Abstract
Loss of tolerance in systemic lupus erythematosus (SLE) leads to the generation of autoantibodies, which accumulate in end-organs where they induce disease. Here we show that immunoglobulin (Ig)G2a and 2b autoantibodies are the pathogenic isotypes by recruiting FcγRIV expressing macrophages. Class switching, but not development, of IgM anti-self B cells to these pathogenic subclasses requires the innate immune receptor Toll-like receptor (TLR)9 and MyD88 signaling. In their absence, switching of autoreactive B cells to the IgG2a and 2b subclasses is blocked, resulting in reduced pathology and mortality. In contrast, switching of anti-self B cells to IgG1 is not perturbed and generation of nonautoreactive IgG2a and 2b antibodies is not impaired in TLR9-deficient mice. Thus, the TLR9 pathway is a potential target for therapeutic intervention in SLE.
Genetic susceptibility and environmental factors are responsible for the development of systemic lupus erythematosus (SLE). Mouse models of SLE, in particular, have provided significant insights into the identification of critical checkpoints and the molecular pathways that mediate the generation of this autoimmune disease (1–12). These models have demonstrated that the loss of tolerance that initiates SLE results from the accumulated effect of multiple genetic defects, which culminates in the deposition of pathogenic IgG autoantibodies in end-organs such as the kidney, where they induce inflammation resulting in pathological events (6). IgG anti-DNA autoantibodies are a general feature of lupus and the molecular mechanisms that result in the selection and expansion of anti-DNA autoantibodies have been suggested to involve the Toll-like receptors (TLRs). In particular, TLR9 and its signaling molecule MyD88 can provide a costimulatory signal in vitro for B cell stimulation by DNA (13, 14). The DNA ligand for TLR9 may be provided in vivo by apoptotic bodies that are incompletely cleared in lupus and could thus lead to uncontrolled activation of the TLR9–MyD88 pathway and promote anti-DNA autoantibody generation (13–15).
It has recently been shown that pediatric SLE patients have a failure to establish B cell tolerance early during B cell development, leading to increased numbers of antinuclear, anticytoplasmic, and polyreactive cells in the naive peripheral B cell pool (16, 17). The increased frequency of autoreactive, naive B cells is thus suggested to be a prerequisite for the generation of autoantibodies and the development of lupus (17). A similar situation has been discussed in murine models of SLE with polyreactive autoantibodies deposited in the kidneys of these mice (18, 19). These polyreactive antibodies recognize DNA as well as glomerular antigens (18, 19) and are believed to be responsible for inducing proteinurea and thereby contribute to the pathology of SLE (19).
We have recently described a strain-specific SLE model in which loss of the IgG inhibitory Fcγ receptor RIIB molecule on the C57BL/6 background resulted in the accumulation of pathogenic autoantibodies in the kidney with the development of glomerulonephritis and premature mortality (2). Through the use of a B cell–intrinsic, anti-DNA knockin model (the 56R VDJ4 heavy chain transgene on the C57BL/6 background), we have shown that the C57BL/6 strain provided a susceptible background by virtue of its inability to completely edit the light chain repertoire associating with the 56R heavy chain, thus allowing for the emergence of anti-DNA B cells into the periphery (6). Although these mice developed circulating anti-DNA antibodies of the IgM isotype, they did not develop disease, consistent with previous observations in which IgG autoantibodies were frequently associated with disease in SLE models (6). Combining the 56R+.B6 mice with the FcγRIIB deficiency was sufficient to result in the accumulation of pathogenic IgG antibodies by permitting the expansion of anti-DNA IgG plasma cells with subsequent tissue deposition of autoantibodies in the glomeruli and glomerulonephritis (6).
Using these lupus models, we have examined the contribution of the TLR9/MyD88 pathway to the generation of anti-DNA/polyreactive autoantibodies and have observed a crucial role for these pathways in the class switching of anti-DNA–expressing B cells to the pathogenic IgG2a and 2b subclasses. The loss of TLR9 protected lupus-susceptible mice from the spontaneous development of these pathogenic anti-DNA subclasses, while not affecting the ability to class switch to exogenous antigens. The specificity of the TLR9–MyD88 pathway to regulating a B cell–intrinsic, anti-DNA class switch to IgG2a and 2b is likely the result of the ability of this pathway to induce the transcription factor T-bet in B cells.
RESULTS
Pathogenic IgG subclasses in SLE
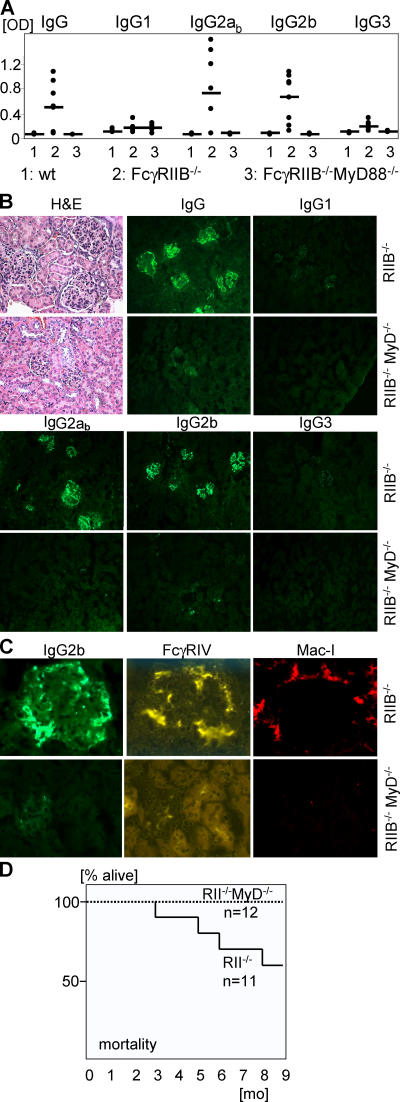
To determine if specific IgG subclasses were responsible for the pathological manifestations of the autoimmune disease in FcγRIIB−/−.B6 mice, we analyzed the IgG subclasses of the autoantibodies in the serum and IgG subclasses deposited in the kidneys of these mice (Fig. 1, A and B). IgG autoantibodies in the serum as well as IgG depositions in the kidney of these mice were mainly of the IgG2a and IgG2b subclasses. IgG1 and IgG3 autoantibodies were underrepresented. IgG depositions were undetectable in wt.B6 mice (unpublished data). The observation that these subclasses dominated in this systemic autoimmune disease suggested a mechanism that could account for the severity of tissue destruction observed in this model. IgG2a and 2b are the most pathogenic subclasses in vivo by virtue of their unique ability to engage the recently described activation Fcγ receptor FcγIV (20). In contrast, IgG1 antibodies are significantly less pathogenic, resulting from their preferential engagement of the inhibitory FcγRIIB molecule (20). FcγRIV-expressing macrophages accumulated in the kidneys of FcγRIIB−/−.B6 mice, but not in the kidneys of wt.B6 mice (Fig. 1 C and not depicted) and are likely to have induced the kidney pathology in SLE.
