Abstract
Immunoglobulin (Ig)α and Igβ initiate B cell receptor (BCR) signaling through immune receptor tyrosine activation motifs (ITAMs) that are targets of SH2 domain–containing kinases. To examine the function of Igβ ITAM tyrosine resides in mature B cells in vivo, we exchanged these residues for alanine by gene targeting (IgβAA). Mutant mice showed normal development of all B cell subtypes with the exception of B1 cells that were reduced by fivefold. However, primary B cells purified from IgβAA mice showed significantly decreased steady-state and ligand-mediated BCR internalization and higher levels of cell surface IgM and IgD. BCR cross-linking resulted in decreased Src and Syk activation but paradoxically enhanced and prolonged BCR signaling, as measured by cellular tyrosine phosphorylation, Ca++ flux, AKT, and ERK activation. In addition, B cells with the ITAM mutant receptor showed an enhanced response to a T-independent antigen. Thus, Igβ ITAM tyrosines help set BCR signaling threshold by regulating receptor internalization.
B lymphocyte survival, development, and function are dependent on signals produced by the B cell receptor (BCR), which comprises membrane-bound Ig and a dimer of Ig superfamily signal transducers Igα and Igβ (CD79a and CD79b) (1–6). Although neither Igα nor Igβ has enzymatic function, transfection and transgenic mouse experiments showed that the cytoplasmic domain of either Igα or Igβ was sufficient to initiate BCR signaling (7–13). Gene targeting revealed that the cytoplasmic domains of either Igα or Igβ were not absolutely required for early stages of B cell development, but a fully intact BCR was essential for complete B cell maturation (14–16) and survival in vivo (17).
The Igα-Igβ dimer is noncovalently associated with membrane Ig through polar residues in the transmembrane domain of Ig (10, 11, 18–21), and it initiates BCR signaling through immune receptor tyrosine activation motifs (ITAMs) (22). Tyrosine residues imbedded in the ITAMs serve as substrates for Src and Syk kinases and as a platform for recruiting and organizing other activated SH2 domain–containing tyrosine kinases (3–6). Syk has two SH2 domains, both of which must be engaged by the BCR for efficient activation (23). Once activated, Syk binds cooperatively to the ITAMs of Igα and Igβ and phosphorylates downstream adaptors and kinases triggering a cascade that leads to nuclear effectors (3–6). Syk is an essential kinase in the BCR pathway. In the absence of Syk, there is no BCR signaling in DT40 cells, and Syk−/− mouse B cells fail to develop beyond the pro–B cell stage (24–26).
Despite the importance of the Igα and Igβ cytoplasmic domains in initiating BCR signaling and Syk recruitment, loss of either produced hyperresponsive IgHEL transgenic B cells, suggesting an unexpected negative regulatory function for Igα and Igβ (16, 27, 28). Experiments with Igα ITAM mutant B cells (IgαFF) demonstrated that this unexpected phenomenon was mediated by Igα ITAM tyrosines, but the mechanism of negative regulation by Igα was not established (15).
Here we show that Igβ ITAM tyrosines modulate ligand-induced signaling by regulating BCR internalization.
RESULTS
IgβAA mice
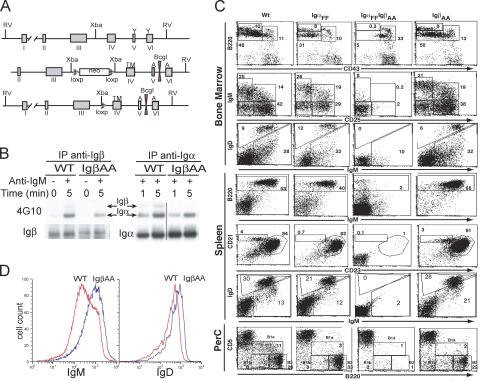
To determine the function of Igβ ITAM tyrosines in mature B cells, we replaced these residues with alanine residues by gene targeting (Fig. 1 A). Expression of the mutant protein was confirmed by immunoprecipitation and Western blotting on B cell lysates using antibodies specific for the cytoplasmic domains of Igα and Igβ (Fig. 1 B) (29, 30). Mutant Igβ was coimmunoprecipitated with Igα and vice versa (Fig. 1 B and Fig. S1 A, which is available at http://www.jem.org/cgi/content/full/jem.20060221/DC1). After BCR cross-linking, Igα was tyrosine phosphorylated in both wild-type and mutant B cells (Fig. 1 B). In the wild-type, small amounts of phosphotyrosine were also found on Igβ in response to receptor cross-linking, but we found no phosphorylation of mutant Igβ in IgβAA B cells (Fig. 1 B and Fig. S1 B). We conclude that IgβAA B cells produce the mutant protein and that it is associated with Igα.
