Abstract
Chronic inflammation is a well-known risk factor for cancer. Proinflammatory mediators such as prostaglandin E2 (PGE2) promote colorectal tumor growth by stimulating angiogenesis, cell invasion, and cell growth, and inhibiting apoptosis. Molecules that regulate tumor-associated angiogenesis provide promising therapeutic targets for treatment of colorectal cancer (CRC) as indicated by the recent development of the novel anti-angiogenic agent bevacizumab (Avastin). However, use of this drug only prolongs survival by several months, highlighting the importance of finding more effective treatment regimens. We report here that PGE2 induces expression of CXCL1 (growth-regulated oncogene α), a pro-angiogenic chemokine, in human CRC cells. More importantly, CXCL1 released from carcinoma cells induces microvascular endothelial cell migration and tube formation in vitro. Furthermore, PGE2 promotes tumor growth in vivo by induction of CXCL1 expression, which results in increased tumor microvessel formation. These results have potential clinical significance because we found that CXCL1 expression correlates with PGE2 levels in human CRCs. Collectively, our findings show for the first time that CXCL1 is regulated by PGE2 and indicate that CXCL1 inhibitors should be evaluated further as potential anti-angiogenic agents for treatment of CRC.
Chronic inflammation caused by infectious or autoimmune diseases is clearly associated with increased cancer risk. It has been estimated that chronic inflammation contributes to the development of ∼15% of malignancies worldwide (1) and postulated that it promotes tumor growth, in part, through stimulation of angiogenesis. Chronic inflammation results in up-regulation of various cytokines, including IL-1α/β, IFN-γ, and TNF-α in inflammatory cells. Because these nonspecific proinflammatory cytokines induce expression of proinflammatory mediators, including cyclooxygenase-2 (COX-2) and proinflammatory chemokines (2–4), we sought to determine whether there was a connection between COX-2 and chemokine signaling pathways in colorectal cancer (CRC).
Prostaglandin E2 (PGE2) plays a role in promoting progression of CRC. PGE2 levels are elevated in human CRCs and adenomas in familial adenomatous polyposis patients (5–8). We have previously shown that PGE2 accelerates intestinal adenoma growth in ApcMin mice (9). PGE2 is thought to mediate the effect of COX-2 during angiogenesis because treatment with PGE2 reversed the anti-angiogenic effects of a selective COX-2 selective inhibitor (10). However, the mechanism(s) by which PGE2 contributes to tumor-associated angiogenesis and modulates endothelial cell biology remains unclear. Therefore, we tested the hypothesis that PGE2, a proinflammatory mediator, stimulates colon carcinoma cells to produce angiogenic factors that modulate endothelial cell migration and tube formation.
Chemokines are known to play a major role in regulating inflammation and wound healing. Growth-regulated oncogene (GRO) is a member of the CXC chemokine family that is crucial for the recruitment of neutrophils to inflammatory sites. Three distinct GRO isoforms (α, β, and γ) have been isolated, characterized, and now are referred to as CXCL1, CXCL2, and CXCL3, respectively. All three ligands bind to the CXC chemokine receptor CXCR2, with CXCL1 (GROα) having the highest affinity (11). The CXCL1 gene was first identified by subtractive hybridization from tumorigenic versus nontumorigenic Chinese hamster embryonic fibroblasts (12), and the CXCL1 protein was originally purified as an autocrine growth factor from supernatants of a human melanoma cell line (13, 14). The mouse CXCL1-3 homologues macrophage inflammatory protein (MIP)-2 and KC have also been cloned (15, 16). CXCL1 is expressed at high levels constitutively in melanoma and in several disorders that involve acute and chronic inflammation. For example, CXCL1 is expressed in 70% of the human melanomas, but very low levels of CXCL2 (GROβ) or CXCL3 (GROγ) are found in these tumors (17). Overexpression of CXCL1 in immortalized melanocytes results in cell transformation (18). Furthermore, this chemokine is a potent mediator of tumor-associated angiogenesis in Kaposi's sarcoma and non-small cell lung cancer (19–21). However, the role of CXCL1 in CRC has not been reported.
To examine tumor-associated angiogenesis in vivo, we generated the Rag2(−/−)/γc(−/−)/Flk1(+/−)lacZ triple mutant mice. These triple mutant mice serve as a powerful tool to examine human tumor-associated angiogenesis by exploiting β-galactosidase activity in endothelial cells as a marker for Flk1 promoter activity and a measure of angiogenesis. We investigated whether PGE2 could regulate tumor-associated angiogenesis by causing tumor cells to produce pro-angiogenic factors. Here we report that CXCL1 mRNA and protein expression is elevated in 85 and 65% of human CRCs, respectively, and correlates well with PGE2 levels in CRC tissues. We also demonstrate that exogenous PGE2 induces CXCL1 expression and release in CRC cells in vitro and in vivo. Induction of CXCL1 by PGE2 is dose dependent and involves activation of the epidermal growth factor receptor (EGFR)–mitogen-activated protein kinase (MAPK) pathway. Furthermore, we found that the conditioned medium from PGE2-treated CRC cells enhanced endothelial cell migration and tube formation, and that CXCL1 released from CRC cells mediates PGE2-induced endothelial cell migration and tube formation in vitro. The in vitro effect of PGE2 on endothelial cells via CXCL1 stimulation was confirmed in vivo. Treatment with an anti-CXCL1–neutralizing antibody resulted in a reduction of tumor growth with decreased microvessel formation. These results demonstrate that PGE2-induced CXCL1 expression is involved in CRC tumor growth and angiogenesis.
RESULTS
CXCL1 expression correlates with PGE2 levels in human CRC
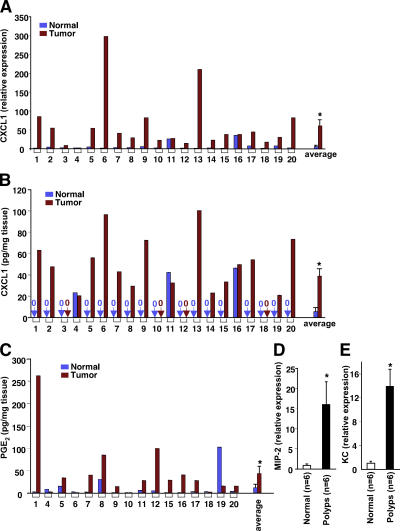
Because CXCL1 is highly expressed in human melanomas, we determined whether CXCL1 levels are also increased in human CRCs. Real-time quantitative PCR and ELISA assays were performed to measure CXCL1 levels in human colon carcinomas at grade II–III compared with the matched normal tissues. CXCL1 mRNA levels were elevated in 17 of 20 (85%) cancer specimens (Fig. 1 A). CXCL1 protein expression was also elevated in 13 of 20 (65%) cancer specimens and was undetectable in 13 matched normal tissues (Fig. 1 B, see pairs 1, 2, 5, 6, 7, 8, 9, 13, 14, 15, 17, 19, and 20). In paired samples 3, 10, 12, and 18, the CXCL1 was not detected. Because differences in CXCL1 levels were less than twofold in pairs 4, 11, and 16, these changes were not considered significant. CXCL1 mRNA and protein levels correlate well in these human biopsies (Fig. 1, A and B). It has been established that PGE2 levels correlate well with progression of human colorectal tumors and adenomas in ApcMin mice (5–8, 22). Therefore, we determined whether CXCL1 expression correlates with PGE2 levels in CRCs (Fig. 1, B and C). The spearman rank coefficient was used to test if the correlation was significant in all 16 paired samples. A positive correlation of PGE2 and CXCL1 was found in these samples (r = 0.37, P = 0.019). Similarly, analysis by quantitative PCR revealed that expression of mouse CXCL1 homologues MIP-2 and KC are much higher in intestinal adenomas than normal matched tissues in ApcMin mice (Fig. 1, D and E). To our knowledge, this is the first report that CXCL1 expression is increased in CRC and that it correlates with PGE2 levels.
Figure 1.
CXCL1 expression is elevated in human colorectal tumors and adenomas of Apcmin mice. CXCL1 expression is associated with PGE2 levels. The CXCL1 expression in 20 tumors and the matched normal tissues is measured by quantitative real-time PCR (A) and ELISA (B), and the levels of PGE2 in these tissues were quantified using gas chromatography negative ion chemical ionization mass spectrometry (C). The levels of MIP-2 (D) and KC (E) mRNA in the normal and intestinal adenoma tissues from six Apcmin mice were also analyzed by quantitative real-time PCR. The relative expression of target gene is averages of triplicates that are normalized against the transcript levels of h-β-actin or mGAPDH. Data are represented as the mean ± SE of the relative expression from six mice. Asterisks represent statistical differences (P < 0.05; Student's t test).
