Abstract
A male zebra finch, Taeniopygia guttata, kept with its father until adulthood develops an imitation of its father’s song motif. We report here that the completeness of this imitation was sensitive to the social or auditory context in which the bird grew up: the greater the number of male siblings in a clutch, the shorter the mean duration of the song motif and the fewer the mean number of song notes imitated from the father; the latter shortfall was not compensated by other, improvised notes. We call this effect fraternal inhibition. Fraternal inhibition was avoided by members of a clutch that developed the song first. To our surprise, this role commonly fell to one of the younger birds in the clutch. Early song learning may influence fitness since individuals that produced the most complete imitations also tended to induce more egg laying.
Song learning in songbirds has provided classical material to demonstrate how the predispositions that members of different species bring to a learning task affect the “when” and “what” of learning. Initially, much attention was devoted to the nature of the songs imitated and to the sensitive period when this occurred (1–3). Subsequently, it was shown that other variables could matter too, as when Baptista and Petrinovich (4) emphasized that live models can be more effective than playbacks, or when King and West (5) described the importance of female responses in coaching the development of male song, or when Williams (6) described the extent to which an individual borrowed material from more than one tutor. Our study adds a new facet to this well-established field in that it looks at the influence of the family milieu on vocal learning, using as an example a very social species, the zebra finch.
Song develops in male zebra finches during the first 90 days after hatching and a relatively stable adult song is in place by the end of that period, which coincides with the onset of sexual maturity (7). Even though different male zebra finches can produce songs that are quite dissimilar, the songs of father and son are remarkably alike when the son grows up in the company of its father. This close similarity is not determined by genetic relatedness because juvenile zebra finches can develop an accurate imitation of a foster father’s song, even when the foster father is a member of a different species (8, 9).
We investigated song imitation in clutches with different numbers of male siblings. We show that (i) the greater the number of male siblings in a clutch, the shorter the mean duration of their song motif and the fewer the mean number of song notes imitated from the father; (ii) the order in which male siblings from the same clutch hatched was inversely related to the completeness of imitations and song duration; (iii) individuals that produced more complete imitations tended to induce an earlier ovulation response in females than was the case for males with less complete imitations. We do not know how these observations apply to vocal learning in free-ranging zebra finches, but they suggest ways in which vocal learning can affect fitness.
METHODS
Animals.
Thirty-four clutches of one to five male siblings from 32 pairs of zebra finches that had bred before were used in the work reported here. An animal attendant checked the nest boxes daily and entered in the record sheet for that cage the day when each offspring was first noticed. As soon as a new hatchling was discovered, one of its toes was clipped and that bird’s hatching date was recorded, so that we knew (on a day-by-day basis) which nestlings hatched first and which last. Yet, it was not infrequent for two or even three nestlings to hatch on a same day and as a result we were able to individually identify only 51% of the first hatched and 47% of the last hatched male siblings (female siblings are not included in this analysis). Because of this imprecision in birth dating, our “early-hatched” and “late-hatched” categories included all male siblings hatched, respectively, on the first or last hatching day for a given clutch. When more than one male sibling of a particular clutch was included in either of these subgroups, we averaged the behavioral observations for siblings in that subgroup, so that each clutch had only one measurement for the first and another for the last day of hatching. Clutches in which all (one or more) male siblings hatched on the same day where not included in this analysis. Each juvenile received a numbered color band at approximately 20 days, when the young left the nest. All siblings of both sexes were kept with their parents up to the age of 30 days.
Experimental Groups.
Group 1. Ten clutches with a variable number of male siblings were used to establish our baseline for optimal song imitation. Only one, early-hatched male in each of these clutches—and no other siblings—was kept singly with its father after day 30 and until day 90. Each father/son pair was housed in its own cage in a soundproof chamber. We thank Uwe Hahmann, a member of our laboratory, for providing these data.
Group 2.
Another five clutches of two to five male siblings (total = 18) and their sisters were kept with their parents up to day 90, one family per soundproof room. To keep track of song development, each of these families was video recorded through a transparent plexiglass cage wall for 2 h every other day between the age of 40 and 90 days. Double banding with black or white bands was used to facilitate bird identification in video playbacks.
Group 3.
