Abstract
Epstein-Barr virus (EBV) establishes lifelong persistent infections in humans by latently infecting B cells, with occasional cycles of reactivation, virus production, and reinfection. Protective immunity against EBV is mediated by T cells, but the role of EBV-specific T helper (Th) cells is still poorly defined. Here, we study the Th response to the EBV lytic cycle proteins BLLF1 (gp350/220), BALF4 (gp110), and BZLF1 and show that glycoprotein-specific Th cells recognize EBV-positive cells directly; surprisingly, a much higher percentage of target cells than those expressing lytic cycle proteins were recognized. Antigen is efficiently transferred to bystander B cells by receptor-mediated uptake of released virions, resulting in recognition of target cells incubated with <1 virion/cell. T cell recognition does not require productive infection and occurs early after virus entry before latency is established. Glycoprotein-specific Th cells are cytolytic and inhibit proliferation of lymphoblastoid cell lines (LCL) and the outgrowth of LCL after infection of primary B cells with EBV. These results establish a novel role for glycoprotein-specific Th cells in the control of EBV infection and identify virion proteins as important immune targets. These findings have implications for the treatment of diseases associated with EBV and potentially other coated viruses infecting MHC class II–positive cells.
Epstein-Barr virus (EBV) is a ubiquitous human γ-herpesvirus implicated in the pathogenesis of several malignancies of lymphoid and epithelial origin (1–3). Primary infection with EBV usually occurs within the first three years of life by parent-to-child oral transmission in an almost always asymptomatic fashion. Delayed primary infection in adolescence or adulthood may cause the syndrome of infectious mononucleosis (4). After oral transmission, the virus replicates in the oropharynx from where it colonizes the host by latently infecting B cells. The tropism for B cells is at least partly a reflection of the B cell lineage–specific, high level expression of the principal EBV receptor CD21, which is the ligand for the outer membrane glycoprotein BLLF1, also referred to as gp350/220 (5). In infected B cells, EBV is able to establish different types of latency based on the set of viral genes expressed. During the primary phase of B cell infection, as well as in lymphoblastoid cell lines (LCL) generated by infection of B cells with EBV in vitro, the full range of nine viral latent proteins is expressed and these drive the activation and proliferation of the infected B cell. After immune control of primary infection, the numbers of infected B cells fall and the pattern of EBV latency changes. Expression of most, if not all, latent proteins is down-regulated, allowing EBV to evade immune recognition and elimination and to persist in the memory B cell compartment for life (6). As yet poorly defined signals may cause reactivation of EBV after terminal differentiation of the infected cells into plasma cells, followed by virus production and reinfection of B cells (7, 8).
The critical importance of the immune system in controlling primary and persistent EBV infection is highlighted by the frequency and severity of EBV-associated disease in immunocompromised individuals. The development of EBV-positive posttransplant lymphoproliferative disorders (PTLDs) in immunosuppressed bone marrow and solid organ transplant recipients and the successful treatment of such lymphomas by the infusion of EBV-specific T cell lines generated by repeated stimulation of peripheral blood T lymphocytes with autologous LCL in vitro have established T cells as critical immune effector cells in EBV immunity (1, 9). The polyclonal LCL-stimulated T cell preparations used to treat PTLDs contain CD4+ as well as CD8+ components, and both components may be necessary for the clinical effectiveness of this adoptive T cell therapy (10). Although the targets of the EBV-specific CD8+ cytotoxic T cell response have been studied in detail, the CD4+ Th cell response to EBV remained ill defined (11). In recent years, the search for the targets of the EBV-specific Th response has gained momentum after observations underlining the importance of CD4+ T cells in the initiation and maintenance of adaptive immune responses to viruses (12) and tumors (13). Although Th responses to some latent antigens of EBV have been described, information on the Th immune response to lytic antigens of EBV is still scarce (11). During lytic replication, a large array of >80 EBV proteins is expressed and exposed to the immune system (8). However, EBV has evolved mechanisms by which lytically infected cells can evade immune recognition, including down-regulation of MHC class I and II molecules, and secretion of gp42, a glycoprotein interfering with antigen recognition by CD4+ T helper cells (14, 15). Thus, although Th responses to the lytic cycle proteins BZLF1, BMRF1, BHRF1, and BLLF1 have been described, and T cells specific for the latter two antigens have been isolated from latently infected donors, it is still largely unknown which lytic cycle proteins of EBV elicit T helper responses, which are the dominant targets of the EBV-specific T helper response, and how Th cells specific for lytic cycle antigens contribute to EBV immunity (16–18).
In the infected host, the reservoir of latently infected memory B cells can seed foci of virus replication at mucosal sites, and this reactivation of the virus and subsequent reinfection of B lymphocytes that reenter the periphery has been suggested to contribute to the maintenance of persistence (19). Thus, immune responses directed against lytic antigens may aid at controlling persistent infection by preventing a recrudescence of viremia associated with this cyclic pattern of transmission between compartments and, in addition, by preventing the host from superinfection with further strains of orally transmitted virus. Circumstantial evidence in support of this scenario has been provided by studies demonstrating that healthy virus carriers are consistently positive for IgG antibodies to lytic antigens of EBV (1). Because Ig isotype switching requires cognate T cell help (20), the presence of IgG antibodies to lytic cycle antigens implies that these antigens are also targets of the CD4+ Th cell response. Moreover, healthy virus carriers maintain CTL memory to lytic cycle epitopes during the persistent phase of infection, and the frequencies of these T cells often exceed those seen for CTL memory to latent cycle epitopes (21). Such lifelong CTL and antibody responses are probably a reflection of continuous antigenic stimulation after virus reactivation and replication. Here, we sought to examine the T helper cell response to lytic antigens of EBV and to assess the role of these T cells in establishing protective immunity against EBV.
RESULTS
Generation of EBV lytic cycle antigen-specific T helper cell lines from a healthy virus carrier
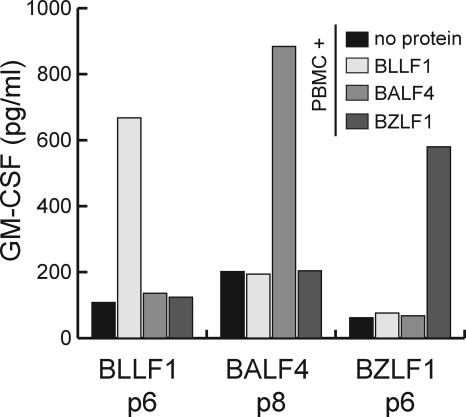
To study the Th cell immune response against lytic antigens of EBV, we sought to isolate and characterize Th cells specific for the lytic cycle proteins BZLF1, BALF4, and BLLF1 from peripheral blood of a healthy virus carrier. BZLF1 and BLLF1 were chosen because Th responses against these antigens had been detected in peripheral blood of patients with infectious mononucleosis and healthy virus carriers, respectively (16, 18). In addition, the glycoprotein BALF4 (also referred to as gp110) was included in this analysis because humoral but no Th cell responses against this antigen have been described (22). BZLF1, BLLF1, and BALF4 proteins were expressed and purified as histidine-tagged proteins from Sf9 insect cells infected with recombinant baculoviruses. PBMCs from the healthy EBV-infected donor JM were pulsed with the recombinant proteins and used to stimulate autologous CD4+ T cells. After several rounds of stimulation, the CD4+ T cell lines specifically responded against the protein used for stimulation, but not against control proteins (Fig. 1). These results indicated that CD4+ T cell memory to lytic antigens does exist and can be reactivated from peripheral blood of healthy virus carriers with this approach in vitro.
