contained heavy chains of ∼75 kD as opposed to ∼50 kD for unmodified mouse IgG1 (Fig. S1). Relative to the original anti–DEC-205 mAb, these fusion mAbs bound identically to DEC-205 transfectants (not depicted).
To use fusion mAbs as vaccines, we needed to overcome the capacity of DEC-205–bearing DCs to induce tolerance (28, 29, 31). To do so, we injected a stimulus for DC maturation together with the engineered mAb into 7–8-wk-old mice. We used a combination of the TLR3 ligand poly IC and an agonistic anti-CD40 mAb. In preliminary experiments, poly IC by itself did not lead to a primary immune response to anti–DEC-p24, but in keeping with prior observations (33), the combination of a TLR ligand and anti-CD40 did elicit stronger immunity. To detect T cell immunity, we used a library of 15-mer “mimetope” peptides staggered every 4 aa along the gag p24 sequence (34, 35). This library was divided into five peptide pools (each containing 9–12 peptides), each of which was used to recall IFN-γ secretion in spleen cells from mice vaccinated with a single dose of anti–DEC-p24.
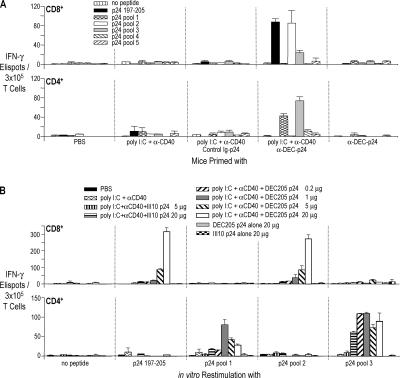
In initial experiments, an ELISPOT assay showed that BALB/c mice made T cell responses after vaccination with the combination of fusion mAb and maturation stimulus, but not with either alone (Fig. 1 A). The CD8+ T cell response was directed to peptides in p24 pool 2 (Fig. 1 A, top, white bar), which contained a previously defined gag 197–205 peptide sequence presented on H-2Kd (Fig. 1 A, top, black bar; reference 36). In addition, CD4+ T cell responses were noted to peptides in p24 pools 1 and 3 (Fig. 1 A, bottom), and these involved IFN-γ production but no detectable IL-4 (not depicted). Interestingly, we had to administer higher doses of fusion mAb to elicit a CD8+ T cell response than a CD4+ T cell response. The former response to p24 pool 2 peptides was increasing when the dose of mAb was increased from 5 to 20 μg/mouse (Fig. 1 B, top), whereas the helper responses to p24 pools 1 and 3 peptides could plateau between 1 and 5 μg/mouse (and some responses began to be detected with the control Ig-p24 vaccine; Fig. 1 B, bottom). We then turned to intracellular cytokine staining to monitor immunization because the response to a single dose of vaccine was large enough to be detected by this approach, and we concentrated our studies on the CD4+ T cell response because helper cells energize many components of immunity.
Figure 1.
Immunization of T cells with one dose of anti–DEC-p24 fusion mAb vaccine. (A) BALB/c mice were injected i.p. with PBS, maturation stimulus alone (25 μg αCD40 mAb and 50 μg poly IC), 5 μg control Ig-p24 or anti–DEC-p24 mAbs and maturation stimulus, and 5 μg anti–DEC-p24 without maturation stimulus. After 17 d, splenic CD8+ T cells (top) or CD4+ T cells (bottom) were restimulated with CD11c+ DCs and peptide (AMQMLKETI, p24 197–205, 2 μg/ml), HIV gag p24 peptide pools (2 μg/ml), or medium alone for 2 d. IFN-γ secretion was evaluated by ELISPOT. (B) As in A, but immunization of BALB/c mice with graded doses of anti–DEC-p24 and a maturation stimulus. Data are representative of two to four similar experiments with two mice pooled in each experiment.
A strong IL-2– and IFN-γ–producing CD4+ T cell response to anti–DEC-p24
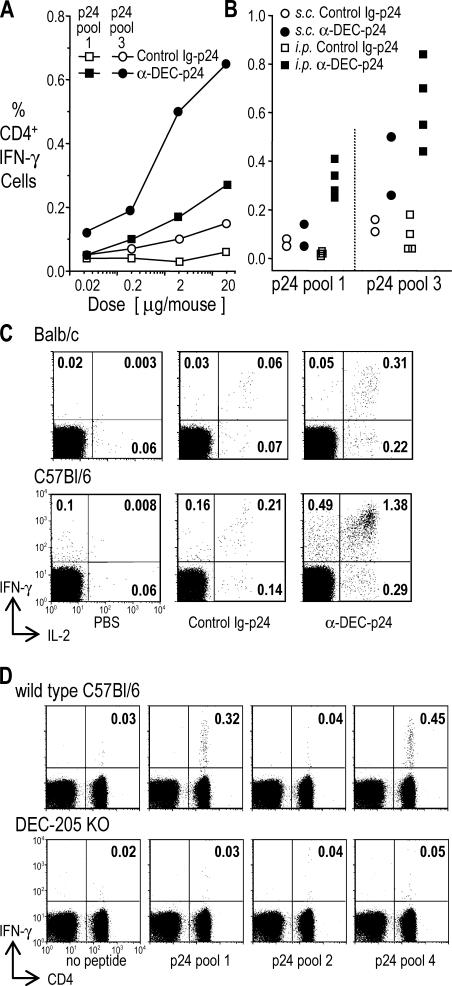
To establish the efficiency of DEC-205 targeting, we immunized BALB/c mice with increasing doses of anti–DEC-p24 mAb plus maturation stimuli or control Ig-p24. Targeting via DEC-205 was at least 100 times more effective at initiating IFN-γ–producing CD4+ T cell immunity (Fig. 2 A, compare filled and open symbols) than control Ig-p24 or soluble gag protein (see below). When we assessed the route of injection, the i.p. route led to slightly higher immune responses than the s.c. route (Fig. 2 B, compare filled squares to circles), possibly because the delivery of the injected antibody solution was more reliable in the former case. When we monitored both IFN-γ and IL-2 production in BALB/c and C57BL/6 mice, the majority of primed cells made both cytokines (Fig. 2 C, top right quadrants), whereas lower frequencies made only one of the cytokines. We also tested DEC-205−/− mice to show that DEC-205 was essential for immunization (Fig. 2 D). Thus, the use of anti–DEC-205 mAb targeting greatly increases the efficiency with which protein antigens elicit T cell immunity, and when given as a single dose together with stimuli for DC maturation, the frequency of primed CD4+ T cells is high.