This group consisted of 16 clutches (from 14 pairs). The siblings from each clutch were housed with their parents in the same cage. These cages were kept in a room with many other zebra finches and each family stayed in its cage until day 90. No attempt was made to prevent any bird from seeing or hearing birds in adjacent cages.
Group 4.
Three clutches of three male siblings were kept in three separate rooms. Between day 30 and day 90 the siblings were housed in separate cages around their parents’ cage so that each sibling could approach its father and other siblings and interact with them across the wire wall of the cages, yet could not attack the other birds or be attacked by them.
Birds in groups 1, 2, and 4 were housed in small cages (18 inches × 9 inches × 10 inches). Birds in group 3 were housed in larger cages (23 inches × 14 inches × 20 inches).
Song Recording.
At least 10 renditions of a bird’s song motif, produced in a female-directed manner (10), were recorded digitally from each male at 90 days or soon thereafter and directed song from each of the fathers was also recorded at that time. After the initial recording, the siblings were held singly in separate cages in a communal room where they could hear other birds, and their song was recorded again 2 wk later to confirm that its development had reached a stable, mature state. Songs were recorded with a SHURE 33–1070C omnidirectional dynamic microphone and were digitized (16 bits, 44100 Hz) using Canary 1.12 song analysis software (11). This equipment, jointly, gave a fairly flat response in the range of 100–8000 Hz that contains most of the amplitude of a zebra finch song. Sound recordings were converted into a visual display (soundspectrogram) using a KAY Digital Sona-Graph 7800.
Scoring Note Similarity.
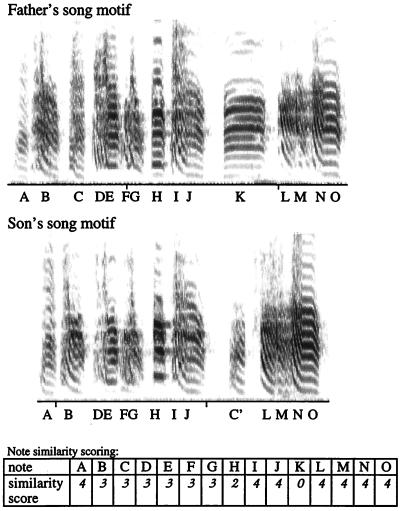
A song “note” is a song sound preceded or followed by a silent interval or by a sharp change in frequency modulation (e.g., refs. 6 and 12). Song notes are usually delivered in a stereotyped order. A song “motif” is defined as a complete set of sequentially delivered notes; only rarely is a song note repeated within the motif. A song bout consists of a series of introductory notes followed by several repetitions of the bird’s song motif. For purposes of analysis, the father’s song motif (exclusive of introductory notes) was segmented into its constituent notes and each note was labeled with a different letter. The son’s song motif was also segmented into its constituent notes and notes that were similar to those of the father received the same letter. The position of a note within the father and son’s song was used as partial evidence for that note’s identity. Notes that received the same letter were scored for degree of similarity. A score of 1 denoted very poor similarity except, for example, similar position, duration, and overall frequency envelope; a score of 2 denoted similarity in position and duration, but only partial similarity in fundamental frequency and frequency modulation; a score of 3 indicated that both versions of the note were quite similar but not identical; a score of 4 stood for virtual identity, but if that note was translocated in the son’s motif then it got a score of 3 (Fig. 1). If a note in the son’s song could not be related to any of the father’s notes, it was assigned a score of 0. Only notes with scores of 3 or 4 were said to be shared by father and son. The person who scored the songs did not know how many male siblings each juvenile male had.
Figure 1.
An example of how we scored the similarity of the song motifs produced by a father and one of its sons. Song notes considered to be similar received the same letter; degree of similarity between each of the notes of the father’s song and the corresponding note in the son’s song is given by the similarity score, which appears under the son’s song motif. In this case 14 of the father’s 15 notes were present in the son’s song, but only 13 of them received a score high enough to qualify as imitation. The son’s note C′ is very similar to the father’s note C but transposed in order. Overall similarity between the two songs was 86.6%. See text for details.
Whole Song Comparisons.
