Abstract
Abnormalities in CD4+CD25+Foxp3+ regulatory T (T reg) cells have been implicated in susceptibility to allergic, autoimmune, and immunoinflammatory conditions. However, phenotypic and functional assessment of human T reg cells has been hampered by difficulty in distinguishing between CD25-expressing activated and regulatory T cells. Here, we show that expression of CD127, the α chain of the interleukin-7 receptor, allows an unambiguous flow cytometry–based distinction to be made between CD127lo T reg cells and CD127hi conventional T cells within the CD25+CD45RO+RA− effector/memory and CD45RA+RO− naive compartments in peripheral blood and lymph node. In healthy volunteers, peripheral blood CD25+CD127lo cells comprised 6.35 ± 0.26% of CD4+ T cells, of which 2.05 ± 0.14% expressed the naive subset marker CD45RA. Expression of FoxP3 protein and the CD127lo phenotype were highly correlated within the CD4+CD25+ population. Moreover, both effector/memory and naive CD25+CD127lo cells manifested suppressive activity in vitro, whereas CD25+CD127hi cells did not. Cell surface expression of CD127 therefore allows accurate estimation of T reg cell numbers and isolation of pure populations for in vitro studies and should contribute to our understanding of regulatory abnormalities in immunopathic diseases.
CD4+ regulatory T (T reg) cells expressing the IL-2Rα chain (CD25) and the master regulator Foxp3 transcription factor play a vital role in controlling adaptive immune responses and maintaining self tolerance (1). Although the best evidence for their importance comes from mouse models, an increasing number of reports have outlined disturbances in T reg cell numbers and/or function in patients with a wide variety of autoimmune (2–8) and allergic diseases (9, 10), in addition to the severe IPEX (immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance) syndrome in which Foxp3 itself is defective (11). Although some studies have demonstrated a reduction in CD4+CD25+ T reg cell numbers in autoimmune conditions (2, 3, 8, 12), others have shown normal or even increased numbers of this same subset of T cells (5, 13–15). This may be due at least in part to the difficulty in accurately distinguishing T reg cells from CD25+ conventional T cells, particularly in human peripheral blood where up to 20% of CD4+ T cells can express CD25 (16, 17).
Production of mAbs reactive with Foxp3 has improved the specificity of T reg cell detection over and above that provided by the combination of anti-CD4 and anti-CD25 (18). However, detection of Foxp3 requires fixation and permeabilization of the cells, so that the technique cannot be used to isolate viable T reg cell populations for functional studies and ex vivo expansion as a prelude to therapeutic administration. Recently, CD4+CD25+ conventional T cells that have down-regulated the costimulatory receptor CD27 after activation were shown to be distinguishable from T reg cells that continue to express CD27 (19). However, the vast majority of peripheral blood antigen-experienced CD25+ conventional T cells also continue to express high levels of CD27 (17), therefore limiting the applicability of CD27 as a T reg cell–specific marker.
In this study, we show that surface expression of CD127, the α chain of the IL-7 receptor, in combination with CD25, the α chain of the IL-2 receptor, can distinguish between human regulatory and conventional CD4+ T cells in adult and cord blood, lymph nodes and thymus. Because IL-2 is critical for survival of regulatory T cells (20), we reasoned that they may not require IL-7, in contrast with many nonregulatory T cell subsets that are IL-2 independent but require IL-7 (21). According to our findings, human T reg cells consistently express lower levels of CD127 than the majority of other CD4+ T cells. By virtue of its cell surface expression, CD127 provides a flexible alternative to the transcription factor FoxP3 for identifying and isolating human T reg cells for functional analysis.
