Abstract
X-linked lymphoproliferative disease is caused by mutations affecting SH2D1A/SAP, an adaptor that recruits Fyn to signal lymphocyte activation molecule (SLAM)-related receptors. After infection, SLAM-associated protein (SAP)−/− mice show increased T cell activation and impaired humoral responses. Although SAP−/− mice can respond to T-independent immunization, we find impaired primary and secondary T-dependent responses, with defective B cell proliferation, germinal center formation, and antibody production. Nonetheless, transfer of wild-type but not SAP-deficient CD4 cells rescued humoral responses in reconstituted recombination activating gene 2−/− and SAP−/− mice. To investigate these T cell defects, we examined CD4 cell function in vitro and in vivo. Although SAP-deficient CD4 cells have impaired T cell receptor–mediated T helper (Th)2 cytokine production in vitro, we demonstrate that the humoral defects can be uncoupled from cytokine expression defects in vivo. Instead, SAP-deficient T cells exhibit decreased and delayed inducible costimulator (ICOS) induction and heightened CD40L expression. Notably, in contrast to Th2 cytokine defects, humoral responses, ICOS expression, and CD40L down-regulation were rescued by retroviral reconstitution with SAP-R78A, a SAP mutant that impairs Fyn binding. We further demonstrate a role for SLAM/SAP signaling in the regulation of early surface CD40L expression. Thus, SAP affects expression of key molecules required for T–B cell collaboration by mechanisms that are distinct from its role in cytokine regulation.
X-linked lymphoproliferative (XLP) disease is a complex disorder characterized by severe immune dysregulation that is exacerbated by EBV infection, often resulting in fatal infectious mononucleosis (1). Individuals with XLP who survive EBV infection frequently develop dysgammaglobulinemia and B cell lymphomas. The presence of these phenotypes in XLP patients in the absence of EBV exposure, however, suggests a more basic immune dysfunction associated with this disease.
Genetic studies have demonstrated that XLP is associated with mutations affecting SH2D1A/SAP/DSHP, which encodes a 128–amino acid protein comprised largely of an SH2 domain (hereafter referred to as signal lymphocyte activation molecule [SLAM]-associated protein [SAP]) (1). SAP is expressed in T cells, NK cells, NKT cells, and some B cell populations. SAP binds to a conserved tyrosine-containing motif found in the intracellular domain of CD150/SLAM and related family members, including CD84, CD229/Ly9, CD224/2B4, CRACC, and NTB-A/Ly108 (1). After ligation of SLAM-related receptors, SAP recruits and activates the Src family kinase Fyn, thereby permitting receptor tyrosine phosphorylation and binding of several downstream proteins (2–4). Overexpression studies indicate that SAP may also competitively interfere with recruitment of phosphatases (5, 6).
To provide insight into the pathophysiology of XLP, several groups have generated mice that lack SAP expression (7–9). Studies of these mice and XLP patients demonstrated that SAP is involved in a diverse array of lymphocyte functions, including Th cell signaling and differentiation, 2B4-mediated NK and CD8 cell killing, generation of NKT cells, and germinal center (GC) formation, as well as the generation of memory B cells and long-lived plasma cells (1). Initial examination of SAP−/− mice suggested that SAP expression is critical for CD4 T cell–mediated help necessary for regulating long-term humoral immunity to lymphocytic choriomeningitis virus (LCMV) (10). However, more recent data argue that B cells also contribute to defects in humoral immunity (11, 12). Thus, the factors leading to humoral defects associated with SAP deficiency remain poorly understood.
In this paper, we have further examined immune responses in SAP−/− mice. We demonstrate that SAP−/− mice can mount a normal T-independent response to 4-hydroxy-3-nitrophenylacetyl (NP)-LPS but show impaired B cell proliferation in addition to defective GC formation in response to T-dependent antigens. These defects are largely T cell dependent because transfer of WT, but not SAP-deficient, CD4 cells into SAP−/− or RAG2−/− reconstituted hosts markedly improved defects in B cell proliferation, GC formation, and antibody titers.
To identify defects that contribute to the impaired humoral responses in SAP−/− mice, we assessed CD4 T cell functions. Although SAP-deficient CD4 cells have defective TCR-mediated Th2 cytokine production in vitro (7, 8, 13), we provide evidence using in vivo challenge with a robust Th2 inducing agent, as well as transfer of in vitro–polarized cells, that the humoral defects can be separated from the cytokine production defects. In contrast, we observed defective regulation of both inducible costimulator (ICOS) and CD40L (CD154), two critical regulators of GC formation. Using retroviral reconstitution with WT and mutant forms of SAP, we demonstrate that in contrast to cytokine defects, the regulation of ICOS and CD40L expression as well as long-term humoral defects in SAP−/− mice can be rescued by retroviral reconstitution with either SAP or SAP-R78A, a mutant previously shown to prevent SAP-mediated recruitment of Fyn to SLAM. Consistent with these observations, we also demonstrate that Fyn−/− mice can form GCs and develop antibody responses to immunization. Finally, we provide evidence that SLAM/SAP-mediated pathways help regulate early surface CD40L (sCD40L) expression. Our results demonstrate that SAP deficiency affects the expression of key molecules required for T cell–mediated B cell help and suggest that the humoral defects in SAP−/− mice occur by mechanisms that are at least partially independent of SAP's regulation of cytokine production.
RESULTS
SAP−/− mice exhibit abnormalities in T-dependent immune responses
SAP is expressed in T, NK, NKT, and some B cells including human GC B cells (12, 14, 15). Although initial adoptive transferred data indicated a T cell–intrinsic defect in long-term humoral immunity (10), recent data using a cell transfer model into RAG2−/− mice argued that SAP-deficient B cells are also defective (11, 12). To further examine these issues, SAP−/− mice were immunized with the T-independent antigen, NP-LPS. Consistent with data using NP-Ficoll (16), SAP−/− mice mounted a normal T-independent response (Fig. 1 A). In addition, SAP-deficient B cells proliferated comparably to WT B cells when stimulated with anti-CD40, LPS, or anti-IgM in vitro (not depicted). Similar findings have been reported for B cells isolated from XLP patients (17).
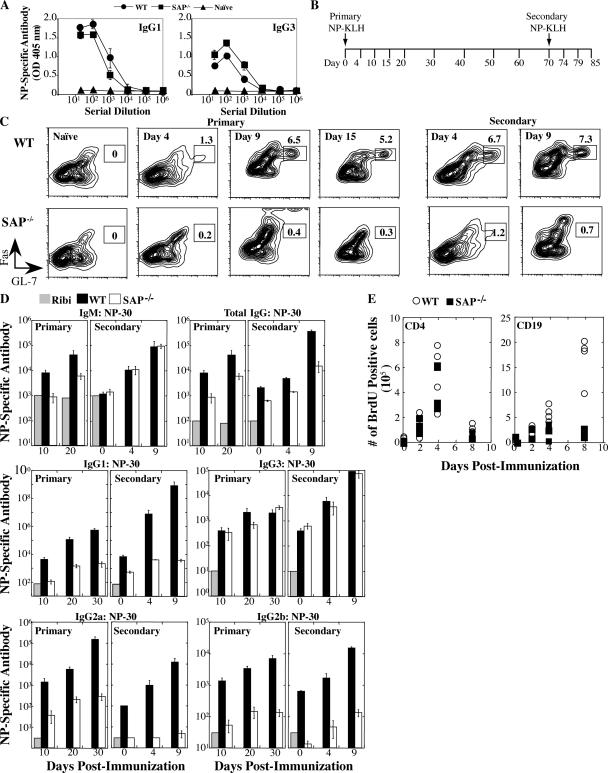
Figure 1.
SAP−/− mice exhibit impaired immune responses to T-dependent antigens. (A) Production of NP-specific antibodies 21 d after NP-LPS (T-independent) immunization by ELISA. (B) Mice were immunized with a T-dependent antigen (NP-KLH) and challenged 70 d after primary immunization (n = 4–5 mice/time point/group). (C) GC development evaluated by gating on B220+IgDlo cells: GC B cells are FashiGL-7hi. (D) NP-specific [NP-(30)] antibody detected by ELISA. Ribi refers to adjuvant control. Day 0 of secondary response is 70 d after primary immunization. Note that y-axis NP-specific antibody dilution refers to the midpoint of the dilution curve for each genotype. Axes for each isotype are different. (E) Mice were immunized with SRBCs (Fig. S1) and injected with BrdU 5 h before they were killed. The numbers of dividing splenic CD19+ B cells and CD4+ T cells were determined by staining with anti-BrdU antibody.
