Abstract
Collagens are the most abundant proteins in the human body, important in maintenance of tissue structure and hemostasis. Here we report that collagens are high affinity ligands for the broadly expressed inhibitory leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1). The interaction is dependent on the conserved Gly-Pro-Hyp collagen repeats. Antibody cross-linking of LAIR-1 is known to inhibit immune cell function in vitro. We now show that collagens are functional ligands for LAIR-1 and directly inhibit immune cell activation in vitro. Thus far, all documented ligands for immune inhibitory receptors are membrane molecules, implying a regulatory role in cell–cell interaction. Our data reveal a novel mechanism of peripheral immune regulation by inhibitory immune receptors binding to extracellular matrix collagens.
Collagens represent the most abundant type of proteins in vertebrates and play crucial roles in the development, morphogenesis, and growth of many tissues (1). Besides their mechanical properties, collagens serve as substrates for cell attachment, migration, coagulation, and mediate signaling events by binding to several cell surface receptors, such as integrins, discoidin domain receptors, glycoprotein VI (GpVI), and proteoglycan receptors (2).
Leukocyte-associated Ig-like receptor-1 (LAIR-1) is a member of the Ig superfamily (IgSF), which is expressed on the majority of PBMCs and thymocytes (3). Antibody-induced cross-linking of the receptor in vitro delivers a potent inhibitory signal that is capable of inhibiting cellular functions of NK cells, effector T cells, B cells, and dendritic cell precursors (3–6). This inhibitory signal is dependent on phosphorylation of tyrosine residues located in immunoreceptor tyrosine-based inhibitory motifs (ITIMs) present in the cytoplasmic tail of LAIR-1 (7).
ITIM-bearing receptors are important for an appropriate immune response that needs to be tightly controlled by the opposing action of activating and inhibitory signals. Immune cells are potentially exposed to multiple activating signals in the tissues, and inhibitory receptors are required to set a threshold for cell activation and thus prevent unwanted immune reactions (8). Although all immune cells express multiple inhibitory receptors, these receptors have crucial nonredundant functions, as underlined by receptor knockout mice that demonstrate enhanced sensitivity to autoimmune-like diseases caused by an over-activated immune system (9). The expression pattern of the receptors and the identity of their ligand determine at what stage of an immune response they are effective. Thus far, all documented ligands for immune ITIM-bearing receptors are membrane molecules, implying a regulatory role in cell–cell interaction. Our finding that collagens are ligands for an ITIM-bearing receptor reveals a novel mechanism of peripheral immune regulation by extracellular matrix proteins.
RESULTS AND DISCUSSION
Collagen XVII is a ligand for LAIR-1
By expression cloning, immunoprecipitation, and subsequent protein sequencing, we identified transmembrane collagen XVII as a ligand for LAIR-1 (Fig. S1 and supplemental Materials and methods, available at http://www.jem.org/cgi/content/full/jem. 20052554/DC1). The interaction was confirmed by specific binding of human (h) LAIR-1-IgG to Ba/F3 cells stably transfected with hcollagen XVII (Fig. 1 A). Furthermore, rat (r) and mouse (m) LAIR-1–IgG bound to hcollagen XVII–transfected cells but not to the untransfected parental cell line. Binding of hLAIR-1–IgG and mLAIR-1–IgG to hcollagen XVII was blocked by anti–hLAIR-1 antibodies (8A8) or polyclonal anti–mLAIR-1 antibodies, respectively, demonstrating the specificity of these interactions (Fig. 1, B and C). The association was divalent cation independent; EDTA did not affect LAIR-1 fusion protein binding (not depicted). In addition, human LAIR-2, a putatively secreted protein that is 84% homologous to hLAIR-1 (10), interacted with hcollagen XVII (Fig. 1 A). Thus, collagen XVII is a ligand for LAIR-1 and LAIR-2, and, as we observed previously, ligand recognition occurs cross-species (11, 12).
Figure 1.
Collagen XVII is a ligand for LAIR-1. (A) Ba/F3 cells transfected with hcollagen XVII (filled histograms) or the parental cell line (open histograms) were stained with the indicated LAIR fusion proteins (LAIR-IgGs), hIgG isotype control (isotype), or anti–collagen XVII antibodies (anti-hColXVII). (B) Anti–hLAIR-1 mAb (8A8) completely abrogated the hcollagen XVII/hLAIR-1–IgG interaction. (C) Polyclonal anti–mLAIR-1 antibodies abrogated hcollagen XVII/mLAIR-1–IgG interaction, whereas control serum did not. Data shown are representative of three independent experiments.
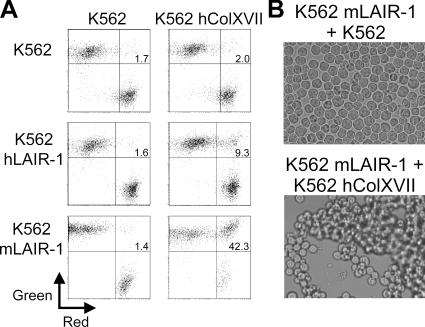
To confirm that LAIR-1 expressed on cells can bind to collagen XVII, we measured formation of conjugates between LAIR-1 and collagen XVII–transfected K562 cells by flow cytometry. We observed profound aggregation between mLAIR-1 and hcollagen XVII–expressing cells, an interaction that was formed within minutes and remained stable for at least 24 h (Fig. 2, A and B). mLAIR-1–transfected cells were more efficient in forming conjugates with hcollagen XVII–transfected cells than hLAIR-1–transfected cells (Fig. 2 A). This difference was evident both in the percentage of cells present in a conjugate (Fig. 2 A) and in the time after which optimal conjugate formation was observed (not depicted). This may indicate an intrinsic difference between mouse and hLAIR-1 in affinity to the collagen XVII trimer.
