Abstract
Oral tolerance induction is a key feature of intestinal immunity, generating systemic nonresponsiveness to ingested antigens. In this study, we report that orally applied soluble antigens are exclusively recognized in the intestinal immune system, particularly in the mesenteric lymph nodes. Consequently, the initiation of oral tolerance is impeded by mesenteric lymphadenectomy. Small bowel transplantation reveals that mesenteric lymph nodes require afferent lymph to accomplish the recognition of orally applied antigens. Finally, oral tolerance cannot be induced in CCR7-deficient mice that display impaired migration of dendritic cells from the intestine to the mesenteric lymph nodes, suggesting that immunologically relevant antigen is transported in a cell-bound fashion. These results demonstrate that antigen transport via afferent lymphatics into the draining mesenteric lymph nodes is obligatory for oral tolerance induction, inspiring new therapeutic strategies to exploit oral tolerance induction for the prevention and treatment of autoimmune diseases.
The intestinal epithelium is constantly exposed to a multitude of foreign material that may either be harmful or beneficial for the organism. Consequently, the intestinal immune system has to balance between either protective immune responses that are induced upon encounter of intestinal pathogens and toxins or tolerance against commensal bacteria and food antigens. Inadequate protective immune reactions can cause severe immunopathology and are actively prevented in healthy individuals. Food antigens and commensal bacteria constitute the majority of the antigenic load in the intestine and the “default” reaction of the immune system confronted with them leads to systemic unresponsiveness. This phenomenon is known as oral tolerance and represents a key feature of intestinal immunity (1). The unique propensity of the intestinal immune system to evoke tolerance to orally administrated antigens offers attractive strategies to prevent or treat autoimmune diseases (2, 3). Such approaches would exploit effective yet selective natural immunosuppressive mechanisms, thereby avoiding unwanted side effects caused by an exhaustive and livelong treatment with immunomodulatory drugs.
The intestinal immune system is classically divided into effector and inductor sites to emphasize the particular functions of both compartments. Inductive sites comprise organized lymphoid tissues in the intestinal mucosa (4) and the intestine draining mesenteric LNs (MLN). In contrast, intestinal effector sites consist of a network of immune cells scattered in a less organized fashion throughout the intestinal epithelium and lamina propria (LP). However, the role of these and further distal lymphoid and nonlymphoid compartments in the initiation and maintenance of oral tolerance remains elusive (1). This is exemplified by the ongoing discussion whether and how ingested antigens gain access to particular compartments and distribute throughout the body to trigger tolerance induction.
The original hypothesis that intestinal immune responses exclusively depend on antigen uptake by M cells in Peyer's patches (PP) epithelium has been challenged by more recent reports that observed induction of oral tolerance in PP-deficient intestines (5–7). M cells have been identified in lymphoid aggregations other than PP (8) and even in histologically inconspicuous villi (9), suggesting that a conclusive study of M cell function for oral tolerance induction will have to consider additional M cell–competent structures other than PP.
In addition to the uptake of antigens by M cells in PP, some intestinal antigens appear to enter the LP where antigens might be taken up by resident DCs that could present antigens both in situ in the LP as well as in the draining MLN. Indeed, LP DCs have been shown to present orally applied antigens (10) and the majority of DCs entering the MLN appears to originate from the LP and not from PP (11, 12). Furthermore, DCs in the LP might directly contribute to antigen uptake by extending dendrites through the epithelium, thereby sampling luminal antigens (13). Importantly, antigen entering the LP might also disseminate via the circulation and after antigen feeding intact food proteins have been reported to be present in minute amounts in serum of mice and humans (14, 15), corroborating the general assumption that distal compartments might contribute to oral tolerance induction.
In this study, we demonstrate that, after oral administration, the initial recognition of antigen is restricted to the intestinal immune system, in particular the MLN. Vice versa, we do not observe any overt signs of oral tolerance induction in peripheral LN (pLN) and spleen. Furthermore, we show that antigen recognition in the intestinal immune system is obligatory for oral tolerance induction and depends on CCR7-mediated cell migration.
RESULTS
Interactions of antigen-presenting cells and naive T cells are thought to induce tolerance as well as immunity (16). Upon first-time encounter of an antigen, both conditions trigger the activation and clonal expansion of antigen-specific T cells (17). Shortly thereafter, only T cells being activated under inflammatory conditions will continue to establish a protective immune response, whereas T cells expanded under tolerogenic circumstances fail to gain classical effector functions. The fact that activated antigen-specific T cells could be found throughout the immune system of antigen-fed animals, along with other observations, led to the hypothesis that tolerance induction is triggered simultaneously in all lymphoid organs (18, 19). Alternatively, proliferating T cells present in peripheral lymphoid tissues may have been primed in the intestinal immune system and subsequently emigrated to seed the peripheral lymphoid tissues (20, 21).