To evaluate the nature of the humoral defect in greater detail, WT and SAP−/− mice were immunized with a defined T-dependent antigen, NP-KLH, or a complex antigen, sheep red blood cells (SRBCs), and formation of GCs and antibody production were determined during primary and secondary responses (Fig. 1 B and Fig. S1, which is available at http://www.jem.org/cgi/content/full/jem.20052097/DC1). After immunization, B cells down-regulate IgD and express activation markers including Fas. A small fraction of precursor B cells within the follicles will up-regulate differentiation markers including PNA and GL-7, down-regulate CD38, and undergo a rapid clonal expansion, forming GCs. Accordingly, in WT mice we observed GC staining after 4 d and maximal GC development by 8–9 d after NP-KLH immunization (Fig. 1 C). SAP−/− mice, however, showed dramatic defects in GC development after both primary and secondary immunization (Fig. 1 C). Similar results were observed by examining the B220+IgDloFashi B cells for peanut agglutinin (PNA)hi and CD38int expression as well as in response to SRBCs (not depicted and Fig. S1). Thus, SAP-deficient B cells fail to differentiate into GCs during a robust T-dependent response.
Although NP-specific IgM levels were similar in WT and SAP−/− mice, NP-specific total IgG levels were 5–50-fold lower in SAP−/− mice relative to WT (Fig. 1 D). The reduction in IgG1 was most dramatic: SAP−/− mice had 10–100-fold lower levels in the primary and 1,000–100,000-fold lower levels in the secondary response. Levels of NP-specific IgG3 were less affected, consistent with extrafollicular differentiation of IgG3-producing cells. Intermediate level defects were observed for IgG2a and IgG2b. Despite the lack of detectible GC development, SAP−/− mice were able to produce high affinity antibodies as indicated by reactivity with [NP-(3)] (not depicted) and increased antibody titres in responses to secondary challenge, although most isotype titres were still far below those of WT mice (Fig. 1 D). Consistent with these observations, we observed dramatic defects in numbers of long-term antibody-secreting cells in the bone marrow (not depicted and see Fig. 4 C).
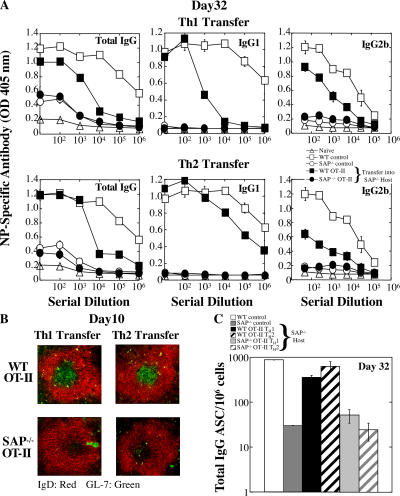
Figure 4.
SAP-deficient Th2-differentiated antigen-specific CD4 T cells fail to rescue humoral defects. Th1- or Th2-skewed WT OT-II and SAP−/− OT-II CD4 cells were adoptively transferred to SAP−/− hosts that were subsequently immunized with NP-OVA. WT and SAP−/− controls did not receive transferred cells but were immunized. Data represent one of three independent experiments (n = 3–4 mice/time point/group). (A) [NP-(30)]-specific antibodies detected 32 d after immunization by ELISA. (B) GCs were detected in SAP−/− hosts reconstituted with WT but not SAP-deficient OT-II cells via staining with IgD (red) and GL-7 (green) 10 d after immunization. (C) Total bone marrow IgG+ plasma cells (ASC) were quantified by ELISPOT.
To examine the impaired humoral responses in SAP−/− mice in greater detail, animals were immunized with SRBCs and GC formation and cell proliferation were assessed. Notably, although SAP-deficient splenic CD4 cells proliferated similarly to WT cells after immunization, marked reductions in splenic B cell proliferation were observed in SAP−/− mice (Fig. 1 E) in addition to the GC formation defects (Fig. S1). These proliferation defects were present by day 4, but most dramatic by day 8. Thus, SAP-deficient B cells display defects in proliferation as well as differentiation.
Rescue of Ig production by WT CD4 cells
The profound defects in T-dependent responses in SAP−/− mice support previous data arguing that a major component of their humoral defects to LCMV lies in T cells (10). To further address this issue, either WT or SAP-deficient OT-II transgenic (OVA-specific) CD4 cells were transferred into SAP−/− hosts and immunized with NP-OVA. WT OT-II and SAP−/− OT-II CD4 cells proliferated equivalently as assessed via CFSE dilution (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20052097/DC1), consistent with our BrdU analyses (Fig. 1 E). However, only WT OT-II cells supported efficient antigen-specific antibody production in the SAP−/− host (Fig. S2 B).
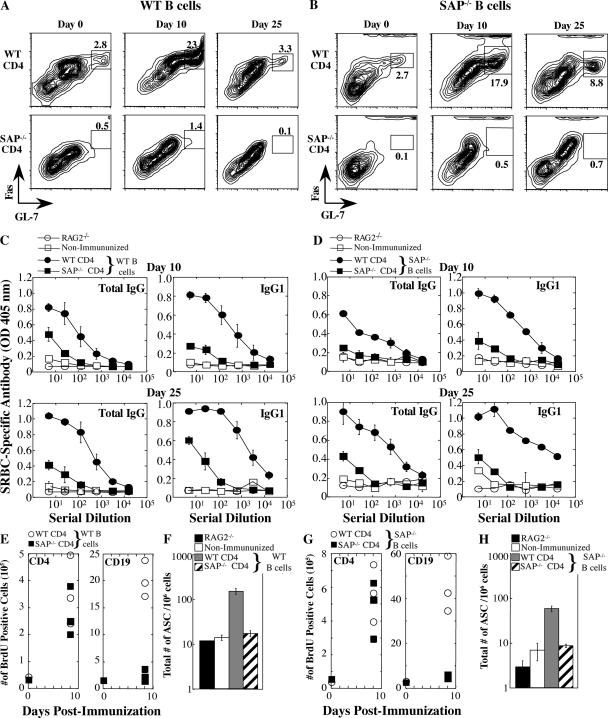
To clarify T verses B cell contributions to the humoral defects, either WT or SAP-deficient naive CD4 cells were adoptively transferred in conjunction with either SAP-deficient or WT B cells into RAG2−/− hosts, which were permitted to equilibrate 30 d after transfer to avoid effects of homeostatic proliferation. Before immunization, mice were assessed for equivalent cell transfer, cell survival, and baseline serum antibody levels. Interestingly, when WT CD4 T cells were transferred along with either SAP-deficient or WT B cells, GC differentiation markers were observed in the RAG2−/− mice before immunization (Fig. 2, A and B, Day 0, and Fig. S3 A, which is available at http://www.jem.org/cgi/content/full/jem.20052097/DC1). After SRBC immunization, mice reconstituted with WT CD4 cells displayed a robust increase in the expression of GC markers (Fig. 2, A and B, top, and Fig. S3 A) accompanied by B cell proliferation (Fig. 2, E and G) and antibody production (Fig. 2, C and D, and Fig. S3 B). However, transfer of SAP-deficient CD4 T cells in conjunction with either SAP-deficient or WT B cells failed to result in GC development, high antibody titers, or the development of long-lived plasma cells (Fig. 2, A–D, F, and H). Moreover, proliferation of either WT or SAP-deficient B cells was markedly impaired in the presence of SAP-deficient CD4 cells (Fig. 2, E and G), strongly supporting our hypothesis that the B cell proliferation defect is due to impaired T cell–mediated B cell help. In contrast to a previous study (11), we did not observe a substantial B cell component to the humoral defects under these conditions (Fig. 2 and Fig. S3). Thus, WT CD4 T cells can rescue many of the humoral defects in SAP−/− mice.
Figure 2.