Figure 2.
Strong CD4+ T cell responses to a single dose of anti–DEC-p24 fusion mAb vaccine. (A) BALB/c mice were immunized s.c. with graded doses of anti–DEC-p24 or control Ig-p24 mAbs and maturation stimulus. 19 d later, we assessed the percentage of IFN-γ+ CD4+ cells in gated CD3+ splenic T cells using gag p24 peptide pools. One of three similar experiments with two mice pooled in each experiment is shown. (B) As in A, but several experiments showing responses to 5 μg anti–DEC-p24 or control Ig-p24 mAbs with maturation stimulus given either s.c. or i.p. to BALB/c mice. Background activity (<0.1%) of nonvaccinated mice boosted with the HIV gag p24 peptide pools was subtracted from the percentage of IFN-γ+ CD4+ cells. Each point represents two mice pooled in each experiment. (C) BALB/c or C57BL/6 mice were treated s.c. with PBS or 2 and 5 μg anti–DEC-p24 or control Ig-p24 mAbs, respectively, each with maturation stimulus. IFN-γ and IL-2 production was monitored at 19 d. Numbers are the percentage of CD4+ cells producing cytokines to HIV gag p24 peptide pool 3 in BALB/c or pool 1 in C57BL/6 mice. These experiments were repeated two to four times with similar results. (D) Comparison of immune responses of wild-type and DEC-205−/− mice to 3 μg anti–DEC-p24 vaccine and maturation stimulus. IFN-γ secretion in response to HIV gag p24 peptide pools by CD4+ splenocytes as in A. Background frequencies (vaccinated mice boosted in medium only) were <0.01% in the top right quadrants. Data are representative of two similar experiments.
Anti–DEC-p24 immunization enhances CD4+ T cell immunity relative to other immunization approaches
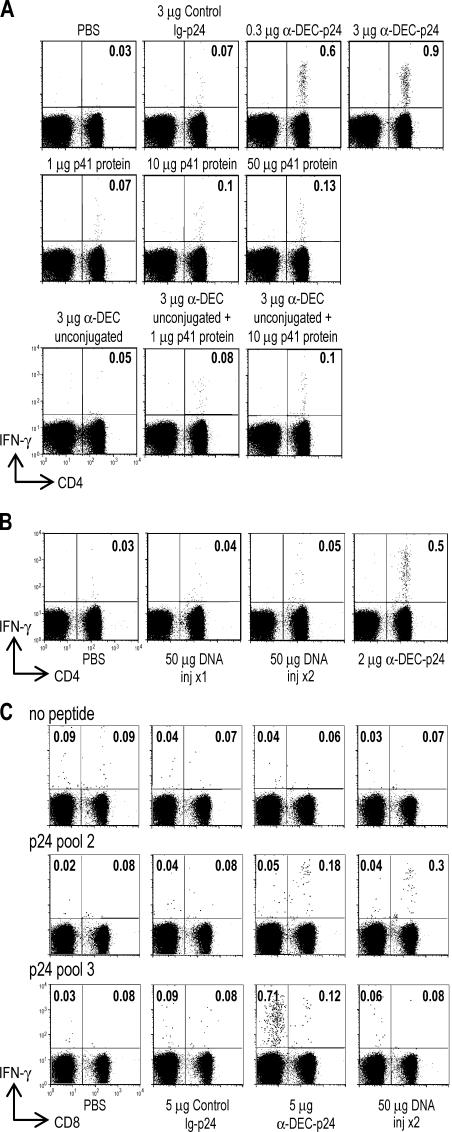
We compared the response to DC-targeted HIV gag p24 with other modes of immunization. When we injected 1–50 μg of soluble HIV p41 protein with anti-CD40 and poly IC maturation, or the combination of HIV protein with unconjugated anti–DEC-205 mAb, the immune responses were weak compared with DC-targeted HIV gag p24 with the same maturation stimulus (Fig. 3 A). A single dose of HIV protein likewise did not induce immunity by these assays when we tested other standard adjuvants, complete Freund's adjuvant and alum (not depicted). We then tested plasmid DNA vaccines, which are currently in trials for immunization of healthy volunteers (37–39). The CD4+ T cell response to one or two injections of 50 μg of a gag p24 DNA vaccine was either weak or undetectable for each of the p24 peptide pools (e.g., p24 pool 3 in Fig. 3 B). The response was likewise weak if the mice were injected with anti-CD40 and poly IC along with the DNA vaccine (not depicted). In contrast, the DNA vaccine did elicit a CD8+ T cell response to a dominant gag peptide for BALB/c mice in p24 peptide pool 2, comparable to that observed with the DC-targeted p24 protein vaccine (Fig. 3 C, top right quadrants). However, we could not detect IFN-γ–producing CD4+ T cell responses to two doses of DNA vaccine by intracellular cytokine staining relative to DC-targeted HIV gag p24 (Fig. 3 C, compare IFN-γ production by CD8− cells in the top left quadrants in the DNA- and anti–DEC-p24–vaccinated mice). Lastly, we tested a recombinant adenovirus-gag vaccine. A single dose i.m. induced clear-cut CD8+ T cell responses, clearly greater than anti–DEC-p24, but the CD4+ T cell responses remained low relative to anti–DEC-p24 (Table I and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20052005/DC1). We conclude that the targeting of a vaccine protein to DCs improves CD4+ T cell immunization relative to other approaches including DNA and recombinant adenovirus-gag vaccines, which are currently undergoing testing in humans.
Figure 3.
Enhanced efficacy of anti–DEC-p24 plus anti-CD40/poly IC relative to DNA and nontargeted protein vaccines. (A) C57BL/6 mice were injected s.c. with maturation stimulus and the proteins indicated above each panel. IFN-γ secretion was evaluated after 18 d in the spleen by intracellular cytokine staining as the percentage of IFN-γ CD4+ cells among CD3+ lymphocytes in response to HIV gag p24 peptide pool 4. Data are one of two to four similar experiments. (B) BALB/c mice were injected with 2 μg anti–DEC-p24 s.c. plus maturation stimulus, one or two doses of 50 μg DNA encoding HIV gag p24 protein i.m., or PBS. IFN-γ secretion in response to HIV gag p24 peptide pool 3 was evaluated as in A. Data shown are one of two to four similar experiments. (C) BALB/c mice were immunized i.p. with 5 μg anti–DEC-p24 or control Ig-p24 antibodies plus maturation stimulus, two doses of 50 μg DNA encoding HIV gag p24 protein i.m., or PBS. IFN-γ secretion was evaluated as in A, but the response to HIV gag p24 peptide pool 2 (containing a dominant peptide presented on MHC class I) and p24 pool 3 (containing a peptide presented on MHC class II) are shown for T cells directly stained for CD8 expression. Data shown are from one of two similar experiments.