Figure 1.
MyD88 deficiency protected against generation and deposition of pathogenic IgG2a and 2b autoantibodies and SLE. (A) IgG subclass analysis of autoantibodies in the serum of 5-mo-old wt.B6 (n = 4), FcγRIIB−/−MyD88+/−.B6 (n = 8), and FcγRIIB−/−MyD88−/−.B6 (n = 6) mice. Shown are anti-DNA and antinuclear antigen titers as determined by ANA ELISA. IgG2ab (haplotype: b) and 2b autoantibodies were up-regulated in FcγRIIB−/−MyD88+/−.B6 mice compared with wt.B6 mice (P = 0.02 and P = 0.004, respectively), whereas IgG2a and 2b titers were reduced to baseline in the absent of MyD88 (P = 0.02 and 0.004, respectively). Horizontal bars represent the average. (B) Histological and immunofluorescence analysis of kidney sections of 9.5-mo-old mice stained with hematoxylin and eosin (H+E) or with anti–mouse IgG subclasses. Compared with the FcγRIIB−/−MyD88+/−.B6 mice, FcγRIIB−/− MyD88−/−.B6 mice showed no IgG2ab and 2b immune complex deposition and pathology in the kidney. (C) Kidney sections in B were costained with anti–mouse IgG2b and anti-FcγRIV (reference 20) or with Mac-I. Sections are representative of three independent mice from each group. (D) Kaplan-Meier survival curves for FcγRIIB−/−MyD88+/+ or FcγRIIB−/−.B6 mice.
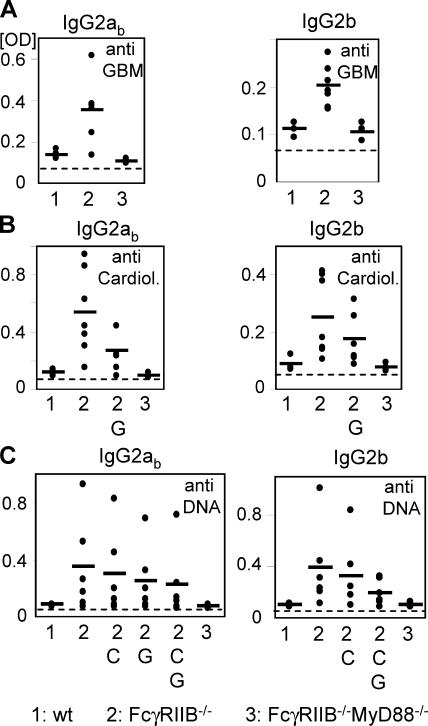
In addition to the anti-DNA antibodies that accumulated in the FcγRIIB−/−.B6 background, we detected IgG2a and 2b subclass–restricted autoantibodies in the serum capable of recognizing both cardiolipin and glomerular basement membrane antigens (Fig. 2). Preabsorbing the serum with glomerular basement membrane (GBM) and cardiolipin antigens reduced the level of autoantibodies recognizing DNA, suggesting that at least a component of the subclass-restricted autoantibodies were polyreactive (Fig. 2).
Figure 2.
MyD88 deficiency protected against generation of IgG2a and 2b polyreactive autoantibodies. IgG2ab and 2b analysis of autoantibodies in the serum of 5-mo-old wt.B6 (n = 4), FcγRIIB−/−MyD88+/−.B6 (n = 8), and FcγRIIB−/−MyD88−/−.B6 (n = 6) mice. Shown are antiglomerular basement membrane (GBM) (A), anticardiolipin (Cardiol.) (B), and anti-DNA (C) titers as determined by ELISA. Polyreactivity was analyzed by preabsorbing the samples on the indicated ELISA plates; G: GBM; C: cardiolipin; G,C: GBM + cardiolipin. IgG1 and IgG3 titers were not significantly elevated over baseline (not depicted). Horizontal bars represent the average.
MyD88 deficiency protected against class switching to pathogenic IgG2a and 2b anti-DNA/polyreactive autoantibodies in SLE
We further investigated the molecular basis for the selective switching of autoantibodies to the pathogenic IgG2a and 2b subclasses by focusing on the role of the TLR–MyD88 pathways. Because the MyD88 pathway had been shown, in vitro, to provide a costimulatory signal for B cell stimulation by DNA (13, 15) as well as potentially influence IgG class switching (21), we examined its in vivo role in the defined models of murine lupus we have established. To determine whether the MyD88 pathway contributed to the pathogenesis of a spontaneous lupus-like autoimmune disease, we generated the FcγRIIB−/−MyD88−/−.B6 strain and compared autoantibody production and disease progression for this strain in comparison with the parental FcγRIIB−/−.B6 strain. MyD88 deficiency resulted in the abrogation of IgG2a and 2b polyreactive anti-DNA and anti-GBM and anticardiolipin autoantibodies (Fig. 1 A and Fig. 2). Susceptible mice lacking MyD88 did not demonstrate evidence of antibody deposition or tissue injury (Fig. 1, B and C) and were protected from renal disease, as measured by accumulation of blood urea nitrogen (not depicted). In contrast with the parental FcγRIIB−/−.B6 strain, the FcγRIIB−/−MyD88−/−.B6 strain was protected from the premature mortality associated with FcγRIIB−/−.B6 mice (2) and all animals survived beyond 9 mo of age (Fig. 1 D).