RESULTS AND DISCUSSION
Expression of CD127 distinguishes between two populations of human CD25+CD4+ T cells
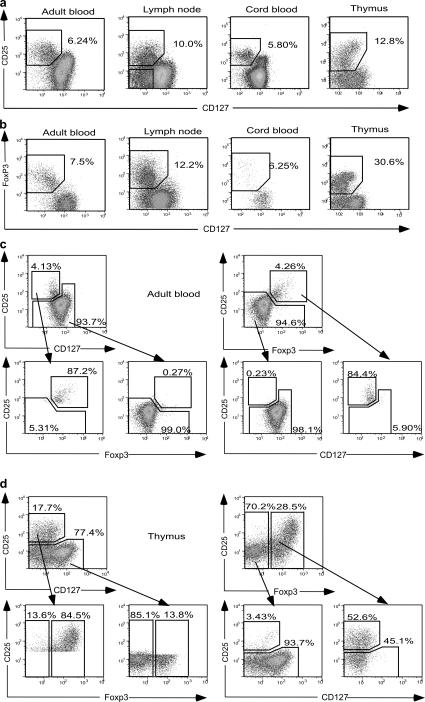
The capacity of CD127 expression to distinguish two populations of CD25+CD4+ T cells in a variety of lymphoid tissues was tested by staining samples of normal adult blood, lymph node, cord blood, and thymus with mAbs to CD4, CD25, and CD127. Adult blood contained a population of CD25+CD127lo cells distinct from the majority population of CD127hi cells (Fig. 1 a). In addition to the CD25+CD127lo population, lymph nodes also contained a significant number of CD25−CD127lo T cells, which were prominent in blood from a minority of normal adults (unpublished data). In cord blood, staining with anti-CD127 revealed that the CD25+ population was not homogeneous, as previously claimed (22), but rather consisted of a mixture of CD25+CD127lo and CD25+CD127hi cells. In the thymus, where antigen-experienced cells expressing CD25 are absent, cells with the highest levels of CD25 retained intermediate expression of CD127 (Fig. 1 a).
Figure 1.
Expression of CD127 and FoxP3 in adult blood, lymph node, cord blood, and thymus. (a) Plots are gated for CD4+CD8− T cells. CD25+CD127lo cells are boxed and the percentage of cells in the box is shown. In the lymph node sample, CD25−CD127lo cells are also boxed. (b) Plots are gated for CD4+CD8− T cells. FoxP3+CD127lo cells are boxed and the percentage of cells in the box is shown. (c) Correlation between FoxP3+CD25+ and CD25+CD127lo phenotypes in peripheral blood. Gating of CD4+ cells for each subset is shown, followed by the distribution of gated cells according to the reciprocal subset. (d) Correlation between FoxP3+ and CD25+CD127lo phenotypes in thymus.
Inverse correlation between expression of FoxP3 and CD127 in CD4+CD25+ T cells
To measure expression of Foxp3 protein within the CD25+CD127lo population, cells from adult and cord blood, lymph node and thymus were costained with mAbs to Foxp3 and CD127 (Fig. 1 b). In blood and lymph node, the population of FoxP3+ cells was CD127lo and similar in size to that of CD25+CD127lo cells in Fig. 1 a. In contrast, the thymic FoxP3+ population was considerably larger than the CD25+CD127lo population. In peripheral blood, 87% of CD4+CD127lo cells (gated as in Fig. 1 c, top left) fell within the CD25+Foxp3+ gate (Fig. 1 c, bottom left), and conversely, 84% of CD25+Foxp3+ cells were detected within the CD4+CD127lo gate (Fig. 1 c, right). In thymic CD4+CD8− T cells, however, 45% of Foxp3+ cells were CD25− (Fig. 1 d, bottom right), so that CD25+CD127lo cells comprised a significantly smaller population than Foxp3+ cells. Nonetheless, all thymic CD4+CD8−Foxp3+ cells were CD127lo. Thus the expression of CD25, CD127, and FoxP3 differed between thymus and peripheral blood.
We (17) and others (23) have recently described a subset of naive CD4+CD45RA+CD25+ cells with regulatory activity in adult as well as cord blood. To test whether these cells also had a FoxP3+CD127lo phenotype, adult blood, lymph node, and cord blood cells were stained with mAbs to CD3, CD4, CD45RA, CD25, CD127, and FoxP3 (Fig. 2 a). CD3+CD4+ cells were separated into CD45RA− and CD45RA+ subsets, and the percentage of CD25+CD127lo cells within the Foxp3+ gate was calculated. In all tissues, >90% of total Foxp3+ cells were CD25+CD127lo, whereas the remaining cells were CD25intCD127hi. Moreover, the proportion of CD127hi cells was similar within the CD45RA− and CD45RA+Foxp3+ subsets.