Figure 1.
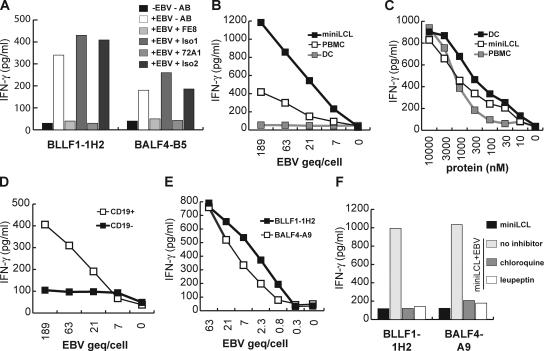
CD4+ T cell memory to lytic cycle proteins of EBV in the peripheral blood of healthy virus carriers. 106/ml PBMCs from the latently EBV-infected donor JM were incubated in separate wells with purified recombinant BLLF1, BZLF1, and BALF4 proteins, and then used to stimulate autologous CD4+ T cells from peripheral blood. The specificity of the T cell lines was assayed by GM-CSF ELISA. After six rounds of stimulation (p6), the lines began to show reactivity to PBMCs pulsed with the stimulator protein, but not against control proteins.
Characterization of the lytic antigen-specific CD4+ T cells
For the purpose of characterizing these T cells in more detail, we cloned the CD4+ T cell lines by limiting dilution. Antigen-specific CD4+, CD8−, TCRα/β+, and TCRγ/δ-negative T cell clones were obtained from all three T cell lines (unpublished data). Clonality of the single cell outgrowths was verified by RT-PCR analysis in conjunction with Southern blot hybridization of the variable part of the T cell receptor β chain (TCR-Vβ). The various antigen-specific T cell clones established from each antigen-specific line expressed at least two different Vβ chains, indicating that they derived from different precursors. The restricting MHC molecules were identified by testing the T cells against antigen-pulsed PBMCs from various donors sharing different MHC class II alleles with donor JM. The epitopes recognized by the T cells were identified using the DEPI method (23). T cell clones expressing the same Vβ chain recognized the same epitope presented on the same MHC II molecule. Six representative clones specific for two different epitopes in each of the three antigens were chosen for further analysis (Table I).
Table I.
Characterization of the CD4+ T cell clones specific for BLLF1, BALF4, and BZLF1
| T cell clone | Epitope | Restriction | TCR-Vβ |
|---|---|---|---|
| BLLF1-1H2 | BLLF1 AA130–144 -VYFQDVFGTMWCHHA- | HLA-DQB1*0402 | Vβ5.2 |
| BLLF1-1D6 | BLLF1 AA65–79 -FGQLTPHTKAVYQPR- | HLA-DRB1*1301 | Vβ21 |
| BZLF1-3E4 | BZLF1 AA207–221 -KSSENDRLRLLLKQM- | HLA-DQB1*0402 | Vβ21 |
| BZLF1-3H11 | BZLF1 AA174–188 -ELEIKRYKNRVASRK- | HLA-DRB1*1301 | Vβ4 |
| BALF4-B5 | BALF4 AA482–496 -AWCLEQKRQNMVLRE- | HLA-DPB1*1301 | Vβ6 |
| BALF4-A9 | BALF4 AA575–589 -DNEIFLTKKMTEVCQ- | HLA-DRB1*0801 | Vβ19 |
Six T cell clones recognizing two different epitopes in each of the three antigens were further characterized. The amino acid sequences and positions of the epitopes within the EBV B95.8 protein sequence are given, together with the restricting MHC molecules and the Vβ chains (TCR-Vβ) expressed by the different T cell clones.
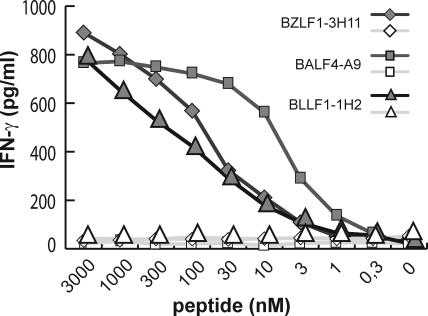
To define the affinities of the T cells for their cognate antigen, peptides spanning the epitopes were synthesized, pulsed at various concentrations onto autologous PBMCs, and recognition by the T cell clones was assayed in cytokine secretion experiments. As shown in Fig. 2, the various clones recognized target cells pulsed with 1–3 nM of the cognate peptide, whereas responses to control peptides were not observed even at much higher concentrations. Along with GM-CSF, all clones secreted IFN-γ in response to antigenic stimulation, suggesting that they are of Th1 subtype.
Figure 2.
Affinity of the T cell clones for their cognate antigen. Peptides at various concentrations were pulsed onto autologous PBMCs for 2 h at 37°C. Subsequently the cells were irradiated (40Gy), unbound peptide was removed by extensive washing, and the cells were used as stimulators for the T cells. IFN-γ secretion by the T cells was measured 24 h later by ELISA. As shown for three T cell clones, all T cells described in this work recognized their cognate peptide (closed symbols) at a minimum concentration of 1–3 nM, whereas no response against PBMCs pulsed with an irrelevant peptide (open symbols) was observed.
Recognition of EBV-positive target cells by the lytic antigen-specific T cells
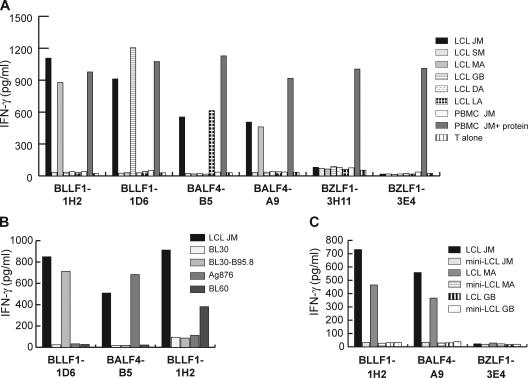
When tested against LCL, some T cells even responded against target cells that had not been pulsed with the cognate peptide or protein. This was unexpected because only a small proportion of cells within an LCL culture spontaneously become permissive for lytic replication (8). However, not all T cells were able to recognize EBV-positive target cells. Although BALF4- and BLLF1-specific T cell clones recognized autologous and HLA-matched LCL (Fig. 3 A) as well as type I and type III EBV-positive Burkitt's lymphoma (BL) cell lines (Fig. 3 B), BZLF1-specific T cell clones failed to recognize EBV-positive targets. To investigate antigen expression, processing, and presentation in more detail, we took advantage of a genetically engineered EBV strain called miniEBV. This virus mutant is still able to infect and transform B cells into so-called miniLCL, but is unable to enter the lytic cycle (24, 25). Although miniLCL are identical to LCL generated by infection of B cells with B95.8 virus in terms of antigen presentation and T cell costimulation (24), miniLCL established from donor JM were not recognized by the BALF4- and BLLF1-specific T cell clones and neither were miniLCL counterparts of HLA-matched allogeneic LCL that were recognized by the T cells (Fig. 3 C).
Figure 3.