CXCR2 expression is elevated in CRC
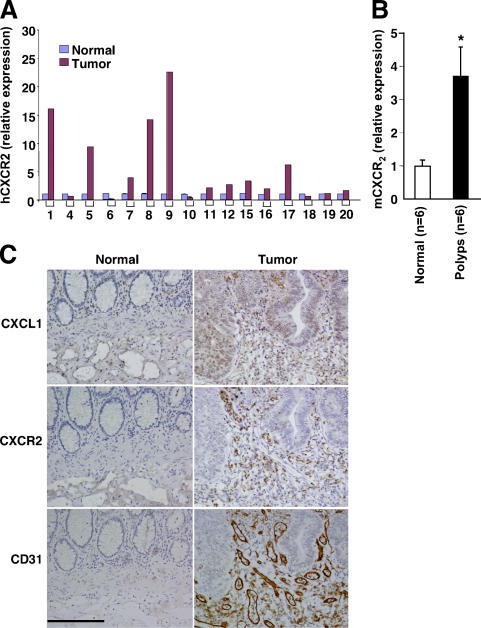
To establish the importance of CXCL1 in the CRC, we determined whether its receptor, CXCR2, is also elevated in human tumors. Analysis of real-time quantitative PCR shows that CXCR2 expression is elevated in 11 of 16 (69%) cancer specimens (Fig. 2 A) and in all intestinal adenomas of ApcMin mice we tested (Fig. 2 B). Furthermore, we examined cell-specific expression of CXCL1 and its receptor CXCR2 in human colorectal carcinomas using immunohistochemistry. As shown in Fig. 2 C, intense staining for CXCL1 in epithelial and stromal cells was observed in tumor tissues but not in matched normal tissues (Fig. 2 C, top), demonstrating that CXCL1 localizes to epithelial and stromal compartments of solid tumors. Strong CXCR2 immunostaining was also observed only in human tumor biopsies and not in matched normal samples (Fig. 2 C, middle). Interestingly, CXCR2 was expressed mainly in stromal endothelial cells as verified by staining with CD31 (Fig. 2 C, middle and bottom). These results indicate that CXCL1 secreted from human tumor epithelial cells may promote tumor-associated angiogenesis by binding to CXCR2 expressed on endothelial cells.
Figure 2.
CXCR2 expression is elevated in human colorectal tumors and adenomas of Apcmin mice. (A and B) The CXCL1 mRNA in human tumor samples listed above (A) and mouse intestinal adenoma tissues (B) were analyzed by quantitative real-time PCR. (C) Sections of formalin-fixed and paraffin-embedded tissues from 16 paired human tumors and the matched normal tissues were immunostained with anti-CXCL1 (top), anti-hCXCR2 (middle), or anti-CD31 antibody. A set of representative images is shown. Bar, 150 μm.
PGE2 stimulates production of CXCL1
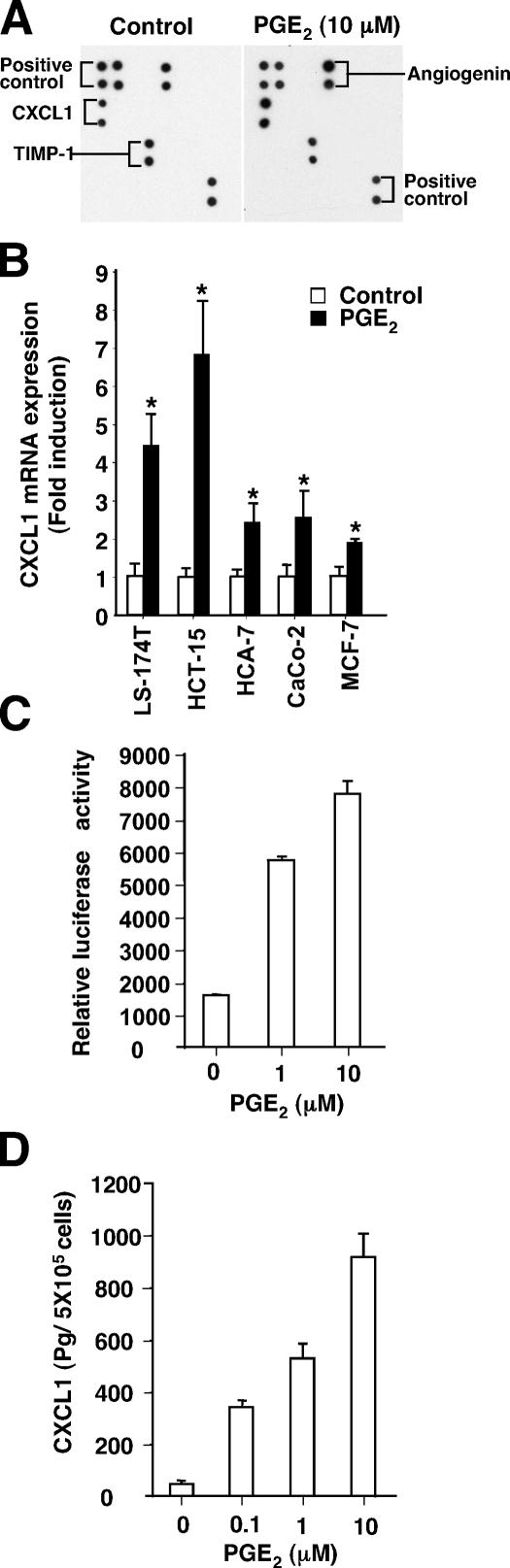
To determine whether PGE2 up-regulates CXCL1 and other angiogenic factors in CRC cells, a commercially available antibody array of pro-angiogenic factors was used. The array was incubated with conditioned medium taken from LS-174T cells treated with either vehicle or PGE2. By comparing the signal intensities, elevation of CXCL1 was seen in media obtained from LS-174T cells treated with PGE2 (Fig. 3 A). A quantitative PCR assay was performed to show that PGE2 induced CXCL1 mRNA levels in three additional colorectal carcinoma cell lines and one breast cancer cell line (Fig. 3 B). Because LS-174T and HCA-7 cell lines have been extensively used to investigate PGE2-dependent tumorigenesis, we further examined whether PGE2 could regulate CXCL1 promoter activity and protein secretion in these two cell lines. Compared with the control cells, PGE2-treated LS-174T cells showed an increase in both CXCL1 promoter activity (Fig. 3 C) and CXCL1 protein secretion (Fig. 3 D). The induction of CXCL1 was dependent on PGE2 concentration (Fig. 3, C and D). Similar results were observed in HCA-7 cells (not depicted). Collectively, these results demonstrate that PGE2 up-regulates CXCL1 transcription, expression, and release, establishing a link between a proinflammatory lipid mediator and a pro-angiogenic chemokine.
Figure 3.
PGE2 promotes CXCL1 expression and release. (A) Human antibody array analysis was used to determine the differences in the release of human angiogenic factors from the conditioned medium of LS-174T cells treated with vehicle (left) or PGE2 (right). The positive controls produced by biotin-conjugated IgG can be used to compare the relative expression levels among the different membranes. (B) Quantitative real-time PCR analysis of the mRNA level of CXCL1 in four CRC cell lines and one breast cancer cell line. The cells were incubated in serum-free media for 24 h and treated with 1 μM PGE2 for 4 h. The relative expression of target gene is averages of triplicates that are normalized against the transcript levels of hActin. Data are represented as the mean ± SE of the relative expression from three independent experiments. Asterisks represent statistical differences (P < 0.05; Student's t test). (C) PGE2 stimulates CXCL1 promoter activity. The LS-174T cells were transiently transfected with CXCL1 (−306 to +45) luciferase reporter gene and pRL-SV40 plasmids followed by treatment with PGE2 for 24 h. The dual luciferase assays were performed as described in Materials and methods. Data are represented as the mean ± SE of relative luciferase activity from three independent experiments. (D) The levels of CXCL1 protein in cell supernatants were determined by ELISA. Bar graphs show the values of CXCL1 concentration in the conditioned medium of LS-174T cells treated with indicated amounts of PGE2 for 24 h, adjusted for cell number. Three independent experiments in duplicate were performed.