Oral administration of antigen does not induce proliferation of antigen-specific T cells in pLN and spleen in the presence of FTY720
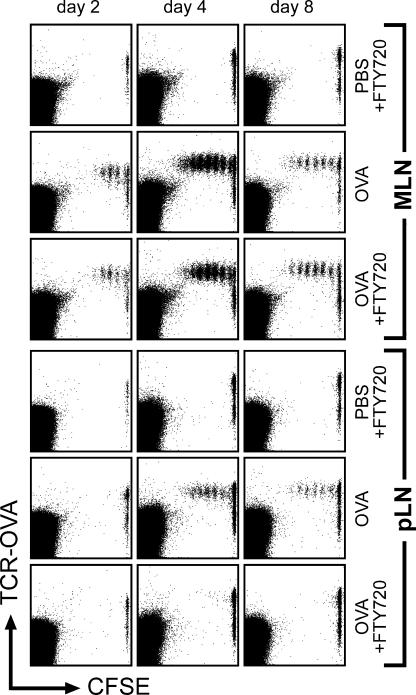
Productive antigen recognition can be tracked in vivo by analyzing the induction of cell proliferation of adoptively transferred antigen-specific T cells. Applying this experimental system, we observe proliferation of antigen-specific T cells in PP and MLN 2 d after antigen feeding. In spleen and other peripheral lymphoid organs, divided T cells appear 1 d later and are abundantly present 4 d after antigen feeding (Fig. 1, not depicted, and references 18, 22). To determine the potential sites of antigen recognition and T cell activation, we prevented the recirculation of lymphocytes by use of the immunomodulatory drug FTY720. FTY720 has been shown to interfere with sphingosine-1–phosphate receptor signaling that is essential for the emigration of lymphocytes from lymphoid organs into efferent lymphatics (23). Therefore, FTY720-mediated shutdown of lymphocyte egress interrupts lymphocyte recirculation and causes a rapid induction of blood lymphopenia (24). In particular, lymphocyte egress from PP into the lymph connecting PP to the MLN is also shut down under FTY720 influence (unpublished data), demonstrating that T cell proliferation in these two lymphoid compartments does not depend on T cell trafficking to the MLN. We observed that FTY720 treatment did not interfere with proliferation of adoptively transferred antigen-specific transgenic T cells in PP and MLN after antigen feeding (Fig. 1 and not depicted). In contrast, proliferating T cells were completely absent in pLN and spleen at all time points analyzed (Fig. 1 and not depicted), identifying the intestinal immune system as the genuine compartment of oral tolerance induction. To test for possible shortcomings in peripheral T cell induction caused by FTY720 treatment, antigen was also given intravenously. Notably, T cell proliferation after systemic antigen administration was not influenced by FTY720 treatment (unpublished data and reference 24), demonstrating an unimpaired capability of pLN and spleen to sustain a response to antigen under these conditions.
Figure 1.
Oral administration of antigen does not induce proliferation of antigen-specific T cells in pLN and spleen in the presence of FTY720. 1 d after adoptive transfer of CFSE-labeled DO11.10 cells, wild-type recipients were fed 100 mg ovalbumin in PBS or PBS alone. Subsequently, mice were either treated with FTY720 (starting 16 h after antigen feeding) or mock treated. 2, 4, and 8 d after feeding of ovalbumin, mice were killed and proliferation of ovalbumin-specific T cells was analyzed. Dot plots are gated on CD4-positve lymphocytes.
Recirculation of lymphocytes is dispensable for oral tolerance induction
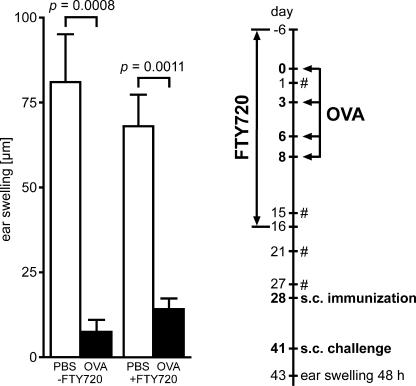
We next analyzed the consequences of FTY720 treatment on the induction of functional oral tolerance. To this end, unmanipulated wild-type mice were continuously treated with FTY720 throughout the period of antigen feeding. Absence of freely disposable antigen as indicated by cessation of antigen presentation was verified experimentally before withdrawal of FTY720 (see Materials and methods). 9 d later, reestablishment of normal peripheral blood lymphocyte counts was confirmed and induction of tolerance was determined by measuring delayed type hypersensitivity (DTH) reactions. We observed significantly reduced DTH reactions in animals fed with antigen compared with unfed controls irrespective of FTY720 treatment (Fig. 2). These data suggest that antigen-specific activation of lymphocytes in the intestinal immune system is sufficient for the induction of systemic unresponsiveness and that lymphocyte recirculation during antigen presentation is not required for the process of oral tolerance induction.
Figure 2.
Recirculation of lymphocytes is dispensable for oral tolerance induction. Wild-type mice were fed four doses of 25 mg ovalbumin in PBS (black bars) or PBS alone (white bars) under continuous treatment with FTY720 (+FTY720) or mock treatment (−FTY720). Subsequently, on day 28, mice were immunized by subcutaneous injection of ovalbumin in PBS/CFA emulsion and challenged on day 41 (see Materials and methods). Tolerance induction was analyzed by measurement of DTH reactions 48 h after challenge. The experimental procedure is depicted in the flow chart. #, control of peripheral blood cell counts to monitor FTY720-induced lymphopenia. Data shown have been obtained for 11 or 12 mice in each group. Error bars represent SEM.