Figure 2.
Collagen XVII– and LAIR-1–transfected cells form aggregates. K562 cells transfected with hLAIR-1, mLAIR-1, or hcollagen XVII were either red or green fluorescently labeled, coincubated in various indicated combinations at 37°C, and analyzed by flow cytometry (percentage of double-positive cell conjugates is indicated) (A) or by visual inspection for cell clustering (B). Data shown are representative of three independent experiments.
LAIR-1 is a general collagen receptor
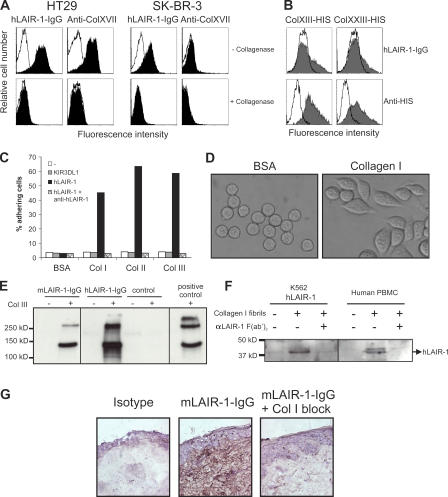
We investigated whether the previously observed binding of LAIR-1 to human tumor cell lines (11) correlated with collagen XVII expression. LAIR-1 ligand+ (11) HT29 colon carcinoma cells expressed collagen XVII, and pretreatment of these cells with Clostridium histolyticum collagenase abrogated both LAIR-1–IgG and anti–collagen XVII mAb binding (Fig. 3 A). The collagen XVII− breast carcinoma cell line SK-BR-3, however, also expressed a ligand for LAIR-1 that was removed after collagenase treatment, suggesting that LAIR-1 may bind to another collagen family member on these cells (Fig. 3 A). Indeed, transient expression of transmembrane collagens XIII and XXIII in 293T cells resulted in the binding of LAIR-1–IgG (Fig. 3 B). In addition, immobilized nontransmembrane collagens I, II, and III were ligands for LAIR-1 (Fig. 3 C). LAIR-1–transfected K562 cells firmly adhered to collagens, which coincided with cell spreading (Fig. 3 D). Both mouse and human LAIR-1–IgG immunoprecipitated hcollagen III from solution (Fig. 3 E). Furthermore, insoluble collagen I fibrils specifically precipitated LAIR-1 from hLAIR-1–transfected K562 cell lysates as well as from human PBMC lysates expressing endogenous LAIR-1 (Fig. 3 F). Additionally, mouse and human LAIR-1–IgG bound specifically to human skin tissue sections (Fig. 3 G). The collagen I– and III–rich dermis stained brightly with LAIR-1–IgG, which was completely blocked by preincubation of the fusion proteins with hcollagen I. We conclude that LAIR-1 is a receptor for multiple transmembrane and extracellular matrix collagens.
Figure 3.
LAIR-1 is a collagen receptor. (A) SK-BR3 cells express a collagen ligand for LAIR-1 that is not collagen XVII. HT29 or SK-BR3 cells were stained with hLAIR-1–IgG or anti–collagen XVII mAb (filled histograms) with or without pretreatment with collagenase. Open histograms represent staining with isotype-matched control Ig. (B) 293T cells were transiently transfected with control vector (open histograms) or indicated HIS-tagged collagens (gray histograms) and stained with hLAIR-1–IgG (top) or anti-HIS antibody (bottom). Similar results were obtained using hLAIR-2–IgG, mLAIR-1–IgG, and rLAIR-1–IgG. (C) Fluorescently labeled wt K562 cells (open bars) or K562 cells expressing hLAIR-1 (black bars) or KIR3DL1 (gray bars) were monitored for their capacity to bind immobilized collagens I, II, and III. Where indicated, cells were preincubated with anti–hLAIR-1 F(ab′)2 (8A8) fragments (hatched bars). Two other members of the IgSF (CD48 and CD80 transfected in K562 cells) did not associate with collagens (not depicted). Percentage of adhering cells relative to input is shown. One of three independent experiments is shown. (D) mLAIR-1–transfected K562 cells spread upon interaction with immobilized collagen I. Spreading was also observed upon interaction with immobilized (GPO)10, but not (GPP)10 (not depicted). Nontransfected cells did not adhere to collagen I or (GPO)10 (not depicted). (E) Immunoprecipitation using mLAIR-1–IgG, hLAIR-1–IgG, or control protein with (+) or without (−) purified hcollagen III. Interacting proteins were Western blotted using anti–hcollagen III–specific mAbs. Positive control represents purified collagen III. (F) Cell lysates from human PBMCs or hLAIR-1–transfected K562 were preincubated with or without anti–hLAIR-1 F(ab′)2 fragments before incubation with insoluble collagen I fibrils. Collagen fibrils and interacting proteins were centrifuged, washed, and Western blotted using anti–hLAIR-1 mAbs. (G) Tissue sections of human skin stained with biotinylated mLAIR-1–IgG (middle) or control Ig (left) followed by streptavidin horseradish peroxidase and counterstaining with hematoxylin. Staining was blocked by preincubation of mLAIR-1–IgG with purified collagen I (right). Staining with hLAIR-Ig gave similar results.