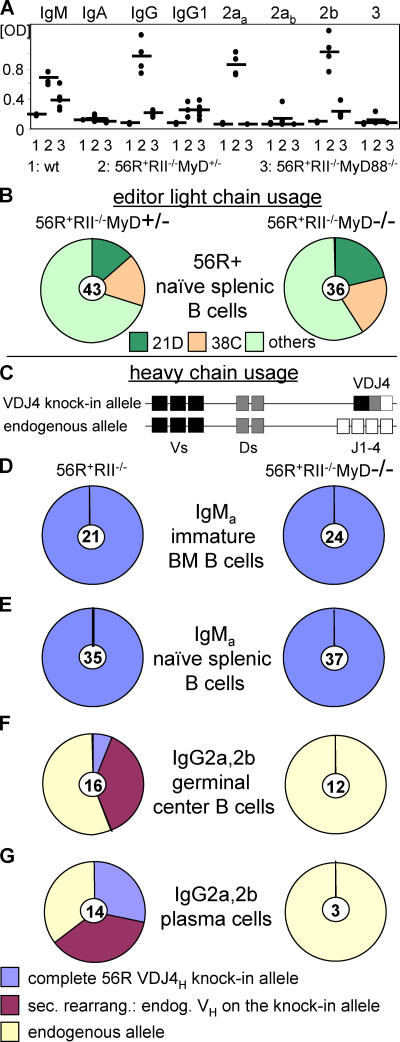
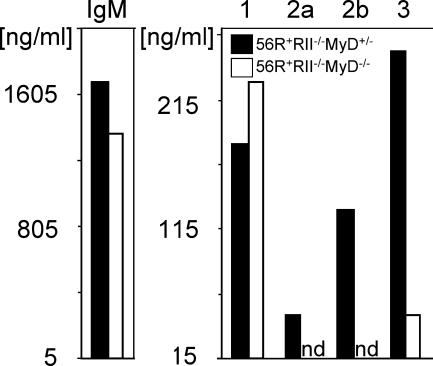
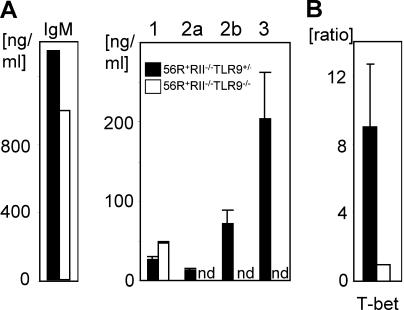
Because MyD88 is common to many toll signaling pathways and is expressed in myeloid, lymphoid, and dendritic cell types (and to determine the contribution of specific TLRs and cell types to the protection seen in MyD88-deficient FcγRIIB−/−.B6 mice), we turned to a spontaneous lupus model in which the development of autoimmunity is driven by intrinsic B cell defects, the anti-DNA knockin model 56R+FcγRIIB−/−.B6 (6, 22, 23). These mice already develop autoantibodies after 5 wk. The triple transgenic strain 56R+FcγRIIB−/−MyD88−/−.B6 was thus compared with its heterozygous counterpart for the development of autoantibodies. As was the case with the FcγRIIB−/−.B6 model, deficiency of MyD88 resulted in strong reduction of IgG autoantibodies in the serum of these mice (Fig. 3 A). In contrast, IgM anti-DNA titers derived from the 56R transgene (6) were only partially reduced in the MyD88-deficient mice (Fig. 3, A). Among the autoreactive IgG subclasses and IgG1 was unaffected by the absence of MyD88, whereas IgG2a and 2b were reduced to background levels (Fig. 3 A).
Figure 3.
IgG2a and IgG2b anti-DNA autoantibodies were absent in the germinal center and plasma cell compartments of MyD88-deficient mice. (A) Isotype and subclass analysis of anti-DNA autoantibodies in the serum of 7–11-wk-old wt.B6 (n = 3), 56R+RIIB−/−MyD88+/− (n = 4), and 56R+RIIB−/−MyD88−/−.B6 (n = 5) mice as determined by ANA ELISA. IgG2aa (haplotype: a) and 2b autoantibodies were strongly and IgG1 slightly up-regulated in 56R+FcγRIIB−/−MyD88+/−.B6 mice compared with wt.B6 mice (P = 0.002, P = 0.004, and P = 0.02, respectively). IgG2ab autoantibodies of B cells without 56R+ were only slightly up-regulated until 7–11 wk in 56R+FcγRIIB−/−MyD88+/−.B6 mice. Among the autoreactive IgG subclasses, IgG1 was unaffected by the absence of MyD88, whereas IgG2a and 2b titers were reduced to baseline (P = 0.002 and 0.03, respectively). Horizontal bars represent the average. (B) MyD-deficient and -sufficient naive splenic B cells used the same light chains, resulting in retention of DNA binding by the 56R VDJ4 heavy chain. Single IgMa + splenic B cells were sorted and single cell PCR was used to identify 56R VH positive cells (knockin allele haplotype: IgMa; C57BL/6 allele haplotype: IgMb). These cells were further analyzed for 21D and 38C κ light chain usage by single cell PCR (reference 6). Naive 56R B cells using the 21D light chain show no DNA binding, whereas 56R B cells using the 38C light chain can still bind DNA (reference 6). (C) Schematic presentation of the anti-DNA 56R knockin VDJ4H transgene. The 56R knockin VDJ4H has replaced the endogenous Js, can be secondarily rearranged with other endogenous Vs, and can class switch to all Ig isotypes (references 22, 23). All 56R+ mice had only one knockin allele. (D and E) MyD88-sufficient (56R+RIIB−/−MyD88+/−) and -deficient IgMa + immature bone marrow (BM) and naive splenic B cells retained the intact 56R VDJ4H transgene. Single IgMa + cells were sorted and analyzed by single cell PCR using a random VH forward primer (reference 34) and an IgM constant region reverse primer (reference 34). (F and G) IgG2a and 2b positive MyD88-deficient GC and splenic plasma cells used the endogenous allele, whereas a fraction of the MyD88-sufficient mice (56R+RIIB−/−MyD88+/− and +/+) still expressed the intact 56R VDJ4H transgene. Single GL7+FAS+ GC or CD138high plasma cells were sorted and analyzed by single cell PCR using a random VH forward primer (reference 34) and specific reverse primers for the different IgG subclass constant regions. A fraction of 56R+RIIB−/− MyD88−/− IgG1 positive GC and plasma cells retained the intact 56R VDJ4 heavy chain (not depicted). No IgG3 cells were found in the GC compartment and no 56R+RIIB−/−MyD88+/− or −/− IgG3 positive plasma cells showed the intact 56R VDJ4 heavy chain (not depicted). The number of cells analyzed is indicated in the center of each pie graph.