Table I.
CD4+ and CD8+ T cell responses to HIV gag p24 in BALB/c mice after a single immunization via anti−DEC-205 antibody or recombinant adenovirus
| Experiment | Spleen | IFN-γ+ cells/104 CD4+ T cells (no peptide/pool 3 restimulation) |
IFN-γ+ cells/104 CD8+ T cells (no peptide/pool 2 restimulation) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No vaccine |
Control Ig p24 |
anti-DEC p24 |
adeno 5 × 105 |
adeno 2 × 107 |
No vaccine |
Control Ig p24 |
anti-DEC p24 |
adeno 5 × 105 |
adeno 2 × 107 |
|||
| 1 | A | 8/5 | 2/4 | 2/101 | 4/14 | ND | 1/14 | 4/9 | 4/13 | 7/40 | ND | |
| B | 4/5 | 3.4 | 7/50 | 6/7 | ND | 8/8 | 6/11 | 11/14 | 14/74 | ND | ||
| 2 | A | 9/15 | 13/19 | 16/125 | 14/26 | 16/24 | 16/14 | 16/14 | 15/17 | 15/59 | 13/326 | |
| B | 2/3 | 2/5 | 4/72 | 3/7 | 3/12 | 3/4 | 2/4 | 5/17 | 2/160 | 2/432 | ||
BALB/c mice were immunized with a single dose of anti–DEC-205 or control Ig-gag p24 fusion antibody i.p. or the indicated dose of recombinant adenovirus gag vaccine i.m. The mAb fusion vaccine was given together with poly IC and anti-CD40 mAb. 18 d later, the frequency of IFN-γ–producing CD4+ and CD8+ T cells was measured by intracellular cytokine staining assay using the dominant peptide pools for these responses, pool 3 and pool 2, respectively. The underlined data contrasts the development of strong CD4+ T cell immunity to anti–DEC-205 gag fusion mAb and CD8+ T cell immunity to recombinant adenovirus.
DC-targeted HIV gag protein induces broad CD4+ T cell immunity
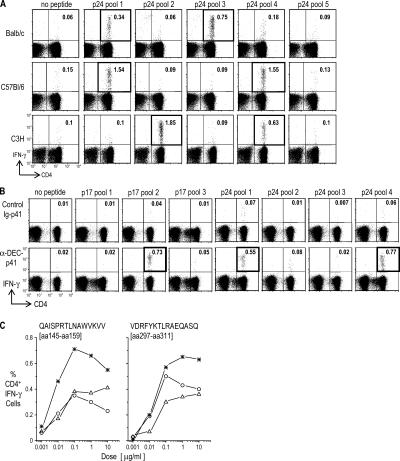
To ensure that the DEC-205 targeting strategy could induce immunity in different MHC haplotypes, we studied three strains: BALB/c (H-2d), C57BL/6 (H-2b), and C3H (H-2k). In each, ∼0.2–2% of the CD4+ T cells became responsive to gag for at least two different p24 peptide pools (Fig. 4 A). We are unable to find reports in the literature of CD4+ T cell responses of this magnitude or breadth with other candidate vaccines. When the reactive p24 pools were broken down into individual peptides, at least two different mimetope peptides were reliably recognized in each of the strains (Table II). We also engineered the larger gag p41 protein into the anti–DEC-205 heavy chain and showed that this protein in C57BL/6 mice broadened the immune response further to include a third highly immunogenic peptide from the p17 portion of gag (Fig. 4 B). When we used graded doses of these individual peptides in the assay for T cell immunity, the mimetope peptides gave optimal responses with 0.1 μg/ml, which indicate a good functional affinity of the T cells (Fig. 4 C, three separate experiments). Therefore, a single dose of HIV protein targeted to DCs results in a broad spectrum of peptides being efficiently recognized by CD4+ T cells, features that should reduce the chances of immune evasion by the virus.
Figure 4.
Breadth and strength of responses to DC-targeted HIV gag vaccine. (A) 5 μg anti–DEC-p24 mAb in combination with maturation stimulus was administered either i.p. to BALB/c mice (top) or s.c. to C57BL/6 (middle) and C3H (bottom) mice. 18 d later, IFN-γ secretion in spleen CD3+ T cells was measured to medium or different HIV gag p24 peptide pools. Data are representative of two to four experiments with two mice pooled in each experiment. (B) C57BL/6 mice were immunized s.c. with 5 μg anti–DEC-p41 or control Ig-p41 mAbs and maturation stimulus. IFN-γ secretion in spleen CD3+ T cells was measured at 15 d in response to medium or different HIV gag p24 and p17 peptide pools. Data shown are from one of three experiments. (C) In three experiments C57BL/6 mice were vaccinated s.c. with 2 μg anti–DEC-p24 with maturation stimulus, and 19 d later, splenocytes were restimulated with graded doses of HIV gag p24 peptide number 6 from pool 1 (QAISPRTLNAWVKVV, p24 aa 145–159) or peptide number 8 from pool 4 (VDRFYKTLRAEQASQ, p24 aa 297–311).
Table II.
HIV gag p24 mimetope peptides identified from 15-mer peptide pools to stimulate IFN-γ secretion by CD4+ T cells in three MHC haplotypes after anti–DEC-p24 immunization
| Strain | HIV gag p24 Peptide pool |
Reactive peptide |
|---|---|---|
| C3H | 2 | AAEWDRLHPVHAGPI (aa 209–223) |
| 4 | YSPTSILDIRQGPKE (aa 277–291) | |
| BALB/c | 1 | SPEVIPMFSALSEGA (aa 165–179) |
| 3 | PVGEIYKRWIILGLN (aa 257–271) | |
| C57BL/6 | 1 | QAISPRTLNAWVKVV (aa 145–159) |
| 4 | VDRFYKTLRAEQASQ (aa 297–311) |
5 μg anti–DEC-p24 antibody in combination with 25 μg anti-CD40 and 50 μg poly IC was administered either i.p. to BALB/c mice or s.c. to C57BL/6 or C3H mice as in Fig. 4 A. After 17 d, splenic CD4+ T cells were restimulated with CD11c+ DCs and single peptides from the different reactive HIV gag p24 peptide pools (2 μg/ml) for 2 d. IFN-γ secretion was evaluated by ELISPOT.