LAIR-1 is a high affinity collagen receptor
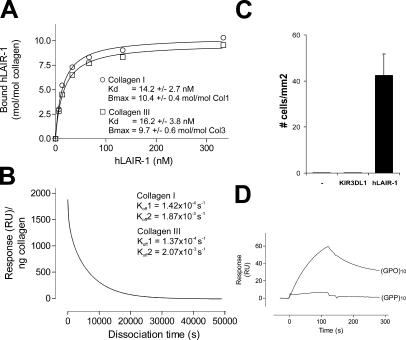
We measured affinity of the collagen–LAIR interaction by surface plasmon resonance (BIAcore). hLAIR-1–IgG bound with high affinity to collagens I and III (Fig. 4 A), and its relatively slow dissociation was characterized by a rapid initial phase and a slower secondary phase (Fig. 4 B). LAIR-1 bound to collagen with ∼40 times higher affinity than the well-studied collagen receptor GpVI (13). Furthermore, the interaction was of 20–1,000-fold higher affinity as compared with most IgSF members interacting with their ligands (14). Although purified LAIR-1–IgG bound directly to collagens I and III (Fig. 4 A), we cannot exclude that additional proteins might modulate the binding in vivo. A single triple-helical collagen I or III molecule interacted with ∼10.4 and 9.7 LAIR-1 proteins, respectively (Fig. 4 A), and this high affinity interaction was sufficient to arrest K562 cells expressing hLAIR-1 on collagen III–coated coverslips under flow conditions (Fig. 4 C).
Figure 4.
LAIR-1 is a high affinity collagen receptor. (A) Indicated concentrations hLAIR-1–IgG were injected at 20 μl/min sequentially through a BIAcore flow cell containing ∼2,000-3,000 RU of directly immobilized collagen I (◯), collagen III (□), or nothing. Each symbol represents the resonance unit at equilibrium and the corresponding concentration of the fusion protein. These data were used to determine the indicated Kd values. Control fusion proteins did not associate with collagen. (B) Rate of dissociation of hLAIR-1–IgG from collagens I and III (not depicted) as monitored by surface plasmon resonance. (C) K562 cells transfected with hLAIR-1 bind collagen III under flow conditions. The indicated transfectants were perfused at wall shear rates of 0.76 dynes/cm2 over collagen III–coated coverslips. The number of binding cells was counted after 5 min (mean ± SEM, n = 6). (D) hLAIR-1 binds immobilized (GPO)10, but not (GPP)10. 780 nM hLAIR-1–IgG was injected at 5 μl/min through a BIAcore flow cell containing ∼250 RU of immobilized (GPO)10 or (GPP)10.
LAIR-1 binds Gly-Pro-Hyp collagen repeats
Because LAIR-1 interacted cross-species with multiple collagen molecules, we hypothesized that LAIR-1 bound to a common collagen-restricted structure. The collagens are a large family of trimeric molecules composed of three polypeptide α chains, which contain the sequence repeat (Gly-X-Y)n, X being frequently proline (P) and, after posttranslational modification, Y being hydroxyproline (O). The GPO triplet is almost exclusively present in collagenous molecules and allows the formation of a triple helix, which is the main characteristic feature of collagens (15). Immobilized triple-helical peptides composed of 10 repeated GPO triplets ((GPO)10, also known as collagen-related peptide) (16) bound hLAIR-1-IgG (Fig. 4 D), whereas a corresponding triple-helical (GPP)10 peptide did not. Thus, LAIR-1 binds a common collagen motif in a hydroxyproline-dependent manner. hLAIR-1–IgG bound less efficiently to (GPO)10 peptide as compared with collagen, suggesting that apart from the GPO sequence, additional structural components are required for optimal interaction. Interestingly, (GPO)10, but not (GPP)10, is also a selective ligand for GpVI (16), a major player in platelet–collagen adhesive interactions leading to thrombus formation. Like LAIR-1, GPVI is a member of the IgSF and is encoded in the leukocyte receptor cluster on human chromosome 19q13.4 (11).