The point at which the MyD88 pathway was required for development of anti-DNA antibodies of the IgG2a and 2b subclasses was investigated by FACS and single cell PCR analysis on B cells sorted for their state of maturation from MyD88-deficient and -sufficient backgrounds. We found no differences in B cell development or survival (Fig. 3, B–E and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20052438/DC1). A high and equivalent percentage of heavy chains in immature and naive B cells expressed the anti-DNA 56R VDJ4H transgene and showed comparable editor light chain usage in both MyD88-sufficient and -deficient animals (Fig. 3 and Fig. S1). In contrast, IgG2a and 2b switched heavy chains with the anti-DNA 56R VDJ4H transgene could not be detected in either germinal center (GC) (Fig. 3 F) or splenic plasma cells (Fig. 3 G) isolated from the MyD88-deficient strain. Switching to the IgG2a and 2b subclasses was restricted to the endogenous alleles in the MyD88-deficient strain. The selective abrogation of the anti-DNA response in the MyD88-deficient 56R+FcγRIIB−/−.B6 strain was consistent with a failure of anti-self antibodies to switch to the IgG2a and 2b subclasses.
The loss of IgG2a and 2b autoantibodies in MyD88-deficient mice is a B cell–intrinsic defect
To confirm that the specific reduction in IgG2a and 2b autoantibody titers in MyD88-deficient mice resulted from a defect of B cells to class switch to these specific subclasses, we examined the ability of 56R-expressing naive IgM+ B cells to class switch to IgG subclasses in vitro (Fig. 4). Consistent with previous reports (21, 24), we observed that stimulation of MyD88+/− B cells with the TLR9 ligand CpG together with the B cell stimuli anti-CD40 and IL-4 resulted in the secretion of IgM and IgG antibodies (Fig. 4). In contrast, MyD88−/− B cells were specifically defective in their ability to secrete IgG2a and 2b antibodies, whereas IgM secretion was unchanged and IgG1 secretion even increased (Fig. 4), thus indicating a cell intrinsic specific defect in the ability of MyD88-deficient B cells to class switch to the IgG2a and 2b subclasses (25).
Figure 4.
Loss of IgG2a and 2b autoantibodies in MyD88-deficient mice was B cell intrinsic. Naive splenic B cells from the indicated genotypes were stimulated with 15 μg/ml CpG1826, 10 μg/ml anti-CD40, and 10 ng/ml rmIL-4 in vitro for 5 d and total Ig subclass concentrations in the supernatants were determined. Class switching to IgM was unchanged and to IgG1 increased in MyD88-deficient backgrounds. In contrast, switching to IgG2a, 2b, and 3 was significantly impaired.
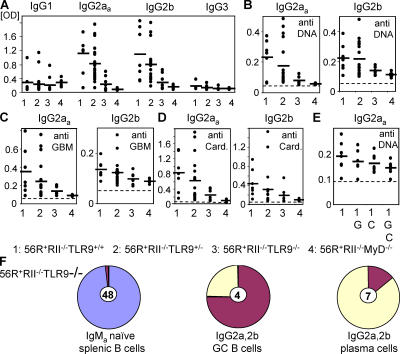
TLR9 deficiency abrogated class switching to pathogenic IgG2a and 2b anti-DNA/polyreactive autoantibodies
The toll receptor mainly responsible for the abrogation of IgG2a and IgG2b anti-DNA antibodies was TLR9 because deficiency in this receptor largely recapitulated the reduction of IgG2a and 2b anti-DNA autoantibodies observed for MyD88 deficiency on the susceptible 56R+FcγRIIB−/−.B6 background as shown in Fig. 5. The TLR9 pathway was therefore required for the generation of specific anti-DNA IgG2a and 2b subclasses by providing a costimulatory signal for B cell stimulation by DNA (13). Recently, it has been suggested that TLR9 deficiency abrogates anti-DNA antibodies, but not other autoantibodies, and does not protect from disease in another SLE mouse model (14). In contrast with those results, we observed that not only IgG2a and 2b anti-DNA autoantibodies but also anti-GBM and anticardiolipin autoantibodies of the IgG2a and 2b subclasses were reduced in 56R+FcγRIIB−/−TLR9−/−.B6 mice as compared with 56R+FcγRIIB−/−TLR9+/+ and +/−.B6 mice (Fig. 5). Preabsorbing the serum of 56R.FcγRIIB−/−.B6 mice with GBM and cardiolipin antigens reduced the amount of autoantibodies that recognized DNA suggested that at least a component of the autoantibodies was polyreactive in the 56R.FcγRIIB−/−B6 mice (Fig. 5 E) and that TLR9 deficiency reduced polyreactive IgG2a and 2b autoantibodies.
Figure 5.
The TLR9 pathway was required for generation of IgG2a and 2b anti-DNA/polyreactive autoantibodies. IgG subclass analysis of autoantibodies in the serum of 7–11-wk-old 56R+RIIB−/−TLR9+/+.B6 (n = 7), 56R+RIIB−/−TLR9+/−.B6 (n = 19), 56R+RIIB−/−TLR9−/−.B6 (n = 7), or 56R+RIIB−/−MyD88−/−.B6 (n = 6) mice as determined by ANA (A), anti-DNA (B), antiglomerular basement membrane (anti-GBM) (C), and anticardiolipin (D) ELISA, indicated a reduction of the autoreactive IgG2aa and IgG2b subclasses for both genotypes (P = 0.007 and P = 0.03, respectively, for the ANA ELISA). (E) Polyreactivity was analyzed by preabsorbing the samples on the indicated ELISA plates; G: GBM; C: cardiolipin; G,C: GBM + cardiolipin. (F) TLR9-deficient IgMa + naive splenic B cells retained the intact 56R VDJ4H transgene, whereas IgG2a and 2b positive TLR9-deficient GC and splenic plasma cells did not use the intact 56R VDJ4H transgene (compare with Fig. 3).