Figure 1.
Targeting the Igβ locus. (A) Diagram shows the endogenous Igβ locus (top), targeting construct (middle), and the targeted locus (bottom). Boxes labeled with roman numerals indicate exons, and the transmembrane domain (TM), diagnostic BcgI site, and position of alanine (A) substitutions are shown. (B) Purified splenic B cells from wild-type and IgβAA mice were stimulated with anti-IgM, and extracts were immunoprecipitated with anti-Igα or anti-Igβ and blotted with anti-phosphotyrosine 4G10 antibodies or anti-Igα or anti-Igβ antibodies. (C) Flow cytometry analysis of bone marrow, spleen, and peritoneal cavity B cells from wild-type, IgαFF, IgαFF/IgβAA, and IgβAA mice. Staining antibodies are indicated. Numbers show relative percentages of cells within indicated gates. (D) Histogram plots show expression of surface IgM and IgD by splenic B220+ B cells in wild-type (red) and IgβAA (blue) mice.
B cell development in IgβAA mice
We used flow cytometry to examine the effect of the IgβAA mutation on B cell development and to compare it with IgαFF, in which the ITAM tyrosine residues of Igα were mutated to phenylalanine (15). We found normal numbers of pro–B (IgM−B220lowCD25), pre–B (IgM−B220lowCD25+), immature B (IgM+B220lowIgD−/low), and recirculating B cells (IgM+B220hiIgDhi) in the bone marrow of IgβAA mice (Fig. 1 C). The only reproducible difference between developing B cells in IgβAA and wild-type mice was in the higher levels of surface IgM and IgD on immature and recirculating B cells (Fig. 1 C).
In the spleen, the number of IgβAA B cells was similar to wild-type, as was the proportion of marginal zone (CD21hiCD23lo) and follicular B cells (CD21hiCD23hi) (Fig. 1 C). This is in contrast to IgαFF mice that appear to have fewer marginal zone B cells (Fig. 1 C) (15). In the spleen, as in the bone marrow, IgβAA B cells showed higher levels of surface IgM and IgD expression than wild-type B cells, but normal CD19 levels (Fig. 1, C and D, and not depicted). Finally, in the peritoneal cavity, IgβAA mice showed a fivefold reduction in the number of B1a B cells similar to that found in IgαFF mice (Fig. 1 C) (31–36). Thus, Igβ ITAM tyrosines are essential for normal levels of cell surface BCR expression but not required for most other aspects of B cell development.
To determine whether the combined ITAMs in Igα and Igβ were required for B cell development, we crossed IgαFF and IgβAA to obtain double mutant mice. We found that B cells that express a BCR with no cytoplasmic ITAM tyrosine residues fail to develop beyond the pro–B cell stage (Fig. 1 C; IgM−B220lowCD25−). We conclude that a single intact ITAM in either Igα or Igβ is both necessary and sufficient for normal B cell development with the exception of B1a cells.