Figure 2.
Correlation between expression of FoxP3 and CD127lo phenotype. (a) Leukocytes from adult blood, lymph node, and cord blood were gated into CD3+CD4+CD45RA+ and CD45RA− populations. FoxP3+ cells are boxed and the percentage of cells in the box is shown, together with expression of CD25 versus CD127 within the FoxP3+ gate. (b) Correlation between the percentages of CD25+CD127lo and CD25+FoxP3+ cells within CD4+CD45RA+ and CD45RA− populations in nine peripheral blood samples from healthy volunteers.
To determine the strength of the correlation between the percentage of cells within CD25+CD127lo and CD25+FoxP3+ populations, peripheral blood samples from nine healthy volunteers were analyzed (Fig. 2 b). In both CD45RA− and CD45RA+ subsets, the cell numbers within the two gates were very similar, indicating that the number of CD25+CD127lo cells correlates strongly with the number of CD25+FoxP3+ cells in peripheral blood.
CD4+CD25+CD127lo cell numbers in peripheral blood of healthy volunteers
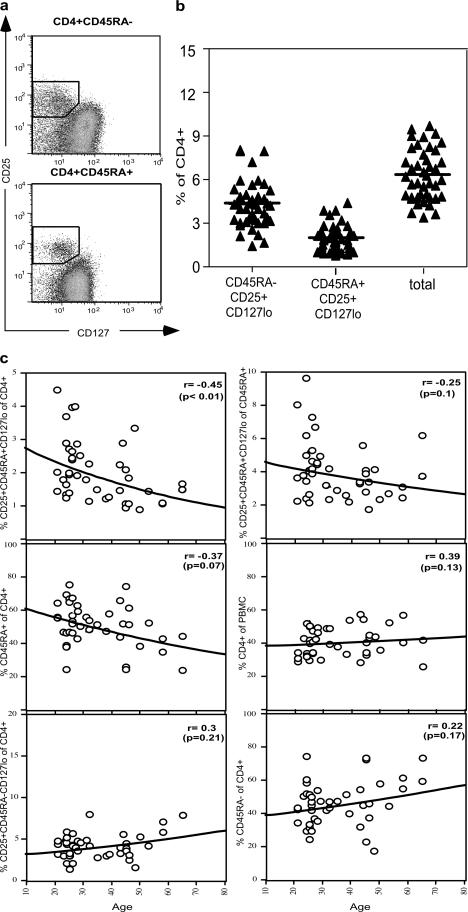
To define normal levels of circulating CD4+CD25+CD127lo cells, peripheral blood samples from a cohort of 43 healthy volunteers were examined (Fig. 3). The mean number (± SEM) of CD45RA−CD25+CD127lo cells as a percentage of CD4+ T cells was 4.29 ± 0.24, while the percentage of CD45RA+CD25+CD127lo cells was 2.05 ± 0.14, giving a total of 6.35 ± 0.26% of CD4+ T cells (Fig. 3 b). This was consistent with our figure of 6.42 ± 0.50% of CD4+ T cells in murine blood (unpublished data), and contrasts with the conventional estimate of 1–2% in human peripheral blood (16). Moreover the ratio of effector/memory to naive T reg cell (Fig. 3 a) was similar to the 2:1 ratio of effector to naive T reg cells that we have previously determined for mice (unpublished data). We (17) and others (23) have previously shown using CD4/CD25/CD45RA staining that the number of naive T reg cells in peripheral blood declines as a function of age, suggestive of an effect of thymic involution. This trend was confirmed with the new staining strategy (Fig. 3 b) and was only partially attributable to the well-described loss of CD45RA+ T cells with age. In contrast, the percentage of CD45RA− T reg cells was stable throughout life, as was the percentage of CD4+ T cells within peripheral blood leukocytes.
Figure 3.