Comparisons between the song of a father and those of its sons used two measures. The first one, “relative duration,” looked at the absolute duration of the father’s and son’s song motifs and expressed the latter as a percentage of the former. Therefore, relative duration = 100 × son’s motif duration/father’s motif duration. This measure makes no assumption about imitation and in principle, though not in practice, is independent of imitation. The second measure, “similarity,” corresponds to the percentage of father’s motif notes present in the son’s song; therefore, similarity = 100 × number of shared notes/number of notes in father’s song. A son with a 100% similarity score might include in its song other sounds, but these additional sounds would not affect the similarity score, though relative duration would in this case be higher than 100%. If there were some song notes that were not included in all repetitions of a bird’s song motif, they were still included in our estimates of similarity because they were part of what the father offered for imitation and what the son might have achieved in terms of imitation.
Song Development.
To assess song development in the five clutches that were observed during ontogeny (group 2), sonograms of early plastic song for each young male were extracted from the sound channel of the video recordings that had been made every other day and were compared with the mature song of the same bird. The male in a clutch of siblings that was first to produce all notes of the mature song in a recognizable, albeit variable manner, on two successive recordings was said to lead in the process of song maturation, and other male siblings in that clutch were ranked in the same manner, yielding an “order of song maturation.”
Statistical Analysis.
Our data are presented as means ± SE. We used nonparametric statistics because we could not assume linearity of our measurements nor could we assume normality for the distribution of our measures (relative duration and similarity). The Mann–Whitney U test was used to test for the significance of behavioral differences between groups. For completeness of presentation, we included the values of similarity and relative duration for all experimental groups/subgroups even when statistical significance was not calculated for some of these measures to avoid using the same sample in multiple tests of significance. Differences between paired samples were examined using Wilcoxon-matched pairs test. We used the Pearson correlation coefficient to test for the significance of observed correlations, but the correlation between song imitation and reproductive success was examined nonparametrically using the γ correlation: γ statistic is preferable here since there were many cases in which the females showed no reproductive response. We used the Faulse discovery rate method (13) to adjust P values when multiple tests were performed on the same data; however, we did not adjust the P values when we examined the possible effect that all siblings in a clutch (male and female) might have on song imitation (group 3) because this analysis was done a posteriori, at the request of a reviewer, and the results were nonsignificant.
RESULTS
Imitation by Single Versus Multiple Male Siblings Kept in Group Isolation.
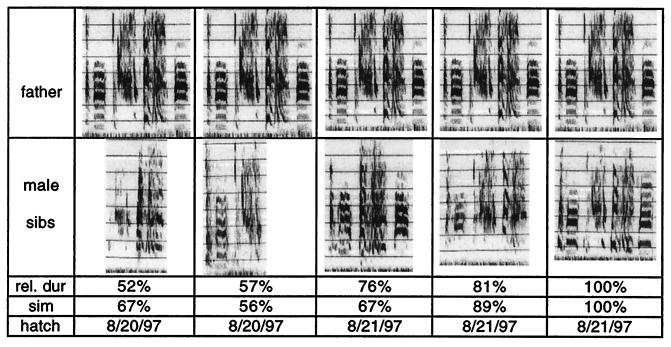
In 10 cases of male zebra finches kept singly in isolation with their fathers between days 30 and 90 after hatching (group 1), the mean relative duration of their song motif was 101 ± 3% and the mean similarity was 91 ± 3%. The correlation between father’s and son’s motif duration was high (r = 0.96; P < 0.001). These results form the baseline for what we call “optimal imitation.” In contrast, the song motifs of 18 male zebra finches raised in five clutches of two to five male siblings until the age of 90 days (group 2) had lower mean relative durations (84 ± 6%, U = 46; P < 0.05) and lower mean similarity (73 ± 5%, U = 43; P < 0.05) than those of the single male siblings. Moreover, the correlation between the father’s and son’s motif duration in those 18 birds was remarkably low (r = 0.21; not significant). As an example, Fig. 2 shows sonograms of songs of five siblings in one of the group 2 families. Each sibling had a distinct motif that included all or just a subset of the father’s notes and none of the siblings improvised notes.
Figure 2.
An example of song imitation in a clutch of five male siblings raised by their parents to the age of 90 days. The song motif of the father and that of its sons was recorded when the latter were 108–110 days old. Relative duration, similarity and hatching date are shown under the song motif of each sibling. The father’s song motif is shown five times, aligned with each son’s song to facilitate comparisons between notes.
Influence of Multiple Siblings When Clutches Were Kept in a Colony Room.