Recognition of EBV-positive target cells by lytic antigen-specific T cells. (A) Autologous LCL, allogeneic LCL sharing distinct MHC II alleles with donor JM (MA: DR8, DQ4; LA: DQ6, DP13; GB: DR13, DQ6, DP4), and MHC II–mismatched LCL (SM, DA) were cocultured with the T cell clones specific for lytic antigens for 24 h. Subsequently, IFN-γ secretion by T cells was measured by ELISA. T cells specific for the glycoproteins BALF4 and BLLF1, but not the immediate early protein BZLF1, recognized autologous and MHC II–matched LCL. Autologous PBMCs pulsed with the specific protein were used as specificity control and T cells were cultivated without target cells (T alone) were included as controls to detect nonspecific secretion of cytokines by the T cells. (B) The glycoprotein-specific T cells recognized EBV-positive BL cell lines expressing the restriction element of the T cell clones (Ag876: DP13; BL30: DR13; BL60: DQ4). BL30-B95.8 is an EBV-positive convertant of the parental, EBV-negative BL30 cell line. (C) The glycoprotein-specific T cells failed to recognize miniLCL generated by infection of B cells with an EBV mutant unable to enter the lytic cycle.
Transfer of antigen occurs between cells in culture
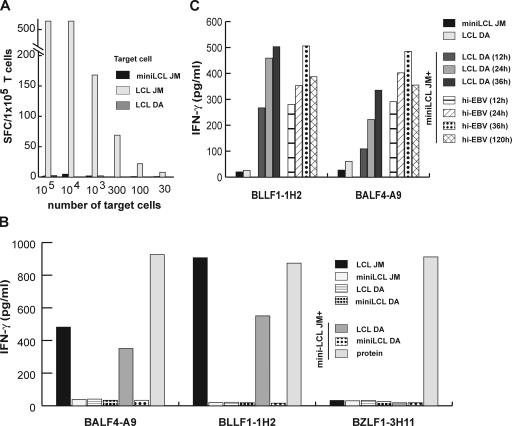
The difference in the recognition of LCL and miniLCL by the T cells suggested that the glycoprotein-specific T cells recognize EBV-positive target cells undergoing lytic replication. However, the strong response to LCL by the T cell clones in cytokine secretion assays was unexpected considering the low percentage of cells that spontaneously become permissive for viral replication (8). Immunofluorescence staining of LCL with BZLF1- and BLLF1-specific monoclonal antibodies demonstrated that <1% of cells had entered the lytic cycle, whereas no staining was seen with miniLCL (unpublished data). Furthermore, cells undergoing lytic replication are known to down-regulate expression of MHC molecules and to secrete gp42, an EBV glycoprotein that inhibits T helper cell recognition (14, 15). To assess the percentage of cells in LCL cultures that were recognized by the T cells, we assayed a fixed number of antigen-specific T cells with serial dilutions of autologous LCL, miniLCL, or MHC-mismatched LCL by IFN-γ ELISPOT. As shown in Fig. 4 A, a surprising 20% of the cells in an LCL culture were recognized by glycoprotein-specific T cells. Wells containing miniLCL or MHC-mismatched LCL showed background number of spots. No spots above background were detected with BZLF1-specific T cells, irrespective of the target cells used (unpublished data).
Figure 4.
Antigen is transferred from lytically infected B cells to bystander B cells by virions. (A) Different numbers of autologous LCL, miniLCL, and MHC II–mismatched LCL were incubated with 105/well BLLF1-1H2 T cells. The number of target cells recognized by the T cells was determined by IFN-γ ELISPOT and are depicted as spot-forming cells (SFC). Approximately 20% of the autologous LCL were detected by the T cells, whereas miniLCL and MHC II–mismatched LCL gave background numbers of spots. (B) MiniLCL JM were cocultured with MHC II–mismatched LCL DA or miniLCL DA for 24 h and probed with lytic antigen-specific T cells. MiniLCL JM cocultured with LCL DA, but not miniLCL DA, were recognized by glycoprotein-specific, but not BZLF1-specific, T cells. MiniLCL JM incubated with the cognate antigen were included as specificity control. (C) MiniLCL JM were cocultured with allogeneic LCL or incubated with heat-inactivated purified virus (hi-EBV) supernatant for different periods of time up to 120 h. Subsequently, the cells were fixed with paraformaldehyde and probed with BLLF1- and BALF4-specific T cells. 12 h of coculture or incubation with heat-inactivated virus was sufficient for T cell recognition.
The discrepancy in the number of cells positive for BLLF1 in immunofluorescence studies and the number of cells positive in the ELISPOT experiments suggested that antigen is transferred between cells. To address this possibility, we performed cell-mixing experiments. Autologous miniLCL were cocultured with MHC-mismatched allogeneic LCL for various periods of time and then probed with the lytic antigen-specific T cells. Although none of the lines was recognized alone, the cell mix was recognized by the glycoprotein-specific, but not BZLF1-specific, T cell clones (Fig. 4 B).
Potential sources of the transferred antigens were either fragments of cells in which EBV had replicated or released virus particles. To test the latter possibility, we purified virus from supernatant of the B95.8 marmoset cell line, pulsed it onto miniLCL, and subsequently probed the cells with the T cells. The virus-pulsed target cells were recognized by the BLLF1- and BALF4-specific T cells, demonstrating that viral particles are capable of transferring antigen. To investigate whether antigen was transferred directly in the form of viral proteins in the virions or indirectly by superinfecting miniLCL, heat-inactivated virus supernatant incapable of immortalizing primary B cells in control experiments was pulsed onto miniLCL. As shown in Fig. 4 C, heat inactivation of the virus supernatant did not affect T cell recognition. Moreover, T cell recognition occurred already 12 h after incubation of miniLCL with purified virus or coculture with allogeneic LCL. This excluded superinfection of miniLCL as the mechanism of antigen transfer because expression of glycoproteins after induction of lytic replication requires 48–72 h (8). Instead, these results indicated that virus particles can function as a passive vector for the transfer of immunogenic viral structural proteins.
B cells are the major antigen-presenting cells for virion proteins
The efficient recognition of miniLCL cocultured with allogeneic LCL was surprising because LCL supernatant is known to contain few infectious virus particles. Infection of B lymphocytes by EBV is receptor mediated and involves adsorption of BLLF1 to CD21, followed by aggregation of CD21 on the plasma membrane and the internalization of EBV into cytoplasmic vesicles (5, 26). To test whether this receptor-mediated uptake of EBV is essential for the antigen transfer by virions, the EBV supernatant was incubated with the anti-BLLF1 monoclonal antibody 72A1 and added to miniLCL. In parallel, miniLCL were incubated with the anti-CD21 monoclonal antibody FE8 before addition of the viral supernatant. Both antibodies had been shown to prevent EBV infection of B cells (27, 28). Treatment with these, but not with isotype control antibodies, completely abrogated T cell recognition, suggesting that the transfer of virion antigens is dependent on receptor-mediated virus uptake (Fig. 5 A). To test whether only APC-expressing CD21 were able to present virion antigens on MHC class II, we pulsed EBV supernatant onto autologous miniLCL, monocyte-derived DCs, and PBMCs. As shown in Fig. 5 B, miniLCL and PBMCs, but not DCs, were able to present virion antigens to T cells when incubated with purified virus supernatant. Of note, DCs efficiently stimulated the T cells when pulsed with BLLF1 peptides and, importantly, when incubated with purified BLLF1 protein, demonstrating that uptake, processing, and presentation of exogenous antigens was not impaired in these cells. In fact, of all cell types tested, DCs presented exogenous antigens most efficiently on MHC class II (Fig. 5 C). Within PBMCs, the CD19-positive but not the CD19-negative population was able to stimulate the glycoprotein-specific T cells, suggesting that the B cell fraction presented the virion-derived antigens (Fig. 5 D). To assess efficiency of this receptor-mediated antigen presentation, we determined the concentration of EBV genome equivalent (geq) in the viral supernatant, pulsed increasing amounts of geq onto miniLCL, and subsequently assessed T cell recognition. MiniLCL incubated with as little as 0.8 geq per cell were recognized by BLLF1- and BALF4-specific T cells, demonstrating that this receptor mediated uptake and that subsequent presentation of virion antigens is extremely efficient (Fig. 5 E). When target cells were treated with chloroquine or leupeptin during incubation with viral supernatant, T cell recognition was almost completely abrogated, indicating that antigen processing occurred in the lysosomal compartment (Fig. 5 F).