WT CD4 cells rescued antibody production in RAG2−/− recipients. RAG2−/− reconstitution experiments: SAP-deficient or WT CD4 T cells and either WT B cells (left panels A, C, E, and F) or SAP-deficient B cells (right panels B, D, G, and H) were cotransferred into RAG2−/− hosts (n = 4 mice/time point/group). Note that transfer experiments with WT or SAP-deficient B cells are separate experiments (see Fig. S3 for direct comparison). (A and B) GC development assessed by gating on B220+IgDlo cells and staining for FashiGL-7hi cells. (C and D) SRBC-specific antibody response evaluated by ELISA. Nonimmunized control refers to mice that received T and B lymphocytes but were not immunized. (E and G) Mice were injected with BrdU 5 h before they were killed to assess CD19+ B cell and CD4+ T cell proliferation. (F and H) Total bone marrow IgG+ plasma cells (ASC) on day 25 were quantified by ELISPOT.
Impaired GC formation and antibody production during a Th2 response
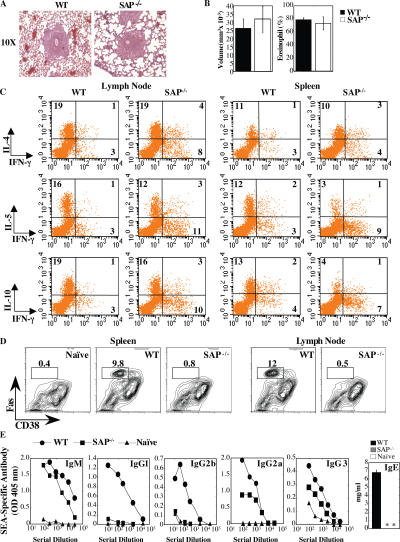
CD4 T cells produce cytokines such as IL-4, IL-10, and IL- 21 that help promote GC formation and antibody secretion. In vitro, SAP-deficient T cells demonstrate impaired TCR-induced Th2 cytokine production yet respond normally to polarizing cytokines (7, 8, 13). These data, in conjunction with low IgE levels in SAP−/− mice, suggest that Th2 defects contribute to their humoral defects. To investigate this issue, we challenged SAP−/− mice with eggs from Schistosoma mansoni, a helminth that induces a strong Th2 response. Upon i.v. injection, these eggs lodge in the lungs and induce a robust eosinophilic granuloma response as well as the production of Th2 cytokines from multiple cell types (18). Consistent with the normal responses of SAP-deficient T cells to polarizing cytokines in vitro (7, 8, 13), WT and SAP−/− mice responded equally to this agent with respect to granuloma size and content (Fig. 3, A and B). Furthermore, CD4 cells from the draining lymph nodes of both WT and SAP−/− mice produced IL-4, IL-5, IL-10, and IL-13 in response to a schistosome egg antigen (SEA) parasite preparation (Fig. 3 C and not depicted). SAP-deficient splenic CD4 cells stimulated with SEA also produced and secreted Th2 cytokines, albeit at lower levels than WT cells.
Figure 3.
Impaired humoral immunity in response to S. mansoni egg injection. (A) Giemsa-stained lung section: granuloma formation 8 d after secondary injection. (B) Granuloma volume and percent eosinophil content. (C) Pooled mediastinal lymph nodes or splenocytes were cultured with 20 μg SEA for 72 h. For intracellular cytokine analysis, cells were rested for 18 h and restimulated with 3 ng/ml PMA and 1 μg/ml ionomycin. (D) Lymph nodes and splenocytes were stained for GC markers by gating on B220+IgDlo cells: GC B cells are FashiCD38int. (E) SEA-specific antibodies evaluated 8 d after challenge with S. mansoni eggs by ELISA. Note the different scales of the axes. *, antibody levels not detected. Data represent one of two independent experiments (n = 5 mice/group).
Despite the production of Th2 cytokines in mice challenged with S. mansoni eggs, SAP−/− mice failed to develop GCs in the spleen and draining lymph node as evaluated by Fashi CD38int expression (Fig. 3 D). In addition, although SAP−/− mice had relatively normal SEA-specific IgM and only slightly reduced IgG3, they failed to secrete SEA-specific IgG1, IgG2b, and IgE isotypes (Fig. 3 E). Although, IgG2a levels were intermediate, these levels were quite low even in WT mice, consistent with the Th2 response. Thus, SAP−/− mice are unable to produce effective antibody titres even in the presence of Th2 cytokine production.
Nonetheless, although SAP-deficient T cells secreted Th2 cytokines when challenged in vivo with S. mansoni, they still produced elevated IFN-γ as compared with WT cells (Fig. 3 C and not depicted). To address whether an imbalance in Th cytokine production or inappropriate kinetics and/or levels of cytokines influenced B cell activation and differentiation in these mice, WT and SAP-deficient OT-II CD4 cells were polarized into either Th1 (IFN-γ–secreting) or Th2 (IL-4–, IL-5–, and IL-10–secreting) cells in vitro and transferred into SAP−/− hosts. As demonstrated previously, both WT and SAP-deficient cells polarized equivalently upon exposure to cytokines in vitro (references 7, 8, and 13, and not depicted). After NP-OVA immunization, WT OT-II–differentiated Th1 and Th2 CD4 cells provided B cell help, exemplified by the production of NP-specific antibodies (Fig. 4 A), GC formation (Fig. 4 B), and development of long-lived bone marrow plasma cells (Fig. 4 C). However, both the SAP-deficient Th1 and Th2 OT-II cells failed to induce humoral responses (Fig. 4). Thus, neither a defect in Th2 cytokine production nor an imbalance in Th1/Th2 differentiation is exclusively responsible for the humoral defects observed in SAP−/− mice.
Elevated and prolonged expression of CD40L
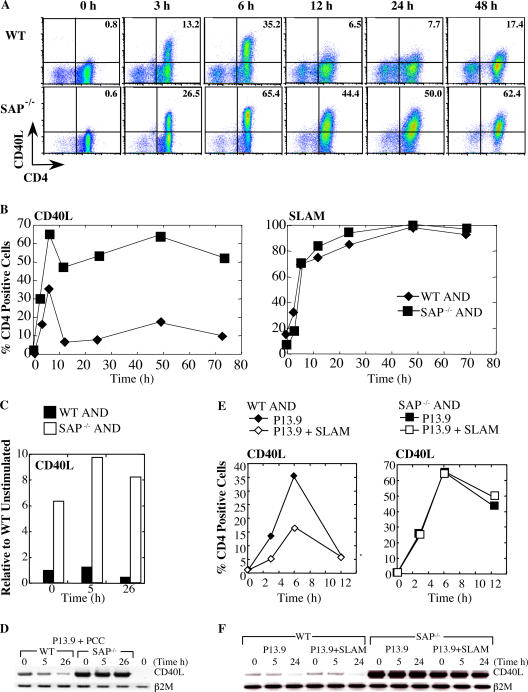
Another integral function of CD4-mediated B cell help is the expression of activation markers, including CD40L, OX40, and ICOS. CD40L is a critical activation marker required for B cell Ig class switching and GC formation, mutations of which cause the X-linked hyper-IgM syndrome (19). However, overexpression of CD40L and/or prolonged stimulation of CD40 on B cells can also impair GC formation and promote early plasma cell differentiation (20, 21). To monitor the expression of activation markers, naive AND TCR transgenic CD4 cells were stimulated with pigeon cytochrome-c (PCC)-pulsed P13.9 APCs. CD40L has a complex pattern of expression characterized by both early and late phases (22, 23). Within 6 h of stimulation, approximately one third of WT AND CD4 cells expressed sCD40L, which was down-regulated 6–12 h after stimulation and subsequently reexpressed at lower levels at 48 h (Fig. 5, A and B). Surprisingly, SAP-deficient AND cells exhibited a marked increase in the number and intensity of cells expressing sCD40L. Although sCD40L levels were decreased after 6 h, they were maintained at exaggerated levels on SAP-deficient cells throughout the entire duration of the time course examined (Fig. 5, A and B, and Fig. S4, which is available at http://www.jem.org/cgi/content/full/jem.20052097/DC1). Increased expression of CD40L was observed at the mRNA level. SAP-deficient cells both showed elevated CD40L mRNA at baseline and failed to down-regulate it upon activation (Fig. 5, C and D). This elevated expression pattern was specific to CD40L, as other activation markers, such as CD69, SLAM, OX40, and CXCR5, were expressed at levels and kinetics comparable to WT cells (Fig. 5 B and not depicted). Similar results were observed using OT-II CD4 cells stimulated with peptide-pulsed dendritic cells and nontransgenic CD4 cells stimulated with anti-CD3 plus anti-CD28 (not depicted).
