Abstract
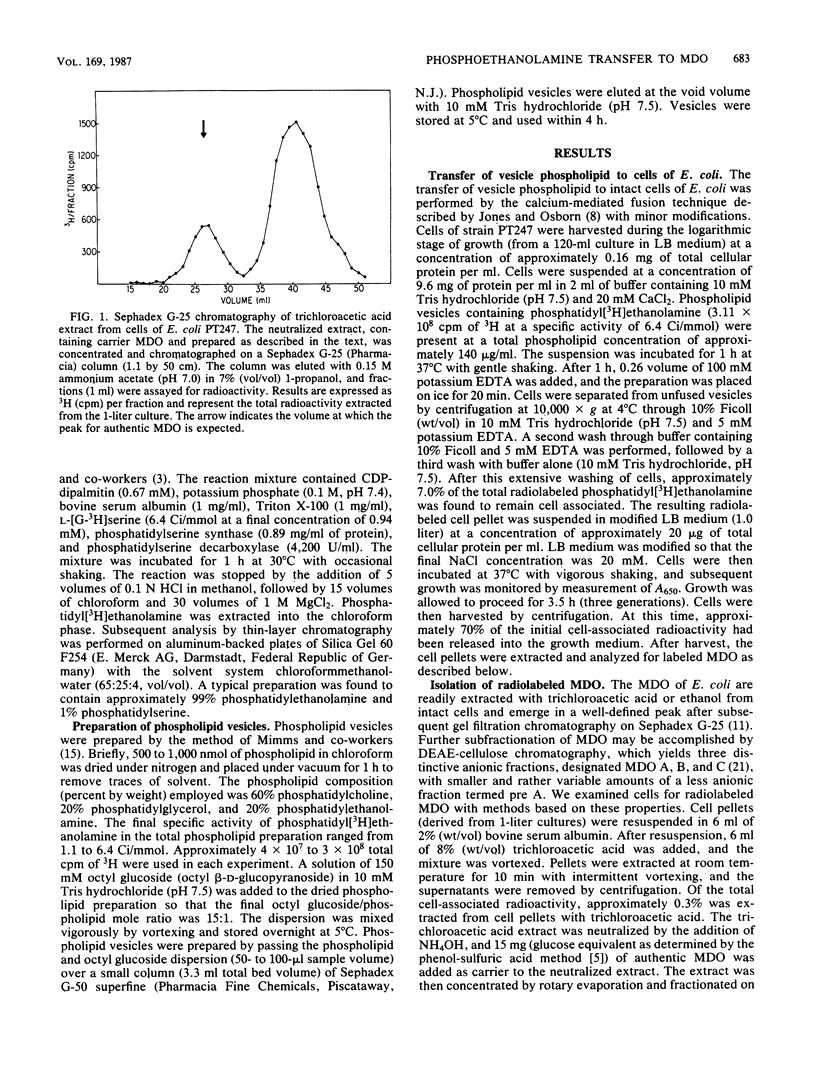
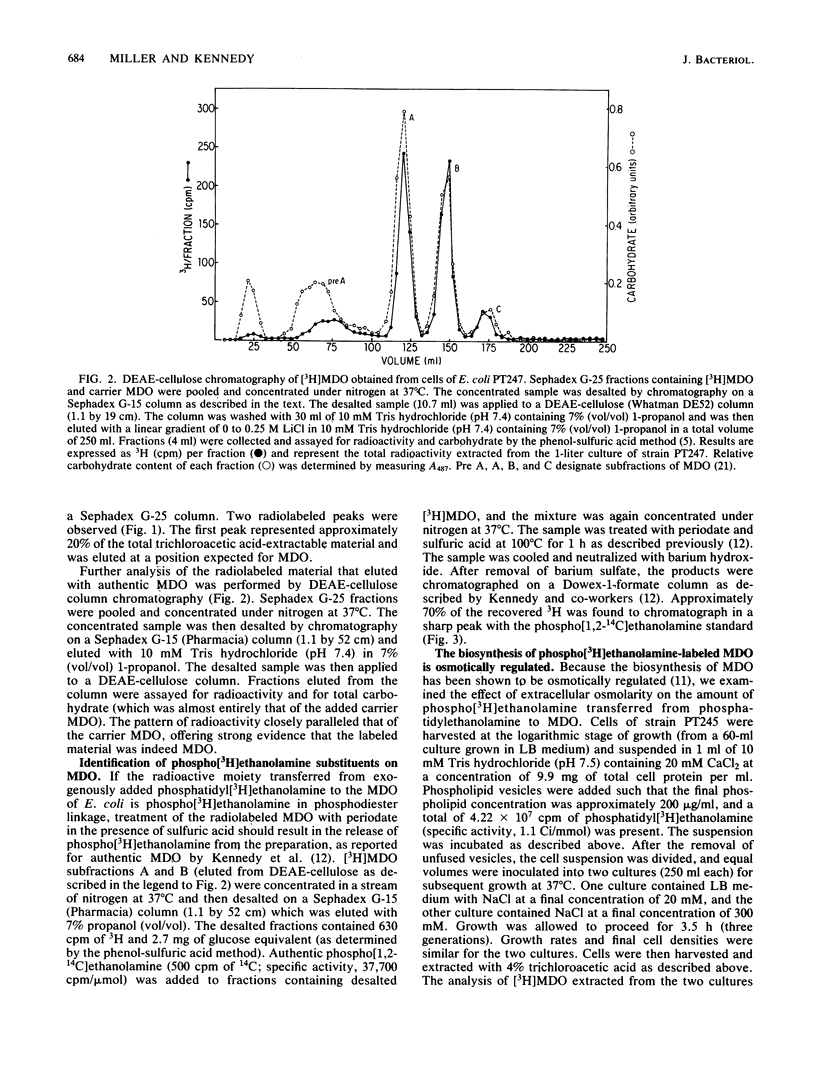
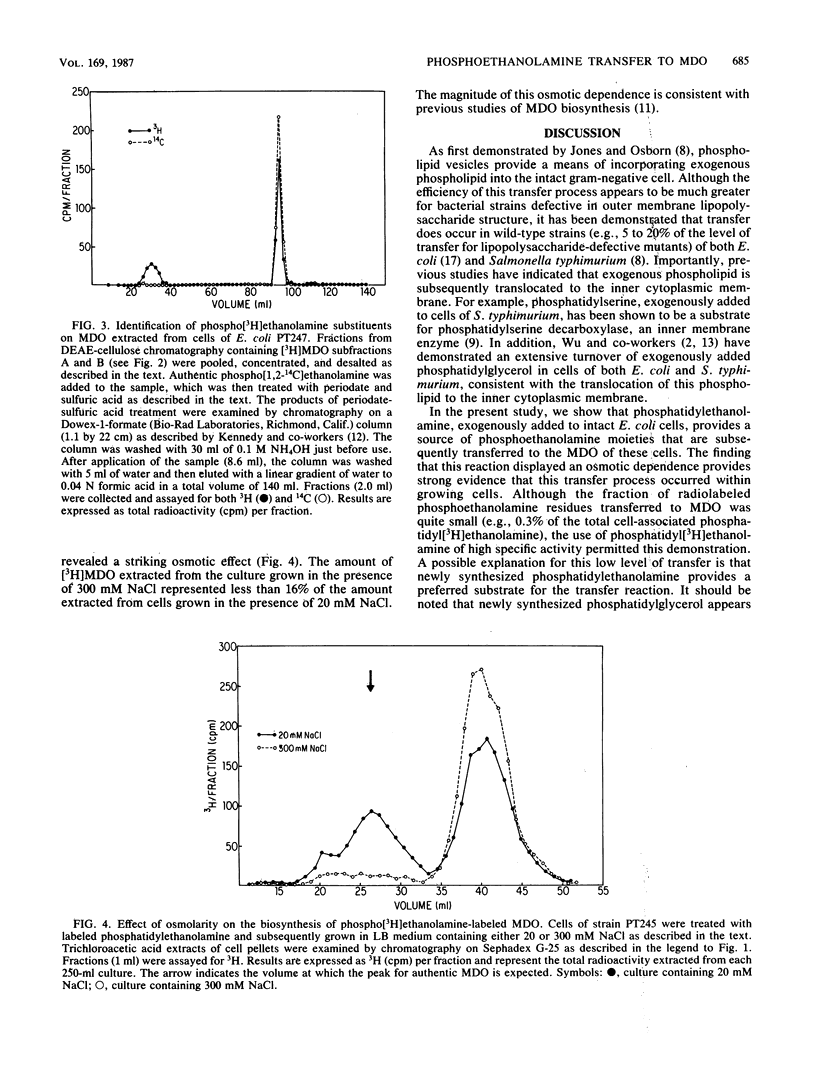
The membrane-derived oligosaccharides (MDO) of Escherichia coli are periplasmic constituents composed of glucose residues linked by beta-1,2 and beta-1,6 glycosidic bonds. MDO are substituted with phosphoglycerol, phosphoethanolamine, and succinic acid moieties. The phosphoglycerol residues present on MDO are derived from phosphatidylglycerol (B. J. Jackson and E. P. Kennedy, J. Biol. Chem. 258:2394-2398, 1983), but evidence as to the source of the phosphoethanolamine residues has been lacking. We now report that phosphatidylethanolamine, exogenously added to intact cells of E. coli, provides a source of phosphoethanolamine residues that are transferred to MDO. The biosynthesis of phosphoethanolamine-labeled MDO is osmotically regulated, with maximum synthesis occurring during growth in medium of low osmolarity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohin J. P., Kennedy E. P. Regulation of the synthesis of membrane-derived oligosaccharides in Escherichia coli. Assay of phosphoglycerol transferase I in vivo. J Biol Chem. 1984 Jul 10;259(13):8388–8393. [PubMed] [Google Scholar]
