Abstract
In the early 1990s, Richard (Randy) Hardy and colleagues divided B cell precursors into subpopulations—the Hardy fractions—based on the cells' expression of various cell surface proteins. This classification helped lay the groundwork for our present-day understanding of the molecular events that control early B cell development.
B cell precursors progress through an ordered series of developmental steps in the bone marrow before reaching maturity. The details of this process are now well defined, but a mere two decades ago the picture was far less clear, in part because it was difficult to follow single cells from birth to maturity.
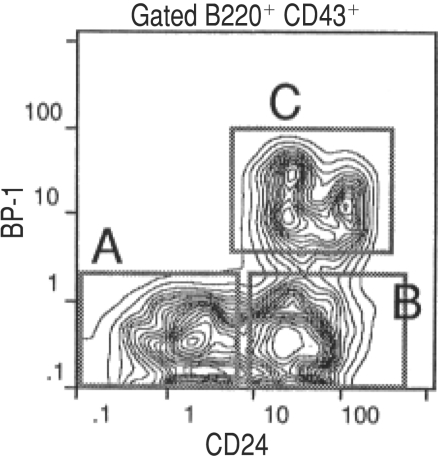
Figure 1.
FACS plot of Hardy fractions A-C. Adapted from reference 7.
Tracking these cells would require detailed fractionation of the cells using monoclonal antibodies (mAbs) specific for a panoply of cell surface markers. There are those who curse the alphabet soup of immune cell surface markers. But those long lists—which started with the ingenuity of Hardy—allow us to make sense out of otherwise identical looking collections of cells.
FACS revolution
In the 1970s, Max Cooper, Dennis Osmond, and others discovered that immunoglobulin (Ig)-bearing B cells arose from Ig-negative precursor cells in the bone marrow. These precursor cells—dubbed pre-B cells by Cooper—had synthesized part of the receptor (Ig heavy chains), which could be detected in the cytoplasm, but had yet to assemble a complete receptor on the cell surface (1).
Osmond later pinpointed a more primitive population of cells (pro-B cells) that had not yet synthesized μ heavy chains (2). But the cellular phenotypes that might be lurking between the pro-B and pre-B cell stages remained mysterious, as there was no way to pick apart this mixed bag of cells.
Also problematic was the inability to grow B cell precursors in culture. As a result, many of the early insights into B cell development—including the ordered rearrangement of the V, D, and J gene segments of the Ig locus—came from studies using transformed B cell lines. The discovery that stromal cells could support the growth and differentiation of primary B cell precursors soon overcame this obstacle (3, 4).
Then came multicolor (multilaser) flow cytometry, a technique that revolutionized the study of B cell development (and immunology as a whole) by allowing single cells to be stained simultaneously with multiple fluorescently tagged mAbs. Hardy—a chemist by training—used one of the first multilaser FACS (fluorescence activated cell sorter) machines to investigate mature B cell subpopulations as a post-doc with Leonore and Leonard Herzenberg in the early 1970s (5, 6).
Partitioning pre- and pro-B
With these techniques in hand, plus a battery of lymphocyte-specific mAbs (generated by the Herzenbergs, John Kemp, and others), Hardy—by then a new faculty member at Fox Chase Cancer Center (Philadelphia)—set out to explore the diversity of B cell precursors. He started by dividing B220+ bone marrow cells based on their expression of CD43, BP-1 and CD24.
Sorting the CD43+ populations of cells and culturing them in vitro revealed their order of differentiation: fraction A (BP-1−/CD24−) begot fraction B (BP-1−/CD24+), which in turn begot fraction C (BP-1+/CD24+). Later (CD43−) populations could be distinguished by their expression of surface IgM and IgD. “It was so striking the way you could sort these cells and see the progression,” says Hardy.
Hardy confirmed the developmental order of the cells by amplifying the Ig genes from each fraction. The Ig genes from fraction A cells were in a germline configuration, whereas those from fractions B and C had D-J, but not V-D-J rearrangements, consistent with previous studies. Hardy published these data, along with his fractionation scheme, in 1991 in The Journal of Experimental Medicine (7).
Markers multiply
Hardy was not alone in his attempt to bring order to early B cell development. Fritz Melchers and colleagues in Basel, Switzerland, who discovered the surrogate light chain, added c-kit and CD25 to the growing list of B cell markers (8). That list now goes on and on (and FACS is up to 17 colors), with each new marker revealing new layers of diversity. As Melchers puts it, “It's a mess, but it's a beautiful mess.” Hardy prefers to call it a “beautiful complexity,” adding, “It's like a fractal—the deeper you look, the more you see.”
References
- 1.Raff, M.C., M. Megson, J.J.T. Owen, and M.D. Cooper. 1976. Nature. 259:224–226. [DOI] [PubMed] [Google Scholar]
- 2.Park, Y.-H., and D.G. Osmond. 1984. J. Exp. Med. 165:444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock, C.A., and O.N. Witte. 1982. Proc. Natl. Acad. Sci. USA. 79:3608–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman, R.L., and I.L. Weissman. 1981. Nature. 289:681–683. [DOI] [PubMed] [Google Scholar]
- 5.Hardy, R.R., K. Hayakawa, J. Haaijman, and L.A. Herzenberg. 1982. Nature. 297:589–591. [DOI] [PubMed] [Google Scholar]
- 6.Hardy, R.R., K. Hayakawa, D.R. Parks, and L.A. Herzenberg. 1983. Nature. 306:270–272. [DOI] [PubMed] [Google Scholar]
- 7.Hardy, R.R., C.E. Carmack, S.A. Shinton, J.D. Kemp, and K. Hayakawa. 1991. J. Exp. Med. 173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolink, A., U. Grawunder, T.H. Winkler, H. Karasuyama, and F. Melchers. 1994. Int. Immunol. 6:1257–1264. [DOI] [PubMed] [Google Scholar]