Enhanced B cell responses in vivo
IgβAA mutant mice showed a threefold increase in serum IgM levels, a fivefold decrease in IgG1, normal levels of IgG2a, IgG2b, IgG3, and IgA, and no increase in anti-DNA antibodies (Fig. 2 A and not depicted). To determine whether IgβAA B cells respond normally to antigen in vivo, we immunized mice with a T-independent antigen 4-hydroxy-3-nitrophenylacetyl (NP)-Ficoll or a T-dependent antigen NP coupled to chicken γ globulin (NP-CGG) and measured anti-NP–specific antibody responses by ELISA (Fig. 2, B–D). IgβAA mice showed a 10-fold increase in anti-NP–specific IgM, a small increase in IgG3 responses to T-independent antigen, no increase in proliferative responses to anti-IgM in vitro, and slightly decreased T-dependent antibody responses (Fig. 2, B–D, and not depicted). In contrast, there was no apparent difference in antibody responses to the same antigens in IgαFF mice (15). We conclude that IgβAA B cells express increased levels of surface IgM and are hyperresponsive to stimulation with T-independent antigen in vivo.
Figure 2.
Steady-state antibody levels and antibody responses. Individual wild-type and IgβAA mice are represented by open circles and filled triangles, respectively. Bold lines indicate mean values for each group. Plots show total serum Ig or relative binding to NP2-BSA. (A) Steady-state serum Ig concentrations (μg/ml) in age- and sex-matched wild-type and IgβAA mice. (B) IgM response to immunization with NP-Ficoll. (C) IgG3 response to immunization with NP-Ficoll. (D) IgG1 response to immunization with NP-CGG.
Decreased BCR internalization
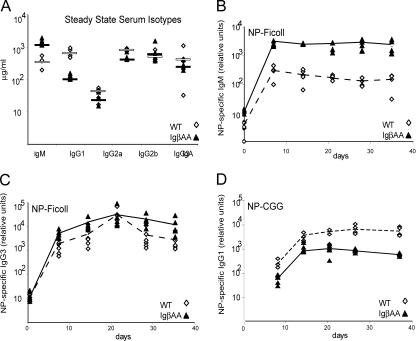
Increased BCR surface expression in IgβAA B cells was not due to increased mRNA expression (not depicted). To determine whether the increased surface levels of BCR found on IgβAA B cells was associated with altered BCR internalization, we measured spontaneous internalization (Fig. 3 A). Cell surface BCRs were labeled with monovalent biotinylated Fab′ anti-IgM to avoid receptor cross-linking, incubated at 37°C, and visualized with streptavidin. Wild-type B cells internalized 30% of their receptors in 20 min, but IgαFF and IgβAA internalized only 5–7% of their receptors during the same time (Fig. 3 A). Thus, both IgαFF and IgβAA B cells were severely impaired in constitutive BCR internalization compared with wild-type. IgβAA B cells were more impaired than IgαFF B cells (Fig. 3 A).
Figure 3.
BCR internalization. (A) Endocytosis. Plots show relative rates of BCR internalization by purified IgαFF, IgβAA, and wild-type B cells at 37°C as measured with a biotinylated Fab′ anti-IgM antibody. (B) Ligand-mediated internalization. Internalization of a biotinylated F(ab′)2 anti-IgM antibody by IgαFF, IgβAA, and wild-type B cells at 37°C. Experiments in A and B were repeated three times with similar results. (C) Electron micrographs show BCR labeling on IgβAA and wild-type B cells with 10 nm of gold-labeled anti–rabbit antibodies 10 min after cross-linking by Fab′2 anti-IgM at 37°C.
To examine BCR internalization in response to BCR cross-linking, we incubated B cells with biotinylated F(ab′)2 anti-IgM. BCR internalization in response to receptor cross-linking was both decreased and delayed in IgβAA B cells (Fig. 3, B and C). Although wild-type cells internalized 50% of the cell surface BCR in 5 min, IgβAA B cells reached this level of BCR internalization only after 20 min. In contrast, IgαFF B cells displayed a more modest decrease in receptor internalization in response to cross-linking. They internalized 50% of their receptors in 9 min (Fig. 3 B). By ultrastructural analysis, wild-type cells (n = 65 cells) internalized 65% of the gold-labeled surface-bound F(ab′)2 in 10 min, whereas only 20% of the same tracer was internalized by IgβAA B cells (n = 64 cells) at this time point (Fig. 3 C). Thus, the ITAM tyrosine residues in both Igα and Igβ are required for constitutive and ligand-mediated BCR internalization. However, the IgβAA mutation results in a more profound block in BCR internalization than the IgαFF mutation.