Percentages of CD4+CD25+CD127lo cells in peripheral blood from 43 healthy volunteers. (a) Gating strategy for CD4+ cells subdivided into CD45RA− and CD45RA+ subpopulations. Boxes indicate the placement of the analysis gates for each cell population. (b) CD45RA− and CD45RA+CD25+CD127lo cells, expressed as a percentage of total CD4+ T cells. Total T reg cell percentages were derived by adding together the values for CD45RA− and CD45RA+ T reg cell subsets. Horizontal bars represent the group means. (c) Relationship between various CD4+ T cell subpopulations and age.
Measurement of mRNA for transcription factors in CD4+ T cell subsets sorted on the basis of CD127 and CD25 expression
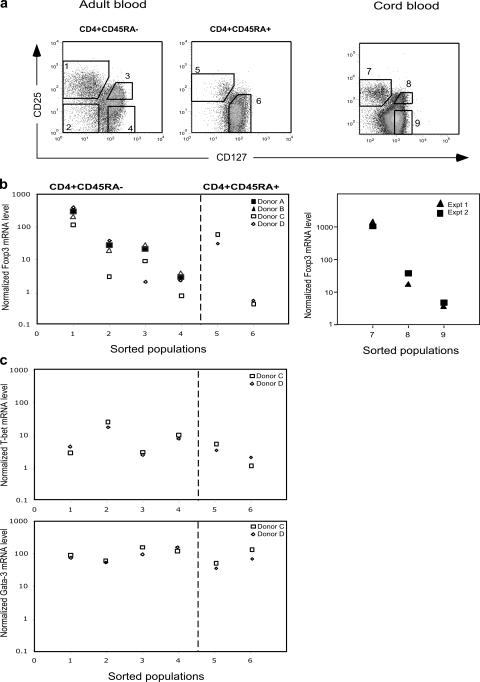
It has been reported that the level of FoxP3 protein does not always correlate with the mRNA level (22). We therefore measured the level of Foxp3 mRNA within sorted subsets of peripheral blood CD4+ T cells (Fig. 4 a). CD25+CD45RA−CD127lo cells (population 1) expressed 100-fold more Foxp3 mRNA than CD25−CD45RA−CD127hi cells (population 4, Fig. 4 b). Intermediate levels of Foxp3 mRNA were present in CD25+CD45RA−CD127hi cells (population 3) and CD25−CD45RA−CD127lo cells (population 2), as previously shown for CD25intCD45RA− cells (17). Population 2 expressed the highest levels of mRNA for T-bet, a master regulator of Th1 effector function, whereas GATA3 (a master regulator of Th2 function) was expressed equally by all populations (Fig. 4 c). These results confirm that population 2 contains CD127lo effector cells. Within the CD45RA+ fraction, CD25+CD127lo cells expressed 100-fold more Foxp3 than naive CD25−CD127hi cells (Fig. 4 b), as previously shown for CD25+CD45RA+ cells (17).
Figure 4.
Quantitative analysis of Foxp3 mRNA expression in sorted populations of CD4+ T cells. (a) Sorting strategy for isolation of subsets of CD4+ T cells from adult and cord blood. Dot plots are gated for lymphocytes expressing CD4, together with CD45RA in the case of adult blood. Numbered boxes indicate the placement of the flow sorting gates for each cell population. (b) RT qPCR for Foxp3 was performed in duplicate using RNA prepared from sorted cell populations. Sorted CD45RA− cells from four donors were compared, whereas sufficient CD45RA+ cells were available from only two donors. (c) RT qPCR for T-bet and GATA3 using RNA prepared from sorted cell populations from two adult donors.
In cord blood, CD25+CD127lo cells expressed 500-fold more Foxp3 mRNA than the corresponding naive CD4+CD25− cells (Fig. 4 b, right), consistent with our previously published results (17). The CD25intCD127hi population (population 8) of antigen-experienced T cells expressed an intermediate level of Foxp3, as demonstrated for the corresponding adult population (population 3, Fig. 4 b).