PGE2 induction of CXCL1 involves the EGFR–MAPK cascade
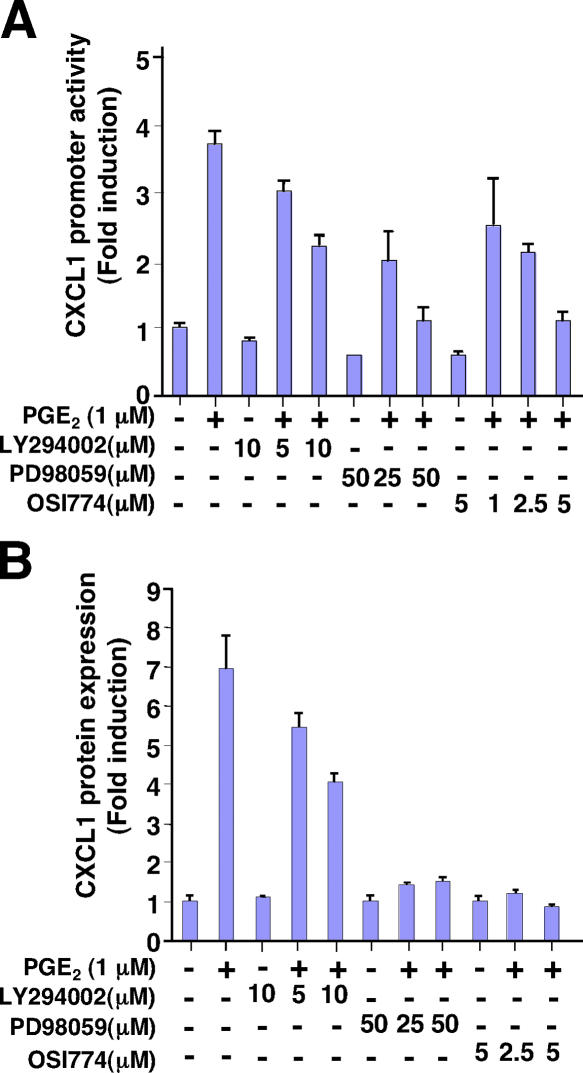
Because PGE2 can activate both the MAPK and PI3K–Akt pathways through EP4-EGFR receptor activation in LS- 174T cells (23, 24), we investigated potential signaling pathways affected by PGE2 that contribute to the regulation of CXCL1. Pretreatment of LS-174T cells with a specific MAPK inhibitor or EGFR tyrosine kinase inhibitor (Erlotinib, OSI774) blocked PGE2-induced CXCL1 promoter activity and protein expression, respectively, whereas a specific PI3K inhibitor (LY294002) had no effect (Fig. 4). These data suggest that an EGFR-MAPK pathway is involved in the induction of CXCL1 by PGE2.
Figure 4.
PGE2–up-regulated CXCL1 expression is dependent on MAPK pathway. (A) The effects of a MAPK inhibitor on PGE2–up-regulated CXCL1 promoter activity. The LS-174T cells were cultured and treated in the same conditions as noted in Fig. 3 C. The cells were pretreated with vehicle or increasing doses of inhibitors for 1 h before the PGE2 (1 μM) treatment after transfection. Data are represented as the mean ± SE of fold induction from three independent experiments. (B) The effects of a MAPK inhibitor on CXCL1 protein expression. LS-174T cells were cultured and treated as described in A. CXCL1 levels in cell supernatants were determined by ELISA. Data are represented as the mean ± SE of fold induction from three independent experiments.
The supernatants from PGE2-treated CRC cells induce endothelial migration and tube formation
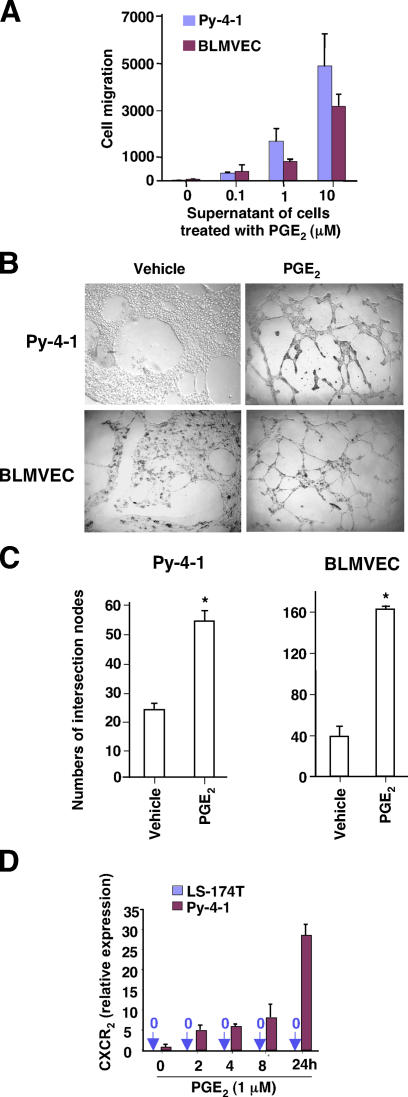
Migration of endothelial cells and assembly into tubes are two critical steps in tumor-associated angiogenesis. Because the conditioned medium from COX-2–overexpressing CRC cells stimulates both endothelial migration and tube formation (25), we assessed whether PGE2 mediates tumor-associated angiogenesis. As shown in Fig. 5 A, the supernatants from PGE2-treated LS-174T CRC cells induced the migration of mouse endothelial cells (Py-4-1) and bovine lung microvascular endothelial cells (BLMVECs). Furthermore, LS-174T–conditioned media enhanced the formation of extensive capillary-like structures in vitro (Fig. 5 B). Quantitative evaluation of tube formation revealed that conditioned media from PGE2-treated cells increased the intersection number of tubular structures by one- to threefold in Py-4-1 and BLMVECs, respectively (Fig. 5 C). Importantly, PGE2 also induced CXCR2 expression in Py-4-1 cells (Fig. 5 D), suggesting that CXCL1 mediates the downstream effects of PGE2 on endothelial cells. A similar result was observed in BLMVECs (not depicted). Two potential mechanisms that could explain the ability of PGE2 to enhance endothelial cell migration and tube formation were considered: (a) PGE2 directly stimulates endothelial cell migration and tube formation; or, (b) PGE2 stimulates CRC cells to produce pro-angiogenic factors that then affect endothelial cell biology. Our studies demonstrated that exogenous PGE2 alone in serum-free medium failed to induce endothelial cell migration or tube formation (not depicted). Collectively, these results indicate that PGE2 enhances microvascular endothelial cell migration and tube formation.
Figure 5.
Supernatants from LS-174T cells treated with PGE2 induce endothelial migration and tube formation. (A) Cell movement was evaluated by counting the number of cells that migrated toward conditioned medium in the undersurface of the filter. The data were represented as the mean ± SE of cell numbers of three independent experiments performed in triplicate. (B and C) The Py-4-1 cells (top) and BPMVECs (bottom) form capillary-like structures on growth factor–reduced Matrigel in vehicle- or 1 μM PGE2-treated conditioned media (B). The number of the intersections between branches of assembled endothelial cell networks was counted in whole field. Data were represented as the mean ± SE of intersection numbers of three independent experiments (C). Asterisks represent statistical differences (P < 0.05; Student's t test). (D) Quantitative real-time PCR analysis of the levels of CXCR2 mRNA in LS-174T and Py-4-1 cell lines was performed.
CXCL1 is required for PGE2-mediated stimulation of endothelial cell migration and tube formation
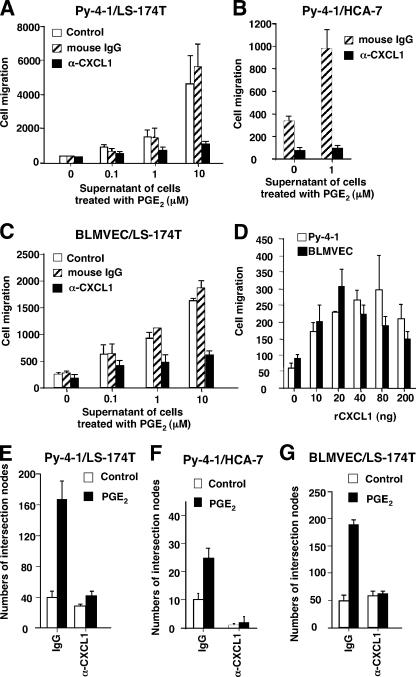
We next investigated whether CXCL1 mediates the effects of PGE2-induced endothelial cell migration and tube formation. We found that treatment with a CXCL1 neutralizing antibody inhibited Py-4-1 cell migration induced by the conditioned media taken from PGE2-treated LS-174T cells (Fig. 6 A) or PGE2-treated HCA-7 cells (Fig. 6 B). Similarly, the CXCL1 neutralizing antibody also inhibited the migration of BLMVECs induced by treatment with conditioned media (Fig. 6 C). We further examined whether CXCL1 alone can induce endothelial cell migration. As shown in Fig. 6 D, human rCXCL1 also stimulated migration of both endothelial cell lines. Consistent with the observations described above, blocking CXCL1 by adding a neutralizing antibody also completely inhibited Py-4-1 endothelial tube formation induced by treatment with conditioned media from LS-174T (Fig. 6 E) or HCA-7 (Fig. 6 F) cells. Furthermore, the observation that CXCL1 mediates Py-4-1 tube formation was confirmed in BLMVECs as well (Fig. 6 G). These results demonstrate that CXCL1 produced by PGE2-treated CRC cells can regulate angiogenesis in this in vitro model.