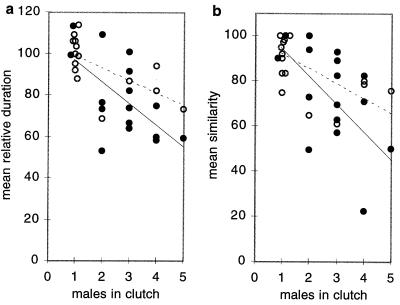
Results similar to those described for group 2 were obtained when clutches were kept in larger cages (see Methods) in a room that housed many other birds. We used this setting to obtain data from a larger number of animals (group 3; n = 16 clutches of one to five males, a total of 46 males). Although imitation was often incomplete, 96% of the notes in the sons’ song motif were very similar to the notes in the father’s motif. As the number of male siblings in a clutch increased, the mean relative duration of the siblings’ song motifs decreased (Fig. 3a, r = −0.54, P < 0.05, n = 16), as did the mean similarity (Fig. 3b, r = −0.55, P < 0.05, n = 16). In clutches with more than one male sibling, the duration of the father’s song was an additional significant factor: the longer the father’s motif, the less complete imitations tended to be. Together, the number of male siblings and father’s song duration explained with little redundancy most of the variability (r2 = 65%) in mean relative duration observed across clutches (r = −0.81; partial correlations: with clutch size, r = −0.72, with father’s song duration, r = −0.71, tolerance >0.99 for both). When instead of looking just at the influence of male siblings on song we looked at the influence of all siblings in a clutch (male and female) on song, the correlations were no longer significant (r{clutch size, similarity} = −0.11, P = 0.7; r{clutch size, relative duration} = −0.43, P = 0.09), suggesting that female siblings do not play a major role in affecting the completeness of imitation. This limited exploration of the influence of female siblings was done a posteriori at the suggestion of one of our reviewers.
Figure 3.
Correlation between the number of males in a clutch and the mean relative duration (a) and mean similarity (b) of their song when compared with that of their father. Each circle stands for the values from one clutch. Filled circles and the solid line correspond to group 3 birds; open circles and dashed line correspond to birds in groups 1 and 2.
Order of Hatching Effect.
We speculated that siblings hatched earlier would tend to acquire their song motif earlier and produce better imitations of the father’s song. We therefore compared the relative duration and similarity of the song of early-hatched and late-hatched individuals that were members of 13 clutches of two to four siblings (part of group 3, excluding two clutches with only one male per clutch and a third clutch for which order of hatching of male siblings was not determined). Surprisingly, imitation was incomplete (lower than the optimal level defined by group 1) in the early-hatched siblings of each clutch (relative duration = 65 ± 6%, Mann-Whitney U = 7; P < 0.001; similarity = 67 ± 7%, Mann-Whitney U = 17; P < 0.01) but not in late-hatched ones (relative duration = 88 ± 6%, not significant; similarity = 81 ± 7%, not significant). Group 2 birds yielded comparable results. In them early-hatched males had songs with a similarity of 64 ± 8%; the songs of the late-hatched ones had a similarity of 89 ± 11%.
Incomplete Imitation Persists When Siblings Cannot Attack Each Other.
We wondered whether the incomplete imitation seen in early-hatched birds resulted from agonistic interactions between siblings. We therefore examined the songs of nine birds (from three clutches, group 4) that were housed singly from day 30 to day 90 after hatching in cages that were adjacent to those holding their father and other siblings. As did Böhner (8), we found that most of a young male’s song notes (95%) were very similar to those of its father. However, all nine siblings had motifs shorter than their father’s (Wilcoxon-matched pairs test, P < 0.01; relative duration = 67 ± 5%; similarity = 56 ± 6%). We conclude that incomplete imitation occurs also when siblings cannot attack each other. This sample was too small to determine whether order of hatching had an effect on relative duration or similarity.
Order of Song Development Effect.