Collagens directly cross-link LAIR-1 and inhibit degranulation of RBL-2H3 cells
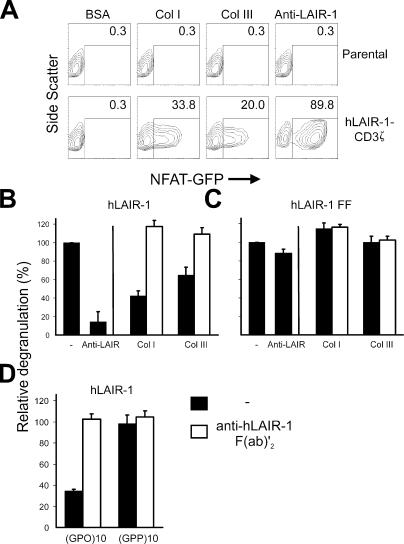
To analyze whether collagen induces functional cross-linking of LAIR-1, we generated 2B4 NFAT-GFP reporter cells (17) expressing a chimeric protein consisting of the extracellular domain of hLAIR-1 and the transmembrane and intracellular domain of CD3ζ. Receptor engagement of cells expressing the hLAIR-1-CD3ζ chimera, but not the parental cells, via plate-bound collagens I, III, or anti–hLAIR-1 mAbs resulted in expression of GFP (Fig. 5 A). Pretreatment of reporter cells with anti–hLAIR-1 F(ab′)2 fragments abrogated the NFAT activation (not depicted). Collagens I and III are thus capable of cross-linking hLAIR-1.
Figure 5.
Collagens directly cross-link LAIR-1 and inhibit degranulation of RBL-2H3 cells. (A) NFAT-GFP reporter cells (reference 17) transfected with hLAIR-1–CD3ζ chimera (bottom) or not (top) were incubated with immobilized collagen I, collagen III, BSA, or anti–hLAIR-1 mAbs for 20 h, and GFP expression was analyzed by flow cytometry. Percentage of GFP+ cells is indicated. (B) hLAIR-1–transfected RBL-2H3 (reference 7) cells were sensitized with IgE anti-TNP and incubated at 37°C in plates coated with TNP conjugated to BSA (0.8 μg/ml) in the absence or presence of plate-bound collagen I, collagen III, or anti–hLAIR-1 mAb (3.3 μg/ml, filled bars). Where indicated, cells were pretreated with 50 μg/ml anti–hLAIR-1 F(ab′)2 fragments (8A8, open bars). (C) The same experiment was performed using RBL-2H3 cells transfected with a LAIR-1 mutant in which both tyrosines in the intracellular tail are mutated to fenylalanines (hLAIR-1 FF). (D) hLAIR-1–transfected RBL-2H3 cells were sensitized with IgE anti-TNP and incubated at 37°C in plates coated with TNP conjugated to BSA (0.8 μg/ml) in the presence of plate-bound trimeric (GPO)10 or (GPP)10 peptides (coated at 3.3 μg/ml; filled bars). Where indicated, cells were pretreated with 50 μg/ml anti–hLAIR-1 F(ab′)2 fragments (8A8, open bars). Degranulation was measured as described before. Typical degranulation values ranged from 9 to 26% of the amount observed when all RBL cells were lysed by addition of 10% Triton. The relative percentage of degranulation was calculated as: 100*[(OD405 TNP + collagen − OD405 spontaneous release)/(OD405 TNP alone − OD405 spontaneous release)]. Spontaneous release was measured after coating with BSA alone. Mean values ± SEM of three independent experiments are shown.
We next investigated whether cross-linking of LAIR-1 by extracellular matrix collagens leads to inhibition of immune cell function in vitro. As a model, we used LAIR-1–transfected RBL-2H3 cells, which express endogenous IgE receptor FcɛRI (7). Incubation of RBL-2H3 hLAIR-1 transfectants with TNP-specific IgE and subsequent triggering with plate-bound TNP-conjugated BSA resulted in degranulation of the cells and release of β-glucuronidase (Fig. 5 B). Simultaneous cross-linking of hLAIR-1 using plate-bound anti–hLAIR-1 mAb or collagen I or III caused marked inhibition of degranulation (Fig. 5 B). A LAIR-1 mutant that is unable to signal because the tyrosine residues in the ITIM were changed to phenylalanine (LAIR-1-FF) (7), could not inhibit the degranulation upon collagen interaction (Fig. 5 C). Furthermore, plate-bound triple-helical (GPO)10 alone, but not (GPP)10, was capable of specifically inhibiting the degranulation of RBL cells (Fig. 5 D), suggesting that other GPO repeat–bearing collagens also can inhibit immune cell function by binding to LAIR-1. The effect was specifically due to the LAIR-1–collagen interaction because preincubation of the cells with blocking anti–hLAIR-1 F(ab′)2 fragments completely abolished the inhibition (Fig. 5, B and D). Thus, extracellular matrix collagens are functional ligands for the inhibitory LAIR-1 that can directly down-regulate immune responses.
When immune cells migrate into the tissues, they are potentially exposed to multiple activating signals. To ensure that they respond appropriately to these stimuli, inhibitory receptors are required to set a threshold for cell activation (8). Our results show that collagen–LAIR-1 interactions can inhibit cell activation and, as such, may contribute to a dampening of the response. Under physiological conditions, immune cells present in the blood are not exposed to collagens (18). Their extravasation, however, results in interaction with collagen-rich subendothelial structures, which may increase the threshold for activation needed to keep these potentially dangerous cells in check. When immune cells reach an inflammatory locus, the presence of specific and strong activating stimuli given by antigen-presenting cells, cytokines, or pathogens will override the threshold and allow cells to become activated and mediate their function. Indeed, we observed that suboptimal activation via the FcɛR was efficiently down-regulated via the LAIR-1–collagen interaction, whereas maximal activation was not (not depicted). Regulation of the LAIR-1–collagen interaction can also occur by modulating LAIR-1 expression at different stages of differentiation or activation of immune cells, as was previously demonstrated for B cells (4), T cells (6), neutrophils (19), and dendritic cells (unpublished data). In addition, secreted LAIR-2 might serve as a regulator of LAIR-1 function by binding collagen, thereby circumventing the inhibitory potential of LAIR-1.