Anti–DEC-p24 induces long-term memory CD4+ T cells
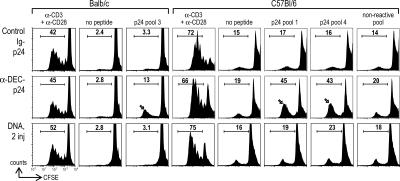
To test if long-term memory could be detected 19–30 wk after priming, we measured CD4+ T cell proliferation by CFSE dilution in response to antigen. Effector–memory cells were no longer detected; i.e., T cells making IFN-γ within 6 h of antigen rechallenge. However, memory T cells responding by proliferation to HIV gag were found in both BALB/c and C57BL/6 mice immunized with a single dose of anti–DEC-p24 (Fig. 5, arrows), relative to the background responses seen with no peptide and a nonreactive peptide pool from gag p17 (this background proliferation, termed the syngeneic mixed leukocyte reaction, is observed when DCs are cocultured with T cells in the absence of added antigens; reference 40). No memory cells were observed after vaccination with control Ig-p24 or with two injections of 50 μg of a gag p24 DNA (Fig. 5, first and third rows). Therefore, long-term memory is detected 4–7.5 mo after a single-dose DC-targeted HIV vaccine.
Figure 5.
Long-lived gag p24-memory to DC-targeted vaccine. BALB/c mice were immunized i.m. with two doses of 50 μg HIV gag p24 DNA or i.p. with 5 μg anti–DEC-p24 and control Ig-p24 mAb with maturation stimulus. C57BL/6 mice were similarly immunized, but the fusion antibodies and maturation stimulus were given s.c. CD4+ T cells were enriched by depleting MHC II+ and CD8+ cells from the lymph node (30 wk after BALB/c immunization) or spleen (at 19 wk for C57BL/6), CFSE labeled, and restimulated with CD11c+ DCs plus the peptide pools as indicated, anti-CD3 and anti-CD28, or medium. Proliferation was evaluated by CFSE dilution at 4 d. Numbers are the percentage of CD4+ proliferating T cells. Control PBS-immunized mice showed similar proliferative responses to control Ig-p24 mAb or two doses of DNA gag p24 vaccine. Data are representative of two to three experiments on the same batch of immunized mice.
Anti–DEC-p24 elicits protective immunity at a mucosal surface
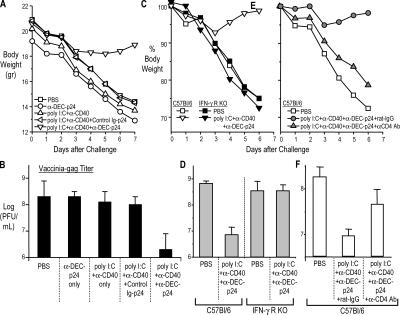
To determine if immunity to gag p24 leads to protection, especially at a mucosal surface distal to the skin vaccination site, we challenged the mice with recombinant vaccinia-gag intranasally, first at 2 wk after vaccination. The mice lost weight and developed high titers of virus in the lung over 7 d (>108 PFU/ml of tissue). By both criteria, reduced weight loss (Fig. 6, A, C, and E) and reduction in viral titers (Fig. 6, B, D, and F), both BALB/c and C57BL/6 mice were protected by the combination of anti–DEC-p24 plus a maturation stimulus, but were not protected when immunized with anti–DEC-p24 alone, maturation stimulus alone, or control Ig-p24 plus a maturation stimulus (Fig. 6, A and B). Vaccinated mice were protected against recombinant vaccinia-gag but not vaccinia-OVA, indicating that protection was gag specific (not depicted). We repeated the challenges but at longer time points, 12 and 24 wk after one dose of vaccine. Again, the DC- targeted vaccine protected the mice from a serious infection at a mucosal surface (not depicted). To evaluate the need for IFN-γ in protection, we studied IFN-γR knockout mice. The latter were not protected (Fig. 6, C and D), although the knockout mice did generate CD4+ T cell immunity comparably to wild-type mice (not depicted). Likewise, when we depleted CD4+ T cells from vaccinated mice before the challenge with vaccinia-gag, protection was ablated (Fig. 6, E and F) as expected from prior work on CD4+ protection to vaccinia (41). Because we were observing resistance at a mucosal surface after s.c. injection of vaccine, we verified prior information that anti–DEC-205 mAb is able to target systemically to DCs, including DCs in lymphoid tissues that drain mucosal surfaces (30). Specifically, we observed that p24 protein, when injected as a fusion anti–DEC-205 mAb, was primarily targeted to the CD8αhigh DC subset (which is also the DEChigh subset) in the spleen and mesenteric lymph node (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20052005/DC1). Thus, IFN-γ–producing CD4+ T cells help to protect at a mucosal surface, and this resistance follows one dose of DC-targeted HIV gag fusion mAb vaccine.
Figure 6.
A single dose of anti–DEC-p24 vaccine elicits CD4- dependent protection at a mucosal surface. (A and B) Groups of four to six BALB/c or C57BL/6 mice were immunized as in Fig. 1 and challenged at 2–3 wk with 5 × 104 PFU of vaccinia-gag intranasally. Weight was measured for 6–7 d, and then lung virus titers were evaluated by plaque assay. Data are representative of two experiments in BALB/c and one in C57BL/6. (C and D) C57BL/6 or IFN-γR knockout mice were immunized s.c. with 2 μg anti–DEC-p24 with maturation stimulus or with PBS and challenged with 2.5 × 104 PFU of vaccinia-gag 20 d later. (E and F) CD4+ T cells were depleted from C57BL/6 immunized mice with three doses of anti-CD4 mAb or control rat Ig 1–3 d before challenge. Data shown are one of three similar experiments. Weights are expressed as average body weight, whereas vaccinia plaque-forming titers (in CV-1 cells) are shown as a mean ± SD.