In vitro suppression by subsets sorted on the basis of CD127 staining
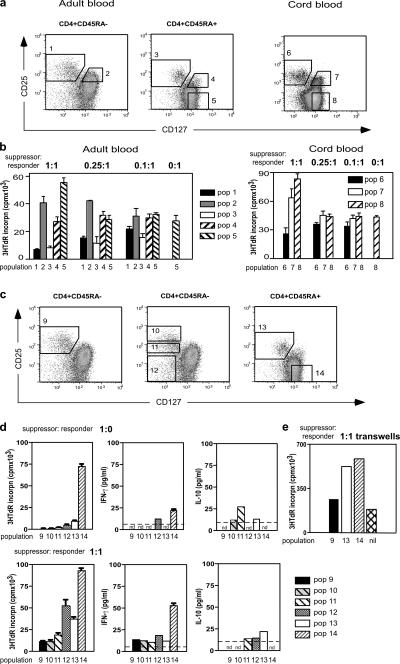
Adult blood CD4+ T cells divided into CD45RA+ and CD45RA− subsets were sorted according to the gates illustrated in Fig. 5 a (left). Autologous sorted CD45RA+CD25− cells (population 5) were used as responder cells in cocultures to measure suppressive activity. Assays using either thymidine (Fig. 5 b) or CFSE (not depicted) as the indicator of cell proliferation showed that only the CD25+CD127lo T cells within each CD45 subset (populations 1 and 3, Fig. 5 b) mediated in vitro suppression. CD45RA+ T reg cells were as potent as their CD45RA− counterparts, in agreement with recently published studies (17, 23). For cord blood assays, CD45 isoform expression was not used to subdivide cells, as the vast majority of cord blood cells express CD45RA to some extent. CD25+CD127lo and CD25+CD127hi subsets sorted according to the gates in Fig. 3 a (right) were cocultured with autologous responder CD4+CD25−CD127hi cells (population 8). Once again, both thymidine (Fig. 5 b) and CFSE assays (not depicted) indicated that the suppressive activity of CD4+CD25+ cells was confined to the CD127lo subset (population 6, Fig. 5 b).
Figure 5.
Suppression of in vitro proliferation by T reg cells from adult and cord blood. (a) Sorting strategy for isolation of subsets of CD4+ T cells. Dot plots are gated for lymphocytes expressing CD4, together with CD45RA in the case of adult blood. Numbered boxes indicate the placement of the flow sorting gates for each cell population. (b) Suppression by flow sorted populations (nos. 1–5) from adult blood and populations (nos. 6–8) from cord blood. Responder cells were sorted autologous CD4+CD45RA+CD25− cells (population 5) for adult blood and autologous CD4+CD25− cells (population 8) for cord blood. Ratios of suppressor to responder cells are shown above the figure. Bars represent the mean ± SEM of three to four replicate cultures. Assays of adult blood are representative of two independent experiments and the cord blood data are derived from a single experiment. (c) Strategy for isolation of subsets of CD4+CD127lo T cells from adult blood, sorted on the basis of CD25 expression. (d) Suppression and cytokine production by flow sorted populations (nos. 9–14) from adult blood. Responder cells were sorted autologous CD4+CD45RA+CD25− cells (population 14). Limit of detection in the cytokine assays is indicated by the dotted line. nd, not detected. (f) Transwell cultures of flow sorted populations (nos. 9, 13–14, and nil) at a 1:1 ratio.
Previous reports have indicated that CD25bright but not CD25int cells have suppressive activity (16). However, in those studies the majority of cells in the CD25int gate would have been CD45RA−CD127hi conventional T cells (population 2, Fig. 5, a and b), compromising the efficiency of suppression in the assay. To compare the suppressive activity of CD45RA−CD127lo cells expressing different levels of CD25, adult blood CD4+ T cells divided into CD45RA+ and CD45RA− subsets were sorted according to the gates illustrated in Fig. 5 c. All three CD45RA−CD25+CD127lo populations (populations 9–11) manifested suppressive activity (Fig. 5 d, bottom left), consistent with their high level of FoxP3 expression (Fig. 2). In addition, all three populations suppressed IFNγ production by responder cells, and populations 10 and 11 secreted a small amount of IL-10. No secretion of IL-4 or IL-5 was detected in any cultures (unpublished data). Interestingly, CD45RA−CD25−CD127lo cells (population 12) showed some suppression of proliferation and IFNγ production, and secreted a detectable level of IL-10, although they do not express FoxP3 protein (Figs. 1 and 2).