Loss of inhibitory immune receptors or down-regulation of ligands for these receptors can result in a hyperactivated immune system, leading to chronic inflammation and autoimmunity (9). Collagens are implied in several human autoimmune diseases. Collagen XVII, which we initially identified as a LAIR ligand, is an autoantigen in acquired blistering disorders, e.g., bullous pemphigoid (20). Collagen II is an autoantigen in rheumatoid arthritis and systemic lupus erythematosus (21), and collagen VII is an autoantigen in epidermolysis bullosa acquisita (22). Potentially, autoantibodies targeting the various collagen molecules could interfere with the collagen–LAIR-1 interaction and thereby play a role in the pathology of these diseases.
Inhibitory receptors can be used by tumors and viruses to evade immune responses (23). Expression of several members of the collagen family, including collagens I, III, V, VI, XIII, XVII, XVIII, and XXIII, by neoplastic cells is associated with tumor progression (24–28). It is tempting to speculate that overexpression of collagens by tumor cells may enable these cells to suppress antitumor responses via the inhibitor, LAIR-1.
All previously documented ligands for ITIM-bearing receptors are membrane molecules, implying a regulatory role in cell–cell interaction. The functional interaction between extracellular matrix collagens and an inhibitory immune receptor presents a novel mechanism of immune regulation.
MATERIALS AND METHODS
Cells, transfectants, and cDNA.
All cells were obtained from American Type Culture Collection. cDNA encoding hLAIR-1a and mLAIR-1a was cloned into the pMX-neo retroviral vector. Full-length mouse collagen XXIII was amplified by PCR from mouse lung cDNA and cloned in-frame with a COOH-terminal His6-tag and FLAG-tag in the pCEP4 vector. Full-length hcollagen XVII, HIS-tagged hcollagen XIII, and hKIR3DL1 were provided by P.A. Khavari (Stanford University, Stanford, CA), T. Väisänen (University of Oulu, Oulu, Finland), and L.L. Lanier (University of California San Francisco, San Francisco, CA), respectively. The chimeric reporter construct was generated by fusing the extracellular domain of hLAIR-1a to the transmembrane and intracellular domain of hCD3ζ.
Retroviral-based constructs were packaged by the pCL-eco or pCL-ampho system (29), and virus was used to infect Ba/F3, K562, or 2B4 NFAT-GFP T cell hybridoma reporter cells (provided by L.L. Lanier). 3 d after transduction, transfectants expressing either hLAIR-1, mLAIR-1, hcollagen XVII, hKIR3DL1, or hLAIR-1-CD3ζ were sorted for high expression on the cell surface by using a flow cytometer (FACSAria; BD Biosciences). hCollagen XVII expression was assessed by using the 233 mAb (provided by K. Owaribe, Nagoya University, Nagoya, Japan). mLAIR-1 was detected by using a biotinylated anti–mLAIR-1 mAb.
Detection of LAIR ligand.
Chimeric proteins of the extracellular domain of rat, mouse, and hLAIR-1 or hLAIR-2 fused to the Fc region of human IgG1 were prepared, and cell lines were stained with these reagents in the absence or presence of blocking antibodies as described previously (11). When indicated, cells were incubated for 1 h with 100 U/ml of chromatography-purified C. histolyticum collagenase type VII (Sigma-Aldrich) before staining with the fusion proteins.
Conjugate analysis.
K562 transfectants were labeled for 5 min at room temperature with either PKH67 (green) or PKH26 (red; Sigma-Aldrich) according to the manufacturer's protocol. Cells were mixed at a ratio of 1:1 and incubated at 37°C for 1.5 h for mLAIR-1–expressing cells and 5 h for hLAIR-1–expressing cells. Cells were gently resuspended before flow cytometric analysis or analysis by light microscopy.
Analysis of the binding of K562 transfectants to plate-bound collagens.
96-well MAXIsorp (Nunc) flat-bottom plates were coated overnight at 4°C with purified collagens I, III (Sigma-Aldrich), II (Chemicon International), or BSA (100 μl/well, 20 μg/ml in PBS, 2 mM acetic acid). After washings, wells were blocked with 1% (wt/vol) BSA. Meanwhile, 5 × 106 cells/ml of wild-type K562 cells or K562 transfectants were fluorescently labeled for 30 min at 37°C with 5 μM calceine AM (Invitrogen) in PBS. Cells were washed twice with RPMI 1640 containing 1% FCS, 1.5 × 106 cells/ml in 100 μl medium were added to each well, and plates were incubated at 37°C for 2 h. Where indicated, cells were preincubated with 50 μg/ml anti–hLAIR-1 F(ab′)2 (8A8) fragments for 15 min at room temperature before addition to the wells. Input fluorescence was determined by using a fluorescence plate reader (Fluoroskan Ascent; Thermo Labsystems). After incubation, the plates were firmly flicked and washed four times in culture medium. The retained fluorescence was determined for each well as a percentage of input fluorescence.