FACS and single cell PCR analysis of B cells showed that a high and equivalent percentage of heavy chains in naive B cells expressed the anti-DNA 56R VDJ4H transgene in TLR9-deficient animals (Fig. 5 F [compare with Fig. 3] and not depicted). In contrast, IgG2a and 2b switched heavy chains with the 56R VDJ4H transgene could not be detected in either splenic GCs or plasma cells isolated from the TLR9-deficient strain (Fig. 5 F). Switching to the IgG2a and 2b subclasses was restricted to the endogenous or secondarily rearranged knockin allele in the TLR9-deficient strain.
No evidence of kidney pathology was observed in the 56R+FcγRIIB−/−TLR9−/−.B6 mice and all mice survived beyond 8 mo of age in contrast with the premature mortality observed for 56R+FcγRIIB−/−TLR9+/+ (unpublished data and reference 6). Thus, TLR9 deficiency protected from pathology by inhibiting the generation of IgG2a and 2b polyreactive autoantibodies.
The loss of IgG2a and 2b autoantibodies in TLR9-deficient mice is also a B cell–autonomous defect
To confirm that the specific reduction in IgG2a and 2b autoantibody titers in TLR9-deficient mice resulted from a defect of B cells, we examined the ability of 56R-expressing naive IgM B cells to class switch to IgG subclasses in vitro (Fig. 6 A). As was observed for MyD88−/− B cells (Fig. 4), TLR9−/− B cells were specifically defective in their ability to secrete IgG2a, 2b, and 3 antibodies in response to CpG, anti-CD40, and IL-4, whereas IgM secretion was unchanged and IgG1 secretion increased (Fig. 6 A), thus indicating a cell-autonomous specific defect in the ability of TLR9-deficient B cells to class switch to the IgG2a, 2b, and 3 subclasses.
Figure 6.
Loss of IgG2a and 2b autoantibodies in TLR9-deficient mice was B cell autonomous. (A) Naive splenic B cells from the indicated genotypes were stimulated with 15 μg/ml CpG1826, 10 μg/ml anti-CD40, and 10 ng/ml rmIL-4 in vitro for 5 d and total Ig subclass concentrations in the supernatants were determined. Class switching to IgM was unchanged and to IgG1 increased in TLR9-deficient backgrounds. In contrast, switching to IgG2a, 2b, and 3 was significantly impaired. (B) T-bet mRNA is not induced by CpG stimulation of B cells in the TLR9-deficient background. A portion of the cells in (A) were collected after 12 h of the indicated stimulation. mRNA and cDNA were prepared and used for real-time PCR with specific primers for T-bet and β-actin as an internal control. The ratio between TLR9-sufficient and -deficient B cells for T-bet mRNA was calculated after normalizing for β-actin. nd, not detected. Results from three independent experiments are shown as mean ± SD.
The selective defect in in vitro class switching of TLR9 and MyD88-deficient B cells, with increased IgG1 and decreased IgG2a and 2b, has also been observed in B cells isolated from mice deficient in the transcription factor T-bet (26). CpG has been shown in vitro to up-regulate T-bet expression in B cells in a TLR9–MyD88-dependent pathway (24). Our results suggested that one mechanism by which the TLR9–MyD88 pathway was specifically regulating the IgG2a and 2b anti-DNA/polyreactive autoantibody response could be through T-bet regulation. To determine if T-bet was induced in B cells isolated from the 56R+RIIB−/−TLR9+/− or −/− strains, we examined the levels of T-bet transcripts in B cells derived from these two strains upon stimulation with CpG, anti-CD40, and IL-4. T-bet expression was ninefold higher in B cells derived from the 56R+RIIB−/−TLR9+/−.B6 strain compared with B cells derived from the 56R+RIIB−/− TLR9−/−.B6 strain (Fig. 6 B). Therefore, absence of TLR9 signaling resulted in less T-bet transcription (24, 26). Given the ability of T-bet to induce class switching in a B cell autonomous mechanism (26), our results suggest that this pathway is a likely route for TLR9–MyD88 regulation of autoantibody class switching in vivo as well.
The loss of IgG2a and 2b autoantibodies in TLR9-deficient mice was restricted to anti-self antibodies
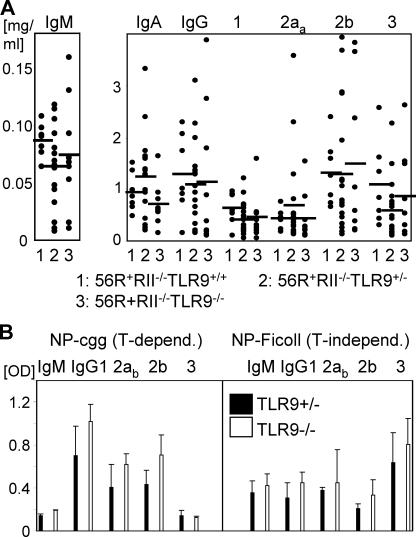
The effect of TLR9 deficiency on the reduction of IgG2a and 2b antibodies was restricted to autoantibodies because the number of plasma cells (CD138+) in spleen and bone marrow and the total serum level of all IgG subclasses were not reduced in 56R+FcγRIIB−/−TLR9−/−.B6 mice compared with 56R+FcγRIIB−/−TLR9+/+ and +/−.B6 mice (Fig. 7 A and not depicted). Furthermore, the specific IgG subclass response to NP-CGG (T-dependent) or NP-Ficoll (T-independent) immunization was not reduced in TLR9−/−.B6 mice compared with TLR9+/−.B6 mice (Fig. 7 B).
Figure 7.