Tolerogenic potential is largely confined to MLN
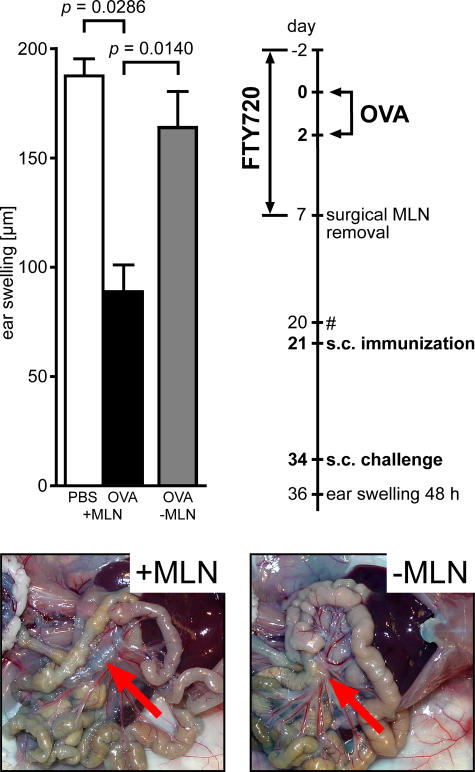
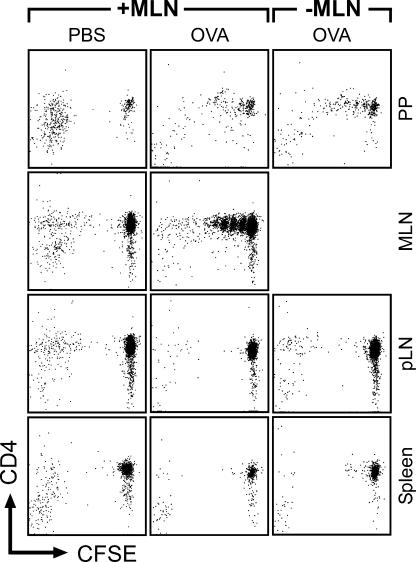
Because the MLN drain the small intestine, it is conceivable that these particular LNs play a pivotal role in the induction of oral tolerance. To test this hypothesis, we tolerized wild-type mice under continuous FTY720 treatment and surgically removed the MLN before withdrawal of FTY720. Under these conditions, excision of the MLN almost completely prevented the establishment of systemic tolerance as indicated by DTH reactions observed in these mice (Fig. 3).This finding reveals that, even though proliferating T cells are present in PP, the majority of the tolerogenic potential was generated in the MLN and resided in this organ until its surgical removal. Furthermore, we also noted that mesenteric lymphadenectomy before antigen feeding counteracted oral tolerance induction (unpublished data).
Figure 3.
Tolerogenic potential is largely confined to MLN. Wild-type mice were fed two doses of 25 mg ovalbumin under continuous FTY720 treatment and subsequently underwent surgical removal of MLN. Color images demonstrate absence of MLN in mice that underwent mesenteric lymphadenectomy (−MLN) compared with unmanipulated controls (+MLN). Arrows indicate (original) position of MLN. The experimental procedure is depicted in the flow chart. #, control of peripheral blood cell counts to monitor FTY720-induced lymphopenia. Data shown have been obtained with 10 mice that underwent mesenteric lymphadenectomy and four mice in each of the unmanipulated control groups. Error bars represent SEM.
Oral administration of antigen does not induce proliferation of antigen-specific T cells in transplanted PP and MLN that are connected to the host circulation, but not to afferent lymphatics of the host intestine
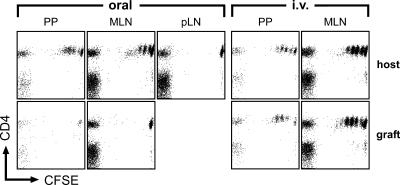
To specifically address the contribution of afferent lymphatic drainage to MLN function, we performed vascularized small bowel transplantations that included the draining MLN. In these mice, both the host and the graft MLN are connected to the blood circulation of the host, but the two types of MLN are distinguished by the fact that they receive afferent lymph in a mutually exclusive manner from their original intestine only. These transplanted mice were adoptively transferred with TCR-transgenic T cells and challenged orally with antigen. After 2 d, we observed antigen-specific T cell proliferation in PP and MLN of the antigen-challenged host intestine, but not in grafted PP or MLN, nor in pLN and spleen (Fig. 4). In contrast, intravenous administration of antigen activated adoptively transferred T cells in both endogenous and transplanted PP and MLN (Fig. 4 and see next paragraph). In conclusion, our observations indicate that the grafted MLN react like distal pLN, and suggest a decisive role for antigen transport via lymphatic vessels from the intestine into draining MLN.
Figure 4.
Oral administration of antigen does not induce proliferation of antigen-specific T cells in transplanted PP and MLN that are connected to the host circulation but not to afferent lymphatics of the host intestine. Intestinal segments together with completely intact donor MLN were transplanted into syngenic wild-type recipients. Grafts were connected to the host blood circulation and both graft ends were exteriorized as stomata. Subsequently, recipient mice were adoptively transferred with 107 CFSE-labeled OTII cells. 2 d after feeding of 100 mg ovalbumin (oral), proliferation of ovalbumin-specific TCR-transgenic T cells occurred only in endogenous but not in transplanted organs, whereas systemic administration of antigen by intravenous injection (i.v.) resulted in comparable reactions in grafted and host gut-associated lymphoid tissue. Note that naive transgenic cells were present in the grafted MLN/PP, indicating a successful coupling to the recipients' lymphocyte recirculation. Dot plots are gated on Vα2/Vβ5-positive lymphocytes.