DISCUSSION
Vaccine design has for some time depended on the discoveries of Pasteur that attenuated and inactivated microorganisms can induce specific and protective immunity (for review see references 11 and 42). It has been difficult to extend this strategy to many global infections because attenuated and inactivated vaccines are either difficult to produce, potentially unsafe, or poorly immunogenic. As a result, alternative vaccines are being tested, such as naked plasmid DNA and recombinant modified vaccinia-Ankara and adenoviruses (37–39). Efforts are underway to increase the immunogenicity of these safe vaccines. We have been taking a different approach, which is to develop protein-based vaccines that directly rely on principles of the immune system, to induce stronger T cell immunity and protection.
We are emphasizing DCs, which are pivotal cells for initiating adaptive immunity and memory, particularly T cell–based responses. To better identify and harness the functions of DCs in intact lymphoid tissues, antigens are delivered within mAbs to specific DC receptors, beginning with a mAb to the endocytic receptor DEC-205 or CD205. The DEC-205 target represents a beginning because the biological outcome may be influenced by the specific DC receptor, the maturation stimulus used to allow DCs to elicit immunity, and the subset of DCs expressing the receptor in question. Previously, we immunized mice with the model protein OVA that was chemically (but not stoichiometrically) conjugated to anti–DEC-205, and we studied the primary CD4+ T cell response to a single previously defined MHC class II–binding peptide in H-2b mice (30). Here we immunized mice of different MHC haplotypes with a homogenous engineered mAb carrying the HIV gag protein, and we studied the breadth and quality of the CD4+ T cell response, including memory and CD4-dependent protection at a mucosal surface. Our data reveal the potential of DCs in situ to elicit a CD4+ T cell response of a quality and quantity that has not been observed previously by other approaches to either study DC function or to develop safe vaccines.
The quality of the CD4+ T cell response was indicated first by its breadth; i.e., the development of immunity in all three MHC haplotypes that were tested, including recognition of at least two gag peptides in each haplotype. This is in contrast to most analyses of protein vaccination in mice, which typically lead to immunization to a single peptide in one haplotype. We used mimetope peptides derived from an overlapping library of 15 mers spanning the full length of the gag p41 protein rather than naturally processed peptides. But even so, relatively low doses of the mimetope peptides (0.1 μg/ml) were required to activate the population of primed T cells in the immune assays. In addition, the observed responses were high even after a single dose of vaccine and included the induction of memory and CD4+ T cell–based protection at a mucosal surface. We have emphasized a single dose of vaccine because single episodes of certain infections induce protective immunity, and it would be important to mimic this and identify principles that quickly lead to strong immunity with safe vaccines. The use of a single dose also provides a means to compare the efficacy of individual vaccines for their capacity to induce a primary immune response and ultimately memory. Additional research is required to study memory and secondary responses after targeted delivery of antigens to DCs in situ. Nonetheless, booster responses are evident, because in a companion paper in this issue (p. 599), the priming of helper CD4+ T cells with anti-DEC fusion mAbs sets the stage for a strong antibody response to a boost with free antigen or anti-DEC fusion mAb (43). In this latter study of antibody formation, we introduced a circumsporozoite protein from a malaria sporozoite into the anti–DEC-205 mAb.
We noted that the DEC-targeted vaccine was superior in inducing CD4+ T cell responses relative to unconjugated protein with standard adjuvants, plasmid DNA, or recombinant adenovirus. Interestingly, the gag DNA vaccines we prepared induced similar or better CD8+ T cell responses when compared with low doses of anti–DEC-205 gag vaccine, but the CD4+ T cell response to DNA vaccine was weak and lacked detectable memory (by a T cell proliferation assay). Likewise, the recombinant adenovirus that we tested induced strong CD8+ T cell responses but again much lower numbers of IFN-γ–producing CD4+ T cells relative to anti–DEC-gag vaccine. DNA vaccines are capable of inducing some CD4+ T cell immunity in humans (44), but our data from mice suggest that the efficiency and breadth of CD4+ T cell immunization can be increased substantially using DC-targeted protein vaccines. It will be important to combine anti–DEC-205 delivery with other modalities like plasmid DNA and recombinant adenovirus because the improved CD4+ T cell help from DEC-205 targeting could greatly improve the antibody and cytotoxic T cell immunity induced by these other vaccines.
Another interesting consequence of the DEC-targeted vaccine was that protective CD4+ T cell immunity was elicited at a mucosal surface, the airway, which was distal to the skin vaccination site. This was observed in two mouse strains that were tested, C57BL/6 and BALB/c. Previously, we had noted that immunization of mice with anti–DEC-OVA allowed for protection to a challenge with vaccinia-OVA, but we did not pursue long-term memory or the contribution of CD4+ T cells and IFN-γ to protection (30). Our results at first glance seemed surprising because there is evidence that DCs capturing antigen from the skin immunize T cells with skin-homing properties, whereas DCs capturing antigen in a mucosal-associated lymphoid organ prime T cells that home to the mucosa (45). We suspect that our observations reflect the capacity of mAb targeting to load DCs with antigen in lymph nodes that drain mucosal surfaces (Fig. S3; reference 30). It is also somewhat surprising to detect a role for CD4+ T cells in vaccinia protection because prior studies have demonstrated a role for CD8+ T cells in defense of mice against vaccinia (46) or the pathogenic mouse pox, ectromelia virus (47). Nevertheless, Xu et al. (41) reported CD4+ T cell– dependent protection via antibody formation to vaccinia. It is also possible that the strong CD4+ T cell responses induced by DEC-205 targeting provides direct resistance, e.g., through high level IFN-γ production and lysis of MHC class II– bearing infected epithelial cells.
Given the normal tolerogenic function of at least some immature DCs in vivo in the steady-state (28, 29, 48, 49), a DC maturation stimulus becomes an important component of a vaccine. Here we have used a combination of agonistic anti-CD40 mAb and poly IC, the latter to ligate TLR3 receptors expressed by the DEC-205+ subset of DCs in mice (50). Interestingly, we did not observe a primary response to a single dose of DEC-205–targeted vaccine when poly IC was the only maturation stimulus, even though the DCs responded by remodeling cell surface markers in a way that is characteristic of DC maturation; i.e., increased expression of MHC class II and CD86 and decreased expression of CD119 IFN-γ receptor (not depicted). It has been shown previously that these changes in surface phenotype are not by themselves sufficient to generate CD4+ and CD8+ T cell immunity by antigen-presenting DCs (51). However, poly IC did enhance the adjuvant effect of anti-CD40, as was also observed with tumor cell antigens when they were targeted to DCs in situ (52). Further research is needed to identify appropriate maturation stimuli for DC-targeted vaccines, including DCs in humans and nonhuman primates, and these studies will need to include analyses of the induction of memory as well as more relevant protection assays than the vaccinia-gag model used here.