Figure 5.
Heightened and sustained CD40L surface expression on SAP-deficient CD4 cells. (A–C) WT and SAP−/− AND CD4 cells were stimulated with peptide-pulsed (1,000 nM PCC) P13.9 cells and evaluated for activation markers. (A) SAP−/− AND cell display enhanced and sustained levels of CD40L. (B) Percentage of Vα11+CD4+ cells stained for CD40L (left) and SLAM (right) expression. Mean fluorescence intensity for CD40L and SLAM are shown in Fig. S4. (C and D) SAP-deficient cells display elevated CD40L mRNA. RNA was isolated from WT and SAP-deficient AND CD4 cells after stimulation with peptide-pulsed P13.9 APCs and subjected to (C) real-time quantitative PCR and (D) RT-PCR analysis. (E) SLAM expression on P13.9 APCs reduced expression of CD40L on WT AND cells. (F) SLAM expression on P13.9 APCs did not affect mRNA levels after stimulation as assessed by RT-PCR.
SLAM/SAP-dependent mechanisms of T cell–mediated B cell help
SLAM is up-regulated on activated T cells and is expressed constitutively on B cells, macrophages, and dendritic cells (1). SLAM interacts homophilically and plays a role in T cell activation (1, 13, 24, 25). To evaluate if SLAM–SLAM interactions could influence T cell–mediated B cell help, a variant of the P13.9 fibroblast surrogate APC line that expresses SLAM (13) was used to stimulate AND CD4 cells. Expression of SLAM on the APCs reduced the kinetics and expression of sCD40L on WT CD4 cells at early times after stimulation (Fig. 5 E), but it did not affect later surface levels (not depicted) or CD40L mRNA levels (Fig. 5 F). Moreover, expression of SLAM on the APCs had no impact on the kinetics or duration of sCD40L expression on SAP-deficient cells (Fig. 5 E). Expression of other activation markers including SLAM and CXCR5 on either WT or SAP-deficient CD4 cells was also unaffected by SLAM (not depicted). These results indicate that SAP expression and SLAM engagement potentially contribute to the tight regulation of early sCD40L expression.
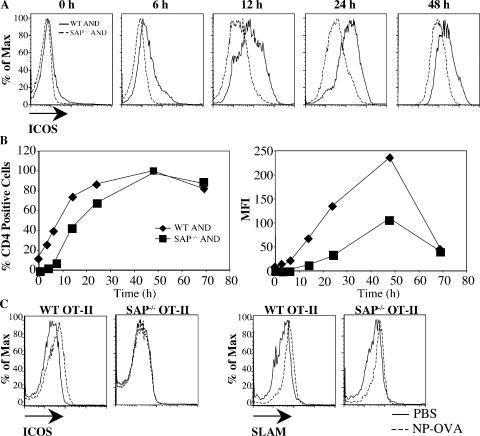
Delayed and impaired ICOS expression
Another T cell activation marker that has an important role in enhancing T cell–dependent B cell help is ICOS, which is induced rapidly on T cells after TCR engagement. WT AND CD4 cells stimulated with peptide-pulsed APCs showed a rapid increase in ICOS expression. Although SAP-deficient T cells induced surface expression of ICOS, the kinetics of induction and maximal intensity of expression were considerably diminished (Fig. 6, A and B). Impaired ICOS expression was also observed with low peptide concentrations (not depicted) as well as when OT-II cells were stimulated with peptide-pulsed dendritic cells (not depicted). However, unlike CD40L, expression of SLAM on the APCs had no noticeable impact on ICOS up-regulation on either WT or SAP-deficient CD4 cells (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20052097/DC1). These results suggest that some SAP-mediated phenotypes contributing to impaired B cells are SLAM independent.
Figure 6.
Impaired ICOS expression on SAP-deficient CD4 cells in vitro and in vivo. (A–C) WT and SAP−/−AND CD4 cells were stimulated with peptide-pulsed (1,000 nM PCC) P13.9 cells and evaluated for ICOS expression. (A) Expression and kinetics of ICOS measured by flow cytometry. (B) Percentage of Vα11+CD4+ cells stained positive for ICOS expression and mean fluorescence intensity of ICOS. (C) CFSE-labeled WT OT-II or SAP−/− OT-II T cells were adoptively transferred to SAP−/− hosts that were subsequently immunized with either PBS control or NP-OVA. Data represent one of three independent experiments (n = 2 mice/group). One representative example is shown for each genotype. Labeled cells were evaluated for expression of SLAM and ICOS 2 d after immunization.
T cells from XLP patients also show reduced ICOS expression in vitro (17). To evaluate if these defects are observed in vivo, CFSE-labeled WT and SAP-deficient OT-II CD4 cells were adoptively transferred into SAP−/− recipients. 2 d after NP-OVA immunization, both WT and SAP-deficient OT-II had divided as assessed by CFSE dilution (not depicted) and up-regulated SLAM expression, indicative of activation (Fig. 6 C). However, SAP−/− OT-II cells demonstrated impaired ICOS expression compared with WT OT-II CD4 cells (Fig. 6 C), verifying their defect in ICOS regulation in vivo.
Rescue of humoral responses by SAP independent of a Fyn interaction
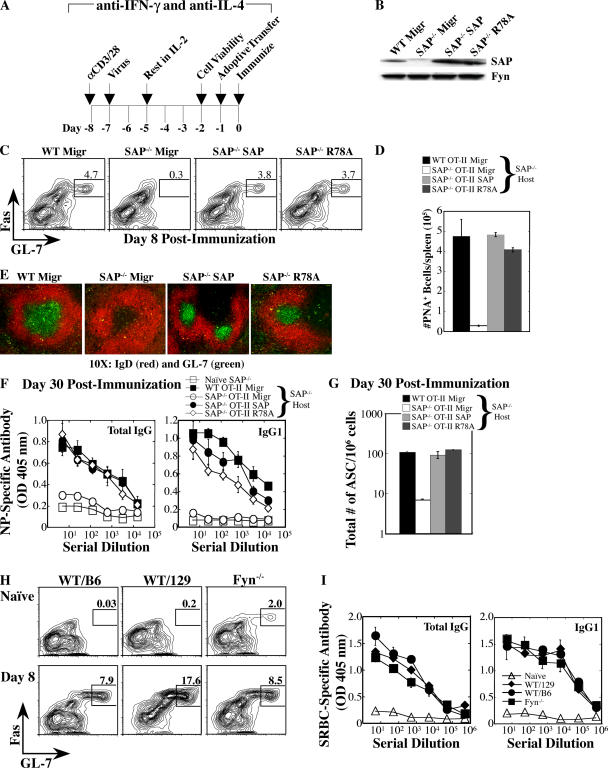
The ability of SAP to recruit the tyrosine kinase Fyn is critical for Th2 cytokine regulation. IL-4 production can be rescued in SAP-deficient CD4 cells by reexpression of human SAP, but not by a SAP mutant (R78A) that exhibits decreased binding to the Fyn SH3 domain and fails to effectively recruit Fyn to SLAM (13, 25). The requirement for SAP in humoral responses, independent of T cell cytokine production, prompted us to investigate the role of SAP's interaction with Fyn. SAP, SAP-R78A, or a control (Migr) construct was expressed in SAP-deficient OT-II CD4 cells under neutral (anti–IFN-γ and anti–IL-4) conditions. Reconstituted cells were transferred into SAP−/− hosts (Fig. 7, A and B), which were then immunized with NP-OVA to evaluate humoral responses. GC formation (Fig. 7, C–E), antibody production (Fig. 7 F), and the generation of long-lived plasma cells (Fig. 7 G) were considerably improved with the transfer of either WT OT-II Migr (control vector) cells or SAP-deficient OT-II cells that expressed WT human SAP, but not SAP-deficient cells that expressed the vector control. Interestingly, SAP-deficient CD4 cells that expressed SAP-R78A also markedly improved humoral responses (Fig. 7). Similar results were obtained using nontransgenic T cells (not depicted). Consistent with Fyn- independent functions of SAP, Fyn−/− mice could respond to the complex T-dependent antigen (SRBCs), as exemplified by GC formation (Fig. 7 H) and antigen-specific antibody production (Fig. 7 I). Thus, SAP can mediate T cell help for B cells by a mechanism that is less dependent on the ability of SAP to recruit Fyn to SLAM.