Loss of IgG2a and 2b autoantibodies in TLR9-deficient mice was restricted to anti-self antibodies. (A) Analysis of total antibody isotypes and subclasses in the serum of 7–11-wk-old 56R+RIIB−/− TLR9+/+.B6 (n = 7), 56R+RIIB−/−TLR9+/−.B6 (n = 19), or 56R+RIIB−/− TLR9−/−.B6 (n = 7) mice as determined by ELISA. Horizontal bars represent the average. (B) The specific IgG subclass responses to NP(24)-CGG or NP(24)-Ficoll immunization were not reduced in TLR9−/−.B6 mice compared with TLR9+/−.B6 mice. The indicated anti-NP IgM and IgG subclasses were analyzed on day 11 after injection. Results from three independent experiments are shown as mean ± SD.
DISCUSSION
The TLR9–MyD88 pathway is required for the generation of pathogenic anti-DNA/polyreactive autoantibodies of the IgG2a and 2b subclasses in vivo, based on the analysis of the spontaneous SLE models described in this study. These subclasses efficiently trigger inflammatory responses by their ability to preferentially engage the activation receptor FcγRIV on macrophages (20). This skewing of autoantibody responses through the TLR9–MyD88 pathway promotes class switching to these pathogenic subclasses and provides an explanation for the predominance and pathogenicity of those antibodies in vivo. In the absence of TLR9, IgG2a and 2b autoantibodies were nearly as strongly reduced as in MyD88-deficient mice, suggesting that TLR9 is the main MyD88-dependent inducer of IgG2a and 2b autoantibodies in our lupus-prone mice (Fig. 5). However, activation of other TLRs may be partly involved as described previously (15).
No changes in the accumulation of IgG1 autoantibodies were observed in mice deficient in either MyD88 or TLR9. The fact that the number of IgG1-positive GCs or splenic plasma cells, which retained the intact 56R VDJ4 heavy chain, was not increased in 56R+RIIB−/−TLR9−/− mice as compared with 56R+RIIB−/− mice, argues against an IgG1 default pathway in TLR9-deficient mice that could compensate for the loss of IgG2a and 2b autoantibodies (unpublished data).
Recently, the role of TLR9 in SLE was investigated by generating F2 crosses of TLR9−/− mice (on a mixed genetic background containing both B6 and 129Sv genomes) and Fas-deficient MRL/Mplpr/lpr mice (14). Consistent with our results, this TLR9 deficiency abrogated anti-DNA antibodies; however, in contrast with our study, other autoantibodies persisted and the animals were not protected from disease. These differences most likely result from the differential contributions of genetic background to autoimmunity and disease progression in the mixed MRL/B6/129 background, thus accounting for the incomplete penetrance observed in those studies. We further suggest that the increased escape of polyreactive B cells from central tolerance we observed in FcγRIIB−/−.B6 mice (Figs. 3 and 5 and reference 6) more closely resembles the phenotype seen in pediatric SLE patients (16, 17) than the Fas-deficient phenotype, where a broadly based number of specific self-reactive B and T cells are activated and protected from death in the periphery. The activation of the immune system in Fas-deficient mice probably depends to a greater extent on other MyD88 costimulators such as IL-1, IL-18, and other TLRs (27) than on TLR9.
A likely mechanism by which TLR9 signaling can break tolerance and promote specific class switching to IgG2a and 2b is suggested by the in vitro induction of the transcription factor T-bet by CpG DNA through the TLR9–MyD88 pathway in B cells (Fig. 6 B and references 3, 24). Our results are thus consistent with the observation that T-bet deficiency results in decreased IgG2a, 2b, and 3 levels in the lupus-susceptible strain MLR/lpr (26). However, in contrast with our results, IgG1 levels are increased in MRL/lpr mice deficient in T-bet (26). This difference could reflect the differences in the mouse models used in the two studies.
Our data thus suggest a model (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20052438/DC1) in which anti-DNA/polyreactive naive IgM+ B cells that have escaped central tolerance can be induced to switch to the pathogenic IgG2a and 2b subclasses by stimulation of the TLR9–MyD88 pathway. This stimulation may occur through pathogen-derived or apoptotic DNA delivered to the endosomal TLR9 molecule by the anti-DNA B cell receptor (28), thus triggering TLR9/MyD88-dependent induction of T-bet expression and subsequent switching to IgG2a and 2b. These pathogenic subclasses are normally prevented from accumulation by the action of the inhibitory receptor FcγRIIB, which limits the expansion of plasma cells and secretion of autoreactive IgGs (6). The reduction of FcγRIIB in other autoimmune-susceptible strains (7, 29–32) removes this checkpoint and allows for the expansion of plasma cells secreting these pathogenic subclasses. Deposition of immune complexes results in tissue pathology and premature mortality that occurs through engagement of FcγRIV expressed on myeloid effector cells (20). The identification of the TLR9–MyD88 pathway as a critical pathway in determining the development of pathogenic polyreactive autoantibody subclasses in vivo provides the rationale for its targeting in disease like SLE.
MATERIALS AND METHODS
Mice.
MyD88−/− and TLR9−/− mice were provided by S. Akira (Osaka University, Osaka, Japan) (27, 33) and backcrossed >12 and 9 generations, respectively, onto the C57BL/6 background. The 56R knockin mouse that is transgenic for an anti-DNA B cell receptor heavy (H) chain has been described previously (6, 22, 23) (Fig. 3 C). MyD88−/− and TLR9−/− mice were crossed to FcγRIIB−/− or 56R+FcγRIIB−/− mice on a C57BL/6 background to produce FcγRIIB−/−MyD+/−, FcγRIIB−/−MyD−/−, 56R+FcγRIIB−/−MyD+/−, 56R+FcγRIIB−/−MyD−/−, 56R+FcγRIIB−/− TLR9+/+, 56R+FcγRIIB−/−TLR9+/−, and 56R+FcγRIIB−/−TLR9−/− mice. Littermates were used as controls in each experiment. C57BL/6 mice were purchased from The Jackson Laboratory. All experiments were done in compliance with federal laws and institutional guidelines and have been approved by The Rockefeller University. Genotypes were determined by PCR on tail DNA (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20052438/DC1). All mice were fed with antibiotic (Sulfatrim) food to reduce the influence of infections.
NP-cgg and NP-Ficoll immunization.