Figure 5.
Efficient presentation of virion-derived antigens after receptor-mediated uptake. (A) MiniLCL JM were incubated with the anti-CD21 antibody FE8 or an isotype control (Iso1) antibody and pulsed with virus supernatant (EBV). In parallel, virus supernatant was incubated with the anti-BLLF1 antibody 72A1 or an isotype control antibody (Iso2) and pulsed onto miniLCL. After 24 h of incubation, virus-pulsed miniLCL were probed with the T cells. (B) MiniLCL, PBMCs, and dendritic cells (DC) were incubated with increasing amounts of purified virus for 24 h and probed with BLLF1-1H2 T cells. (C) The three types of APCs as in B were incubated with increasing amounts of purified BLLF1 mutant protein lacking the CD21 binding domain for 24 h and subsequently probed with the BLLF1-1H2 T cells. (D) PBMCs were separated into CD19+ and CD19− cell fractions by magnetic sorting and subsequently incubated for 24 h with increasing amounts of purified viral particles before addition of glycoprotein-specific T cells. (E) MiniLCL were pulsed for 24 h with increasing amounts of EBV geq and probed with BLLF1- and BALF4-specific T cells as indicated. (F) MiniLCL JM were incubated for 24 h with purified viral supernatant in the absence or presence of leupeptin or chloroquine. After the incubation period, unbound virus and inhibitors were removed by washing, and the cells were fixed and probed with the glycoprotein-specific T cells.
No detectable transfer of antigen from cell fragments and released proteins
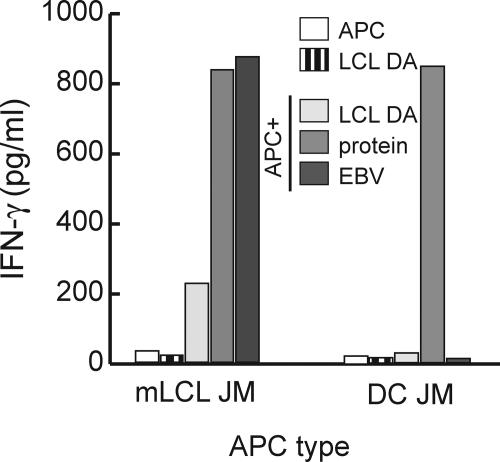
These experiments illustrated that receptor-mediated uptake of virions plays a central role in the transfer of antigen, but did not exclude that proteins released by lysed cells contributed to this process. The failure of T cells specific for the transcription factor BZLF1 to recognize LCL had suggested that the amount of antigen released by lysed cells is insufficient for T cell stimulation. However, in lytically infected cells, the virion proteins BLLF1 and BALF4 are expressed at much higher levels than the transcription factor BZLF1 (8). Therefore, released virion proteins might reach levels sufficient for T cell detection. Because DCs were incapable of receptor-mediated uptake of virion-derived antigens, we used DCs to assess to what extent released proteins and cell debris contributed to the transfer of antigen. Autologous DCs were cocultured with MHC-mismatched LCL and probed with the lytic antigen-specific T cells. None of the T cell clones recognized the DCs, even after extended periods of coculture, demonstrating that cells debris or proteins released from lytically EBV-infected cells contributed insignificantly, if at all, to the transfer of antigen (Fig. 6).
Figure 6.
The amount of antigen released from lytically infected cells is insufficient for T cell recognition. MiniLCL JM (5 × 105/ml) and DC JM (5 × 105/ml) were cultured either alone or together with the MHC II–mismatched LCL DA (ratio 1:1) for 24 h. After the cocultures, the cells were probed with BLLF1-1H2 T cells and cytokine secretion determined 24 h later by ELISA. MiniLCL and DC pulsed with the cognate protein were included as controls.
Glycoprotein-specific CD4+ T cells are cytolytic and suppress the growth of EBV-infected cells
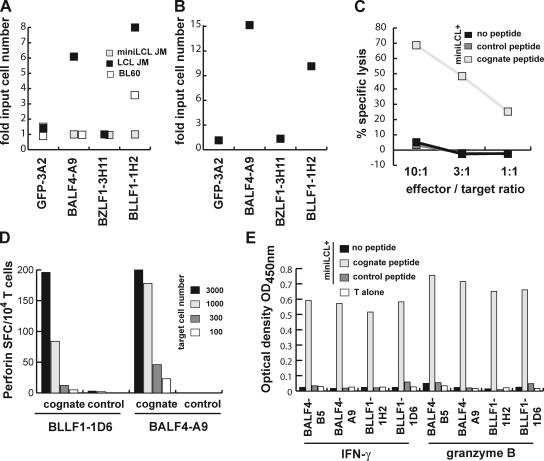
To assess direct effector functions of the glycoprotein-specific T cells on the growth of EBV-infected target cells, serial dilutions of LCL and mini-LCL were plated with or without 10,000 monoclonal T cells, and proliferation of the cultures was followed over time. The GFP-specific CD4+ T cell clone 3A2 (also established from donor JM) was included as a control (29). After 4 wk, the cultures were assayed microscopically for the outgrowth of cells, and CD19 staining demonstrated that the outgrowing cells were exclusively B cells (unpublished data). As compared with irrelevant GFP-specific T cells, proliferation of LCL but not miniLCL was strongly impaired in the presence of glycoprotein-specific T cells. Approximately sevenfold higher numbers of LCL were required to achieve continuous proliferation of the cells than in control wells (Fig. 7 A). The glycoprotein-specific T cells BLLF1-1H2 also inhibited proliferation of the MHC II–matched EBV-positive type I BL cell line BL60. Approximately three- to fourfold more BL60 cells were required to obtain proliferating cultures than in the presence of GFP-3A2 and BALF4-A9 T cells. Proliferation of miniLCL was not affected by any of the T cells used (Fig. 7 A). These experiments indicated that T cells specific for virion proteins may play an important role in limiting virus spread by inhibiting or eliminating B cells that have become infected by EBV. To investigate whether glycoprotein-specific T cells could limit the number of productively infected B cells and consequently the generation of LCL in vitro, B cells from donor JM were incubated with B95.8 virus for 4–24 h. Subsequently, the cells were washed to remove free virus and plated at various dilutions in 96-well microtiter plates together with 10,000 BLLF1-, BALF4-, BZLF1-, or GFP-specific T cells. Cultures were maintained by weekly refeeding and assayed for LCL outgrowth after 4 wk. By that time, outgrowth was immediately apparent from microscopic inspection of the wells, and CD19 staining confirmed that the outgrowing cells are of B cell origin and not surviving T cells. Fig. 7 B depicts the results of a representative experiment expressed as x-fold input cell number at which outgrowth of LCL occurred. Addition of the BLLF1- and BALF4-specific T cells greatly diminished the number of wells with LCL outgrowth. Compared with control wells to which BZLF1- or GFP-specific T cells had been added, ∼10-fold higher numbers of EBV-infected B cells were required to obtain LCL outgrowth. In accordance with the finding that virus-pulsed B cells are able to present virion glycoproteins even beyond 120 h of pulsing, delaying the time of exposure of the virus-pulsed B cells to T cells did not affect the outcome of the experiments. Addition of cyclosporin A to the cultures also did not abrogate this inhibition, indicating that cytokines secreted by the T cells were unlikely to mediate this effect (unpublished data). To investigate whether the T cells were able to lyse target cells upon recognition, miniLCL pulsed with cognate or control peptides were incubated with the various T cells for 3 h. Subsequently, lysis of the target cells was measured in a europium release assay. Upon antigen recognition, glycoprotein-specific T cells efficiently lysed target cells (Fig. 7 C). The T cells also secreted perforin and granzyme B in an antigen-specific manner, suggesting that cytolysis is mediated by the granule exocytosis pathway (Fig. 7, D and E).