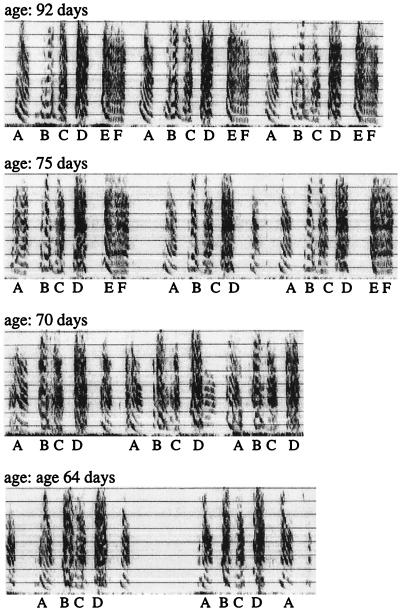
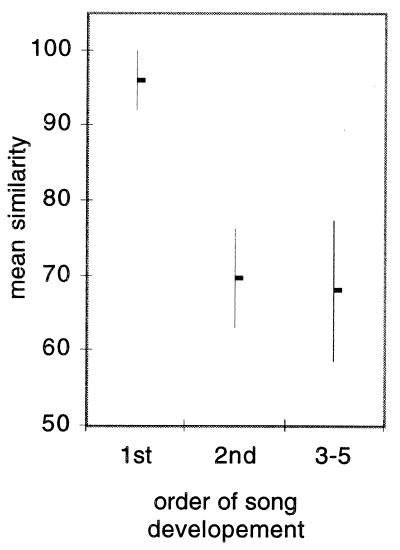
It was mentioned above that late-hatched siblings tend to produce more complete imitations than early-hatched ones. How does this come about during ontogeny? To answer this question we made every other day recordings of song in five clutches of two to five male siblings raised by their parents in soundproof rooms (group 2). We found that the first siblings to produce recognizable versions of their adult motif (example of motif development shown in Fig. 4) also produced the most accurate imitation of their father’s motif (Fig. 5, P < 0.05).
Figure 4.
Example of motif development in a juvenile male shown in reverse order. All notes of the adult motif were identifiable on day 75 though the temporal order was still variable. Note E could not be identified on day 70 or earlier.
Figure 5.
Relationship between order of song development and song similarity; values for birds that developed their song in order 3, 4, and 5 were lumped together (P < 0.05 for comparisons between order 1 and 2 and between 1 and 3–5).
The results presented up to here make it clear that the birds that hatched last and those that started to develop their song first were most likely to produce complete song imitations. In one case, however, these two variables were not in step. This occurred in one of the group 2 clutches, which had three male siblings. One of the two early-hatched siblings was the first to produce a recognizable version of its adult song and it achieved, as adult, a similarity of 100%. The last hatched sibling was second to produce a recognizable version of its adult song and when this bird reached maturity its song had a similarity of 86%. The other early-hatched sibling was the last one to develop song and the similarity of its song as adult was only 14%. Another remarkable case, also in a group 2 clutch, was a late-hatched bird that suffered a severe leg injury at a young age. Although it was clearly the weakest sibling in the clutch, it was the first to produce a recognizable version of its adult song and this injured bird produced the best imitation of the father’s song among the five male siblings in that clutch.
Sibling Interactions May Affect Sex Appeal.
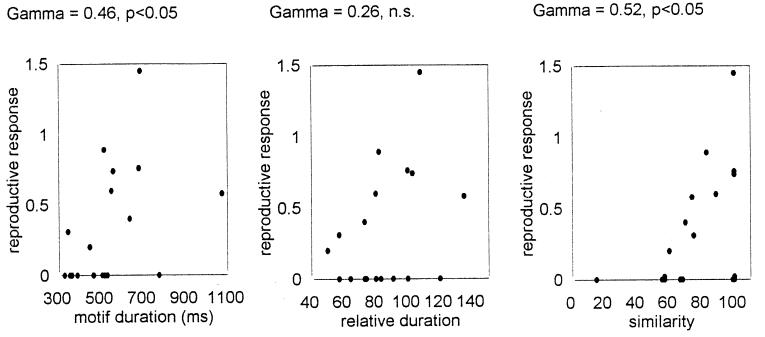
In some songbird species (14, 15), including zebra finches (16), females are more attracted to males with large, complex song repertoires than to males with simple songs. We wondered whether the completeness of a young male zebra finch’s song imitation would affect its breeding success. To explore this question, as each of the 18 males of group 2 reached the age of 110–130 days, it was placed in the same cage with a 90-day-old virgin female that had no prior exposure to any of the young males or their fathers. Each pair received a nest cup and burlap nesting material. Egg laying was observed for 3 wk. The intensity of the reproductive response of each female was assessed by lag to egg laying and number of eggs laid as follows: let D1, D2 ,… , Dn represent days elapsed before laying the first, second,… , nth egg. We define a female’s reproductive response as the summation of the reciprocals, ∑Di−1. Fig. 6 presents the relationship between a female’s reproductive response and her partner’s song similarity, song relative duration, and absolute song duration (in ms). The relationship between the reproductive response and song similarity and that between the reproductive response and absolute song duration were statistically significant; the relation between the reproductive response and song relative duration showed a similar trend but was not significant. Interestingly, half of the 18 females did not respond with ovulation. When only females that laid eggs are considered, the relationship between the reproductive response and song similarity became stronger (γ = 0.7, P < 0.05) while the significance of the relationship with song duration disappeared (γ = 0.3, not significant). Apparently, males with very short songs were not likely to induce ovulation, but when ovulation was induced, the intensity of the female’s response was strongly related to the completeness of her partner’s song imitation.