100 μg of NP(24)-cgg or NP(24)-Ficoll (Biosearch Technologies) were injected i.p. and IgM and IgG subclass titers were analyzed on day 11.
ELISA.
For total immunoglobulin isotype and subclass determination, Nunc-Immuno 96-well MicroWell Maxisorp ELISA plates (Nalgene Nunc International) were coated overnight with goat anti–mouse IgG, IgM, IgA, IgE, IgG1, IgG2a, IgG2b, or IgG3 (Bethyl Laboratories) at a concentration of 10 μg/ml. After blocking with 50 mM Tris, pH 8, 0.14 M NaCl, 1% BSA for 30 min, 100 μl of 1/100 diluted serum or cell culture supernatant was applied for 1 h at room temperature. Captured antibodies were detected with horseradish peroxidase–coupled goat anti–mouse IgG, IgM, IgA, IgE, IgG1, IgG2a, IgG2b, and IgG3 (Bethyl Laboratories), followed by incubation with 3,3′,5,5′-tetramethylbenzidine peroxidase substrate solution. Absorbance was measured at 450 nm.
Detection of antibodies specific for double-stranded DNA was done as described previously (6). In brief, ELISA plates were precoated with 5 μg/ml of methylated BSA (Sigma-Aldrich) followed by incubation overnight at 4°C with 50 μg/ml of calf thymus DNA (Sigma-Aldrich). After washing, the plates were blocked (PBS, 3% BSA, 1 mM EDTA, 0.1% gelatin) and subsequently incubated with 1/100 diluted serum. Bound antibodies were detected as described for the immunoglobulin isotype and subclass determination. IgG2a autoantibodies of 56R+ B cells (haplotype: a) were detected with an antibody against IgG2aa (Bethyl Laboratories) and IgG2a antibodies of B cells without 56R+ (haplotype: b) were detected with an antibody against IgG2ab (Bethyl Laboratories). Antibodies against nuclear proteins and DNA were detected with an ANA ELISA kit (Corgenix) and antibodies against GBM and cardiolipin were detected with kits from Hycor, according to the manufacturer's directions. Serum was diluted 1/100. Antibodies against NP were detected with NP(24)-BSA coated ELISA plates. Serum was diluted 1/300.
Kidney pathology.
Kidneys were fixed with 10% formalin in PBS and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin and eosin. To assay for immune complex deposition and macrophage accumulation, kidneys were embedded in Tissue-Tek OCT compound and snap frozen. Sections (5 μm) were air-dried, fixed with cold acetone, and stained with FITC-conjugated anti–mouse IgG subclass antibodies (anti-IgG1 and IgG3, Sigma-Aldrich; IgG2 ab [recognizing the C57BL/6 IgG2a protein] and IgG2b, Bethyl Laboratories), biotin-conjugated anti–mouse FcγRIV (20), and Cy3-conjugated antibiotin (Sigma-Aldrich) or PE-conjugated anti–mouse Mac-I (Becton Dickinson).
Flow cytometry.
The following antibodies from Beckon Dickinson were used: anti–mouse AA4.1-FITC, anti–mouse B220-allophycocyanin, anti–mouse CD19-PE, anti–mouse IgMa-PE, anti–mouse CD21-FITC, anti–mouse CD23-Pe, anti–mouse GL7-FITC, anti–mouse FAS-PE, anti–mouse CD138-PE, and anti–mouse CD3ɛ-FITC.
Single cell sorting, cDNA synthesis, and PCR.
The single cell PCR methodology has been described previously (6). In brief, immature IgMa +AA4.1+CD19int bone marrow cells (knockin allele: IgMa), IgMa +CD19+B220+ splenocytes, FAS+GL7+ GC splenocytes, and CD138high plasma splenocytes from indicated mice were sorted by a FACSVantage (Becton Dickinson) cell sorter into 96-well PCR plates. Cells were lysed and cDNA was prepared as described. Transcripts were amplified by two rounds of PCR (outer and inner primers; Fig. S3) with the following conditions: denaturating for 5 min at 94°C, 50 cycles of 30 s at 94°C, 30 s at 60°C, 55 s (first PCR) or 45 s (second PCR) at 72°C, followed by final elongation for 10 min at 72°C.
In vitro stimulation.
Naive B cells were enriched by the MACS B cell depletion kit (Miltenyi Biotec). The purity was verified by FACS analyses and found to be >97% B cells. The cells were cultured for 5 d in RPMI 1610, 10% FCS, 150 μg/ml streptomycin, 150 U/ml penicillin, and 5 μM 2-mercaptoethanol with the indicated concentrations of rmIL-4 (PeproTech), anti-CD40 (Becton Dickinson), and CpG1823 (InvivoGen). Supernatants were used for ELISA analyses and RNA for RT-PCR was prepared with the RNeasy Mini kit (QIAGEN).
RT-PCR.
cDNA was prepared with the superscript III polymerase kit (Invitrogen). RT-PCR was performed with the SYBR green PCR Master Mix and the Applied Biosystems machine with the primers in Fig. S3.
Online supplemental material.
Fig. S1 shows B cell development of MyD88-sufficient and -deficient mice characterized by FACS analyses. Fig. S2 represents a model depicted the B cell checkpoints involved in the generation of anti-DNA/polyreactive autoantibodies and systemic autoimmune disease. Fig. S3 lists the primers used for the paper. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20052438/DC1.
Supplemental Material
Acknowledgments
We thank S.L. Peng for assistance with the TLR9−/− mice and K. Velinzon, P. Smith, P. Gell, M. Patt, J. Pagan, A. Miguelez, and P. Curry for technical assistance.
This work was supported by grants from the National Institutes of Health (J.V. Ravetch). M. Ehlers is the Eileen Ludwig fellow at Rockefeller University.
The authors have no conflicting financial interests.
Abbreviations used: GBM, antiglomerular basement membrane; GC, germinal center; SLE, systemic lupus erythematosus; TLR, Toll-like receptor.
References
- 1.Peter, J.B., and Y. Shoenfeld. 1996. Autoantibodies. Elsevier, New York. 910 pp.