Figure 7.
Glycoprotein-specific CD4+ T cells are cytolytic and inhibit outgrowth and proliferation of LCL. (A) Decreasing numbers of LCL JM, miniLCL JM, or BL60 cells were either cultured alone or cocultured with BLLF1-1H2, BALF4-A9, BZLF1-3H11, or GFP-specific 3A2 T cells (10,000/well). Proliferation of the cells was followed over time and the minimum number of cells proliferating was determined after 4 wk. Coculture of LCL and BL60 cells, but not miniLCL, with glycoprotein-specific T cells increased the minimum number of cells necessary for proliferation 3–10-fold. (B) Purified B cells were infected with EBV and increasing cell numbers plated together with BLLF1-, BALF4-, BZLF1-, or irrelevant GFP-specific T cells (10,000/well). The minimum number of EBV-infected B cells required for LCL outgrowth was ∼10-fold higher in the presence of glycoprotein-specific T cells. (C) MiniLCL JM pulsed with the indicated peptides were cocultured with the BALF4-B5 T cells at different effector to target ratios. Upon antigen recognition, the glycoprotein-specific T cells efficiently lysed the target cells. (D) Serial dilutions of miniLCL JM pulsed with cognate or control peptides were cocultured with glycoprotein-specific T cells, and perforin secretion by the T cells was assayed by ELISPOT. (E) In addition to IFN-γ, all glycoprotein-specific T cell clones also secreted granzyme B in response to target cell recognition.
Th cell responses to EBV glycoproteins are consistently detected in healthy virus carriers
If glycoprotein-specific Th cells played an important role in the control of EBV infection in vivo, Th cell responses against these antigens should be detectable in a significant proportion of healthy virus carriers. The last set of experiments, therefore, sought to address whether Th cells specific for BLLF1 and BALF4 are detectable in peripheral blood of latently EBV-infected donors. Peripheral blood CD4+ T cells from four EBV-seropositive donors and one EBV-seronegative healthy adult were repeatedly stimulated using autologous PBMCs pulsed with recombinant BLLF1 and BALF4 proteins. After five to seven rounds of stimulation, T cell lines from all four EBV-seropositive donors, but not from the EBV-seronegative donor, showed specificity for the antigen used for stimulation (Fig. 8). The successful isolation of BALF4- and BLLF1-specific CD4+ Th cells from peripheral blood of all the healthy virus carriers identifies these proteins as important and common targets of the EBV-specific CD4+ T cell response.
Figure 8.
Healthy virus carriers are consistently positive for glycoprotein-specific CD4+ T cells. CD4+ T cells from peripheral blood of one EBV-seronegative (EBV−) and four EBV-seropositive (EBV+) healthy donors were repeatedly stimulated with protein-pulsed autologous PBMCs. After five to seven restimulations, the T cell lines from all healthy virus carriers, but not from the EBV-negative control donor, specifically responded against PBMCs pulsed with the protein used for stimulation.
DISCUSSION
Maintenance of EBV persistence has been suggested to involve cycles of virus reactivation, production, and reinfection of B lymphocytes (19). Immune responses directed against lytic cycle proteins of EBV are sustained during persistent infection and, as indicated by shifts in the virus–host balance in immunocompromised individuals, are likely to interfere with this cyclic pattern of transmission (1).
Here we studied the poorly defined T helper response to lytic cycle proteins of EBV. Because cell systems in which EBV infection leads directly into a fully productive lytic infection are lacking and because it is unknown which of the >80 different EBV proteins expressed during lytic replication are dominant T helper cell targets, we studied the T helper response against defined lytic cycle proteins. BALF4, BLLF1, and BZLF1 were chosen as candidate targets because humoral and/or cellular immune responses against these antigens have been described previously (1, 16, 18, 21, 22). Although all T cell clones recognized their cognate peptides with similar avidity, only CD4+ T cells specific for the glycoproteins BLLF1 and BALF4, but not for the transcription factor BZLF1, recognized autologous as well as HLA-matched allogeneic EBV-infected target cells. MiniLCL generated by infection of B cells with a genetically engineered miniEBV strain incapable of entering the lytic cycle were not recognized, implying that T cell recognition was dependent on sporadic lytic replication occurring in a low percentage of cells in culture. However, a substantially higher percentage of cells was recognized than those positive for lytic cycle proteins in immunofluorescence studies. Coculture experiments of HLA-mismatched LCL and HLA-matched miniLCL indicated that antigen was transferred from one cell type to the other. We provide several lines of evidence that the antigen transferred was derived from released virions. First, miniLCL pulsed with purified virus preparations were recognized by the T cells, demonstrating that virions can serve as a source of antigen. Because miniLCL pulsed with heat-inactivated virus were still recognized, T cell recognition does not depend on productive infection of the target cells. Of note, virus supernatant has been used before as source of antigen to reactivate and expand BLLF1-specific T cells. In fact, these were the first EBV-specific Th cell clones isolated and characterized in vitro (18, 30). The authors showed that these T cells were able to recognize LCL presenting endogenous and exogenous antigen on MHC II, but further implications of such recognition in the control of EBV infection were not addressed. Second, antigen is most efficiently transferred to B cells and this transfer can be blocked by BLLF1- and CD21-specific monoclonal antibodies (27, 28), implying that antigen uptake is receptor mediated. Importantly, this antibody treatment not only prevented subsequent recognition by BLLF1-specific but also by BALF4-specific T cells, further adding to the notion that the antigens presented are derived from virions and not from released proteins. Third, the failure of BZLF1-specific T cells to recognize LCL, and the failure of BALF4- and BLLF1-specific T cells to recognize autologous DCs cocultured with HLA-mismatched LCL demonstrated that the amount of antigen released by dead or dying cells is not sufficient for T cell detection. This finding was unexpected because DCs have been shown to efficiently stimulate EBV-specific cellular immune responses upon coculture with freshly EBV-infected B cells or LCL (31, 32). Two possibilities may account for these discrepant results. First, the study by Bickham et al. focused on T cell responses to latent cycle antigens, which are expressed in all and not just a small percentage of cells in an LCL culture. Therefore, the amount of lytic cycle proteins available for uptake by DCs may be generally lower. This is especially true for BLLF1 and BALF4. These proteins are incorporated into virions that, as we have shown, are efficiently taken up by neighboring B cells but not by DCs. Second, we cocultured DCs with LCL for only 24 h, whereas in the study by Bickham et al. the cells were cocultured for several days. Recently, the lytic cycle protein BHRF1 has been shown to be transferred from lytically to latently infected cells. Interestingly, 30 d of coculture were required to reach levels sufficient for T cell recognition (17). Therefore, efficient presentation of proteins released from LCL by DCs may require coculturing over several days.