Figure 6.
The relationship among intensity of the ovulation response and (i) motif duration (in ms), (ii) relative duration, and (iii) similarity. The γ correlation value and its significance are shown above each graph.
DISCUSSION
The classic studies of King and West on song ontogeny in the cowbird showed that song structure in this parasitic species is affected not just by the availability of a model, leading to imitation, but also by social factors such as stimulation by females (5) and male aggression (17) that cause a male to include or omit, respectively, song features that add or detract from the sex appeal of its song. Böhner (8) suggested that inaccuracies in song imitation might serve a purpose in zebra finches, e.g., by promoting the uniqueness of each juvenile’s own song when several siblings imitated the same model. Our study goes one step further in that it shows that rules seem to govern the relative completeness of song imitation. Incomplete imitations are more common among early-hatched than among late-hatched siblings. Moreover, the completeness of the imitation seems to be influenced by the order of song development, which tends to be inversely related to hatching order. Our greatest surprise came from the observation that the youngest siblings were most likely to develop song first and claim the entirety of the father’s song; older siblings, which developed their song later, settled for less. These results bring to mind Schwabl’s (18) observations on the relationship between order of egg laying and amount of testosterone in egg yolk, with eggs laid first having less yolk testosterone than eggs laid last. Higher levels of yolk testosterone could induce an earlier onset of song development and therefore of imitation. However, it is not known whether Schwabl’s (18) observations, made in canaries, apply to zebra finches.
We do not know whether the relationship among clutch size, order of hatching, completeness of imitation, and reproductive success that we observed in captive birds has parallels in zebra finches kept in a colony milieu or breeding in the wild. Male zebra finches that grow up in a colonial aviary where they have access to more than one adult male often imitate parts of the songs of two or more different males (6). We cannot be sure, from our present results, that the song parameters we quantified were crucial variables in inducing ovulation. But even leaving these matters aside, we are puzzled by what might be the advantage of a vocal ontogeny that, under some circumstances at least, is so narrowly committed to imitation. Male zebra finches reared in auditory isolation from other birds improvise sounds, some of which are zebra finch-like and others that are not. The development of these sounds is influenced by auditory feedback because the sounds produced are very different when the males are deafened early in life (19). Why is it that zebra finches reared in a family milieu make so little use of improvisation, a strategy that under the circumstances of our work could have easily led to greater song diversity and to a lengthening of the song motif? An understanding of the interactions between the strategy used to acquire adult song (e.g., imitation), the contexts that modify this strategy (e.g., the circumstances described in the present study), and reproductive fitness may help clarify the selective pressures that led to the evolution of vocal learning and how the use of vocal learning relates to a species’ life history.
Another study (20) noted, anecdotally, that in a group of three male siblings, the youngest one produced the best song imitation and also had the greatest number of neurons in the high vocal center. High vocal center is a key nucleus in the pathway for song learning (21). The 10 males in our group 1 that were kept singly with their father and showed optimal imitation were early hatchers in clutches that, in most cases, contained one or more other siblings. Severe cases of incomplete imitation were observed only when several male siblings were kept together with their father, and in those cases the incomplete imitation tended to be most pronounced in the early-hatched birds. We therefore suggest that all male siblings in a clutch, regardless of their order of hatching, start with the full potential to produce a complete song imitation. We suggest, too, that interactions among male siblings from the same clutch or among these siblings and their father affect the endocrine and neural development of some of the juveniles so as to hinder the eclosion of their vocal talents and imitation of their father’s song.