- Chattopadhyay P. K., Lai J. S., Wu H. C. Incorporation of phosphatidylglycerol into murein lipoprotein in intact cells of Salmonella typhimurium by phospholipid vesicle fusion. J Bacteriol. 1979 Jan;137(1):309–312. doi: 10.1128/jb.137.1.309-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan W., Wickner W. T., Kennedy E. P. Purification and properties of phosphatidylserine decarboxylase from Escherichia coli. J Biol Chem. 1974 May 25;249(10):3079–3084. [PubMed] [Google Scholar]
- Fiedler W., Rotering H. Characterization of an Escherichia coli mdoB mutant strain unable to transfer sn-1-phosphoglycerol to membrane-derived oligosaccharides. J Biol Chem. 1985 Apr 25;260(8):4799–4806. [PubMed] [Google Scholar]
- Jackson B. J., Bohin J. P., Kennedy E. P. Biosynthesis of membrane-derived oligosaccharides: characterization of mdoB mutants defective in phosphoglycerol transferase I activity. J Bacteriol. 1984 Dec;160(3):976–981. doi: 10.1128/jb.160.3.976-981.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. J., Gennity J. M., Kennedy E. P. Regulation of the balanced synthesis of membrane phospholipids. Experimental test of models for regulation in Escherichia coli. J Biol Chem. 1986 Oct 15;261(29):13464–13468. [PubMed] [Google Scholar]
- Jackson B. J., Kennedy E. P. The biosynthesis of membrane-derived oligosaccharides. A membrane-bound phosphoglycerol transferase. J Biol Chem. 1983 Feb 25;258(4):2394–2398. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- Lai J. S., Wu H. C. Incorporation of acyl moieties of phospholipids into murein lipoprotein in intact cells of Escherichia coli by phospholipid vesicle fusion. J Bacteriol. 1980 Oct;144(1):451–453. doi: 10.1128/jb.144.1.451-453.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Plimmer R. H., Burch W. J. Esters of phosphoric acid: Phosphorylaminoethanol and phosphorylcholine. Biochem J. 1937 Mar;31(3):398–409. doi: 10.1042/bj0310398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx P. Interaction of lipid vesicles with an heptoseless strain of Escherichia coli. Exp Biol. 1985;43(3):191–199. [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Reinhold V., Rumley M. K., Kennedy E. P. Structural studies of the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1979 Oct 25;254(20):10135–10138. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]