At this point, we have targeted a single receptor, DEC-205/CD205, which is expressed by a subset of CD8+ DCs. Isolated and cultured CD8+ DCs produce IL-12 (17, 53), and when loaded with antigen and reinfused into mice, these DCs differentiate IFN-γ–producing T cells (21). Nonetheless, the control of antigen access to DCs and the maturation of the DCs in vivo represent intricate new areas of immunobiology. Other DC receptors, DC subsets, and immune responses—particularly memory—will be important to investigate. DC-targeted vaccines offer potential advantages with respect to safety and ease of production, and we suggest they should be tested to improve efficacy as a stand-alone approach or in combination with other modalities.
MATERIALS AND METHODS
Animals.
We used C57BL/6 (B6), C3H, and BALB/c mice from Taconic, IFN-γR knockouts (B6 background) from The Jackson Laboratory, and DEC-205−/− mice made by us. Mice were maintained under specific pathogen-free conditions and used at 7–8 wk of age according to the guidelines of our Institutional Animal Care and Use Committee.
Fusion HIV gag mAbs and other vaccines.
DNA for HIV-1 gag p24 or gag p41, i.e., aa 133–363 or aa 1–363 of gag p55 protein (from HIV isolate BH10), was cloned in frame into the COOH terminus of anti–DEC-205 and control mAb heavy chains (28). Fusion mAbs were expressed by transient transfection (calcium-phosphate) in 293 cells in serum-free DMEM supplemented with Nutridoma SP (Roche), purified on protein G columns (GE Healthcare), and characterized by SDS-PAGE and Western blotting (horseradish peroxidase sheep anti–mouse IgG [GE Healthcare] or α-p24 [ImmunoDiagnostics]). Unconjugated anti–DEC-205 mAb expressed by stably transfected CHO cells was similarly purified. Plasmid DNA gag p24 vaccine was prepared by Aldevron. The plasmid was derived from consensus B gag (HIV Databases), codon optimized to improve expression, and cloned into the commercial plasmid pVAX1 (Invitrogen). The aa sequence encoding the DNA gag p24 vaccine differed from the p24 protein sequence derived from BH10 isolate at positions 138 (I→L) and 215 (V→L), respectively. Vaccine DNA was eluted into saline at 1 μg/μl, and endotoxin in purified DNA was <5 EU/mg. To construct a recombinant adenoviral vector vaccine, the consensus B gag p24 plasmid was cloned into a commercial E1/E3-deleted, replication-incompetent adenoviral type 5 vector (BD Biosciences). This vector was propagated in HEK 293 cells and purified according to the manufacturer's instructions (ViraKit for adenovirus 5; Virapur, LLC). Infectious units were determined by cytopathic effects and subsequent measurement of HIV gag p24 by ELISA. One or two doses of 50 μg HIV-1 gag p24 DNA or one dose of recombinant adenovirus (5 × 105 or 2 × 107 PFU) was administered i.m.
Antigen targeting and maturation of DCs in vivo.
Mice were injected i.p. or s.c. in the hind footpads with fusion mAb, unconjugated anti-DEC mAb, or HIV gag p41 protein (provided by R. Seder, National Institutes of Health [NIH], Bethesda, MD) without or with a stimulus for DC maturation, which was 50 μg poly IC (InVivoGen) and 25 μg 1C10 agonistic anti-CD40 mAb (54) per mouse.
HIV gag peptides.
Overlapping (staggered by 4 aa) gag p17 and p24 15-mer peptides were obtained from the NIH AIDS Reference Reagent Program (catalog no. 8117). Peptides, including the H-2Kd binding peptide p24 197–205 (36), were also synthesized by the Proteomics Resource Center (The Rockefeller University). The 30- and 60-member gag p17 and p24 libraries were divided into 3 and 5 pools of 9 to 12 peptides, respectively. The respective gag p17 peptide pools span from aa 1–51 (pool 1), aa 41–91 (pool 2), and aa 81–135 (pool 3) of the HIV gag p17 protein, and the respective gag p24 peptide pools span from aa 125–183 (pool 1), aa 173–231 (pool 2), aa 221–279 (pool 3), aa 269–327 (pool 4), and aa 317–363 (pool 5) of the HIV gag p24 protein.
Assays for HIV gag-specific immune T cells.
To identify HIV gag- responsive T cells, spleen cells were stimulated with pools of peptides (2 μg/ml) or medium alone in the presence of 2 μg/ml of costimulatory anti-CD28 (clone 37.51) for 6 h, adding 10 μg/ml brefeldin A (Sigma-Aldrich) for the last 4 h to accumulate intracellular cytokines. Cells were washed, incubated for 15 min at 4°C with 2.4G2 mAb to block Fcγ receptors, and stained with FITC- or PE-conjugated anti-CD3 (145-2C11) and PerCP-conjugated anti-CD4 (RM4-5) or anti-CD8 (S3-6.7) for 20 min at 4°C. The cells were permeabilized (Cytofix/Cytoperm Plus; BD Biosciences) and stained with PE- or APC-anti–IFN-γ (XMG 1.2) and PE- or APC-anti–IL-2 (JESG-5H4) mAbs for 15 min at room temperature (BD Biosciences). We used a FACSCalibur with data analysis in FlowJo (Tree Star). All plots were gated on low forward and side scatter CD3+ cells. For ELISPOT assays, we restimulated for 2 d CD4+ or CD8+ T cells, purified by positive selection, with CD11c+ spleen DCs and HIV gag p24 peptide pools (2 μg/ml), an H-2Kd–restricted peptide (2 μg/ml; AMQMLKETI, p24 197–205), or medium alone. DCs were enriched from the spleens or mesenteric lymph nodes dissociated with collagenase (collagenase D; Roche) using anti-CD11c–coated magnetic beads (Miltenyi Biotec). We used CFSE (107 cells/ml, 1 μM, 10 min, 37°C; Invitrogen) dilution to assess proliferation of primed T cells in response to antigen. CD4+ T cells from the spleen or lymph node were obtained by depleting MHC class II+ and CD8+ cells. After labeling with CFSE, the T cells were added at 10–30 CD4+ T cells per spleen CD11c+ DC in the presence of HIV gag p24 peptide pools (2 μg/ml), nonreactive peptide pool, medium alone, or anti-CD3 (0.1 μg/ml) and anti-CD28 (2 μg/ml; positive control) for 4 d in 1 ml round-bottom tubes.