Mesenteric lymphadenectomy does not result in antigen recognition in peripheral lymphoid organs after oral administration of antigen
The precise role of passively drained antigen in comparison to cell-bound antigen transport remains an unresolved issue in oral tolerance induction. In general, the port of entry into the body creates a bias in the reactivity toward an antigen among the diverse lymphoid compartments. Thus, after adoptive transfer of TCR-transgenic T cells, antigen-specific proliferation of these cells can be induced more efficiently in spleen and pLN than in MLN when low doses of antigen are injected into the tail vein (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20052016/DC1). In contrast, oral administration of a 1,000-fold higher antigen dose fails to induce de novo T cell proliferation of antigen-specific T cells in peripheral lymphoid organs (Fig. 1 and not depicted). Interestingly, we observed that this lack of antigen recognition in peripheral lymphoid organs after antigen feeding is sustained in mice that underwent mesenteric lymphadenectomy (Fig. 5). In such animals, within 5 wk after surgery, pseudo-afferent lymph vessels emerged that would permit the direct transfer of antigen being passively drained from the intestine into the circulation. Regardless of the presence or absence of MLN, free antigen entering the blood circulation after feeding (14) remains immunologically inconspicuous possibly because it might be diluted below threshold levels required for antigen recognition in the peripheral lymphoid organs.
Figure 5.
Mesenteric lymphadenectomy does not result in antigen recognition in peripheral lymphoid organs after oral administration of antigen. 5 wk after mesenteric lymphadenectomy, MLN-explanted mice (−MLN) and unmanipulated wild-type controls (+MLN) were adoptively transferred with 107 CFSE-labeled DO11.10 cells. Oral administration of 100 mg ovalbumin 1 d later did not result in proliferation of ovalbumin-specific TCR-transgenic T cells in pLN and spleen 2 d after feeding, irrespective of the presence of the MLN. Dot plots are gated on DO11.10 TCR-positive lymphocytes.
Induction of T cell proliferation in MLN and induction of oral tolerance upon antigen feeding depend on CCR7
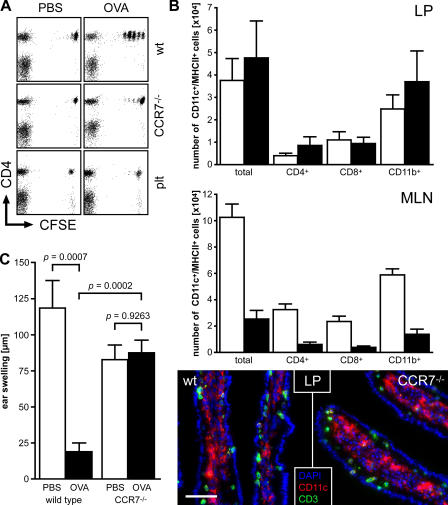
A key regulator of immune cell trafficking is the chemokine receptor CCR7 that is abundantly expressed by subsets of T cells and DCs (25). We have previously reported that skin DCs that lack functional CCR7 fail to migrate to the draining LNs (26), most likely a result of their inability to enter afferent lymphatics. We thus analyzed the proliferation of adoptively transferred antigen-specific T cells in wild-type and CCR7-deficient recipients after antigen feeding. As expected, a robust T cell proliferation was induced in MLN and PP of wild-type recipients, but not in pLN and spleen 2 d after antigen feeding (see also results presented in Fig. 1). In contrast, in CCR7-deficient recipients, almost no proliferation of adoptively transferred transgenic T cells was observed in any of the organs analyzed except in PP (Fig. 6 A and not depicted), whereas intravenous antigen injection resulted in comparable levels of T cell proliferation in MLN of both wild-type and CCR7-deficient mice (not depicted and reference 26). Similar results were observed in plt/plt mice that lack the CCR7 ligands CCL19 and CCL21-Ser (Fig. 6 A and not depicted). CCR7-deficient mice possess normal numbers of PP (not depicted) and harbor unaltered populations of DCs in the LP compared with wild-type mice (Fig. 6 B), suggesting that the uptake of intestinal antigens should not be altered in these mutants. In consequence, the role of CCR7-mediated cell migration in oral tolerance induction can be explored in CCR7-deficient mice. To this aim, we fed ovalbumin to CCR7-deficient and wild-type mice under tolerogenic conditions. Importantly, we failed to observe induction of oral tolerance in CCR7-deficient mice (Fig. 6 C). Assuming that an important path for tolerance induction relies on soluble antigens being passively drained into the MLN, in CCR7 mutant mice these antigens would meet LN-resident DCs that are dramatically reduced in numbers (Fig. 6 B) and possibly defective in function as a result of misplacement (not depicted), wherefore tolerance induction possibly fails to occur. However, as mentioned earlier in this paragraph, the mere presence of soluble antigen diffused into the MLN via the bloodstream after intravenous administration elicits a vigorous T cell response in CCR7-deficient organs, demonstrating the MLN's unhindered capability to drive T cell responses (unpublished data and reference 26). These observations establish a decisive role for CCR7-dependent cell migration from the intestine to the draining MLN in oral tolerance induction. Additionally, we observed that the homeostatic migration of LP DCs stringently depends on CCR7. Intestinal LP DCs express the αE-integrin chain CD103 and MLN resident CD103+ DCs derived from the LP are capable of imprinting intestinal homing on T cells (27). In CCR7-deficient mice, the frequency of these LP-derived DCs in MLN is selectively reduced compared with CD103− DCs (18 ± 7% CD103+ DCs in CCR7-deficient mice compared with 46 ± 12% CD103+ DC in wild-type mice, mean ± SD, n = 6 in each group). In contrast, the frequency of CD103+ DCs is unchanged in the LP, indicating that the homeostatic migration of CD103+ DCs from the intestine into MLN is governed by CCR7 signaling.