Precipitation studies.
For immunoprecipitation using LAIR-1-IgG, protein A/G PLUS agarose beads (Santa Cruz Biotechnology, Inc.) were coated with either mLAIR-1–IgG, hLAIR-1–IgG, or control protein. Immunoprecipitation was performed for 2 h in the presence of 10 μg purified human collagen III (Sigma-Aldrich) and 1% BSA in Triton lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, and 0.02% sodium azide) supplemented with 1 mM phenylmethylsulfonyl fluoride and protease inhibitors (Complete Mini EDTA-free protease inhibitor cocktail tablets; Roche). Immune complexes were washed with Triton wash buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, and 0.02% sodium azide), supplemented with 1 mM phenylmethylsulfonyl fluoride, and boiled in Laemmli sample buffer supplemented with 2-mercaptoethanol. Proteins were resolved by SDS-PAGE and transferred to Immobilon-P membranes (Millipore). Western blot analysis was performed with goat anti–human collagen III antibodies (Southern Biotech) followed by horseradish peroxidase–linked secondary antibodies. Proteins were detected by enhanced chemiluminescence (GE Healthcare).
For precipitations using insoluble collagen I fibrils, collagen fibrils were prepared by dialysis of a nonfibrillar collagen I solution (1 mg/ml in 50 mM acetic acid) against 0.02 M sodium phosphate buffer, pH 7.4, for 24 h at 4°C. Collagen fibrils were separated from nonpolymerized collagen by centrifugation (5 min, 13,000 rpm, 4°C). Human PBMCs or K562 cells transfected with hLAIR-1 were preincubated with blocking anti–human LAIR-1 F(ab′)2 fragments or left untreated, lysed in Triton lysis buffer, and cleared by centrifugation (5 min, 13,000 rpm, 4°C). Cell lysates were incubated with insoluble collagen I fibrils for 1 h at 4°C. Collagen fibrils and interacting proteins were centrifuged, washed three times with Triton wash buffer, and boiled in Laemmli sample buffer supplemented with 2-mercaptoethanol. Proteins were resolved by SDS-PAGE and transferred to Immobilon-P membranes (Millipore). Western blot analysis was performed with mouse anti–hLAIR-1 mAbs (8A8) followed by horseradish peroxidase–linked secondary antibodies. Proteins were detected by enhanced chemiluminescence (GE Healthcare).
Human skin tissue section staining.
Frozen tissue sections from healthy human skin were fixed in 10% acetone in the presence of 1.5% H2O2, and free biotin in the sections was blocked using the avidin/biotin blocking kit (Vector Laboratories). Tissue sections were stained with biotinylated mLAIR-1–IgG, hLAIR-1–IgG, or isotype-matched control Ig in the presence of 2% fetal calf serum and, when indicated, 100 μg/ml purified human collagen I (Sigma-Aldrich). After washing with PBS, sections were treated with streptABComplex/HRP (DakoCytomation) according to the manufacturer's instructions, counterstained with hematoxylin, dehydrated, and mounted in DePeX mounting medium (British Drug House Ltd.). Sections were analyzed by light microscopy.
Reporter cell assay.
2B4 T cell hybridoma cells stably transduced with an NFAT-GFP reporter and hLAIR-1-CD3ζ were analyzed as described previously (17). In brief, 96-well MAXIsorp flat-bottom plates (Nunc) were coated overnight at 4°C with purified collagen I, collagen III, BSA, or anti–hLAIR-1 antibodies (8A8) (100 μl/well, 10 μg/ml in PBS, 2 mM acetic acid). After washings, 3.5 × 105 cells/ml in 200 μl medium were added to each well, and plates were incubated at 37°C for 20 h and analyzed for GFP expression by flow cytometry.
Surface plasmon resonance experiments.
Surface plasmon resonance binding studies were performed by using a BIAcore2000 system (BIAcore). Approximately 2,000–3,000 response units (RU) of acid-soluble human collagen type I or III (Sigma-Aldrich) were immobilized on a CM5 biosensor chip by using the amine coupling kit as instructed by the supplier. Immobilized triple-helical peptides composed of GCO(GPO)10GCOG-NH2 ((GPO)10, also known as collagen-related peptide) and GCP(GPP)10GCPG-NH2 ((GPP)10) were described previously (16). Approximately 250 RU (GPP)10 or (GPO)10 peptide trimers were immobilized by using a cysteine coupling kit according to the manufacturer's instructions. Analysis was performed in buffer (125 mM NaCl, 2.5 mM CaCl2, 0.005% [vol/vol] Tween 20, and 25 mM Hepes, pH 7.4) at 25°C at a flow rate of 20 μl/min for collagen I and III interaction studies and 5 μl/min for the immobilized peptides. Binding of hLAIR-1–IgG to collagens I and III was specific because nonspecific binding to an uncoated control channel was <2% compared with collagen-coated channels. In addition, an irrelevant IgG fusion protein did not bind to the collagen-coated surface. hLAIR-1–IgG dimer concentration was calculated based on a theoretical mass of 85.2 kD (corrected for removal of leader peptide). Increasing concentrations of hLAIR-1–IgG were injected and allowed to reach an equilibrium plateau for 10 min. The delay between injections was 13 min, during which time the biosensor chip was flushed with buffer. In the peptide-binding studies, biosensor chips were regenerated by injection of 0.1 M H3PO4 (2 min, 5 μl/min).