Antibody-mediated delivery of HIV gag p24 to DCs in lymphoid tissues.
CD11c+ DCs were enriched from lymphoid organs of mice given 10 μg anti–DEC-p24 or control Ig-p24 1 d earlier s.c. The cells were preincubated with 2.4G2 culture medium to block Fcγ receptors, washed, and incubated with PE-conjugated CD11c (HL3; BD Biosciences) and APC-conjugated CD8 (53-6.7; BD Biosciences) for 20 min at 4°C. Permeabilized cells (Cytofix/Cytoperm Plus) were stained with FITC–anti-p24 mAb (KC57-FITC; Coulter) for 15 min at room temperature and evaluated by flow cytometry.
Protection experiments to an airway challenge with recombinant vaccinia-gag.
2.5–5 × 104 PFU recombinant vaccinia-gag virus or vaccinia-OVA control were applied intranasally to anesthetized mice (30, 55). In some experiments, vaccinated B6 mice were depleted of CD4+ T cells by injection with 500 μg GK 1.5 mAb 1–3 d before challenge. To assess protection, we monitored body weights daily for 6–7 d, and then the animals were killed (because of considerable weight loss) and the lungs harvested to prepare extracts by physical disruption. Weight data are expressed as average body weight from groups of four to six mice, whereas vaccinia titers in a plaque-forming assay (in CV-1 cells) are shown as a mean ± SD for four to six mice.
Online supplemental material.
Fig. S1 shows the constructs we prepared to express fusion mAbs that carry HIV gag, as well as SDS-PAGE electrophoresis and immunoblot analysis of the control Ig-p24 and anti–DEC-p24 fusion mAbs. Fig. S2 shows that recombinant adenovirus-gag induces a strong CD8+ T cell response, whereas anti–DEC-p24 elicits a robust CD4+ T cell response. Fig. S3 shows the systemic distribution of p24 (in spleen and mesenteric lymph node, CD8α+ and DEC-205+ DCs) after anti–DEC-205 targeting s.c. Figs. S1–S3 are available at http://www.jem.org/cgi/content/full/jem.20052005/DC1.
Supplemental Material
Acknowledgments
We thank S. Yamazaki and L. Munoz for their contribution to this work, H. Zebroski for synthesizing peptides, and J. Adams for help with the graphics. Funding was provided by NIH grants U19 AI062623 to T.M. Moran, RO1 AI41111 to C.B. Lopez, and BM, AI13013 and AI40874 to R.M. Steinman, Direct Effect, as well as a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative.
R.M. Steinman and M.C. Nussenzweig are consultants to Celldex, which is developing human DEC-205–based vaccines.
All other authors do not have a conflict of interest.
References
- 1.Seder, R.A., and J.R. Mascola. 2003. Basic immunology of vaccine development. In The Vaccine Book. B.R. Bloom and P.-H. Lambert, editors. Academic Press, Boston. 51–72 pp.
- 2.Berzofsky, J.A., J.D. Ahlers, J. Janik, J. Morris, S. Oh, M. Terabe, and I.M. Belyakov. 2004. Progress on new vaccine strategies against chronic viral infections. J. Clin. Invest. 114:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantaleo, G., and R.A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10:806–810. [DOI] [PubMed] [Google Scholar]
- 4.Saul, A., V. Nussenzweig, and R.S. Nussenzweig. 2004. Rationale for malaria vaccine development. In Novel Vaccination Strategies. S.H.E. Kaufmann, editor. Wiley-Vch Verlag GmbH & Co. 479–503.
- 5.Frazer, I.H. 2004. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 4:46–54. [DOI] [PubMed] [Google Scholar]
- 6.Bevan, M.J. 2004. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4:595–602. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg, E.S., J.M. Billingsley, A.M. Caliendo, S.L. Boswell, P. Sax, S.A. Kalams, and B.D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 278:1447–1450. [DOI] [PubMed] [Google Scholar]
- 8.Martinez, V., D. Costagliola, O. Bonduelle, N. N'Go, A. Schruriger, I. Theodorou, J.-P. Clauvel, D. Sicard, H. Agut, P. Debre, et al. 2005. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 anitbodies is the best predictor for persistence of long-term nonprogression. J. Infect. Dis. 191:2053–2063. [DOI] [PubMed] [Google Scholar]
- 9.Lichterfeld, M., D.E. Kaufmann, X.G. Yu, S.K. Mui, M.M. Addo, M.N. Johnston, D. Cohen, G.K. Robbins, E. Pae, G. Alter, et al. 2004. Loss of HIV-1–specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1–specific CD4+ T cells. J. Exp. Med. 200:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. [DOI] [PubMed] [Google Scholar]
- 11.Levine, M.M., and M.B. Sztein. 2004. Vaccine development strategies for improving immunization: the role of modern immunology. Nat. Immunol. 5:460–464. [DOI] [PubMed] [Google Scholar]
- 12.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 13.Hart, D.N.J. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 90:3245–3287. [PubMed] [Google Scholar]
- 14.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 15.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell. 106:263–266. [DOI] [PubMed] [Google Scholar]
- 16.Moser, M. 2003. Dendritic cells in immunity and tolerance—do they display opposite functions? Immunity. 19:5–8. [DOI] [PubMed] [Google Scholar]
- 17.Pulendran, B. 2004. Modulating vaccine reponses with dendritic cells and toll-like receptors. Immunol. Rev. 199:227–250. [DOI] [PubMed] [Google Scholar]
- 18.Inaba, K., and R.M. Steinman. 1985. Protein-specific helper T lymphocyte formation initiated by dendritic cells. Science. 229:475–479. [DOI] [PubMed] [Google Scholar]
- 19.Inaba, K., J.P. Metlay, M.T. Crowley, and R.M. Steinman. 1990. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuler-Thurner, B., E.S. Schultz, T.G. Berger, G. Weinlich, S. Ebner, P. Woerl, A. Bender, B. Feuerstein, P.O. Fritsch, N. Romani, and G. Schuler. 2002. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, W., W.J. Swiggard, C. Heufler, M. Peng, A. Mirza, R.M. Steinman, and M.C. Nussenzweig. 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 375:151–155. [DOI] [PubMed] [Google Scholar]