To test whether cell surface interaction between T reg cell and responder cells was required for suppression by CD25+CD127lo cells, transwell cultures were performed (Fig. 5 e). No suppression was seen when cell–cell contact between suppressor and responder cells was prevented, ruling out a role for soluble factors such as IL-10 in suppression by CD25+CD127lo cells. Indeed, in the transwell cultures, the proliferation of responder cells was augmented when compared with the control cultures lacking suppressor cells (Fig. 5 e).
Collectively, these results indicate that suppressive activity was restricted to CD25+CD127lo cells in both cord and adult blood. In contrast, markers such as HLA-DR, which split CD4+CD25+ T cells into two populations, distinguish T reg cell subsets with different spectra of activity in vitro (24). A small proportion (<10%) of CD25+FoxP3+ cells retained high expression of CD127 (Fig. 2). Whether these cells have suppressive function remains unknown because they cannot currently be purified for testing in vitro. Nonetheless the population of CD25+CD127hi cells as a whole does not manifest suppressive activity in standard in vitro assays (Fig. 5). These data are therefore consistent with several recent studies indicating that expression of FoxP3 does not always confer obligatory suppressive function on human T cells (25, 26).
Conclusions
We have shown that human peripheral CD4+CD25+ T reg cells can be accurately identified and purified using surface expression of CD127, as an alternative to the transcription factor FoxP3. Murine T reg cells have also been reported to express low levels of CD127, but the decrease in its expression is insufficient to allow accurate flow-based separation from other CD4+ T cells (27, 28). In a recent publication, differential CD127 expression was demonstrated for CD4+CD25+ T reg cell and naive CD4+CD25− T cells in human fetal lymph node samples (29). However, this report is, to our knowledge, the first demonstration that antigen-experienced CD45RA− (CD45RO+) conventional CD4+ T cells, the major contaminant of CD25+CD4+ T reg cells as normally identified, can be excluded from both neonatal and adult blood and lymph node T reg cell populations in a flow-based strategy using anti-CD127 mAbs.
MATERIALS AND METHODS
Samples.
Peripheral blood was obtained from healthy adult donors (Centenary Institute and Rush University Medical Center). Buffy coats were obtained from the Australian Red Cross Blood Service. Cord blood samples from Nepean Hospital, Australia, were obtained from umbilical cord veins immediately after delivery of the placenta. The neonates were full-term and had no hematologic abnormalities or infectious complications. Normal thymus specimens were from children aged 1–7 mo undergoing corrective cardiac surgery at the Children's Hospital (Australia). Lymph nodes were obtained from patients undergoing colectomy for incontinence. The study was performed with the approval of the Central and Western Sydney Area Health Services Ethics Committees, the Royal Alexandra Hospital for Children Ethics Committee, and the Rush Institutional Review Board. Informed consent was provided in accordance with the declaration of Helsinki.
Mononuclear cell preparations.
Peripheral blood, buffy coat, cord blood, thymus, and lymph node mononuclear cells were prepared as described previously (17).
Antibodies and flow cytometry.
Anti-CD4, anti-CD25, and anti-CD45RO mAbs (clones OKT4, 7GB6 and UCHTL-1, respectively) were labeled with Alexa488 (Invitrogen) and FITC (Sigma-Aldrich) by standard protocols. Additional monoclonal antibodies used in this study were as follows: anti-CD3, -CD4, -CD8, -CD45RA, -CD45RO (BD Biosciences); -CD25 (BD Biosciences); -CD127 (Immunotech); and -Foxp3 (eBioscience). Abs were conjugated with biotin, Alexa488, FITC, PE, PerCpCy5.5, Pacific blue, PECy7, or PECy5.5. Biotin conjugates were developed with streptavidin conjugated with Alexa594 (Invitrogen) or PerCP (BD Biosciences).