The observation that mainly B cells, whether primary or EBV-infected, present virion proteins most efficiently implicated a protective role of such T cells in controlling the spread of infection. Importantly, glycoprotein-specific T cells recognize target cells pulsed with virus supernatant before the EBV genome has circularized and before EBNA2, the protein essential for primary B lymphocyte growth transformation, is expressed (33). EBV infection of B lymphocytes is initiated by BLLF1 adsorption to CD21 on the B cell plasma membrane, followed by BXLF2-mediated envelope fusion with the cell membrane and nucleocapsid exocytosis into the cytoplasm (5, 26). During this process of virus uncoating, proteins of the viral envelope are probably retained at the cell membrane, as indicated by the higher percentage of cells positive for BLLF1 than for BZLF1 in immunofluorescence experiments (30). Because cell membrane proteins efficiently access the MHC class II processing and loading compartment, T helper cells specific for envelope antigens are able to detect EBV-infected cells before viral latency is established. This receptor-mediated virion uptake and subsequent presentation of glycoprotein-derived peptides on MHC class II is extremely efficient. BALF4- and BLLF1-specific T cells were able to recognize miniLCL incubated with less than one EBV geq per cell. Thus, virion proteins retained at the cell surface during virus uncoating render newly EBV-infected B cells vulnerable to immune attack by Th cells specific for virion proteins. Preliminary results with Th cells specific for additional glycoproteins of EBV also recognizing newly infected B cells provide further evidence in support of this concept (unpublished data). This mechanism of immune surveillance may not be limited to EBV, but may also apply to other coated viruses infecting MHC class II–positive target cells.
The glycoprotein-specific CD4+ T cells described here are of Th1 type and cytolytic, and are able to inhibit the proliferation of EBV-positive BL cells as well as LCL in vitro. Importantly, these T cells are also able to prevent the outgrowth of primary B cells infected with EBV in vitro, implicating a role of such T cells in diminishing the pool of EBV-infected B cells in vivo. This notion is further supported by the successful isolation of T helper cells specific for BLLF1 and BALF4 from peripheral blood of five out of five latently infected healthy virus carriers. Consistent with this, subsidence of T cell surveillance after immunosuppression is often associated with increased viral shedding in the throat, and higher numbers of EBV-infected B cells in the peripheral blood (34, 35). In bone marrow and solid organ transplant recipients, PTLD incidence correlates with severity of immunosuppression. In PTLDs as well as in Burkitt's lymphoma, lytic replication has been observed at the site of tumor development (36, 37). Thus, glycoprotein-specific Th cells may not only aid in controlling persistent infection, but may also play a role in the immune control of EBV-associated malignancies.
As compared with blood or LCL cultures, diffusion is impeded in solid tumors leading to the sequestration and accumulation of viral progeny within tumors. Because the transformed cells in PTLDs are considered to be in vivo correlates of LCL, these cells are expected to take up released virions with similar efficiency. Also, BL cells express the principal EBV receptor CD21 and have retained the ability to process exogenous antigen through the HLA class II pathway for presentation to CD4+ T cells (38). Because MHC class I antigen presentation is severely impaired in BL (39), CD4+ T cells may play an important role in the immune response against this tumor. EBNA1-specific Th1 type CD4+ T cells have been shown to kill BL cells (40). However, because EBNA1 is the only EBV latent cycle antigen expressed in BL and the recognition of endogenous antigen by EBNA1-specific CD4+ T cells appears to differ between different epitope specificities, glycoprotein-specific Th cells, which we have found to recognize EBV-positive BL cell lines, may play a critical role in the immune response against this tumor. Evidence in support of this concept has been obtained in preclinical models. Injection with purified, or infection with vaccinia virus–expressing BLLF1, has been shown to protect cottontop tamarins against a lethal, lymphomagenic EBV challenge (41, 42). Although BLLF1 had been identified as the dominant target of the neutralizing antibody response (43), protective immunity in these and other studies did not always correlate with the presence of virus-neutralizing antibodies, inferring a role for BLLF1-specific cell-mediated responses in disease protection (44). Th cell recognition of newly EBV-infected B cells after receptor-mediated cell adhesion, penetration, and uncoating as described in the present study may provide a mechanistic explanation for these findings. Thus, the targets of the EBV-specific T helper cell response may not be limited to a small set of latent cycle proteins expressed in these tumors, but may also include antigens transferred by virions.
MATERIALS AND METHODS
Generation and culture of LCL and miniLCL.
Supernatant from the marmoset B cell line B95.8 was used as the source of wild-type virus to generate LCL from PBMCs using standard protocols. 1 μg/ml cyclosporin A was initially added to the cultures to inhibit T cell growth. MiniLCL were generated similarly, except that a mutant EBV strain unable to enter lytic replication was used (24). LCL and miniLCL were grown as suspension cultures in LCL media consisting of RPMI 1640 supplemented with 10% FCS, 1% nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, and 50 μg/ml gentamicin.
Protein expression and purification.
Generation of recombinant baculovirus and protein expression in Sf9 cells has been described previously (45). Recombinant proteins were purified from cell lysates using Nickel-NTA (QIAGEN). Protein concentration was determined using Bradford reagent (Bio-Rad Laboratories). Purity and integrity of the proteins was verified by Coomassie staining and Western blot analysis using an anti-His6 monoclonal antibody (clone BMG-His-1; Roche).
Culture of T cell lines and clones.
Whole blood was obtained from healthy laboratory members and HLA-typed using PCR-based methods. The HLA class II genotype of donor JM consists of the following: DRB1*0801, DRB1*1301, DRB3*0101, DQB1*0402, DQB1*0603, DPB1*0401, and DPB1*1301. EBV status of the donors was determined serologically and by PCR analysis of throat wash samples. PBMCs were isolated from heparinized venous blood by density gradient centrifugation on Ficoll-Paque (GE Healthcare). CD4+ cells from PBMCs were selected over MACS columns following the guidelines of the manufacturer (Miltenyi Biotec). To generate CD4+ T cell lines specific for lytic antigens of EBV, 106/ml autologous PBMCs in AIM-V medium (Invitrogen) were pulsed with recombinant proteins (500 ng/ml) for 24 h, washed, irradiated (40Gy), and used to stimulate an equal number of CD4+ T cells in 2 ml T cell media (RPMI 1640, 10% heat inactivated human serum, 2 mM l-glutamine, 10 mM Hepes, and 50 μg/ml gentamicin) in 24-well plates. After 48 h, 10 U/ml recombinant IL-2 (Chiron) was added to the cultures. Limiting dilution cloning and expansion of the T cells was performed as described previously (45).
Cytokine secretion by T cells.
Specificity of T cells was tested by incubating 105 target cells with 105 T cells in a final volume of 200 μl T cell media. After 24 h of coculture, the cytokine content in the supernatant was measured by ELISA (R&D Systems). For mixing experiments, LCL and miniLCL were cocultured at a 1:1 ratio for different periods of time before addition of the T cells. Autologous DCs from donor JM were prepared as described previously (29). For peptide titration experiments, target cells were incubated with the various peptides at 37°C for 2 h, followed by extensive washing before the addition of T cells. BLLF1 antibody-blocking studies were performed by incubating virus supernatant with the anti-BLLF1 monoclonal antibody 72A1 (HB-168; American Type Culture Collection) or an isotype control antibody (final concentration 15 μg/ml) for 1 h at 37°C before addition to target cells. For CD21 antibody blocking studies, 5 × 105 miniLCL cells were incubated in 1 ml LCL medium with 5.6 μg/ml anti-CD21 monoclonal antibody FE8 (Upstate Biotechnology) for 1 h and washed extensively before further use in antigen presentation studies. To inhibit lysosomal processing, chloroquine (ICN Biomedicals) and leupeptin (Sigma-Aldrich) were added to the target cells at a final concentration of 100 μM and 200 μg/ml, respectively, together with the virus supernatant. After the incubation period, the inhibitors and excess virus were removed by washing, and the cells were fixed by treatment with 1% paraformaldehyde for 10 min at room temperature, followed by extensive washing before addition of T cells. IFN-γ and perforin ELISPOT assays were performed as described previously (29, 46).