Figure 7.
GC formation and antibody responses in SAP−/− mice are less dependent on SAP-mediated recruitment of Fyn. (A) WT OT-II and SAP−/− OT-II CD4 cells were retrovirally reconstituted with Migr control, WT SAP, or SAP-R78A vectors and transferred to SAP−/− mice that were subsequently immunized with NP-OVA. Data represent three independent experiments (n = 3 mice/time point/group). (B) SAP expression was verified by immunoblot. (C) GC B cells were detected with the transfer of WT (Migr) and SAP−/− cells reconstituted with WT SAP or SAP-R78A, but not Migr control. GC development assessed at day 8 after immunization by gating on B220+IgDlo cells: GC B cells are FashiGL-7hi. (D) Quantification of PNA+ B cells based on flow cytometric analysis.(E) GCs were detected in SAP−/− hosts via staining with IgD (red) and GL-7 (green) 8 d after immunization. (F) [NP-(30)]-specific antibodies detected 30 d after immunization by ELISA. (G) Total bone marrow IgG+ plasma cells (ASC) were quantified by ELISPOT. (H and I) Fyn−/− mice exhibit a productive humoral response to a T-dependent antigen, SRBCs. Data represent two independent experiments (n = 4–5 mice/time point/group). (H) GC development evaluated by gating on B220+IgDlo cells and staining for FashiGL-7hi expression. (I) SRBC-specific antibody response evaluated by ELISA 30 d after immunization. Fyn−/− mice are on a mixed 129/Sv × C57BL/6 background. Controls from 129/Sv and C57BL/6 strains are shown.
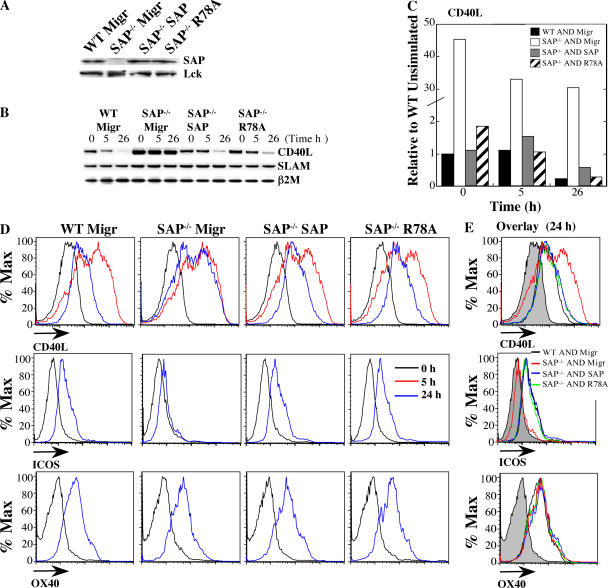
If misregulation of ICOS and CD40L contributes to the humoral defects in SAP−/− mice, one would predict that their regulation is also less dependent on SAP's interaction with Fyn. To address this question, we introduced WT SAP, SAP-R78A, and vector control retroviruses into SAP−/− AND CD4 cells and restimulated the cells in vitro with peptide-pulsed P13.9 APCs (Fig. 8 A). Reconstitution of SAP-deficient AND CD4 cells with either SAP or SAP-R78A, but not the vector control, markedly reduced CD40L mRNA to levels similar to those of WT control cells, particularly upon stimulation (Fig. 8, B and C). Moreover, both WT SAP and SAP-R78A normalized patterns of CD40L expression as well as ICOS up-regulation (Fig. 8, D and E). Thus, SAP is required for both humoral immunity and Th2 cytokine induction. However, in contrast to the cytokine defects, ICOS and CD40L expression as well as long-term humoral defects in SAP−/− mice can be rescued by retroviral reconstitution with either WT SAP or SAP-R78A, a mutant that does not effectively recruit Fyn.
Figure 8.
Reexpression of SAP or SAP-R78A rescued aberrant CD40L and ICOS expression. SAP-deficient AND CD4 cells were reconstituted with WT SAP, SAP-R78A, or the Migr control vector. WT AND CD4 cells were reconstituted with Migr. Cells were restimulated with 100 nM PCC-pulsed P13.9 cells. Data represent four independent experiments. (A) SAP protein expression confirmed by immunoblot. (B and C) Expression of either WT SAP or SAP-R78A reduced basal and activated CD40L mRNA expression. RNA was isolated after stimulation with peptide-pulsed P13.9 APCs and subject to (B) RT-PCR and (C) real-time quantitative PCR analysis. (D and E) SAP and SAP-R78A, but not Migr control, improved CD40L kinetics and ICOS up-regulation in reconstituted SAP-deficient cells. Flow cytometric analysis (gated on CD4+GFP+ cells) of CD40L (top), ICOS (middle), and OX40 (bottom) expression. (E) Overlaid data from the 24-h time point. Filled histograms represent unstimulated cells.
DISCUSSION
XLP is characterized by severe immune dysregulation that is often triggered or exacerbated by EBV infection. Evidence now indicates that the extreme hypersensitivity of these patients to EBV may result from defective NK and CD8 T cell 2B4-mediated cytotoxity of EBV-infected targets (26–28). However, one of the other cardinal features of XLP is the development of dysgammaglobulinemias and, in particular, hypogammaglobulinemia over time. Here we provide evidence supporting a strong T cell–intrinsic component to the humoral defects observed in SAP−/− mice and, moreover, show that these T cell–intrinsic defects can be separated from their defect in Th2 cytokine production. Furthermore, we demonstrate that SAP regulates CD40L and ICOS expression as well as humoral immunity by a mechanism that may be less dependent on SAP's ability to recruit Fyn to SLAM.
Although dysgammaglobulinemias are one of the defining features of XLP, the mechanisms contributing to this defect remain unclear. SAP−/− mice have variable decreases in basal antibody levels compared with WT mice (9), as well as humoral defects associated with impaired GC formation and a lack of B cell memory and long-term plasma cells (10). Recent studies from XLP patients have confirmed a marked reduction in memory (CD27+) B lymphocytes (17, 29). However, whether these defects are secondary to intrinsic B cell defects, T cell help for B cells, or both factors remains controversial. An initial study of SAP−/− mice suggested that CD4 cells were unable to provide help for long-term antibody production during infection with LCMV (10). However, more recent data using immunizations in a RAG2−/− reconstitution model suggested that SAP-deficient B cells also contribute to the observed defects in antibody production (11).
In this paper, we have examined the responses of SAP−/− mice to immunizations and have confirmed that although T-independent responses are normal, T-dependent responses are defective with impaired GC development, decreased antibody production, and altered patterns of class switching. These defects are accompanied by decreased B cell proliferation as well as differentiation, perhaps accounting for the decreased antibody levels early after immunization. Indeed, these early defects in response to immunization may be more severe than those seen in LCMV, perhaps reflecting the ability of LCMV to induce a transient hypergammaglobulinemia (30). Nonetheless, both the humoral responses and B cell proliferation were markedly improved by transfer of WT, but not SAP-deficient, TCR transgenic cells, supporting our previous findings with LCMV (10). In addition, RAG2−/− reconstitution experiments confirm that SAP-deficient CD4 cells could not provide adequate help to either WT or SAP−/− B cells. Furthermore, WT T cells in the presence of either WT or SAP-deficient B cells induced B cell proliferation, GC formation, antibody production, and long-lived bone marrow plasma cells. Although we cannot exclude that B cells contribute to these phenotypes, we have not found evidence for a major B cell component, strongly supporting a critical role for T cells in contrast to a previous report (11).
Thus, key questions remain as to what defects are present in SAP-deficient T cells. Although SAP-deficient CD4 cells proliferate and produce IL-2 normally in response to TCR engagement, they do show defective TCR-induced Th2 cytokine production and variable increases in IFN-γ expression in vitro (7, 8, 13). Interestingly, T cells from XLP patients have decreased production of IL-10 (17), a cytokine produced by Th2 cells that can help promote human B cell antibody secretion. However, despite the implication of Th2 cytokines in the promotion of class switching and B cell help, we clearly demonstrate that humoral defects are still observed in SAP−/− mice that have Th2 responses induced by a strong Th2 polarizing agent, S. mansoni. Although the observation that SAP−/− mice can mount Th2 responses in vivo may seem surprising, there are multiple factors and cell types involved in generating in vivo Th2 responses to S. mansoni (18). Nonetheless, even under these Th2-inducing conditions, SAP−/− mice still fail to produce long-term humoral immunity to this agent. Moreover, when we artificially polarized cells in vitro, SAP-deficient Th2-producing cells also failed to provide adequate B cell help in vivo. Thus, defective Th2 cytokine production cannot be the sole cause of the humoral defects in SAP−/− mice. The same scenario is also likely to be true in XLP because exogenous IL-10 only partially rescued antibody production in vitro (17). Such observations are consistent with phenotypes of IL-4−/− mice, which mainly affect specific Ig isotypes (31).