Figure 6.
CXCL1 mediates the effects of PGE2 on endothelial migration and tube formation. (A–C) The effects of CXCL1 neutralizing antibody on endothelial migration. The Py-4-1 cells (A and B) or BPMVCs (C) were cultured and treated in the same conditions as noted in Fig. 4 A. The supernatants from LS-174T cells (A or C) or HCA-7 cells (B) were pretreated with either 5 μg of normal mouse IgG or anti-hCXCL1 mouse monoclonal antibody for 1 h before being placed in the lower chamber. The data were represented as the mean ± SE of cell numbers of three independent experiments performed in triplicate. (D) Recombinant CXCL1 induces endothelial cell migration. The Py-4-1 cells and BPMVCs were cultured and treated in the same conditions as noted in Fig. 4 A. The serum-free DMEM medium with indicated amounts of CXCL1 was placed in the lower chamber. The data were represented as the mean ± SE of cell numbers of three independent experiments performed in triplicate. (E–G) The effects of CXCL1 neutralizing antibody on endothelial tube formation. The Py-4-1 cells (E and F) and BPMVCs (G) were cultured and treated in the same conditions as noted in Fig. 4 B. The supernatants from LS-174T (E and G) or HCA-7 (F) cells were pretreated with either 5 μg of normal mouse IgG or anti-hGROα mouse monoclonal antibody for 1 h before being placed in the lower chamber. Data were represented as the mean ± SE of intersection numbers of three independent experiments.
CXCL1 mediates the effect of PGE2 to stimulate tumor growth and increase microvessel density
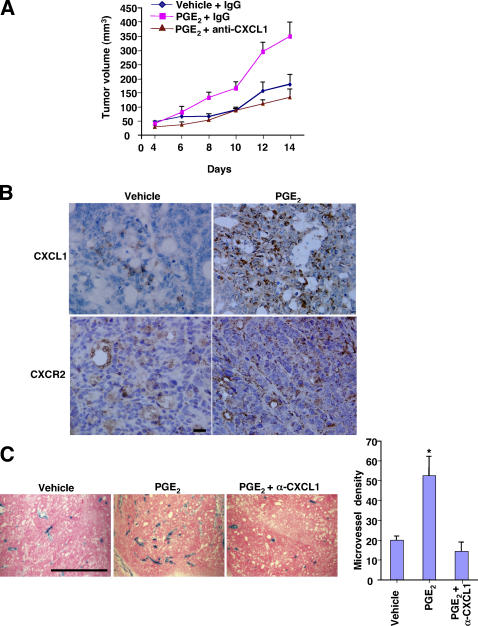
To determine whether PGE2-induced CXCL1 regulates tumor growth or tumor-associated angiogenesis in vivo, female Rag2 −/−/γc −/−/Flk1+/−lacZ triple mutant mice were generated by crossing the heterozygous Flk1 female mice (Flk1+/−lacZ) to mutant males (Rag2 −/−/γc −/y) and engrafting with human LS-174T cells (see Materials and methods for a complete description of how these mice strains were developed). The LS-174T cells were inoculated, and mice were treated with normal mouse IgG or anti-CXCL1 neutralizing mouse antibody (100 μg/mouse). After tumor engraftment (4 d), mice were gavaged with vehicle or PGE2 (300 μg/each mouse) twice daily and antibody treatment was administered every 2 d for 10 d. Tumor growth was recorded every 2 d for a period of 2 wk. We found that PGE2 treatment alone promoted tumor growth (Fig. 7 A) and induced CXCL1 expression (Fig. 7 B). Tumor cells showed diffuse cytoplasmic staining (brown) for CXCL1 (Fig. 7 B, top). Moreover, immunohistochemical staining was performed to determine whether CXCR2 receptors were present on the microvessels in these tumors. As shown in Fig. 7 B (bottom), CXCR2 was expressed on the endothelial cells in tumor microvessels and PGE2 treatment enhanced CXCR2 expression levels. In addition, PGE2-treated tumors exhibited a 2.6-fold higher density of lacZ-stained blood vessels than vehicle-treated tumors (Fig. 7 C). Similar to our observations in vitro, tumor growth and angiogenic response after PGE2 treatment was inhibited by administration of a CXCL1 neutralizing antibody (Fig. 7, A and C). Because CXCL1 is known to stimulate melanoma cell proliferation, it is possible that tumor growth in our studies could depend on the ability of CXCL1 to induce LS-174T cell proliferation. However, we observed that CXCL1 failed to stimulate LS-174T cell proliferation in vitro, even in suboptimal conditions (serum deprivation; not depicted). This is not surprising because LS-174T cells do not express the CXCL1 receptor, CXCR2 (Fig. 5 D). Collectively, these results suggest that PGE2 promotes tumor growth indirectly by inducing CXCL1 expression, which in turn stimulates angiogenesis.
Figure 7.
Blocking CXCL1 inhibited PGE2-enhanced tumor growth and angiogenesis. (A) The effect of PGE2 and anti-CXCL1 antibody on human colorectal xenograft tumor growth. The Rag2 −/−/γc −/−/Flk1 +/−lacZ mice were cotreated with a combination of vehicle and normal mouse IgG (n = 6), PGE2 and normal mouse IgG (n = 6), or PGE2 and anti-GROα (n = 6) as described in Materials and methods. (B) PGE2 up-regulates CXCL1 expression in xenografted tumors. Sections from each group were immunostained with anti-CXCL1 (top) or anti-mCXCR2 (bottom) antibody. A representative section shows strong diffuse cytoplasmic staining (brown) for CXCL1 in the tumor cells taken from mice treated with PGE2. Faint staining was observed in the tumor cells of vehicle-treated mice. In addition, a positive signal is visible when evaluating tumor microvessels. Bar, 50 μm. (C) lacZ-stained tumor blood vessels in Rag2 −/−/γc −/−/Flk1 +/−lacZ mice after PGE2 treatments. Photomicrographs of lacZ staining of representative tissue-sections are shown. Results were presented as the mean of vessel number ± SEM per field. Bar, 300 μm. A total of 120 fields were examined and counted from six tumors of each group. Four sections from six mice in each group were evaluated for quantification of lacZ staining. Results are as mean ± SEM. Values were statistically significant from each other (P < 0.05).
DISCUSSION
It is well established that high COX-2 enzyme levels correlate with poor clinical outcome in cancer patients (26–32), and inhibition of cyclooxygenase is an effective approach to reduce polyp burden in humans (33). Moreover, genetic studies demonstrate that colorectal tumor growth and vascular density are significantly attenuated in COX-2−/− null ApcMin mice (34), indicating that COX-2 plays a crucial role in tumor-associated angiogenesis (35, 36). Our laboratory demonstrated previously that overexpression of COX-2 in CRC cells stimulates endothelial cell migration and tube formation when endothelial cells were cocultured with CRC cells expressing COX-2 (25). However, all of the mechanisms by which COX-2 stimulates angiogenesis are not known. In this study, we extended our previous work to assess the role of COX-2–derived PGE2 in vitro and in vivo in tumor-associated angiogenesis. We identified CXCL1 as a key downstream mediator of PGE2 in regulating tumor-associated angiogenesis. We have established a role for CXCL1 in colorectal tumor–associated angiogenesis by our results demonstrating reduced tumor growth and decreased tumor vascular density in mice treated with a neutralizing CXCL1 antibody. These findings may have clinical relevance because CXCL1 is elevated in the majority of human CRCs and correlates well with tissue PGE2 levels. Importantly, this study delineates a novel molecular mechanism by which COX-2 regulates tumorassociated angiogenesis.