Staining for flow cytometry was performed as previously described (17). A total of 105 events, gated for lymphocytes on the basis of forward and side scatter profiles, were collected using a FACSCalibur, FACSVantage, FACSAria, or LSRII (Becton Dickinson). Analysis was performed using the FlowJo program (Treestar). Sorting was performed on a FACSVantage or FACSAria cell sorter.
Real-time qPCR.
Real-time qPCR for Foxp3, T-bet, and GATA-3 was performed as described previously (17). Relative expression was determined by normalization to β-actin.
In vitro suppression assays.
In vitro suppression assays were performed as previously described (17). The number of putative suppressor cells added to each well was either 2 × 104, 0.5 × 104, 2 × 103, or zero, giving final suppressor to responder ratios of 1:1, 0.25:1, 0.1:1, or 0:1, respectively. After 72 h of culture, 100 μl of supernatant was removed from each well for cytokine assays, before pulsing with tritiated thymidine for 16 h before harvesting. CFSE assays were performed in parallel using labeled responder cells harvested after 72 h of culture. Cytokines (IFN-γ, IL-4, IL- 5, IL-10) were measured using OptEIA kits (BD Biosciences) according to the manufacturer's instructions. Transwell assays were performed in 24-well plates as described previously (16).
Statistical analysis.
Statistical analyses were performed using Prism 4.0 (GraphicPad) or Cricket Graph III (Computer Associates International) software. Parametric statistical analysis (mean and SEM, linear and exponential regression) was performed using standard methods. Significance of correlation coefficients (nonparametric) was calculated using the Spearman test. For all tests, p-values <0.05 were considered significant.
Acknowledgments
The authors would like to thank Prof. A. Basten for his helpful comments on the manuscript, S.-Y. Tan and C. Higgins for providing access to murine data, and A. Smith and C. Brownlee of the Centenary Institute Flow Cytometry Facility for their expert cell sorting.
This project was supported by Program and Project Grant funding from the Australian National Health and Medical Research Council, a Senior Research Award from the Crohn's and Colitis Foundation of America, and National Institutes of Health grant no. AI 55793. The National Centre in HIV Epidemiology and Clinical Research is supported by the Commonwealth Department of Health and Aging through the Australian National Council on AIDS, Hepatitis C, and Related Diseases. B. Fazekas de St. Groth is an NHMRC Principal Research Fellow.
The authors have no conflicting financial interests.
References
- 1.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 2.Kriegel, M.A., T. Lohmann, C. Gabler, N. Blank, J.R. Kalden, and H.M. Lorenz. 2004. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J. Exp. Med. 199:1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispin, J.C., A. Martinez, and J. Alcocer-Varela. 2003. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J. Autoimmun. 21:273–276. [DOI] [PubMed] [Google Scholar]
- 4.Cao, D., R. van Vollenhoven, L. Klareskog, C. Trollmo, and V. Malmstrom. 2004. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res. Ther. 6:R335–R346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenstein, M.R., J.G. Evans, A. Singh, S. Moore, G. Warnes, D.A. Isenberg, and C. Mauri. 2004. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiyama, H., R. Gyulai, E. Toichi, E. Garaczi, S. Shimada, S.R. Stevens, T.S. McCormick, and K.D. Cooper. 2005. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 174:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viglietta, V., C. Baecher-Allan, H.L. Weiner, and D.A. Hafler. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuno, K., T. Yuge, K. Kusuhara, H. Takada, H. Nishio, V. Khajoee, T. Ohno, and T. Hara. 2004. CD25+CD4+ regulatory T cells in patients with Kawasaki disease. J. Pediatr. 145:385–390. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson, M.R., J. Rugtveit, and P. Brandtzaeg. 2004. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J. Exp. Med. 199:1679–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling, E.M., T. Smith, X.D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V.A. Carr, and D.S. Robinson. 2004. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363:608–615. [DOI] [PubMed] [Google Scholar]