Enhanced Ca2+ responses
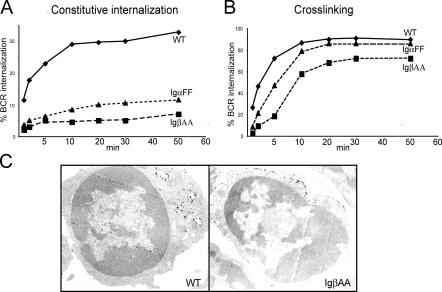
To determine whether signaling is affected by the IgβAA mutation, we initially measured Ca2+ flux responses to BCR cross-linking with F(ab′)2 anti-IgM antibodies. We found that IgβAA B cells showed increased Ca2+ flux responses to anti-IgM when compared with wild-type or IgαFF B cells at all doses of anti-IgM tested (Fig. 4 A).
Figure 4.
Ca2+ flux responses. (A) Ca2+ flux response to BCR cross-linking with 0.1, 5, and 20 μg/ml anti-IgM in splenic B cells from IgβAA (blue), IgαFF (green), and wild-type (red) B cells. Histograms show fluorescence 405/485 nm ratio of Indo-1-AM emission (y axis) in response to BCR cross-linking plotted as a function of time in seconds (x axis). (B) Ca2+ flux analysis in response to BCR stimulation in IgβAA (blue) and wild-type (red) B cells gated based on similar levels of surface IgM. Top panel shows IgM expression by B cells stained with FITC-labeled Fab′ and gating based on the level of IgM expression. Bottom panel shows histogram plots of Ca2+ flux response to BCR cross-linking with 5 μg/ml Fab′2 anti-IgM on the gated populations as indicated by arrows. The time points of anti-IgM addition are indicated by small arrows.
To determine whether the enhanced Ca2+ signaling is simply the result of increased surface IgM expression by IgβAA B cells, we compared Ca2+ responses in wild-type and mutant B cells with low (L), medium (M), and high (H) levels of IgM expression (Fig. 4 B). B cells were stained with Fab′ anti-IgM and gated based on expression of similar levels of surface IgM. Although Ca2+ flux was lower in cells expressing lower levels of surface IgM (Fig. 4 B, L), direct comparison of IgβAA and wild-type B cells expressing equivalent levels of surface IgM showed that Ca2+ flux responses were always higher in IgβAA B cells (Fig. 4 B). We conclude that IgβAA are hyperresponsive to BCR cross-linking and that this effect is not simply due to higher levels of surface BCR expression.
Tyrosine phosphorylation
BCR cross-linking leads to phosphorylation of several cellular substrates. To determine whether tyrosine phosphorylation responses were altered in IgβAA B cells, we measured total cellular tyrosine phosphorylation (Fig. 5 A). Consistent with increased Ca2+ flux responses, we found increased and prolonged general cellular tyrosine phosphorylation in IgβAA B cells compared with wild-type control (Fig. 5 A).
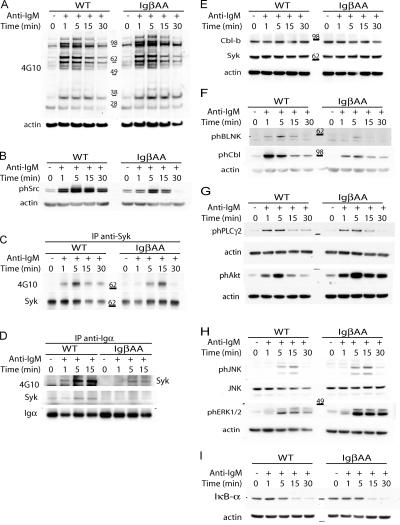
Figure 5.