- 2.Bolland, S., and J.V. Ravetch. 2000. Spontaneous autoimmune disease in FcγRII deficient mice results from strain-specific epistasis. Immunity. 13:277–285. [DOI] [PubMed] [Google Scholar]
- 3.Rifkin, I.R., and A. Marshak-Rothstein. 2003. T-bet: the Toll-bridge to class-switch recombination? Nat. Immunol. 4:650–652. [DOI] [PubMed] [Google Scholar]
- 4.Fields, M.L., and J. Erikson. 2003. The regulation of lupus-associated autoantibodies: immunoglobulin transgenic models. Curr. Opin. Immunol. 15:709–717. [DOI] [PubMed] [Google Scholar]
- 5.Wakeland, E.K., K. Liu, R.R. Graham, and T.W. Behrens. 2001. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 15:397–408. [DOI] [PubMed] [Google Scholar]
- 6.Fukuyama, H., F. Nimmerjahn, and J.V. Ravetch. 2005. The inhibitory Fcγ receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 6:99–106. [DOI] [PubMed] [Google Scholar]
- 7.McGaha, T.L., B. Sorrentino, and J.V. Ravetch. 2005. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 307:590–593. [DOI] [PubMed] [Google Scholar]
- 8.Theofilopoulos, A.N., and F.J. Dixon. 1985. Murine models of systemic lupus erythematosus. Adv. Immunol. 37:269–390. [DOI] [PubMed] [Google Scholar]
- 9.Vyse, T.J., and B.L. Kotzin. 1998. Genetic susceptibility to systemic lupus erythematosus. Annu. Rev. Immunol. 16:261–292. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard, N.R., and K.G. Smith. 2003. B cell inhibitory receptors and autoimmunity. Immunology. 108:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel, L., B.P. Croker, K.R. Blenman, C. Mohan, G. Huang, G. Gilkeson, and E.K. Wakeland. 2000. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc. Natl. Acad. Sci. USA. 97:6670–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotzin, B.L. 1996. Systemic lupus erythematosus. Cell. 85:303–306. [DOI] [PubMed] [Google Scholar]
- 13.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]
- 14.Christensen, S.R., M. Kashgarian, L. Alexopoulou, R.A. Flavell, S. Akira, and M.J. Shlomchik. 2005. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 202:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau, C.M., C. Broughton, A.S. Tabor, S. Akira, R.A. Flavell, M.J. Mamula, S.R. Christensen, M.J. Shlomchik, G.A. Viglianti, I.R. Rifkin, and A. Marshak-Rothstein. 2005. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardemann, H., S. Yurasov, A. Schaefer, J.W. Young, E. Meffre, and M.C. Nussenzweig. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377. [DOI] [PubMed] [Google Scholar]
- 17.Yurasov, S., H. Wardemann, J. Hammersen, M. Tsuiji, E. Meffre, V. Pascual, and M.C. Nussenzweig. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie, C., Z. Liang, S. Chang, and C. Mohan. 2003. Use of a novel elution regimen reveals the dominance of polyreactive antinuclear autoantibodies in lupus kidneys. Arthritis Rheum. 48:2343–2352. [DOI] [PubMed] [Google Scholar]
- 19.Liang Z., C. Xie, C. Chen, D. Kreska, K. Hsu, L. Li, X.J. Zhou, and C. Mohan. 2004. Pathogenic profiles and molecular signatures of antinuclear autoantibodies rescued from NZM2410 lupus mice. J. Exp. Med. 199:381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimmerjahn, F., P. Bruhns, K. Horiuchi, and J.V. Ravetch. 2005. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 23:41–51. [DOI] [PubMed] [Google Scholar]
- 21.Lin, L., A.J. Gerth, and S.L. Peng. 2004. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur. J. Immunol. 34:1483–1487. [DOI] [PubMed] [Google Scholar]
- 22.Chen C., Z. Nagy, E.L. Prak, and M. Weigert. 1995. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 3:747–755. [DOI] [PubMed] [Google Scholar]
- 23.Li, H., Y. Jiang, E.L. Prak, M. Radic, and M. Weigert. 2001. Editors and editing of anti-DNA receptors. Immunity. 15:947–957. [DOI] [PubMed] [Google Scholar]
- 24.Liu, N., N. Ohnishi, L. Ni, S. Akira, and K.B. Bacon. 2003. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat. Immunol. 4:687–693. [DOI] [PubMed] [Google Scholar]
- 25.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature. 438:364–368. [DOI] [PubMed] [Google Scholar]
- 26.Peng, S.L., S.J. Szabo, and L.H. Glimcher. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA. 99:5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 28.Latz, E., A. Schoenemeyer, A. Visintin, K.A. Fitzgerald, B.G. Monks, C.F. Knetter, E. Lien, N.J. Nilsen, T. Espevik, and D.T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190–198. [DOI] [PubMed] [Google Scholar]
- 29.Xiu, Y., K. Nakamura, M. Abe, N. Li, X.S. Wen, Y. Jiang, D. Zhang, H. Tsurui, S. Matsuoka, Y. Hamano, et al. 2002. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J. Immunol. 169:4340–4346. [DOI] [PubMed] [Google Scholar]
- 30.Jiang, Y., S. Hirose, M. Abe, R. Sanokawa-Akakura, M. Ohtsuji, X. Mi, N. Li, Y. Xiu, D. Zhang, J. Shirai, et al. 2000. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 51:429–435. [DOI] [PubMed] [Google Scholar]
- 31.Jiang, Y., S. Hirose, R. Sanokawa-Akakura, M. Abe, X. Mi, N. Li, Y. Miura, J. Shirai, D. Zhang, Y. Hamano, et al. 1999. Genetically determined aberrant down-regulation of FcγRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int. Immunol. 11:1685–1691. [DOI] [PubMed] [Google Scholar]
- 32.Pritchard, N.R., A.J. Cutler, S. Uribe, S.J. Chadban, B.J. Morley, and K.G. Smith. 2000. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcγRII. Curr. Biol. 10:227–230. [DOI] [PubMed] [Google Scholar]
- 33.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 34.Paul, E., J. Lutz, J. Erikson, and M.C. Carroll. 2004. Germinal center checkpoints in B cell tolerance in 3H9 transgenic mice. Int. Immunol. 16:377–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.