Consistent with the cytokine-independent T cell–intrinsic defect in humoral immunity, CD4 cells reconstituted with either WT SAP or a SAP mutant that affects Fyn recruitment to SLAM (R78A) were able to improve GC formation, antibody production, and long-lived plasma cell development in a SAP−/− host (Fig. 7). Although we cannot exclude some residual Fyn binding with the SAP-R78A mutant, retroviral reexpression of this mutant failed to improve Th2 cytokine production (13), yet it rescued humoral responses. Consistent with our SAP-R78A observations, Fyn−/− mice are also able to form GCs upon immunization, despite defects in TCR-induced Th2 cytokine production (13). Thus, SAP-mediated regulation of Th2 cytokine production and humoral immunity may involve distinct pathways. Such effects may result from other potential interactions of SAP with Src family tyrosine kinases, such as Lck in addition to Fyn (32), or alternate mechanisms of SAP signaling, such as competition with phosphatases for SLAM-related receptors (5, 6).
It is therefore relevant that SAP-deficient CD4 cells show aberrant temporal regulation of two key cell surface markers, CD40L and ICOS, required for B cell help. Although the increased CD40L expression on SAP-deficient CD4 cells may seem paradoxical, administration of an agonist anti-CD40 antibody has been shown to induce a pattern of extrafollicular B cell differentiation while abolishing GC formation and memory B cell generation in a T-dependent response (20, 21). Thus, CD40L expression requires tight regulation to generate appropriate humoral responses.
The expression of CD40L on T cells is complex, undergoing an early phase with a rapid induction attributed to TCR regulation that is quickly downmodulated and a later phase regulated partially through the actions of cytokines (22, 23). Although sCD40L levels are in part down-regulated by interactions with CD40 (33), the mechanisms by which expression is controlled remain poorly understood. We provide evidence that SAP-mediated pathways have a profound impact on CD40L mRNA levels, even before cell activation, when sCD40L expression is still quite low. In addition, SLAM ligation plays a novel role in regulating the early phase of sCD40L expression in a SAP-dependent fashion that is independent of its effects on mRNA. Whether these effects are due to SLAM's effects on adhesion or other signaling pathways is not known. Both WT and SAP-deficient AND CD4 cells down-regulated TCR expression comparably in the presence of peptide-pulsed APCs as well as SLAM-expressing APCs, suggesting that at least some receptor endocytosis pathways are not affected (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20052097/DC1). Although naive T cells do express SLAM, SLAM is rapidly up-regulated (within 3–6 h) in response to TCR stimulation and remains high for several days. Conversely, in murine cells SAP expression is down-regulated 24 h after T cell stimulation (25). It is therefore intriguing that the effects of SLAM on sCD40L expression are greatest at 3–12 h after stimulation, a time when both SLAM and SAP are expressed at high levels. Our data thus provides evidence for a new role for SLAM in T cell activation as well as insight into the dynamic regulation of CD40L expression.
Whether the increased sCD40L expression directly contributes to the defect in long-term antibody production is difficult to confirm in vivo. The reported effects of CD40 overstimulation suggest that it should lead to increased early antibody production and increased numbers of early plasma cells; however, this is not observed in SAP−/− mice. It is therefore relevant that both XLP patients and SAP−/− mice show decreased and delayed ICOS expression. Both ICOS deficiency and heterozygosity are associated with defects in GC formation and antibody production (34–36), suggesting that B cells are sensitive to levels of surface ICOS. Thus, together, the extent of ICOS up-regulation and the duration of sCD40L expression may have important biological consequences for balancing the generation of GCs and B cell terminal differentiation. Indeed, the rescue of both ICOS and CD40L expression as well as humoral immunity by SAP-R78A suggests a major link between these phenotypes. However, although both the impaired ICOS up-regulation and the prolonged CD40L expression may prevent effective GC formation, the impairment of both early and late antibody levels suggests the ICOS defect in SAP−/− mice may play a more dominant role in the humoral phenotype.
Recently, a distinct subset of T cells, known as T follicular helpers, has been described, which provide help for GC differentiation and express high levels of SAP, SLAM, CD84, and ICOS mRNA (37, 38). A recently described mutation in the gene roquin results in increased GC development with excessive T follicular helpers displaying elevated ICOS expression (38). Roquin is a RING-type E3 ubiquitin ligase containing a CCCH zinc finger domain found in RNA binding proteins (38). SAP mRNA contains AUUUA sequences in the 3′ UTR that are targeted for ubiquitin-dependent degradation by RNA binding proteins such as AUF1 and HuR (39). It is intriguing to speculate that Roquin may help regulate degradation of SAP and/or ICOS mRNA and thereby GC formation.
Our results provide new insight into the nature of the T cell–intrinsic defects that affect antibody responses in SAP−/− mice. Which SAP-associated receptors are responsible for these phenotypes, what the Fyn-independent signaling pathways downstream of SAP are, and how these phenotypes are affected by T cell– and B cell–intrinsic defects remain important questions. Nonetheless, the examination of SAP−/− mice underscores both the extent and importance of these humoral defects, as well as the potential role of Ig therapy for the treatment of XLP patients.
MATERIALS AND METHODS
Mouse strains and reagents.
SAP−/− mice (7) were backcrossed to C57BL/6J for 8–10 generations and maintained in sterile microisolator cages on autoclaved water and food, according to institutional guidelines. AND transgenic, RAG2−/−, and Fyn−/− mice were from The Jackson Laboratory, and OT-II transgenic mice were from Taconic. All the animals were housed and treated within published guidelines of humane animal care, and all procedures were performed according to National Human Genome Research Institute (NHGRI) Animal Care and Use Committee–approved protocols for animal research. The P13.9 fibroblast cell line expressing I-Ek, CD80, and ICAM (40) as well as the SLAM-expressing variant were described previously (13). PCC peptide was purchased from SynPep.
Immunizations.
Mice were injected i.p. with 100 μg of either NP-KLH or NP-OVA (Biosearch Technologies Inc.) in Ribi (Fisher Scientific). Mice were injected i.p. with 2.5 × 108 SRBCs (Colorado Serum) in 0.2 ml HBSS. For T-independent responses, mice were immunized with 100 μg NP-LPS (Biosearch Technologies Inc.) in 0.2 ml HBSS.
Adoptive transfer.
OT-II CD4 lymphocytes were purified via negative selection from lymph nodes and spleens as described previously (13). In vitro Th1 and Th2 differentiation has been described (13). 3–5 × 106 CD4 cells were adoptively transferred into age- and sex-matched SAP−/− recipients by i.v. tail vein injection, and 24 h later, mice were immunized i.p. with NP-OVA. For RAG2−/− reconstitution experiments, CD4 T cells were purified from spleens and lymph nodes via negative selection and sorted for CD44loCD62Lhi naive cells. Splenic B cells were isolated via negative selection and sorted for CD19+ cells. 5 × 106 naive CD4 T cells and 10 × 106 CD19 B cells from WT or SAP−/− mice were transferred i.v. into RAG2−/− hosts.
Response to S. mansoni eggs.
Groups of mice were i.p. primed with 5,000 S. mansoni eggs, i.v. challenged with 5,000 eggs 2 wk later, and killed after 8 d. Histology was performed as described previously (41). Single cell suspensions from either the mediastinal lymph nodes or spleens were cultured at 3 × 106/ml and 5 × 106/ml, respectively, with 20 μg/ml SEA.
Plasma cell ELISPOT.