Figure 6.
(A) Induction of T cell proliferation in MLN upon antigen feeding depends on CCR7. Wild-type, plt/plt, and CCR7-deficient mice were adoptively transferred with 107 CFSE-labeled OTII cells and fed 100 mg ovalbumin 1 d later. Proliferation of ovalbumin-specific T cells in MLN was analyzed 2 d after antigen administration. Dot plots are gated on Vα2/Vβ5-positive lymphocytes. In contrast with wild-type mice, no induction of T cell proliferation occurred in MLN of plt/plt mutants and CCR7-deficient animals. (B) Analysis of DCs in LP and MLN. DCs in the LP and MLN of wild-type (white bars) and CCR7-deficient mice (black bars) were analyzed by flow cytometry and immunofluorescent microscopy. No significant differences in the number and phenotype of DCs were observed in the LP, whereas MLN of CCR7 mutants harbored severely decreased numbers of DCs compared with wild-type mice. (C) Oral tolerance induction is impaired in CCR7-deficient mice. Wild-type and CCR7-deficient mice were fed 25 mg ovalbumin (black bars) on days 0 and 2 or mock treated (white bars). Subsequently, on day 9, mice were immunized by intraperitoneal injection of ovalbumin in PBS/CFA emulsion and challenged on day 22 (see Materials and methods). Tolerance induction was analyzed by measurement of DTH reactions 48 h after challenge. Data shown have been obtained with the following: n = 7 wild-type mice (PBS); n = 9 wild-type mice (Ova); n = 11 CCR7−/− mice (PBS); n = 12 CCR7−/− mice (Ova). Error bars represent SD in B and SEM in C.
DISCUSSION
Circumstantial evidence fostered the widely accepted concept that the foundation of oral tolerance induction is laid in all the lymphoid tissues present in an organism. We reevaluated this process, dissecting it into the following crucial steps: (a) surgical manipulations removing the MLN or grafting MLN, including intestines, to investigate the importance of MLN and direct intestinal-to-MLN transport of antigen, respectively; (b) applying the drug FTY720, thus preventing regular T cell homeostasis; and (c) analyzing CCR7-deficient mice known for their disturbed DC balance and migration.
Our combined data obtained by these experimental approaches would favor the hitherto unrecognized hypothesis that oral tolerance induction relies solely on mechanisms restricted to cells residing in the intestinal immune system. In particular, the MLN are privileged in triggering oral tolerance because their removal results in devastating consequences with respect to oral tolerance induction. The importance of MLN for oral tolerance induction is also supported by the findings of others, demonstrating that the reconstitution of MLN by an agonistic anti-lymphotoxin–β-receptor antibody is sufficient to regain the capability to induce oral tolerance in lymphotoxin-α–deficient mice (7). In contrast, PP and further M cell–competent compartments of gut-associated lymphoid tissue such as solitary intestinal lymphoid tissue (4) cannot compensate for deficits in oral tolerance induction caused by the absence of MLN (5, 6). A similar reasoning also holds true for the liver that, in addition to classical lymphoid organs, has been suggested to be required for oral tolerance induction (28).
Grafted MLN that do not receive efferent lymph drained from the antigen-challenged intestine react like any distal pLN in respect to their capability to drive T cell proliferation. This suggests that the special role of MLN in oral tolerance induction relies on afferent lymph drained from the intestine and entering these particular LNs. Apparently, antigen passing through the intestinal tract and being taken up into the circulation (14) does not manifest its presence inside the body by triggering T cell responses in spleen and pLN. Therefore, these results imply that the recognition of fed antigen is strictly confined to the intestinal immune system. Consequently, the appearance of divided cells in distal organs in the absence of FTY720 is most likely the result of the peripheral dissemination of cells that have first been activated in the intestinal immune system.
It is generally accepted that oral tolerance induction may involve either anergy or deletion of T cells, or the induction of regulatory T cells. Although the precise contribution of these mechanisms to oral tolerance induction is unknown, high doses of antigen tend to favor deletion and anergy, whereas low doses seem to generate predominantly regulatory T cells (29). Interestingly, we observed that oral tolerance can be induced in mice in the absence of lymphocyte recirculation using a high dose feeding regimen. Under these conditions, a substantial pool of antigen-specific T cells is trapped in the periphery, preventing antigen recognition by these cells. Yet tolerance is maintained after full reconstitution of lymphocyte homeostasis, indicating that potentially reactive T cells are held in check. Consequently, this observation suggests a decisive role for regulatory T cells in a high-dose feeding regimen. We hypothesize that, once initiated in the intestinal immune system, the knowledge of tolerance is communicated shortly thereafter to the periphery by T cells disseminating into distal LNs and spleen.