The molecular events involved in progression of CRC are complex, involving dysregulation of factors that regulate cell growth and cell death, evasion of host defenses, invasion, and metastasis. Tumor growth and metastatic spread of disease are dependent on angiogenesis (37–40), and substantial increases in tumor size require a dependable blood supply. For neoplasms to develop a stable blood supply, the tumor cells and/or the stromal microenvironment must secrete a variety of pro-angiogenic factors, including vascular endothelial growth factor (VEGF) and CXC chemokines that stimulate endothelial cell proliferation, migration, and tube formation (19–21, 25, 41–44). Inhibition of tumor-associated angiogenesis is being pursued as a promising therapeutic strategy for treatment of patients with advanced disease. Recently, Avastin (bevacizumab), which blocks VEGF signaling, was approved for treatment of patients with advanced CRC. However, treatment with bevacizumab plus chemotherapy extends progression-free survival in patients with metastatic CRC by only 5–10 mo (45, 46), and this treatment is very expensive. ELISA analysis revealed that LS-174T cells do secrete VEGF at very low levels compared with the amount of CXCL1 (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20052124/DC1, and Fig. 3 D). Therefore, it is crucial to determine what other pro-angiogenic factors are involved in progression of CRC. Our in vitro and in vivo results here demonstrate that CXCL1 is an important target for PGE2 signaling in CRC cells (Figs. 3 and 7 B).
Although our studies support the role of CXCL1 as an important angiogenic factor in tumor growth, other angiogenic factors are likely to be involved as well. For example, we were unable to fully inhibit basal tumor–associated angiogenesis by only targeting CXCL1 (Fig. 7 C). Moreover, the ability of CXCL1 alone to induce cell migration is less than that seen with conditioned media (Fig. 6 D), indicating that CXCL1 is not the sole factor involved in the regulation of endothelial cell migration. One plausible explanation is that human CRC cells in vivo produce other members of the CXC chemokine family, bFGF, and VEGF, which also promote neovascularization. For example, recombinant hVEGF stimulated endothelial cell migration in both Py-4-1 and BLMVECs (Fig. S1 B), although the ability of VEGF alone to induce cell migration is much less than that seen with conditioned media. However, VEGF failed to synergize with CXCL1 on stimulating endothelial cell migration in our in vitro model (Fig. S1 C). It is possible that VEGF may synergize with CXCL1 to further stimulate angiogenesis in vivo. Further work is needed to determine whether both angiogenic factors coordinate tumor-associated angiogenesis in vivo. We have performed real-time quantitative PCR assays to determine whether VEGF levels correlate with CXCL1 in human tumor samples. As shown in Fig. S1 E and Fig. 1 A, VEGF expression is elevated in 17 of 20 (85%) cancer specimens and positively correlates with CXCL1 expression (r = 0.65, P < 0.0001). In this study, we demonstrate that PGE2 accelerates tumor growth, in part, by inducing CXCL1 secretion by tumor epithelial cells. These results may point out, in part, why treating patients with only one anti-angiogenic agent may not be the most effective clinical regimen.
Chemokines and their respective receptors are classified into the CXC, CC, C, and CX3C families based on the positions of their conserved two NH2-terminal cysteine residues. Although chemokines play a crucial role in immune and inflammatory reactions, such as allergic disorders, autoimmune diseases, and viral infections, recent studies indicate that they have an equally important role in the development of a variety of cancers, such as melanomas, ovarian, breast, lung, and prostate cancers (47). Some of these chemokines are involved in transformation, survival, growth, metastasis, and angiogenesis. The chemokine receptors expressed on tumor cells may contribute to organ-specific metastasis. For example, CXCR4 is expressed and participates in directed migration of cancer cells to sites of metastasis in many cancers, including small cell lung cancer, pancreatic cancer, astrogliomas, myelomas, B cell lymphomas, and chronic lymphocytic leukemias (48). Moreover, tumor cells and/or stromal cells produce inflammatory chemokines, which in turn result in the recruitment of various types of leukocytes into the tumor tissue through binding to their receptors expressed on these leukocytes. These infiltrating leukocytes in tumors promote neoplastic development. In breast cancer, it has been shown that macrophage infiltration promotes tumor invasion and metastasis (49).
PGE2 inhibits production of CC chemokines CCL3 (MIP-1α) and CCL4 (MIP-1β) in dendritic cells through binding to the EP2 receptor (50, 51) and suppresses CC chemokine CCL5 (RANTES [regulated upon activation, normal T cell expressed and secreted]) production in human macrophages through the EP4 receptor (52). Because breast cancer cells produce CCL5, we examined whether PGE2 regulates these CC chemokines in CRC cells. Analysis of real-time quantitative PCR revealed that PGE2 up-regulated expression of CCL3, CCL4, and CCL5 in LS-174T cells (Fig. S1 D). These CC chemokines are crucial for macrophage and lymphocyte infiltration in human breast, cervix, pancreas, and gliomas cancers (53, 54). These results indicate that PGE2 promotes tumor growth and metastasis through, in part, by recruiting these macrophages and lymphocytes into tumor tissues. Moreover, a recent study showed that COX-2 up-regulates expression of CXC chemokines such as CXCL5 (epithelial cell–derived neutrophil activator 78) and CXCL8 (IL-8) in human non-small cell lung cancer cells (55), indicating that PGE2 can regulate the expression of CXC chemokines in certain contexts. In this study, we present a novel mechanism by which PGE2 stimulates expression of the CXC chemokine CXCL1 in CRC cells through activation of an EGFR–MAPK cascade (Fig. 3 and 4). Because our previous results showed that PGE2 can transactivate EGFR through activation of EP4 receptor signaling in LS-174T cells (23), it is likely that the EP4 mediates the effects seen on the EGFR–MAPK signaling cascade.
Recent evidence demonstrates that CXCR2 is expressed in intestinal microvessels (56) and other endothelial cells, such as lung microvascular endothelial cells, dermal microvascular endothelial cells, umbilical vein endothelial cells, and saphenous vein endothelial cells (57). Moreover, inhibition of COX-2 by nonselective or selective nonsteroidal antiinflammatory drugs suppresses αVβ3-mediated Cdc42/Rac-depenent migration and spreading of endothelial cells (58). Interestingly, PGE2 promotes αVβ3-Cdc42/Rac-dependent migration and spreading of endothelial cells (59). There is strong evidence indicating that CXCL1 can promote Rac/cdc42-Pak1–dependent migration via the CXCR2 receptor (60). Thus, it is possible that the Rac/cdc42-Pak1 cascade is required for CXCL1-induced endothelial cell migration and tube formation. Further studies are needed to address these issues more completely.
The biological significance of the PGE2-induced CXCL1 expression and secretion in CRC cells was substantiated by the results of our in vivo experiments. Administration of exogenous PGE2 promoted tumor growth and increased microvessel density. Inhibition of PGE2-induced CXCL1 signaling led to inhibition of tumor growth with decreased microvessel density (Fig. 7). One very interesting finding from both our in vitro and in vivo experiments is that CXCL1 expression correlates with PGE2 levels in human CRCs (Fig. 1).
The key finding of this study is that PGE2 stimulates tumor epithelial cells to secrete the pro-angiogenic chemokine CXCL1 via activation of the EP4-EGFR–MAPK cascade. Furthermore, we reveal the importance of PGE2–up-regulated CXCL1 in tumor-associated angiogenesis. To our knowledge, this represents the first report suggesting that the up-regulation of CXCL1 is directly involved in the pro-angiogenic effects of PGE2 in CRC.
MATERIALS AND METHODS
Cell culture and reagents.
LS-174T, HCA-7, HCT-15, CaCo-2, and MCF-7 cells were maintained in McCoy's 5A medium with 10% FBS (Hyclone). BLMVECs (VEC Technologies) were maintained in EGM medium (Cambrex Corp.) with 5% FBS. A mouse endothelial cell line (Py-4-1) established from hemangiomas of adult transgenic mice carrying the Py virus early region transgene was cultured in 1.5% gelatin-coated plates with DMEM medium with 10% FBS. PD98059 and LY 294002 were obtained from Calbiochem. OSI 774 was provided by Genentech, Inc.
CXCL1 and ELISA.
CXCL1 production from cell-free supernatants and tissues was measured by using a human CXCL1 Quantikine ELISA kit (R&D Systems) according to the manufacturer's instructions. In brief, 0.5 × 106 LS-174T cells were cultured in serum-free medium for 16 h and pretreated with indicated inhibitors for 1 h. After pretreatment, cells were treated with vehicle or indicated concentration of PGE2 for 24 h. The serum-free conditioned media was subjected to ELISA assay. For human tumor and normal tissues, total proteins were extracted by homogenizing and subsequently sonicating in anti-protease buffer (50 mM Hepes, 150 mM NaCl, and 1 mM EDTA) with protease inhibitor cocktail tablets (Boehringer). With Vanderbilt University Internal Review Board approval, human colorectal tumor and matching normal mucosa specimens were obtained from surgical resections.