T cell cytotoxicity assay.
Lysis of target cells by T cells was measured essentially as described previously (47). In brief, target cells were labeled with BATDA reagent (PerkinElmer) for 15 min at 37°C. Subsequently, viable cells were washed and plated at 5,000 cells/100 μl per well in 96-well V-bottom plates. T cells were added at different effector-to-target ratios and incubated for 3 h at 37°C. Subsequently, 40 μl of the culture supernatant from each well was mixed with 200 μl of europium solution and time-resolved fluorometry was measured after 15 min. Means of triplicates were calculated and the specific lysis was calculated using the following formula: percentage specific lysis = (mean release in the presence of T cells − mean minimum release)/(mean maximum release − mean minimum release) × 100.
Preparation of virus rich supernatant.
Supernatant from B95.8 cells was filtered through a 0.8-μm filter and ultracentrifuged at 25,000 g for 3 h in a SW28 rotor (Beckman Coulter). The supernatant was removed and the virus-rich pellet was resuspended in 1/20 volume of the original culture supernatant. The genomic copy number of this virus concentrate was determined by semi-quantitative real-time PCR using primers directed to the BALF5 gene (48). For virus inactivation, the EBV supernatant was heated to 56°C for 1 h. Successful inactivation of virus infectivity was verified by the inability of the heated virus supernatant to transform primary human B cells.
LCL and BL growth regression and LCL outgrowth inhibition assay.
For growth regression assays, serial dilutions of cells were plated in 96-well plates with or without 10,000 T cells/well in LCL media. The cultures were refed weekly and the cultures were inspected for outgrowth of cells after 4 wk. T cell inhibition of LCL outgrowth was assessed by incubating CD19-positive cells from PBMCs for 4 h at 37°C with EBV supernatant (50 geq/cell). Subsequently, the cells were washed, and serial dilutions of the cells plated in 96-well round bottom plates together with T cells (10,000 cells/well) in 200 μl of LCL media. Outgrowth of LCL was analyzed microscopically and verified by CD19 staining.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB455).
The authors have no conflicting financial interests.
Abbreviations used: BL, Burkitt's lymphoma; EBV, Epstein-Barr virus; geq, genome equivalent; LCL, EBV-immortalized lymphoblastoid B cell lines; miniLCL, miniEBV LCL; PTLD, posttransplant lymphoproliferative disorder.
References
- 1.Rickinson, A.B., and E. Kieff. 2001. Epstein-Barr virus. In Fields Virology. Knipe, D.M., and Howley, P.M. editors. Lippincott-Raven, Philadelphia, PA. 2575–2627.
- 2.Kuppers, R. 2003. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 3:801–812. [DOI] [PubMed] [Google Scholar]
- 3.Young, L.S., and A.B. Rickinson. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer. 4:757–768. [DOI] [PubMed] [Google Scholar]
- 4.Papesch, M., and R. Watkins. 2001. Epstein-Barr virus infectious mononucleosis. Clin. Otolaryngol. 26:3–8. [DOI] [PubMed] [Google Scholar]
- 5.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 50:203–213. [DOI] [PubMed] [Google Scholar]
- 6.Thorley-Lawson, D.A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328–1337. [DOI] [PubMed] [Google Scholar]
- 7.Laichalk, L.L., and D.A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kieff, E., and A.B. Rickinson. 2001. Epstein-Barr virus and its replication. In Fields Virology. Knipe, D.M., and Howley, P.M. editors. Lippincott-Raven, Philadelphia, PA. 2511–2573.
- 9.Rooney, C.M., C.A. Smith, C.Y. Ng, S. Loftin, C. Li, R.A. Krance, M.K. Brenner, and H.E. Heslop. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 345:9–13. [DOI] [PubMed] [Google Scholar]
- 10.Heslop, H.E., and C.M. Rooney. 1997. Adoptive cellular immunotherapy for EBV lymphoproliferative disease. Immunol. Rev. 157:217–222. [DOI] [PubMed] [Google Scholar]
- 11.Callan, M.F. 2004. The immune response to Epstein-Barr virus. Microbes Infect. 6:937–945. [DOI] [PubMed] [Google Scholar]
- 12.Kalams, S.A., and B.D. Walker. 1998. The critical need for CD4+ help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung, K., R. Hayashi, A. Lafond-Walker, C. Lowenstein, D. Pardoll, and H. Levitsky. 1998. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating, S., S. Prince, M. Jones, and M. Rowe. 2002. The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules. J. Virol. 76:8179–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ressing, M.E., D. van Leeuwen, F.A. Verreck, S. Keating, R. Gomez, K.L. Franken, T.H. Ottenhoff, M. Spriggs, T.N. Schumacher, L.M. Hutt-Fletcher, et al. 2005. Epstein-Barr virus gp42 is posttranslationally modified to produce soluble gp42 that mediates HLA class II immune evasion. J. Virol. 79:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Precopio, M.L., J.L. Sullivan, C. Willard, M. Somasundaran, and K. Luzuriaga. 2003. Differential kinetics and specificity of EBV-specific CD4+ and CD8+ T cells during primary infection. J. Immunol. 170:2590–2598. [DOI] [PubMed] [Google Scholar]
- 17.Landais, E., X. Saulquin, M. Bonneville, and E. Houssaint. 2005. Long-term MHC class II presentation of the EBV lytic protein BHRF1 by EBV latently infected B cells following capture of BHRF1 antigen. J. Immunol. 175:7939–7946. [DOI] [PubMed] [Google Scholar]
- 18.Wallace, L.E., J. Wright, D.O. Ulaeto, A.J. Morgan, and A.B. Rickinson. 1991. Identification of two T-cell epitopes on the candidate Epstein-Barr virus vaccine glycoprotein gp340 recognized by CD4+ T-cell clones. J. Virol. 65:3821–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitki-Green, D., M. Covington, and N. Raab-Traub. 2003. Compartmentalization and transmission of multiple epstein-barr virus strains in asymptomatic carriers. J. Virol. 77:1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHeyzer-Williams, M., L. McHeyzer-Williams, J. Panus, R. Pogue-Caley, G. Bikah, D. Driver, and M. Eisenbraun. 2003. Helper T-cell-regulated B-cell immunity. Microbes Infect. 5:205–212. [DOI] [PubMed] [Google Scholar]
- 21.Callan, M.F., L. Tan, N. Annels, G.S. Ogg, J.D. Wilson, C.A. O'Callaghan, N. Steven, A.J. McMichael, and A.B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jilg, W., C. Bogedain, H. Mairhofer, S.Y. Gu, and H. Wolf. 1994. The Epstein-Barr virus-encoded glycoprotein gp 110 (BALF 4) can serve as a target for antibody-dependent cell-mediated cytotoxicity (ADCC). Virology. 202:974–977. [DOI] [PubMed] [Google Scholar]
- 23.Milosevic, S., U. Behrends, H. Christoph, and J. Mautner. 2005. Direct mapping of MHC class II epitopes. J. Immunol. Methods. 306:28–39. [DOI] [PubMed] [Google Scholar]