A simple explanation for all of the facts we observed is that model abundance affects the fidelity of imitation. Fidelity is at its peak (optimal imitation) when there is a single model source—in our case the father—and a single, live-in pupil that imitates the model. As more pupils—in our case male siblings from the same clutch—produce recognizable copies of the father’s song, model abundance increases. Birds that under those conditions develop their song last find correspondingly higher levels of model abundance. At this higher level, the factors thought to favor imitation—a live-in, socially interactive model—and those that we now hypothesize discourage imitation—over abundance of model expression—are pitted against each other and this conflict determines how much of the model will be imitated.
Factors other than model abundance, such as the nature of the social interaction among male members of a clutch, could also affect the completeness of song imitation, as suggested by the fact that none of the males kept in cages adjacent to that of their father produced optimal imitations. Perhaps model abundance and social variables interact. Interestingly, young male zebra finches group reared without access to a live-in adult model do not produce a diversity of improvised songs but share many song notes and the order of their delivery, and this seems to result from mutual imitation (22, 23). Thus, the freedom to learn would seem to be curtailed by the tyranny of laws that govern learning. Model imitation, when there is a model, takes precedence over song improvisation. We now suggest that the overabundant expression of a model reduces the completeness of imitation without reinstating the freedom to improvise. Sed lex, dura lex—it’s a hard law, but it is the law.
Acknowledgments
We thank Uwe Hahmann for making his data (group 2) available for us. We thank Heather Williams, David Vicario, Constance Scharff, and Thierry Lints for their very helpful comments. This study was made possible by a grant from Mr. Romie Shapiro and by support from Public Health Service Grant MH18343.
References
- 1.Thorpe W H. Ibis. 1958;100:535–570. [Google Scholar]
- 2.Marler P. J Comp Physiol Psychol. 1970;71:1–25. [Google Scholar]
- 3.Gould J L, Marler P. Sci Am. 1987;256:74–85. [Google Scholar]
- 4.Baptista L F, Petrinovich L. Anim Behav. 1984;32:172–181. [Google Scholar]
- 5.King A P, West M J. Nature (London) 1983;305:704–706. [Google Scholar]
- 6.Williams H. Anim Behav. 1990;39:745–757. [Google Scholar]
- 7.Immelmann K. In: Bird Vocalizations. Hinde R A, editor. Cambridge, U.K.: Cambridge Univ. Press; 1969. pp. 61–74. [Google Scholar]
- 8.Böhner J. Anim Behav. 1983;31:231–237. [Google Scholar]
- 9.Eales L A. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 10.Zann R E. The Zebra Finch: Synthesis of Field and Laboratory Studies. New York: Oxford Univ. Press; 1996. [Google Scholar]
- 11.Charif R A, Mitchell S, Clark C W. Canary 1.12 User’s Manual. Ithaca, NY: Cornell Laboratory of Ornithology; 1995. [Google Scholar]
- 12.Morrison R G, Nottebohm F. J Neurobiol. 1993;24:1045–1064. doi: 10.1002/neu.480240805. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. F R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 14.Kroodsma D E. Science. 1976;192:574–575. doi: 10.1126/science.192.4239.574. [DOI] [PubMed] [Google Scholar]
- 15.Catchpole C K, Dettami J, Leisler B. Nature (London) 1984;312:563–564. [Google Scholar]
- 16.Clayton N S, Pröve E. Anim Behav. 1989;38:352–362. [Google Scholar]
- 17.West M J, King A P. J Comp Psychol. 1980;94:263–270. [Google Scholar]
- 18.Schwabl H. Proc Natl Acad Sci USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price P H. J Comp Physiol Psychol. 1979;93:260–277. [Google Scholar]
- 20.Ward B C, Nordeen E J, Nordeen K W. Proc Natl Acad Sci USA. 1998;95:1277–1282. doi: 10.1073/pnas.95.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 22.Morrison R G. Ph.D. dissertation. New York: The Rockefeller University; 1991. [Google Scholar]
- 23.Volman S F, Khanna H. J Comp Psychol. 1995;109:211–221. doi: 10.1037/0735-7036.109.3.211. [DOI] [PubMed] [Google Scholar]