In addition, our observations suggest that immunologically relevant food antigens need to be transported into the MLN via afferent lymph, most likely by LP-resident DCs. Oral tolerance cannot be induced in CCR7-deficient mice, although CCR7-deficient lymphoid organs are competent to drive T cell proliferation upon systemic antigen administration. This implies a decisive contribution of unimpaired cell-bound antigen transport by DCs migrating from the intestine into MLN. Importantly, free antigen passively drained from the intestine and entering the MLN appears to remain immunologically inconspicuous and does not elicit T cell proliferation in MLN. Notably, this scenario is reminiscent to the situation of antigen entering the circulation without initiating T cell proliferation in pLN and spleen. In support of this hypothesis, it was observed that, after antigen feeding, DCs collected from the thoracic duct of lymph-adenectomized rats are able to stimulate T cells in vitro and in vivo (30). Furthermore, DCs in the intestine have been reported to present orally applied antigens (10), and expansion of the DC pool by treatment with Flt3-ligand facilitates the induction of oral tolerance (31). Oral tolerance inducing DCs entering the MLN might originate from organized lymphoid structures (PP, solitary intestinal lymphoid tissue) or alternatively the LP. However, PP residing at the frontline of antigen uptake (i.e., the intestinal mucosa) are not essential for oral tolerance induction (5) and the majority of DCs entering the MLN has been proposed to originate from the LP (11, 12), suggesting that predominantly LP-derived DCs contribute to oral tolerance induction.
In conclusion, our results reveal that food antigen receives immunological attention exclusively in the intestinal immune system. Consequently, establishment of systemic tolerance to food antigens is inevitably bound to its origin in the intestinal immune system. These results call for a shift in the basic understanding of how the immune system manages tolerance and indicate that intestinal DCs are potential targets for both the therapeutic use of oral tolerance and the prevention of tolerance against oral vaccines.
MATERIALS AND METHODS
Animals.
BALB/c, C57BL/6, C57BL/10, C57BL/6 OTII, C57BL/6 CCR7−/−, C57BL/6 plt/plt, and BALB/c DO11.10 were bred at the central animal facility of the Hannover Medical School under specific pathogen-free conditions; BALB/c and C57BL/6 mice were also purchased from Charles River Laboratories. All animal experiments have been performed in accordance with the institutional guidelines and have been approved by the institutional review board of the Hannover Medical School.
Antibodies.
The following antibodies and conjugates were used in this study: anti-CD4–PerCp, anti-CD8α–allophycocyanin–Cy7, anti-CD11b–PE-Cy7, anti-CD11c–PE, anti-CD11c– allophycocyanin, anti-CD11c–bio, anti-CD103–PE, anti-MHCII (I-Ab)–FITC, anti-MHCII (I-Ab)–bio, anti-Vα2–PE, anti-Vβ5–bio (BD Biosciences), anti-CD8α–bio, and anti-DO11.10 TCR–Cy5 (clone KJ1-26; Caltag). Anti-CD3 (clone 17A2) and anti-CD4 (clone RMCD4) antibodies were provided by E. Kremmer (GSF München, Munich, Germany). Cy5 conjugates of anti-CD3 and anti-CD4 antibodies were prepared as recommended by the manufacturer (GE Healthcare). Biotinylated antibodies were recognized by streptavidin coupled to Cy3, PE (Jackson ImmunoResearch Laboratories), Alexa 405 (Invitrogen), or PerCp (BD Biosciences).
Immunohistochemistry.
Immunohistochemistry was performed according to standard protocols. In brief, sections were rehydrated in TBST (0.1 M Tris, pH 7.5, 0.15 M NaCl, 0.1% Tween 20), preincubated with TBST containing 5% rat or mouse serum, blocked with 0.001% avidin/PBS and 0.001% biotin/PBS, and stained with a cocktail of biotinylated or fluorescent dye–coupled antibodies in 2.5% serum/TBST. Biotinylated antibodies were visualized by fluorescent streptavidin conjugates. Nuclei were visualized by DAPI staining (1 μg/ml DAPI/TBST) and sections were mounted with MOWIOL. Images were acquired using an Axiovert 200 M microscope with Axiovision software (Carl Zeiss MicroImaging, Inc.).
Flow cytometry.
To obtain single cell suspensions of pLN (inguinal, brachial, and axillary LNs), MLN, and PP, organs were minced through a nylon mesh and washed with PBS supplemented with 2% FCS. For the isolation of LP cells, gut content and PP were removed before intestines were opened longitudinally. Intestines were washed twice in cold PBS and once in cold PBS/5% FCS/5 mM EDTA, and incubated twice in 25 ml RPMI 1640 medium/5% FCS/5 mM EDTA at 37°C to remove the epithelial cell fraction. The remaining tissue was washed with PBS, cut into small pieces, and incubated at 37°C for 45 min in RPMI 1640/20% FCS/0.5 mg/ml collagenase A (Roche). The resulting suspension was filtered through a nylon mesh, pelleted, and resuspended in 40% Percoll (GE Healthcare) in RPMI 1640/5% FCS. This cell suspension was overlaid onto 70% Percoll in RPMI 1640/5% FCS and centrifuged at 800 g for 20 min. LP cells were recovered from the interphase and washed twice in PBS/2% FCS before staining with the antibodies described. FACS analysis was performed on a FACSCalibur or LSRII (both obtained from BD Biosciences).
Intestinal surgery.