PGE2 levels.
Human colorectal tumor and matched normal tissues were homogenized, and PGE2 was acidified and extracted from the homogenate as described previously (61). The levels of PGE2 in the tissue were quantified using gas chromatography negative ion chemical ionization mass spectrometry as described previously (61). The concentration of PGE2 in the samples was calculated by comparing the ratios of its peak areas with the internal standard.
Real-time quantitative PCR.
CXCL1, MIP-2, KC, hCXCR2, and mCXCR2 mRNA was quantified by real-time quantitative PCR using iCycler (Bio-Rad Laboratories) and iQ SYBR Green Supermix (Bio-Rad Laboratories). In brief, total RNA was isolated using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. The mRNA was reverse transcribed using SuperScript II RT kit (Invitrogen). The real-time PCR assay was conducted according to the manufacturer's instructions. Primers for CXCL1, MIP-2, KC, and mGAPDH genes were chosen by using the computer program Beacon Designer 4. The sequences of the specific PCR primers were as follows (5′ to 3′): hβ-actin, forward: AGAAAATCTGGCACCACACC; reverse: AGAGGCGTACAGGGATAGCA; CXCL1, forward: AACCGAAGTCATAGCCACAC; reverse: GTTGGATTTGTCACTGTTCAGC; hCXCR2, forward: AACATGGAGAGTGACAGCTTTG; reverse: TTCACATGGGGCGGCATC; mGAPDH, forward: GCCTTCCGTGTTCCTACCC; reverse: TGCCTGCTTCACCACCTTC; mCXCR2, forward: GTGCATAGCCATGTGGTTAC; reverse: GGCAGGATACGCAGTACG; MIP-2, forward: GGGAGAGGGTGAGTTGGG; reverse: GCACACTACTTCCATGAAAGC; and KC, forward: AAAAGGTGTCCCCAAGTA; reverse: AAGCAGAACTGAACTACCATCG. Relative signal intensity (CXCL1/β-actin, hCXCX2/β-actin, MIP-2/ GAPDH, KC/GAPDH, and mCXCR2/GAPDH) was measured and used for quantification.
Human angiogenesis antibody array.
Pro-angiogenic protein levels from cell-free supernatants were determined by using the RayBio Human Angiogenesis Antibody Array I (RayBiotech Inc.) according to the manufacturer's instructions. In brief, 0.5 × 106 LS-174T cells were cultured in serum-free medium for 16 h and treated with vehicle or PGE2 for 24 h. The membranes were exposed to a blocking buffer, and 1 ml of the conditioned media from cells was then treated with either vehicle or PGE2. The membranes were washed and incubated by primary biotin-conjugated antibody. The membranes were then incubated with horseradish peroxidase–conjugated streptavidin, and the protein spots were detected using the ECL Western blotting detection reagents (GE Healthcare) according to the manufacturer's instructions.
Transfection and reporter activity assay.
CXCL1 promoter reporter constructs were obtained from A. Richmond (Vanderbilt University, Nashville, TN) as described previously (62). The LS-174T cells (2.0 × 105) were transiently cotransfected with 0.4 μg CXCL1 (−306 to +45) and 5 ng pRL-SV40 plasmids using LipofectAMINE Plus reagent according to the manufacturer's instructions (Life Technologies). After incubation for 3 h, fresh serum-free media was added and the cells were incubated for an additional 4 h. The cells were then pretreated with inhibitors as indicated for 1 h and treated with either vehicle or/and PGE2 for 24 h. Luciferase activity was measured using a Dual Luciferase kit (Promega) with a Monolight 3010 luminometer (BD Biosciences). The relative luciferase activity was determined and normalized to Renilla luciferase.
Migration assays.
Migration assays were performed using transwells (6.5 mm in diameter and 8-μm pore size; Corning Costar Co.) as described previously (24). After a 24-h incubation in serum-free DMEM medium, cells (4 × 104/well) were suspended in 400 μl of serum-free DMEM medium and placed in the upper chamber. The lower chamber was filled with 1 ml of the conditioned media from LS-174T or HCA-7 cells treated with vehicle or PGE2. For antibody studies, the supernatants from both cells were pretreated with either 5 μg of normal mouse IgG or anti-CXCL1 mouse monoclonal antibody (R&D Systems) for 1 h. The supernatants were then placed in the lower chamber. For recombinant CXCL1 studies, the serum-free DMEM medium with indicated amounts of CXCL1 was placed in the lower chamber. After 24 h at 37°C, the cells on the upper surface of the filter were removed. The filters were fixed and stained with 0.5% crystal violet solution. Cells adhering to the undersurface of the filter were counted, and the data were represented as the mean ± SE of cell numbers of three independent experiments in triplicate.
In vitro vascular assembly assays.
In vitro vascular assembly assays were performed as described previously (25). Transwells (12 mm in diameter and 0.4-μm pore size; Corning Costar Co.) were coated with 100 μl of growth factor–reduced Matrigel (BD Biosciences). After 24 h in OPTI-MEM I Reduced-Serum Medium (Life Technologies), 1.4 × 104 BPMVECs or 3 × 104 Py-4-1 cells were plated in wells, respectively. The lower chamber was filled with 1 ml of the conditioned media from LS-174T or HCA-7 cells treated with vehicle or PGE2. For antibody studies, the supernatants from both cell lines were pretreated with either 5 μg of normal mouse IgG or anti-CXCL1 mouse antibody for 1 h. After incubation for 12 h for Py-4-1 cells or 16 h for BMMVECs, tube-like structures were quantified by counting the number of intersections between branches of endothelial cell networks in whole field. Values were represented as the mean ± SE of intersection numbers of three independent experiments.
Allograft tumor growth.
Rag2−/− knockout mice have been developed that express no T and B lymphocytes, whereas Rag2(−/−)/IL-2Rγ−/− (common γ-chain [γc]) double knockout mice (Rag2(−/−)/γc(−/−)) completely lacked T and B lymphocytes and natural killer cells. It has been reported that Rag2−/−/γc−/− mice accepted the grafts at the greatest rate as compared with SCID and Rag2−/− mice (63). The heterozygous Flk1 female mice (Flk1+/−lacZ) were bred to mutant males (Rag2(−/−)/γc(−/y), homozygous for Rag2 and hemizygous null for the γc). The heterozygous female offspring (Rag2+/−/γc+/−/Flk1+/−lacZ) were identified by PCR with allele-specific primers. These females were then mated back to mutant males (Rag2−/−/γc−/y) to obtain Rag2−/−/γc+/y/Flk1+/−lacZ male mice. The males were then bred back to the Rag2+/−/γc+/− female mice to generate triple mutant female mice (Rag2−/−/γc−/−/Flk1+/−lacZ) and male mice (Rag2−/−/γc−/y/Flk1+/−lacZ). The colony (Rag2−/−/γc−/−/Flk1+/−lacZ) has been established at our facility by crossing triple mutant female mice (Rag2−/−/γc−/−/Flk1+/−lacZ) with triple mutant male mice (Rag2−/−/γc−/y/Flk1+/−lacZ). PCR analysis of tail genomic DNA was used to determine the genotypes.
LS-174T cells were used for tumor growth studies in vivo. A suspension of 2.5 × 106 LS-174T cells in 0.1 ml PBS was placed under the back skin of 7–8-wk-old female Rag2 −/−/γc −/−/Flk1 +/−lacZ mice. The mice (n = 18) were randomly placed into three groups according to the PGE2 and anti-CXCL1 antibody treatment regimen (vehicle/100 μg normal mouse IgG/each mouse, 300 μg PGE2/100 μg normal mouse IgG/each mouse, and 300 μg PGE2/100 μg anti-CXCL1/each mouse). The mice were injected with either normal mouse IgG or anti-CXCL1 neutralizing mouse antibody every 2 d via intraperitoneal administration. The animals were maintained under sterile conditions in laminar flow rooms, and tumors were measured every 2 d by direct measurement of tumor volume in groups of six. After tumor engraftment (4 d), these mice were gavaged with the vehicle or PGE2 twice a day for 10 d. Tumor volume was determined by external measurement and calculated according to the equation (V = 0.5 × [LW2]), where V = volume, L = length, and W = width. A portion of the tumor was fixed in 0.2% paraformaldehyde for lacZ staining and immunohistochemical staining.
lacZ staining and quantification.