- 24.Moosmann, A., N. Khan, M. Cobbold, C. Zentz, H.J. Delecluse, G. Hollweck, A.D. Hislop, N.W. Blake, D. Croom-Carter, B. Wollenberg, et al. 2002. B cells immortalized by a mini-Epstein-Barr virus encoding a foreign antigen efficiently reactivate specific cytotoxic T cells. Blood. 100:1755–1764. [PubMed] [Google Scholar]
- 25.Kempkes, B., D. Pich, R. Zeidler, B. Sugden, and W. Hammerschmidt. 1995. Immortalization of human B lymphocytes by a plasmid containing 71 kilobase pairs of Epstein-Barr virus DNA. J. Virol. 69:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carel, J.C., B.L. Myones, B. Frazier, and V.M. Holers. 1990. Structural requirements for C3d,g/Epstein-Barr virus receptor (CR2/CD21) ligand binding, internalization, and viral infection. J. Biol. Chem. 265:12293–12299. [PubMed] [Google Scholar]
- 27.Wendtner, C.M., C. Kurzeder, H.D. Theiss, D.M. Kofler, J. Baumert, H.J. Delecluse, A. Janz, W. Hammerschmidt, and M. Hallek. 2003. High level of transgene expression in primary chronic lymphocytic leukemia cells using helper-virus-free recombinant Epstein-Barr virus vectors. Exp. Hematol. 31:99–108. [DOI] [PubMed] [Google Scholar]
- 28.Prodinger, W.M., M.G. Schwendinger, J. Schoch, M. Kochle, C. Larcher, and M.P. Dierich. 1998. Characterization of C3dg binding to a recess formed between short consensus repeats 1 and 2 of complement receptor type 2 (CR2; CD21). J. Immunol. 161:4604–4610. [PubMed] [Google Scholar]
- 29.Nimmerjahn, F., D. Kobelt, A. Steinkasserer, A. Menke, G. Hobom, U. Behrends, G.W. Bornkamm, and J. Mautner. 2003. Efficient generation and expansion of antigen-specific CD4+ T cells by recombinant influenza viruses. Eur. J. Immunol. 33:3331–3341. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S.P., L.E. Wallace, M. Mackett, J.R. Arrand, P.F. Searle, M. Rowe, and A.B. Rickinson. 1993. MHC class II-restricted presentation of endogenously synthesized antigen: Epstein-Barr virus transformed B cell lines can present the viral glycoprotein gp340 by two distinct pathways. Int. Immunol. 5:451–460. [DOI] [PubMed] [Google Scholar]
- 31.Bickham, K., K. Goodman, C. Paludan, S. Nikiforow, M.L. Tsang, R.M. Steinman, and C. Munz. 2003. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J. Exp. Med. 198:1653–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subklewe, M., C. Paludan, M.L. Tsang, K. Mahnke, R.M. Steinman, and C. Munz. 2001. Dendritic cells cross-present latency gene products from Epstein-Barr virus-transformed B cells and expand tumor-reactive CD8+ killer T cells. J. Exp. Med. 193:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 181:595–608. [DOI] [PubMed] [Google Scholar]
- 34.Babcock, G.J., L.L. Decker, R.B. Freeman, and D.A. Thorley-Lawson. 1999. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J. Exp. Med. 190:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldanti, F., P. Grossi, M. Furione, L. Simoncini, A. Sarasini, P. Comoli, R. Maccario, R. Fiocchi, and G. Gerna. 2000. High levels of Epstein-Barr virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders. J. Clin. Microbiol. 38:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montone, K.T., R.L. Hodinka, K.E. Salhany, E. Lavi, A. Rostami, and J.E. Tomaszewski. 1996. Identification of Epstein-Barr virus lytic activity in post-transplantation lymphoproliferative disease. Mod. Pathol. 9:621–630. [PubMed] [Google Scholar]
- 37.Tao, Q., K.D. Robertson, A. Manns, A. Hildesheim, and R.F. Ambinder. 1998. Epstein-Barr virus (EBV) in endemic Burkitt's lymphoma: molecular analysis of primary tumor tissue. Blood. 91:1373–1381. [PubMed] [Google Scholar]
- 38.Khanna, R., S.R. Burrows, S.A. Thomson, D.J. Moss, P. Cresswell, L.M. Poulsen, and L. Cooper. 1997. Class I processing-defective Burkitt's lymphoma cells are recognized efficiently by CD4+ EBV-specific CTLs. J. Immunol. 158:3619–3625. [PubMed] [Google Scholar]
- 39.Staege, M.S., S.P. Lee, T. Frisan, J. Mautner, S. Scholz, A. Pajic, A.B. Rickinson, M.G. Masucci, A. Polack, and G.W. Bornkamm. 2002. MYC overexpression imposes a nonimmunogenic phenotype on Epstein-Barr virus-infected B cells. Proc. Natl. Acad. Sci. USA. 99:4550–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paludan, C., K. Bickham, S. Nikiforow, M.L. Tsang, K. Goodman, W.A. Hanekom, J.F. Fonteneau, S. Stevanovic, and C. Munz. 2002. Epstein-Barr nuclear antigen 1-specific CD4(+) Th1 cells kill Burkitt's lymphoma cells. J. Immunol. 169:1593–1603. [DOI] [PubMed] [Google Scholar]
- 41.Morgan, A.J., M. Mackett, S. Finerty, J.R. Arrand, F.T. Scullion, and M.A. Epstein. 1988. Recombinant vaccinia virus expressing Epstein-Barr virus glycoprotein gp340 protects cottontop tamarins against EB virus-induced malignant lymphomas. J. Med. Virol. 25:189–195. [DOI] [PubMed] [Google Scholar]
- 42.Morgan, A.J., and A.D. Wilson. 1997. Epstein-Barr virus gp350 vaccines. EBV Rep. 4:33–39. [Google Scholar]
- 43.Thorley-Lawson, D.A., and C.A. Poodry. 1982. Identification and isolation of the main component (gp350-gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J. Virol. 43:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson, A.D., M. Shooshstari, S. Finerty, P. Watkins, and A.J. Morgan. 1996. Virus-specific cytotoxic T cell responses are associated with immunity of the cottontop tamarin to Epstein-Barr virus (EBV). Clin. Exp. Immunol. 103:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mautner, J., D. Pich, F. Nimmerjahn, S. Milosevic, D. Adhikary, H. Christoph, K. Witter, G.W. Bornkamm, W. Hammerschmidt, and U. Behrends. 2004. Epstein-Barr virus nuclear antigen 1 evades direct immune recognition by CD4+ T helper cells. Eur. J. Immunol. 34:2500–2509. [DOI] [PubMed] [Google Scholar]
- 46.Zuber, B., V. Levitsky, G. Jonsson, S. Paulie, A. Samarina, S. Grundstrom, S. Metkar, H. Norell, G.G. Callender, C. Froelich, and N. Ahlborg. 2005. Detection of human perforin by ELISpot and ELISA: ex vivo identification of virus-specific cells. J. Immunol. Methods. 302:13–25. [DOI] [PubMed] [Google Scholar]
- 47.Granberg, C., K. Blomberg, I. Hemmila, and T. Lovgren. 1988. Determination of cytotoxic T lymphocyte activity by time-resolved fluorometry using europium-labelled concanavalin A-stimulated cells as targets. J. Immunol. Methods. 114:191–195. [DOI] [PubMed] [Google Scholar]
- 48.Kimura, H., M. Morita, Y. Yabuta, K. Kuzushima, K. Kato, S. Kojima, T. Matsuyama, and T. Morishima. 1999. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J. Clin. Microbiol. 37:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]