- 11.Ochs, H.D., S.F. Ziegler, and T.R. Torgerson. 2005. FOXP3 acts as a rheostat of the immune response. Immunol. Rev. 203:156–164. [DOI] [PubMed] [Google Scholar]
- 12.Kukreja, A., G. Cost, J. Marker, C. Zhang, Z. Sun, K. Lin-Su, S. Ten, M. Sanz, M. Exley, B. Wilson, et al. 2002. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 109:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, Y.M., R. Pirskanen, R. Giscombe, H. Link, and A.K. Lefvert. 2004. Circulating CD4+CD25+ and CD4+CD25− T cells in myasthenia gravis and in relation to thymectomy. Scand. J. Immunol. 59:408–414. [DOI] [PubMed] [Google Scholar]
- 14.Putheti, P., A. Pettersson, M. Soderstrom, H. Link, and Y.M. Huang. 2004. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J. Clin. Immunol. 24:155–161. [DOI] [PubMed] [Google Scholar]
- 15.van Amelsfort, J.M., K.M. Jacobs, J.W. Bijlsma, F.P. Lafeber, and L.S. Taams. 2004. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 50:2775–2785. [DOI] [PubMed] [Google Scholar]
- 16.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 17.Seddiki, N., B. Santner-Nanan, S.G. Tangye, S.I. Alexander, M. Solomon, S. Lee, R. Nanan, and B. Fazekas de St Groth. 2006. Persistence of naïve CD45RA+ regulatory T cells in adult life. Blood. 107:2830–2838. [DOI] [PubMed] [Google Scholar]
- 18.Roncador, G., P.J. Brown, L. Maestre, S. Hue, J.L. Martinez-Torrecuadrada, K.L. Ling, S. Pratap, C. Toms, B.C. Fox, V. Cerundolo, et al. 2005. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 35:1681–1691. [DOI] [PubMed] [Google Scholar]
- 19.Ruprecht, C.R., M. Gattorno, F. Ferlito, A. Gregorio, A. Martini, A. Lanzavecchia, and F. Sallusto. 2005. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 201:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A.C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 75:253–261. [DOI] [PubMed] [Google Scholar]
- 21.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey, W.R., D.J. Spoden, Y.G. Ge, S.R. Baker, B. Liu, B.L. Levine, C.H. June, B.R. Blazar, and S.B. Porter. 2005. Cord blood CD4+CD25+-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 105:750–758. [DOI] [PubMed] [Google Scholar]
- 23.Valmori, D., A. Merlo, N. Souleimania, C. Hesdorffer, and M. Ayyoub. 2005. A peripheral circulating compartment of natural naive CD4 Tregs. J. Clin. Invest. 115:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baecher-Allan, C., E. Wolf, and D.A. Hafler. 2006. MHC class II expression identifies functionally distinct human regulatory T cells. J. Immunol. 176:4622–4631. [DOI] [PubMed] [Google Scholar]
- 25.Morgan, M.E., J.H. van Bilsen, A.M. Bakker, B. Heemskerk, M.W. Schilham, F.C. Hartgers, B.G. Elferink, L. van der Zanden, R.R. de Vries, T.W. Huizinga, et al. 2005. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol. 66:13–20. [DOI] [PubMed] [Google Scholar]
- 26.Allan, S.E., L. Passerini, R. Bacchetta, N. Crellin, M. Dai, P.C. Orban, S.F. Ziegler, M.G. Roncarolo, and M.K. Levings. 2005. The role of 2 FOXP3 isoforms in the generation of human CD4 Tregs. J. Clin. Invest. 115:3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavin, M.A., S.R. Clarke, E. Negrou, A. Gallegos, and A. Rudensky. 2002. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 3:33–41. [DOI] [PubMed] [Google Scholar]
- 28.Cozzo, C., J. Larkin III, and A.J. Caton. 2003. Self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J. Immunol. 171:5678–5682. [DOI] [PubMed] [Google Scholar]
- 29.Cupedo, T., M. Nagasawa, K. Weijer, B. Blom, and H. Spits. 2005. Development and activation of regulatory T cells in the human fetus. Eur. J. Immunol. 35:383–390. [DOI] [PubMed] [Google Scholar]