The expression of β-galactosidase was assessed by lacZ staining as described previously (64). Small pieces of tissue were fixed in 0.2% paraformaldehyde solution followed by infusion in 30% sucrose at 4°C overnight. Tissues were embedded in OCT and snap-frozen. Frozen sections were mounted onto glass slides and stained overnight at 37°C using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside as a substrate. Sections were counterstained with eosin. The number of lacZ-stained blood vessels in tumor tissues was quantitated. Random sections of tumor tissues were used for lacZ staining, and measurements were made using the Scion Image program (Scion Corp.). Any distinct area of positive staining for lacZ (blue) was counted as a single vessel. Results were expressed as the mean number of vessels ± SEM per field (100×). 120 fields were examined and counted from six tumors of each groups.
Immunohistochemical staining.
5-μm-thick tissue sections (n = 5 per animal) were stained with anti-CXCL1, mouse monoclonal antibody (R&D Systems), anti-hCXCR2, anti-CD31 (Santa Cruz Biotechnology, Inc.), and anti-mCXCR2 goat antibody at a dilution of 1:250. The immunohistochemical staining was completed by using a Zymed-Histostain-SP kit (Zymed Laboratories) as described previously (9).
Online supplemental material.
Fig. S1 A shows that PGE2 induces VEGF secretion. Fig. S1 B demonstrates that recombinant hVEGF stimulates endothelial cell migration. Fig. S1 C reveals that VEGF does not synergize with CXCL1 on inducing endothelial cell migration. Fig. S1 D illustrates the ability of PGE2 to induce CC chemokines. Fig. S1 E shows VEGF mRNA expression in human colorectal tumor and matched normal tissues. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20052124/DC1.
Supplemental Material
Acknowledgments
The authors gratefully acknowledge Vijaykumar R. Holla and Jason R. Mann for valuable contributions to the RNA samples.
This work is supported, in part, from the United States Public Health Services Grants RO1DK 62112, P01-CA-77839, R37-DK47297, and P30 DK58404 (all to R.N. DuBois). R.N. DuBois is the B.F. Byrd Professor of Molecular Oncology. R.N. DuBois (R37-DK47297) is recipient of a National Institutes of Health MERIT award. We also thank the T.J. Martell Foundation and the National Colorectal Cancer Research Alliance (NCCRA) for generous support (to R.N. DuBois).
The authors have no conflicting financial interests.
Abbreviations used: BLMVEC, bovine lung microvascular endothelial cell; COX-2, cyclooxygenase-2; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; GRO, growth-regulated oncogene; MAPK, mitogen-activated protein kinase; MIP, macrophage inflammatory protein; PGE2, prostaglandin E2; VEGF, vascular endothelial growth factor.
References
- 1.Kuper, H., H.O. Adami, and D. Trichopoulos. 2000. Infections as a major preventable cause of human cancer. J. Intern. Med. 248:171–183. [DOI] [PubMed] [Google Scholar]
- 2.DuBois, R.N., S.B. Abramson, L. Crofford, R.A. Gupta, L.S. Simon, L.B. Van De Putte, and P.E. Lipsky. 1998. Cyclooxygenase in biology and disease. FASEB J. 12:1063–1073. [PubMed] [Google Scholar]
- 3.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847–855. [PubMed] [Google Scholar]
- 4.Anisowicz, A., M. Messineo, S.W. Lee, and R. Sager. 1991. An NF-kappa B-like transcription factor mediates IL-1/TNF-alpha induction of gro in human fibroblasts. J. Immunol. 147:520–527. [PubMed] [Google Scholar]
- 5.Pugh, S., and G.A. Thomas. 1994. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E 2. Gut. 35:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigas, B., I.S. Goldman, and L. Levine. 1993. Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med. 122:518–523. [PubMed] [Google Scholar]
- 7.Mahmoud, N.N., A.J. Dannenberg, R.T. Bilinski, J.R. Mestre, A. Chadburn, M. Churchill, C. Martucci, and M.M. Bertagnolli. 1999. Administration of an unconjugated bile acid increases duodenal tumors in a murine model of familial adenomatous polyposis. Carcinogenesis. 20:299–303. [DOI] [PubMed] [Google Scholar]
- 8.Giardiello, F.M., R.A. Casero Jr., S.R. Hamilton, L.M. Hylind, J.D. Trimbath, D.E. Geiman, K.R. Judge, W. Hubbard, G.J. Offerhaus, and V.W. Yang. 2004. Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis. Gastroenterology. 126:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, D., H. Wang, Q. Shi, S. Katkuri, W. Walhi, B. Desvergne, S.K. Das, S.K. Dey, and R.N. DuBois. 2004. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 6:285–295. [DOI] [PubMed] [Google Scholar]
- 10.Jones, M.K., H. Wang, B.M. Peskar, E. Levin, R.M. Itani, I.J. Sarfeh, and A.S. Tarnawski. 1999. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med. 5:1418–1423. [DOI] [PubMed] [Google Scholar]
- 11.Hammond, M.E., V. Shyamala, M.A. Siani, C.A. Gallegos, P.H. Feucht, J. Abbott, G.R. Lapointe, M. Moghadam, H. Khoja, J. Zakel, and P. Tekamp-Olson. 1996. Receptor recognition and specificity of interleukin-8 is determined by residues that cluster near a surface-accessible hydrophobic pocket. J. Biol. Chem. 271:8228–8235. [DOI] [PubMed] [Google Scholar]
- 12.Anisowicz, A., L. Bardwell, and R. Sager. 1987. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc. Natl. Acad. Sci. USA. 84:7188–7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmond, A., D.H. Lawson, D.W. Nixon, and R.K. Chawla. 1985. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 45:6390–6394. [PubMed] [Google Scholar]
- 14.Richmond, A., and H.G. Thomas. 1986. Purification of melanoma growth stimulatory activity. J. Cell. Physiol. 129:375–384. [DOI] [PubMed] [Google Scholar]
- 15.Cochran, B.H., A.C. Reffel, and C.D. Stiles. 1983. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 33:939–947. [DOI] [PubMed] [Google Scholar]
- 16.Tekamp-Olson, P., C. Gallegos, D. Bauer, J. McClain, B. Sherry, M. Fabre, S. van Deventer, and A. Cerami. 1990. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J. Exp. Med. 172:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luan, J., R. Shattuck-Brandt, H. Haghnegahdar, J.D. Owen, R. Strieter, M. Burdick, C. Nirodi, D. Beauchamp, K.N. Johnson, and A. Richmond. 1997. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J. Leukoc. Biol. 62:588–597. [DOI] [PubMed] [Google Scholar]
- 18.Balentien, E., B.E. Mufson, R.L. Shattuck, R. Derynck, and A. Richmond. 1991. Effects of MGSA/GRO alpha on melanocyte transformation. Oncogene. 6:1115–1124. [PubMed] [Google Scholar]
- 19.Strieter, R.M., P.J. Polverini, S.L. Kunkel, D.A. Arenberg, M.D. Burdick, J. Kasper, J. Dzuiba, J. Van Damme, A. Walz, D. Marriott, et al. 1995. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 270:27348–27357. [DOI] [PubMed] [Google Scholar]
- 20.Lane, B.R., J. Liu, P.J. Bock, D. Schols, M.J. Coffey, R.M. Strieter, P.J. Polverini, and D.M. Markovitz. 2002. Interleukin-8 and growth-regulated oncogene alpha mediate angiogenesis in Kaposi's sarcoma. J. Virol. 76:11570–11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arenberg, D.A., P.J. Polverini, S.L. Kunkel, A. Shanafelt, J. Hesselgesser, R. Horuk, and R.M. Strieter. 1997. The role of CXC chemokines in the regulation of angiogenesis in non-small cell lung cancer. J. Leukoc. Biol. 62:554–562. [DOI] [PubMed] [Google Scholar]
- 22.Kettunen, H.L., A.S. Kettunen, and N.E. Rautonen. 2003. Intestinal immune responses in wild-type and Apcmin/+ mouse, a model for colon cancer. Cancer Res. 63:5136–5142. [PubMed] [Google Scholar]
- 23.Buchanan, F.G., D. Wang, F. Bargiacchi, and R.N. DuBois. 2003. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J. Biol. Chem. 278:35451–35457. [DOI] [PubMed] [Google Scholar]