Mouse-vascularized small bowel transplantation was performed as described previously (32) with some modifications. C57BL/10 mice were used as donors and recipients. In brief, under the combined anesthesia with Ketamine and Rompun, the donor jejunum and proximal ileum together with the MLN were isolated with the superior mesenteric artery and portal vein attached. After luminal irrigation and vascular perfusion, the graft was stored at 4°C in Ringer's solution until implantation. The graft portal vein and superior mesenteric artery were anastomosed to the recipient's inferior vena cava and abdominal aorta, respectively, in an end-to-side fashion. Both ends of the graft were exteriorized as stomata. Mesenteric lymphadenectomy was performed by microdissection along the length of the superior mesenteric artery to aortic root (33). To confirm completeness of mesenteric lymphadenectomy, animals received 150 μL Chicago sky blue (Sigma-Aldrich) solution (1% in PBS) by intraperitoneal injection at the end of the experiment. 10 d later, mice were killed and carefully inspected to reveal remaining MLN.
FTY720 treatment.
Mice received 1 mg/kg body weight of FTY720 dissolved in PBS by gavage every second day. Effect of FTY720 treatment was monitored by regular analysis of peripheral blood lymphocyte counts.
Adoptive transfer of CFSE-labeled lymphocytes.
Either BALB/c DO11.10 (Figs. 1 and 5) or C57BL/6 OTII (Figs. 4 and 6 A) mice were used as cell donors for adoptive transfer into syngenic recipient animals. Lymphocytes were isolated from pLN (inguinal, brachial, and axillary), MLN, and spleen and labeled with 5 μM CFSE (Invitrogen) for 15 min at 37°C. After washing twice with PBS/3% FCS, 107 cells per mouse were injected into the lateral tail vein.
Antigen-feeding regimen.
For analyzing the proliferation of ovalbumin-specific transgenic T cells, mice were fed 100 mg ovalbumin (Grade III; Sigma-Aldrich) in 200 μl PBS by gavage above the lower esophageal sphincter with a stainless steel feeding needle on day 0. For the measurement of DTH responses, mice were fed 25 mg ovalbumin (Grade III, Sigma-Aldrich) in 200 μl PBS on days 0, 3, 6, and 8 (Fig. 2) or on days 0 and 2 (Figs. 3 and 6). In some experiments, cessation of antigen presentation was verified by adoptive transfer of CFSE-labeled DO11.10 transgenic T cells into wild-type “reporter” mice at different time points after antigen feeding. 2 d after T cell transfer, animals were killed and proliferation of transgenic T cells was determined by flow cytometry. Absence of T cell proliferation indicated cessation of functional antigen recognition.
Immunization.
Mice were immunized by subcutaneous (Figs. 2 and 3) or intraperitoneal (Fig. 6 C) injection of 300 μg ovalbumin (Grade VI; Sigma-Aldrich) in 200 μl PBS/CFA emulsion (containing 100 μg MT; Sigma-Aldrich) on day 28 (Fig. 2), day 21 (Fig. 3), or day 9 (Fig. 6 C) after the first oral ovalbumin dose.
Induction and measurement of DTH responses.
13 d after immunization, mice were challenged by subcutaneous injection of 50 μg ovalbumin (Grade VI; Sigma-Aldrich) in 20 μl PBS into the right ear pinna while 20 μl PBS without ovalbumin were injected into the left ear pinna for control purposes. Ear swelling was measured in a blinded fashion before and 48 h after injection with a custom-built spring driven micrometer. Ovalbumin-specific ear swelling was calculated as the following: (right ear thickness − left ear thickness)48h − (right ear thickness − left ear thickness)0h. None of the animals used for the measurement of DTH responses had received adoptively transferred cells.
Statistical analysis.
Statistical analysis was performed with the commercially available software GraphPadPrism. All significant values were determined using the unpaired two-tailed nonparametric Mann-Whitney-test, error bars represent SD (Fig. 6 B) and SEM (Figs. 2, 3, and 6 C), respectively.
Online supplemental material.
Fig. S1 shows that MLN are less effective than peripheral lymphoid tissues in supporting T cell proliferation after intravenous injection of antigen. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20052016/DC1.
Supplemental Material
Acknowledgments
We would like to thank M. Friedrichsen and S. Willenzon for excellent technical assistance and V. Brinkmann for providing FTY720. We are especially grateful to B.J. Lindbom and A.McI. Mowat for discussion of the manuscript.
This work has been supported by Deutsche Forschungsgemeinschaft grant no. SFB621-A01 (to R. Förster).
The authors have no conflicting financial interests.
Abbreviations used: DTH, delayed type hypersensitivity; LP, lamina propria; MLN, mesenteric LNs; pLN, peripheral LN; PP, Peyer's patches.