Goat anti–mouse IgG+M+A (Caltag Laboratories) was used to capture antibody for total antibody-secreting cell ELISPOTs. Plates were blocked with RPMI 10% FCS, and bone marrow cells were added to the plate in threefold serial dilutions in RPMI 10% FCS and incubated at 37°C for 5 h. Biotinylated goat anti–mouse IgGγ (Caltag Laboratories) followed by streptavidin–horseradish peroxidase (Vector Laboratories) was used for detection. AEC was used for spot development. Plates were scanned by an ImmunoSpot Analyzer (Cellular Technology Ltd).
ELISA.
96-well flat-bottom Immuno Plates (Nunc) were coated overnight at 4°C with [NP-(3)-BSA] or [NP-(30)-BSA] (2.5 μg/well; Biosearch Technologies Inc.) in PBS. For SRBC immunization, plates were coated overnight at 4°C with SRBCs. Peroxidase-conjugated goat antibody specific for total mouse IgG (Jackson ImmunoResearch Laboratories) IgG1, IgG2b, IgG3 (SouthernBiotech), or IgG2a (Zymed Laboratories) was used to detect antibodies, and ABTS solution (KPL) was used as a developing substrate. Levels of SEA-specific antibody were determined by ELISA as described previously (42).
Flow cytometry and microscopy.
All antibodies used for were from BD Biosciences, with the exception of anti-IgD (SouthernBiotech), SLAM (Biolegend), and PNA (Vector Laboratories). Samples stained for BrdU were washed in PBS, resuspended, fixed, and stained according to the manufacturer's instructions (BD Biosciences). Data analysis was performed using Flojo or CellQuest software. GCs were identified on 7-μM OCT-embedded frozen sections using anti–GL-7–FITC, CD3-biotin, CD4-biotin, CD8-biotin, IgD-biotin, and sheep anti–mouse IgD (The Binding Site). Secondary antibodies included streptavidin-Alexa568 (Invitrogen), anti–sheep-Cy5, and anti–rat AMCA (Jackson ImmunoResearch Laboratories).
Retroviral transduction and cytokine production.
CD4 cells were retrovirally reconstituted with a vector control (Migr), hSAP, or SAP R78A (provided by K. Nichols, University of Pennsylvania, Philadelphia, PA) as described previously (13). Cytokines were detected by ELISA (R&D Systems). Intracellular cytokine analyses were performed on splenic and/or lymph node cultures as described previously (41).
RT and quantitative PCR.
RNA was isolated with Trizol (Invitrogen Life Technologies). For RT-PCR, PCR amplification was performed for 35 cycles. For RT and quantitative PCR analysis, RNA was added directly to one-step quantitative RT-PCR reactions (Invitrogen Life Technologies) as described previously (13). The following primers were used: for RT-PCR: CD40L forward primer: AAGTCGACAGCGCACTGTTCAGAGT, CD40L reverse primer: CGGAATTCAGTCAGCATGATAGAAAC; and for quantitative PCR: CD40L forward primer: CAAATTGCAGCACACGTTGTAAG, CD40L reverse primer: TCAAGCATTACCAAGTTGCTTTTC, and CD40L probe FAM-CAGCATCCGTTCTACAGTGGGCCAA-BHQ1.
Online supplemental material.
Fig. S1 demonstrates that SAP−/− mice fail to form GCs after immunization with SRBCs. Fig. S2 shows that WT antigen-specific CD4 cells rescued antibody production in the SAP−/− host. Fig. S3 is a direct comparison of WT and SAP-deficient B cells in the RAG2−/− transfer model. Fig. S4 shows the mean fluorescence intensity of sCD40L and SLAM. Fig. S5 demonstrates that SLAM-expressing P13.9 APCs have no detectible impact on the expression or mean fluorescence intensity of ICOS on WT AND or SAP-deficient AND cells. Fig. S6 depicts TCR down-regulation in WT and SAP-deficient AND cells stimulated with peptide-pulsed and SLAM-expressing APCs.
Supplemental Material
Acknowledgments
We would like to thank S. Pierce, J. Wu, L. Finkelstein, and A. Shaffer for their critical comments; R. Handon and Julia Fekecs for their invaluable technical assistance; R. Caspi for use of the ELISPOT reader; and K. Nichols for plasmids.
L.J. Yu was part of the NIH Undergraduate Scholarship Program. Funding was provided by the intramural programs of NHGRI and NIAID.
The authors have no conflicting financial interests.
Abbreviations used: GC, germinal center; ICOS, inducible costimulator; LCMV, lymphocytic choriomeningitis virus; NP, 4-hydroxy-3-nitrophenylacetyl; PCC, pigeon cytochrome-c; PNA, peanut agglutinin; SAP, signal lymphocyte activation molecule–associated protein; SEA, schistosome egg antigen; SLAM, signal lymphocyte activation molecule; SRBC, sheep red blood cell; XLP, X-linked lymphoproliferative.
References
- 1.Nichols, K.E., C.S. Ma, J.L. Cannons, P.L. Schwartzberg, and S.G. Tangye. 2005. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol. Rev. 203:180–199. [DOI] [PubMed] [Google Scholar]
- 2.Latour, S., G. Gish, C.D. Helgason, R.K. Humphreis, T. Pawson, and A. Veillette. 2001. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2:681–690. [DOI] [PubMed] [Google Scholar]
- 3.Latour, S., R. Roncagalli, R. Chen, M. Bakinowski, X. Shi, P.L. Schwartzberg, D. Davidson, and A. Veillette. 2003. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat. Cell Biol. 5:149–154. [DOI] [PubMed] [Google Scholar]
- 4.Chan, B., A. Lanyi, H.K. Song, J. Griesbach, M. Simarro-Grande, F. Poy, D. Howie, J. Sumegi, C. Terhorst, and M.J. Eck. 2003. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 5:155–160. [DOI] [PubMed] [Google Scholar]
- 5.Sayos, J., C. Wu, M. Morra, N. Wang, X. Zhang, D. Allen, S. van Schaik, L. Notarangelo, R. Geha, M.G. Roncarolo, et al. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 395:462–469. [DOI] [PubMed] [Google Scholar]
- 6.Li, C., C. Iosef, C.Y.H. Jai, V.K.M. Han, and S.S.-C. Li. 2003. Dual functional roles for X-linked lymphoproliferative syndrome gene product SAP/SH2D1A in signaling through the signaling lymphocyte activation molecule (SLAM) family immune receptors. J. Biol. Chem. 278:3852–3859. [DOI] [PubMed] [Google Scholar]
- 7.Czar, M.J., E.N. Kersh, L.A. Mijares, G. Lanier, J. Lewis, G. Yap, A. Chen, A. Sher, C.S. Duckett, R. Ahmed, and P.L. Schwartzberg. 2001. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc. Natl. Acad. Sci. USA. 98:7449–7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu, C., K.B. Nguyen, G.C. Pien, N. Wang, C. Gullo, D. Howie, M.R. Sosa, M.J. Edwards, P. Borrow, A.R. Satoskar, et al. 2001. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat. Immunol. 2:410–413. [DOI] [PubMed] [Google Scholar]
- 9.Yin, L., U. Al-Alem, J. Liang, W.-M. Tong, C. Li, M. Badiali, J.J. Medard, J. Dmegi, Z.-Q. Wang, and G. Romeo. 2003. Mice deficient in the X-linked lymphoproliferative disease gene sap exhibit increased susceptibility to murine gammaherpervirus-68 and hypo-gammaglobulinemia. J. Med. Virol. 71:446–455. [DOI] [PubMed] [Google Scholar]
- 10.Crotty, S., E.N. Kersh, J. Cannons, P.L. Schwartzberg, and R. Ahmed. 2003. SAP is required for generating long-term humoral immunity. Nature. 421:282–287. [DOI] [PubMed] [Google Scholar]
- 11.Morra, M., R.A. Barrington, A.C. Abadia-Molina, S. Okamoto, A. Julien, C. Gullo, A. Kalsy, M.J. Edwards, G. Chen, R. Spolski, et al. 2005. Defective B cell responses in the absence of SH2D1A. Proc. Natl. Acad. Sci. USA. 102:4819–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Alem, U., C. Li, N. Forey, F. Relouzat, M.-C. Fondaneche, S.V. Tavtigian, Z.-Q. Wang, S. Latour, and L. Yin. 2005. Impaired Ig class switch in mice deficient for the X-linked lymphoproliferative disease gene sap. Blood. 106:2069–2075. [DOI] [PubMed] [Google Scholar]