- 24.Sheng, H., J. Shao, M.K. Washington, and R.N. DuBois. 2001. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 276:18075–18081. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii, M., S. Kawano, S. Tsuji, H. Sawaoka, M. Hori, and R.N. DuBois. 1998. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 93:705–716. [DOI] [PubMed] [Google Scholar]
- 26.Eberhart, C.E., R.J. Coffey, A. Radhika, F.M. Giardiello, S. Ferrenbach, and R.N. DuBois. 1994. Up-regulation of cyclooxygenase-2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 107:1183–1188. [DOI] [PubMed] [Google Scholar]
- 27.Marnett, L.J., and R.N. DuBois. 2002. COX-2: a target for colon cancer prevention. Annu. Rev. Pharmacol. Toxicol. 42:55–80. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan, K.M., K. Sheahan, D.P. O'Donoghue, F. MacSweeney, R.M. Conroy, D.J. Fitzgerald, and F.E. Murray. 1999. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 282:1254–1257. [DOI] [PubMed] [Google Scholar]
- 29.Khuri, F.R., H. Wu, J.J. Lee, B.L. Kemp, R. Lotan, S.M. Lippman, L. Feng, W.K. Hong, and X.C. Xu. 2001. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin. Cancer Res. 7:861–867. [PubMed] [Google Scholar]
- 30.Ristimaki, A., A. Sivula, J. Lundin, M. Lundin, T. Salminen, C. Haglund, H. Joensuu, and J. Isola. 2002. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 62:632–635. [PubMed] [Google Scholar]
- 31.Gaffney, D.K., J. Holden, M. Davis, K. Zempolich, K.J. Murphy, and M. Dodson. 2001. Elevated cyclooxygenase-2 expression correlates with diminished survival in carcinoma of the cervix treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 49:1213–1217. [DOI] [PubMed] [Google Scholar]
- 32.Buskens, C.J., B.P. Van Rees, A. Sivula, J.B. Reitsma, C. Haglund, P.J. Bosma, G.J. Offerhaus, J.J. Van Lanschot, and A. Ristimaki. 2002. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 122:1800–1807. [DOI] [PubMed] [Google Scholar]
- 33.Gupta, R.A., and R.N. Dubois. 2001. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 1:11–21. [DOI] [PubMed] [Google Scholar]
- 34.Chulada, P.C., M.B. Thompson, J.F. Mahler, C.M. Doyle, B.W. Gaul, C. Lee, H.F. Tiano, S.G. Morham, O. Smithies, and R. Langenbach. 2000. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 60:4705–4708. [PubMed] [Google Scholar]
- 35.Williams, C.S., M. Tsujii, J. Reese, S.K. Dey, and R.N. DuBois. 2000. Host cyclooxygenase-2 modulates carcinoma growth. J. Clin. Invest. 105:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshima, M., J.E. Dinchuk, S.L. Kargman, H. Oshima, B. Hancock, E. Kwong, J.M. Trzaskos, J.F. Evans, and M.M. Taketo. 1996. Suppression of intestinal polyposis in APCΔ716 knockout mice by inhibition of prostaglandin endoperoxide synthase-2 (COX-2). Cell. 87:803–809. [DOI] [PubMed] [Google Scholar]
- 37.Folkman, J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1:27–31. [DOI] [PubMed] [Google Scholar]
- 38.Folkman, J. 1971. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285:1182–1186. [DOI] [PubMed] [Google Scholar]
- 39.Zetter, B.R. 1998. Angiogenesis and tumor metastasis. Annu. Rev. Med. 49:407–424. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan, D., and J. Folkman. 1996. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 86:353–364. [DOI] [PubMed] [Google Scholar]
- 41.Arenberg, D.A., S.L. Kunkel, P.J. Polverini, M. Glass, M.D. Burdick, and R.M. Strieter. 1996. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J. Clin. Invest. 97:2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risau, W. 1997. Mechanisms of angiogenesis. Nature. 386:671–674. [DOI] [PubMed] [Google Scholar]
- 43.Korpelainen, E.I., and K. Alitalo. 1998. Signaling angiogenesis and lymphangiogenesis. Curr. Opin. Cell Biol. 10:159–164. [DOI] [PubMed] [Google Scholar]
- 44.Neufeld, G., T. Cohen, S. Gengrinovitch, and Z. Poltorak. 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13:9–22. [PubMed] [Google Scholar]
- 45.Hurwitz, H., L. Fehrenbacher, W. Novotny, T. Cartwright, J. Hainsworth, W. Heim, J. Berlin, A. Baron, S. Griffing, E. Holmgren, et al. 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 46.Franson, P.J., and D.V. Lapka. 2005. Antivascular endothelial growth factor monoclonal antibody therapy: a promising paradigm in colorectal cancer. Clin. J. Oncol. Nurs. 9:55–60. [DOI] [PubMed] [Google Scholar]
- 47.Balkwill, F. 2004. Cancer and the chemokine network. Nat. Rev. Cancer. 4:540–550. [DOI] [PubMed] [Google Scholar]
- 48.Balkwill, F. 2004. The significance of cancer cell expression of the chemokine receptor CXCR 4. Semin. Cancer Biol. 14:171–179. [DOI] [PubMed] [Google Scholar]
- 49.Lin, E.Y., A.V. Nguyen, R.G. Russell, and J.W. Pollard. 2001. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jing, H., E. Vassiliou, and D. Ganea. 2003. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J. Leukoc. Biol. 74:868–879. [DOI] [PubMed] [Google Scholar]
- 51.Jing, H., J.H. Yen, and D. Ganea. 2004. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E 2. J. Biol. Chem. 279:55176–55186. [DOI] [PubMed] [Google Scholar]
- 52.Takayama, K., G. Garcia-Cardena, G.K. Sukhova, J. Comander, M.A. Gimbrone Jr., and P. Libby. 2002. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J. Biol. Chem. 277:44147–44154. [DOI] [PubMed] [Google Scholar]
- 53.Balkwill, F., and A. Mantovani. 2001. Inflammation and cancer: back to Virchow? Lancet. 357:539–545. [DOI] [PubMed] [Google Scholar]
- 54.Bottazzi, B., N. Polentarutti, R. Acero, A. Balsari, D. Boraschi, P. Ghezzi, M. Salmona, and A. Mantovani. 1983. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 220:210–212. [DOI] [PubMed] [Google Scholar]
- 55.Pold, M., L.X. Zhu, S. Sharma, M.D. Burdick, Y. Lin, P.P. Lee, A. Pold, J. Luo, K. Krysan, M. Dohadwala, et al. 2004. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 64:1853–1860. [DOI] [PubMed] [Google Scholar]
- 56.Heidemann, J., H. Ogawa, M.B. Dwinell, P. Rafiee, C. Maaser, H.R. Gockel, M.F. Otterson, D.M. Ota, N. Lugering, W. Domschke, and D.G. Binion. 2003. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR 2. J. Biol. Chem. 278:8508–8515. [DOI] [PubMed] [Google Scholar]
- 57.Hillyer, P., E. Mordelet, G. Flynn, and D. Male. 2003. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin. Exp. Immunol. 134:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dormond, O., A. Foletti, C. Paroz, and C. Ruegg. 2001. NSAIDs inhibit alpha V beta 3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat. Med. 7:1041–1047. [DOI] [PubMed] [Google Scholar]
- 59.Dormond, O., M. Bezzi, A. Mariotti, and C. Ruegg. 2002. Prostaglandin E2 promotes integrin alpha Vbeta 3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J. Biol. Chem. 277:45838–45846. [DOI] [PubMed] [Google Scholar]
- 60.Wang, D., J. Sai, G. Carter, A. Sachpatzidis, E. Lolis, and A. Richmond. 2002. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry. 41:7100–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DuBois, R.N., J. Awad, J. Morrow, L.J. Roberts, and P.R. Bishop. 1994. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J. Clin. Invest. 93:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nirodi, C., S. NagDas, S.P. Gygi, G. Olson, R. Aebersold, and A. Richmond. 2001. A role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J. Biol. Chem. 276:9366–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenberg, L.H., and O.D. Slayden. 2004. Human endometriotic xenografts in immunodeficient RAG-2/gamma(c)KO mice. Am. J. Obstet. Gynecol. 190:1788–1795. [DOI] [PubMed] [Google Scholar]
- 64.Ma, W., J. Tan, H. Matsumoto, B. Robert, D.R. Abrahamson, S.K. Das, and S.K. Dey. 2001. Adult tissue angiogenesis: evidence for negative regulation by estrogen in the uterus. Mol. Endocrinol. 15:1983–1992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.