- 23.Mahnke, K., M. Guo, S. Lee, H. Sepulveda, S.L. Swain, M. Nussenzweig, and R.M. Steinman. 2000. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II–positive lysosomal compartments. J. Cell Biol. 151:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraal, G., M. Breel, M. Janse, and G. Bruin. 1986. Langerhans cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J. Exp. Med. 163:981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vremec, D., and K. Shortman. 1997. Dendritic cell subtypes in mouse lymphoid organs. Cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 159:565–573. [PubMed] [Google Scholar]
- 26.Inaba, K., W.J. Swiggard, M. Inaba, J. Meltzer, A. Mirza, T. Sasagawa, M.C. Nussenzweig, and R.M. Steinman. 1995. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell. Immunol. 163:148–156. [DOI] [PubMed] [Google Scholar]
- 27.Witmer-Pack, M.D., W.J. Swiggard, A. Mirza, K. Inaba, and R.M. Steinman. 1995. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. II. Expression in situ in lymphoid and nonlymphoid tissues. Cell. Immunol. 163:157–162. [DOI] [PubMed] [Google Scholar]
- 28.Hawiger, D., K. Inaba, Y. Dorsett, K. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonifaz, L., D. Bonnyay, K. Mahnke, M. Rivera, M.C. Nussenzweig, and R.M. Steinman. 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196:1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonifaz, L.C., D.P. Bonnyay, A. Charalambous, D.I. Darguste, S. Fujii, H. Soares, M.K. Brimnes, B. Moltedo, T.M. Moran, and R.M. Steinman. 2004. In vivo targeting of antigens to the DEC-205 receptor on maturing dendritic cells improves T cell vaccination. J. Exp. Med. 199:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawiger, D., R.F. Masilamani, E. Bettelli, V.K. Kuchroo, and M.C. Nussenzweig. 2004. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 20:695–705. [DOI] [PubMed] [Google Scholar]
- 32.Trombetta, E.S., and I. Mellman. 2005. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23:975–1028. [DOI] [PubMed] [Google Scholar]
- 33.Ahonen, C.L., C.L. Doxsee, S.M. McGurran, T.R. Riter, W.F. Wade, R.J. Barth, J.P. Vasilakos, R.J. Noelle, and R.M. Kedl. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kern, F., N. Faulhaber, C. Frommel, E. Khatamzas, S. Prosch, C. Schonemann, I. Kretzschmar, R. Volkmer-Engert, H.D. Volk, and P. Reinke. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 30:1676–1682. [DOI] [PubMed] [Google Scholar]
- 35.Maecker, H.T., H.S. Dunn, M.A. Suni, E. Khatamzas, C.J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T.-M. Fu, E. Sinclair, et al. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods. 255:27–40. [DOI] [PubMed] [Google Scholar]
- 36.Mata, M., P.J. Travers, Q. Liu, F.R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985–2993. [PubMed] [Google Scholar]
- 37.McMichael, A.J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874–880. [DOI] [PubMed] [Google Scholar]
- 38.Emini, E.A., and W.C. Koff. 2004. AIDS/HIV. Developing an AIDS vaccine: need, uncertainty, hope. Science. 304:1913–1914. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly, J.J., B. Wahren, and M.A. Liu. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633–639. [DOI] [PubMed] [Google Scholar]
- 40.Nussenzweig, M.C., and R.M. Steinman. 1980. Contributions of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J. Exp. Med. 151:1196–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, R., A.J. Johnson, D. Liggitt, and M.J. Bevan. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 172:6265–6271. [DOI] [PubMed] [Google Scholar]
- 42.Dubos, R. 1988. Pasteur and Modern Science. Science Tech Publishers, Madison, WI. 168 pp.
- 43.Boscardin, S.B., J.C.R. Hafalla, R.F. Masilamani, A.O. Kamphorst, H.A. Zebroski, U. Rai, A. Morrot, F. Zavala, R.M. Steinman, R.S. Nussenzweig, and M.C. Nussenzweig. 2005. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 203:599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McConkey, S.J., W.H. Reece, V.S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J.M. Vuola, T.J. Blanchard, P. Gothard, K. Watkins, et al. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729–735. [DOI] [PubMed] [Google Scholar]
- 45.Mora, J.R., G. Cheng, D. Picarella, M. Briskin, N. Buchanan, and U.H. von Andrian. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachmann, M.F., P. Wolint, K. Schwarz, and A. Oxenius. 2005. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J. Immunol. 175:4677–4685. [DOI] [PubMed] [Google Scholar]
- 47.Fang, M., and L.J. Sigal. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175:6829–6836. [DOI] [PubMed] [Google Scholar]
- 48.Probst, H.C., J. Lagnel, G. Kollias, and M. van den Broek. 2003. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 18:713–720. [DOI] [PubMed] [Google Scholar]
- 49.Probst, H.C., K. McCoy, T. Okazaki, T. Honjo, and M. van den Broek. 2005. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6:280–286. [DOI] [PubMed] [Google Scholar]
- 50.Schulz, O., S.S. Diebold, M. Chen, T.I. Naslund, M.A. Nolte, L. Alexopoulou, Y.T. Azuma, R.A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus- infected cells. Nature. 433:887–892. [DOI] [PubMed] [Google Scholar]
- 51.Fujii, S., K. Liu, C. Smith, A.J. Bonito, and R.M. Steinman. 2004. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199:1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu, K., J. Idoyaga, A. Charalambous, S. Fujii, A. Bonito, J. Mordoh, R. Wainstok, X.F. Bai, Y. Liu, and R.M. Steinman. 2005. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J. Exp. Med. 202:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moser, M., and K.M. Murphy. 2000. Dendritic cell regulation of Th1-Th2 development. Nat. Immunol. 1:199–205. [DOI] [PubMed] [Google Scholar]
- 54.Heath, A.W., W.W. Wu, and M.C. Howard. 1994. Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur. J. Immunol. 24:1828–1834. [DOI] [PubMed] [Google Scholar]
- 55.Brimnes, M.K., L. Bonifaz, R.M. Steinman, and T.M. Moran. 2003. Influenza virus–induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J. Exp. Med. 198:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.