Dissociation constants (Kd) and the number of binding sites expressed as the response at infinite hLAIR-1–IgG concentration (Bmax) were calculated as follows. First, the response at equilibrium (Req) was calculated for each association curve. Subsequently, Kd and Bmax were determined from the binding isotherms (Req plotted against hLAIR-1–IgG concentration) by the fitting equation Req = Bmax*[hLAIR-1–IgG]/(Kd + [hLAIR-1–IgG]). The fit was calculated by using GraphPad Prism (GraphPad Prism version 3.0 for Windows; GraphPad Software). Bmax values were converted to number of hLAIR-1–IgG molecules interacting with a single collagen trimer by using the theoretical mass of hLAIR-1–IgG (85.2 kD) versus collagens I and III (416.7 and 415.7 kD, respectively).
The dissociation of hLAIR-1–IgG in the presence of buffer was followed for at least 13 h, and Koff values were calculated by using the Biaevaluation software version 3.0.1.
Perfusion studies.
Perfusions were performed in a single-pass perfusion chamber as described previously (30). In brief, collagen type III was solubilized in 50 mM acetic acid and sprayed onto glass coverslips by using a retouching airbrush (Badger model 100; Badger Brush) at a density of 6.5 μg/cm2. Afterward, coverslips were blocked for 1 h at room temperature with 1% human albumin in PBS. Wild-type or transfected K562 cells were then perfused for 5 min at a shear rate of 0.75 dyne/cm2 at 37°C. After perfusion, slides were washed with Hepes buffer (10 mm Hepes, 150 mm NaCl, pH 7.35), fixed in 0.5% glutaraldehyde in PBS, dehydrated in methanol, and stained with May-Grünwald and Giemsa. Adhered K562 cells were counted by using light microscopy and presented as number of cells per mm2.
Online supplemental material.
Fig. S1 shows the identification of mouse collagen XVII as a ligand for mLAIR-1. Supplemental Materials and methods describe the identification of collagen XVII as a ligand for LAIR-1. The online supplemental material is available at http://www.jem.org/cgi/ content/full/jem.20052554/DC1.
Supplemental Material
Acknowledgments
We thank Hans Clevers, Paul Coffer, Philip de Groot, Christine Jansen, Lewis Lanier, Frank Miedema, and Annelies Verbrugge for critically reading the manuscript and useful discussions. We are grateful to Cornelieke Pals for technical assistance with 2D-electrophoresis, Marian Groot Koerkamp for the microarray analysis, and Alain Kummer for providing the human skin sections.
R.J. Lebbink and L. Meyaard were supported by grant 016.026.008 from the Netherlands Organization for Scientific Research (NWO). M. Koch was supported by the Deutsche Forschungsgemeinschaft (SFB 589).
R.J. Lebbink and L. Meyaard are named as inventors on a patent application on the LAIR–collagen interaction. All other authors have no conflicting financial interests.
References
- 1.Myllyharju, J., and K.I. Kivirikko. 2004. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20:33–43. [DOI] [PubMed] [Google Scholar]
- 2.Gelse, K., E. Poschl, and T. Aigner. 2003. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55:1531–1546. [DOI] [PubMed] [Google Scholar]
- 3.Meyaard, L., G.J. Adema, C. Chang, E. Woollatt, G.R. Sutherland, L.L. Lanier, and J.H. Phillips. 1997. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 7:283–290. [DOI] [PubMed] [Google Scholar]
- 4.van der Vuurst de Vries, A.R., H. Clevers, T. Logtenberg, and L. Meyaard. 1999. LAIR-1 is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 29:3160–3167. [DOI] [PubMed] [Google Scholar]
- 5.Poggi, A., E. Tomasello, E. Ferrero, M.R. Zocchi, and L. Moretta. 1998. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by GM-CSF. Eur. J. Immunol. 28:2086–2091. [DOI] [PubMed] [Google Scholar]
- 6.Maasho, K., M. Masilamani, R. Valas, S. Basu, J.E. Coligan, and F. Borrego. 2005. The inhibitory LAIR-1 is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol. Immunol. 42:1521–1530. [DOI] [PubMed] [Google Scholar]
- 7.Verbrugge, A., T. de Ruiter, H. Clevers, and L. Meyaard. 2003. Differential contribution of the ITIMs of human LAIR-1 to inhibitory function and phosphatase recruitment. Int. Immunol. 15:1349–1358. [DOI] [PubMed] [Google Scholar]
- 8.Ravetch, J.V., and L.L. Lanier. 2000. Immune inhibitory receptors. Science. 290:84–89. [DOI] [PubMed] [Google Scholar]
- 9.Pritchard, N.R., and K.G. Smith. 2003. B cell inhibitory receptors and autoimmunity. Immunology. 108:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyaard, L., J. Hurenkamp, H. Clevers, L.L. Lanier, and J.H. Phillips. 1999. LAIR-1 functions as an inhibitory receptor on cytotoxic T cells. J. Immunol. 162:5800–5804. [PubMed] [Google Scholar]