R. Förster and O. Pabst contributed equally to this work.
References
- 1.Mowat, A.M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341. [DOI] [PubMed] [Google Scholar]
- 2.Mayer, L., and L. Shao. 2004. Therapeutic potential of oral tolerance. Nat. Rev. Immunol. 4:407–419. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson, K., and J. Holmgren. 2002. Recent advances in mucosal vaccines and adjuvants. Curr. Opin. Immunol. 14:666–672. [DOI] [PubMed] [Google Scholar]
- 4.Pabst, O., H. Herbrand, T. Worbs, M. Friedrichsen, S. Yan, M.W. Hoffmann, H. Korner, G. Bernhardt, R. Pabst, and R. Forster. 2005. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 35:98–107. [DOI] [PubMed] [Google Scholar]
- 5.Kraus, T.A., J. Brimnes, C. Muong, J.H. Liu, T.M. Moran, K.A. Tappenden, P. Boros, and L. Mayer. 2005. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J. Clin. Invest. 115:2234–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spahn, T.W., A. Fontana, A.M. Faria, A.J. Slavin, H.P. Eugster, X. Zhang, P.A. Koni, N.H. Ruddle, R.A. Flavell, P.D. Rennert, and H.L. Weiner. 2001. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J. Immunol. 31:1278–1287. [DOI] [PubMed] [Google Scholar]
- 7.Spahn, T.W., H.L. Weiner, P.D. Rennert, N. Lugering, A. Fontana, W. Domschke, and T. Kucharzik. 2002. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur. J. Immunol. 32:1109–1113. [DOI] [PubMed] [Google Scholar]
- 8.Hamada, H., T. Hiroi, Y. Nishiyama, H. Takahashi, Y. Masunaga, S. Hachimura, S. Kaminogawa, H. Takahashi-Iwanaga, T. Iwanaga, H. Kiyono, et al. 2002. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168:57–64. [DOI] [PubMed] [Google Scholar]
- 9.Jang, M.H., M.N. Kweon, K. Iwatani, M. Yamamoto, K. Terahara, C. Sasakawa, T. Suzuki, T. Nochi, Y. Yokota, P.D. Rennert, et al. 2004. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA. 101:6110–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirdo, F., O. Millington, H. Beacock-Sharp, and A.M. Mowat. 2005. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 35:1831–1840. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull, E.L., U. Yrlid, C.D. Jenkins, and G.G. Macpherson. 2005. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J. Immunol. 174:1374–1384. [DOI] [PubMed] [Google Scholar]
- 12.Bimczok, D., E.N. Sowa, H. Faber-Zuschratter, R. Pabst, and H.J. Rothkotter. 2005. Site-specific expression of CD11b and SIRPα (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur. J. Immunol. 35:1418–1427. [DOI] [PubMed] [Google Scholar]
- 13.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J.P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361–367. [DOI] [PubMed] [Google Scholar]
- 14.Furrie, E., R.E. Smith, M.W. Turner, S. Strobel, and A.M. Mowat. 2002. Induction of local innate immune responses and modulation of antigen uptake as mechanisms underlying the mucosal adjuvant properties of immune stimulating complexes (ISCOMS). Vaccine. 20:2254–2262. [DOI] [PubMed] [Google Scholar]
- 15.Husby, S., J.C. Jensenius, and S.E. Svehag. 1985. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand. J. Immunol. 22:83–92. [DOI] [PubMed] [Google Scholar]
- 16.Shakhar, G., R.L. Lindquist, D. Skokos, D. Dudziak, J.H. Huang, M.C. Nussenzweig, and M.L. Dustin. 2005. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat. Immunol. 6:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney, E.R., K.A. Pape, D.Y. Loh, and M.K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1:327–339. [DOI] [PubMed] [Google Scholar]
- 18.Smith, K.M., J.M. Davidson, and P. Garside. 2002. T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology. 106:144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutgemann, I., A.M. Fahrer, J.D. Altman, M.M. Davis, and Y.H. Chien. 1998. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 8:667–673. [DOI] [PubMed] [Google Scholar]
- 20.Williamson, E., J.M. O'Malley, and J.L. Viney. 1999. Visualizing the T-cell response elicited by oral administration of soluble protein antigen. Immunology. 97:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanas, E., G.M. Davey, F.R. Carbone, and W.R. Heath. 2000. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J. Immunol. 164:2890–2896. [DOI] [PubMed] [Google Scholar]
- 22.Kunkel, D., D. Kirchhoff, S. Nishikawa, A. Radbruch, and A. Scheffold. 2003. Visualization of peptide presentation following oral application of antigen in normal and Peyer's patches-deficient mice. Eur. J. Immunol. 33:1292–1301. [DOI] [PubMed] [Google Scholar]
- 23.Matloubian, M., C.G. Lo, G. Cinamon, M.J. Lesneski, Y. Xu, V. Brinkmann, M.L. Allende, R.L. Proia, and J.G. Cyster. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann, V., J.G. Cyster, and T. Hla. 2004. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 4:1019–1025. [DOI] [PubMed] [Google Scholar]
- 25.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 26.Ohl, L., M. Mohaupt, N. Czeloth, G. Hintzen, Z. Kiafard, J. Zwirner, T. Blankenstein, G. Henning, and R. Forster. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 21:279–288. [DOI] [PubMed] [Google Scholar]
- 27.Johansson-Lindbom, B., M. Svensson, O. Pabst, C. Palmqvist, G. Marquez, R. Forster, and W.W. Agace. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, R., Q. Liu, J.L. Grosfeld, and M.D. Pescovitz. 1994. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J. Pediatr. Surg. 29:1145–1148. [DOI] [PubMed] [Google Scholar]
- 29.Mowat, A.M., L.A. Parker, H. Beacock-Sharp, O.R. Millington, and F. Chirdo. 2004. Oral tolerance: overview and historical perspectives. Ann. NY. Acad. Sci. 1029:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Liu, L.M., and G.G. MacPherson. 1993. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J. Exp. Med. 177:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viney, J.L., A.M. Mowat, J.M. O'Malley, E. Williamson, and N.A. Fanger. 1998. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J. Immunol. 160:5815–5825. [PubMed] [Google Scholar]
- 32.Zhong, R., Z. Zhang, D. Quan, B. Garcia, J. Duff, C. Stiller, and D. Grant. 1993. Intestinal transplantation in the mouse. Transplantation. 56:1034–1037. [PubMed] [Google Scholar]
- 33.Macpherson, A.J., and T. Uhr. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 303:1662–1665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.