- 13.Cannons, J.L., L.J. Yu, B. Hill, L.A. Mijares, D. Dombroski, K.E. Nichols, A. Antonellis, G.A. Koretzky, K. Gardner, and P.L. Schwartzberg. 2004. SAP regulates TH2 differentiation and PKC-theta-mediated activation of NF-κB1. Immunity. 21:693–706. [DOI] [PubMed] [Google Scholar]
- 14.Nichols, K.E., D.P. Harkin, S. Levitz, M. Krainer, K.A. Kolquist, C. Genovese, A. Bernard, M. Ferguson, L. Zuo, E. Snyder, et al. 1998. Inactivation mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA. 95:13765–13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikhalap, S.V., L.M. Shlapatska, A.G. Berdova, C.-L. Law, E.A. Clark, and S.P. Sidorenko. 1999. CDw150 associates with src-homology 2-containing inositol phosphatase and modulates CD95-mediated apoptosis. J. Immunol. 162:5719–5727. [PubMed] [Google Scholar]
- 16.Hron, J.D., L. Caplan, A.J. Gerth, P.L. Schwartzberg, and S.L. Peng. 2004. SH2D1A regulates T-dependent humoral autoimmunity. J. Exp. Med. 200:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma, C.S., N.J. Hare, K.E. Nichols, L. Dupre, G. Andolfi, M.-G. Roncarolo, S. Adestein, P.D. Hodgkin, and S.G. Tangye. 2005. Impaired humoral immunity in X-linked lymphoproliferative disease associated with defective IL-10 production by CD4+ T cells. J. Clin. Invest. 115:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn, T.A., R.W. Thompson, A.W. Cheever, and M.M. Mentink-Kane. 2004. Immunopathogenesis of schistosomiasis. Immunol. Rev. 201:156–167. [DOI] [PubMed] [Google Scholar]
- 19.Aruffo, A., M. Farrington, D. Hollenbaugh, X. Li, A. Milatovich, S. Nonoyama, J. Bajorath, L.S. Grosmaire, R. Stenkamp, M. Neubauer, et al. 1993. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 72:291–300. [DOI] [PubMed] [Google Scholar]
- 20.Randall, T.D., A.W. Heath, L. Santos-Argumedo, M.C. Howard, I.L. Weissman, and F.E. Lund. 1998. Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity. 8:733–742. [DOI] [PubMed] [Google Scholar]
- 21.Erickson, L.D., B.G. Durell, L.A. Vogel, B.P. O'Connor, M. Cascalho, T. Yasui, H. Kikutani, and R.J. Noelle. 2002. Short-circulating long-lived humoral immunity by the heightened engagement of CD40. J. Clin. Invest. 109:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, B.O., L. Haynes, S.M. Eaton, S.L. Swain, and T.D. Randall. 2002. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J. Exp. Med. 196:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDyer, J.F., Z. Li, S. John, C.-Y. Wu, and J.A. Ragheb. 2002. IL-2 receptor blockade inhibits late, but not early, IFN-γ and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-γ production. J. Immunol. 169:2736–2746. [DOI] [PubMed] [Google Scholar]
- 24.Wang, N., A. Satoskar, W. Faubion, D. Howie, S. Okamoto, S. Feske, C. Gullo, K. Clarke, M.R. Sosa, A.H. Sharpe, and C. Terhorst. 2004. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med. 199:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson, D., X. Shi, S. Zhang, H. Wang, M. Nemer, N. Ono, S. Ohno, Y. Yanagi, and A. Veillette. 2004. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to src-related kinase FynT in TH2 cytokine regulation. Immunity. 21:707–717. [DOI] [PubMed] [Google Scholar]
- 26.Parolini, S., C. Bottino, M. Falco, R. Augugliaro, S. Giliani, R. Franceschini, H.D. Ochs, H. Wolf, J.-Y. Bonnefoy, R. Biassoni, et al. 2000. X-linked lymphoproliferative disease: 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus–infected cells. J. Exp. Med. 192:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tangye, S.G., J.H. Phillips, L.L. Lanier, and K.E. Nichols. 2000. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J. Immunol. 165:2932–2936. [DOI] [PubMed] [Google Scholar]
- 28.Dupre, L., G. Andolfi, S.G. Tangye, R. Clementi, F. Locatelli, M. Arico, A. Aiuti, and M.-G. Roncarolo. 2005. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 105:4383–4389. [DOI] [PubMed] [Google Scholar]
- 29.Malbran, A., L. Belmonte, B. Ruibal-Ares, P. Bare, I. Massud, C. Parodi, M. Felippo, R. Hodinka, K. Haines, K.E. Nichols, and M.M. Bracco. 2004. Loss of circulating CD27+ memory B cells and CCR4+ T cells occuring in association with elevated EBV loads in XLP patients surviving primary EBV infection. Blood. 103:1625–1631. [DOI] [PubMed] [Google Scholar]
- 30.Hunziker, L., M. Recher, A.J. Macpherson, A. Ciurea, S. Freigang, H. Hengartner, and R.M. Zinkernagel. 2003. Hypergamaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 4:343–349. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann, M.F., H. Schorle, R. Kuhn, W. Muller, H. Hengartner, R.M. Zinkernagel, and I. Horak. 1995. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J. Virol. 69:4842–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simarro, M., A. Lanyi, D. Howie, F. Poy, J. Bruggeman, M. Choi, J. Sumegi, M.J. Eck, and C. Terhorst. 2004. SAP increases FynT kinase activity and is required for phosphorylation of SLAM and Ly9. Int. Immunol. 16:727–736. [DOI] [PubMed] [Google Scholar]
- 33.Yellin, M.J., K. Sippel, G. Inghirami, L.R. Covey, J.J. Lee, J. Sinning, E.A. Clark, L. Chess, and S. Lederman. 1994. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. J. Immunol. 152:598–608. [PubMed] [Google Scholar]
- 34.Dong, C., A.E. Juedes, U.-A. Temann, S. Shresta, J.P. Allison, N.H. Ruddle, and R.A. Flavell. 2001. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 409:97–101. [DOI] [PubMed] [Google Scholar]
- 35.McAdam, A.J., R.J. Greenwald, M.A. Levin, T. Chernova, N. Malenkovich, V. Ling, G.J. Freeman, and A.H. Sharpe. 2001. ICOS is critical for CD40-mediated antibody class switch. Nature. 409:102–105. [DOI] [PubMed] [Google Scholar]
- 36.Tafuri, A., A. Shahinian, F. Bladt, S.K. Yoshinaga, M. Jordana, A. Wakeman, L.-M. Boucher, D. Bouchard, V.S.F. Chan, G. Duncan, et al. 2001. ICOS is essential for effective T-helper-cell responses. Nature. 409:105–109. [DOI] [PubMed] [Google Scholar]
- 37.Chtanova, T., S.G. Tangye, R. Newton, N. Frank, M.R. Hodge, M.S. Rolph, and C.R. Mackay. 2004. T follicular helper cells express a distinct transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173:68–78. [DOI] [PubMed] [Google Scholar]
- 38.Vinuesa, C.G., M.C. Cook, C. Angelucci, V. Athanasopoulos, L. Rui, K.M. Hill, D. Yu, H. Domaschenz, B. Whittle, T. Lambe, et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 435:452–458. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto, S., H. Ji, D. Howie, K. Clarke, C. Gullo, S. Manning, A.J. Coyle, and C. Terhorst. 2004. Expression of the SH2D1A gene is regulated by a combination of transcriptional and post-transcriptional mechanisms. Eur. J. Immunol. 34:3176–3186. [DOI] [PubMed] [Google Scholar]
- 40.Ding, L., P.S. Linsley, L.-Y. Huang, R.N. Germain, and E.M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 151:1224–1234. [PubMed] [Google Scholar]
- 41.Schaeffer, E.M., G.S. Yap, C.M. Lewis, M.J. Czar, D.W. McVicar, A.W. Cheever, A. Sher, and P.L. Schwartzberg. 2001. Mutation of Tec family kinases alters T helper cell differentiation. Nat. Immunol. 2:1183–1188. [DOI] [PubMed] [Google Scholar]
- 42.Jankovic, D., M.C. Kullberg, N. Noben-Trauth, P. Caspar, J.M. Ward, A.W. Cheever, W.E. Paul, and A. Sher. 1999. Schistome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J. Immunol. 163:337–342. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.