- 11.Lebbink, R.J., T. de Ruiter, A. Verbrugge, W.S. Bril, and L. Meyaard. 2004. The mouse homologue of LAIR-1 is an inhibitory receptor that recruits SHP-2, but not SHP-1. J. Immunol. 172:5535–5543. [DOI] [PubMed] [Google Scholar]
- 12.Lebbink, R.J., T. de Ruiter, G.J. Kaptijn, and L. Meyaard. 2005. Identification and characterization of the rat homologue of LAIR-1. Immunogenetics. 57:344–351. [DOI] [PubMed] [Google Scholar]
- 13.Miura, Y., T. Takahashi, S.M. Jung, and M. Moroi. 2002. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J. Biol. Chem. 277:46197–46204. [DOI] [PubMed] [Google Scholar]
- 14.Maenaka, K., T. Juji, T. Nakayama, J.R. Wyer, G.F. Gao, T. Maenaka, N.R. Zaccai, A. Kikuchi, T. Yabe, K. Tokunaga, et al. 1999. KIRs and T cell receptors bind peptide-major histocompatibility complex class I with distinct thermodynamic and kinetic properties. J. Biol. Chem. 274:28329–28334. [DOI] [PubMed] [Google Scholar]
- 15.Ricard-Blum, S., and F. Ruggiero. 2005. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol. Biol. (Paris). 53:430–442. [DOI] [PubMed] [Google Scholar]
- 16.Knight, C.G., L.F. Morton, D.J. Onley, A.R. Peachey, T. Ichinohe, M. Okuma, R.W. Farndale, and M.J. Barnes. 1999. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc. Res. 41:450–457. [DOI] [PubMed] [Google Scholar]
- 17.Voehringer, D., D.B. Rosen, L.L. Lanier, and R.M. Locksley. 2004. CD200 receptor family members represent novel DAP12-associated activating receptors on basophils and mast cells. J. Biol. Chem. 279:54117–54123. [DOI] [PubMed] [Google Scholar]
- 18.Farndale, R.W., J.J. Sixma, M.J. Barnes, and P.G. de Groot. 2004. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2:561–573. [DOI] [PubMed] [Google Scholar]
- 19.Verbrugge, A., T. de Ruiter, C. Geest, P.J. Coffer, and L. Meyaard. 2006. Differential expression of LAIR-1 during neutrophil differentiation and activation. J. Leukoc. Biol. 79:828–836. [DOI] [PubMed] [Google Scholar]
- 20.Franzke, C.W., P. Bruckner, and L. Bruckner-Tuderman. 2005. Collagenous transmembrane proteins: recent insights into biology and pathology. J. Biol. Chem. 280:4005–4008. [DOI] [PubMed] [Google Scholar]
- 21.Gioud, M., A. Meghlaoui, O. Costa, and J.C. Monier. 1982. Antibodies to native type I and II collagens detected by an ELISA in rheumatoid arthritis and systemic lupus erythematosus. Coll. Relat. Res. 2:557–564. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt, E., and D. Zillikens. 2000. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv. Dermatol. 16:113–157. [PubMed] [Google Scholar]
- 23.Lanier, L.L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274. [DOI] [PubMed] [Google Scholar]
- 24.Parikka, M., T. Kainulainen, K. Tasanen, A. Vaananen, L. Bruckner-Tuderman, and T. Salo. 2003. Alterations of collagen XVII expression during transformation of oral epithelium to dysplasia and carcinoma. J. Histochem. Cytochem. 51:921–929. [DOI] [PubMed] [Google Scholar]
- 25.Iizasa, T., H. Chang, M. Suzuki, M. Otsuji, S. Yokoi, M. Chiyo, S. Motohashi, K. Yasufuku, Y. Sekine, A. Iyoda, et al. 2004. Overexpression of collagen XVIII is associated with poor outcome and elevated levels of circulating serum endostatin in non-small cell lung cancer. Clin. Cancer Res. 10:5361–5366. [DOI] [PubMed] [Google Scholar]
- 26.Banyard, J., L. Bao, and B.R. Zetter. 2003. Type XXIII collagen, a new transmembrane collagen identified in metastatic tumor cells. J. Biol. Chem. 278:20989–20994. [DOI] [PubMed] [Google Scholar]
- 27.Roepman, P., L.F. Wessels, N. Kettelarij, P. Kemmeren, A.J. Miles, P. Lijnzaad, M.G. Tilanus, R. Koole, G.J. Hordijk, P.C. van der Vliet, et al. 2005. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nat. Genet. 37:182–186. [DOI] [PubMed] [Google Scholar]
- 28.Vaisanen, T., M.R. Vaisanen, H. Autio-Harmainen, and T. Pihlajaniemi. 2005. Type XIII collagen expression is induced during malignant transformation in various epithelial and mesenchymal tumours. J. Pathol. 207:324–335. [DOI] [PubMed] [Google Scholar]
- 29.Naviaux, R.K., E. Costanzi, M. Haas, and I.M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sixma, J.J., P.G. de Groot, H. van Zanten, and M.I. Jsseldijk. 1998. A new perfusion chamber to detect platelet adhesion using a small volume of blood. Thromb. Res. 92:S43–S46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.