Signaling responses. IgβAA or wild-type splenic B cells were stimulated with 20 μg/ml F(ab′)2 anti-IgM at 37°C for the indicated times. Anti-actin, anti-Iga, anti-Syk, and anti-JNK antibodies were used for loading controls as indicated. (A) Tyrosine phosphorylation of total cellular proteins analyzed by immunoblotting with 4G10 antibody. (B) Src kinase phosphorylation in total cellular lysates measured by immunoblotting with anti–phospho-src–specific antibodies. (C) Syk tyrosine phosphorylation analyzed by immunoblotting of anti-Syk immunoprecipitates with 4G10. (D) Association of Syk with the BCR and its phosphorylation. Anti-Igα immunoprecipitates were immunoblotted with anti-Syk or 4G10 antibody. (E) Cbl-b and Syk levels in total cellular lysates were measured by immunoblotting with anti–Cbl-b and anti-Syk antibodies. (F) Phosphorylation of BLNK and c-Cbl in total cellular lysates measured by immunoblotting with anti–phospho-Cbl or anti–phospho-BLNK antibodies. (G) PLC-γ2 and Akt phosphorylation in total cellular lysates measured by immunoblotting with anti–phospho-Akt or anti–phospho-PLC-γ2 antibodies. (H) JNK and ERK phosphorylation in total cellular lysates measured by immunoblotting with anti–phospho-JNK and anti–phospho-ERK. (I) NF-κB activation was measured by degradation of the IkB-α subunit in total cell extracts using anti–IkB-α antibodies. All experiments were repeated three times with similar results.
BCR phosphorylation is mediated by Src and Syk family kinases. To determine whether Src and Syk activation was altered in IgβAA B cells, we measured their phosphorylation in responses to BCR cross-linking by Western blotting with phospho-Src–specific antibodies (Fig. 5 B) and by immunoprecipitation with anti-Syk (Fig. 5 C). In contrast to increased Ca2+ flux and general tyrosine phosphorylation responses, we found a decrease in Src and Syk activation in IgβAA B cells (Fig. 5, B and C). In addition, Syk phosphorylation was delayed (Fig. 5 C).
Syk becomes associated with phosphorylated Igα-Igβ ITAM tyrosines after BCR cross-linking by a mechanism that requires cooperative binding of both of its SH2 domains (23, 37). To determine whether this association was altered in IgβAA B cells, we immunoprecipitated BCR with anti-Igα and immunoblotted it with 4G10 and anti-Syk antibodies. Although Iga phosphorylation was marginally reduced (Fig. 1 B and Fig. S1 B), we found decreased association of phosphorylated Syk with the BCR in IgβAA B cells after receptor cross-linking when compared with wild-type controls despite equivalent levels of cellular Syk expression in wild-type and IgβAA B cells (Fig. 5, D and E). BLNK and c-Cbl are central adaptor proteins that are directly downstream of Syk. Consistent with decreased Syk activation, we found decreased BLNK and c-Cbl phosphorylation in IgβAA B cells after BCR cross-linking but normal levels of BLNK and c-Cbl expression (Fig. 5 F and not depicted). We conclude that phosphorylation of BCR proximal kinases is impaired but overall cellular tyrosine phosphorylation is enhanced and prolonged in IgβAA B cells.
Phospholipase C-γ2 (PLC-γ2) amplifies BCR signals by producing second messengers, diacylglycerol and inositoltriphosphate. To determine whether PLC-γ2 activation was altered in IgβAA B cells, we measured its phosphorylation by immunoblotting with phospho-specific antibodies (Fig. 5 G). Despite the reduced Syk activity and increased Ca2+ flux responses, we found no difference in the level of PLC-γ2 phosphorylation in the mutant B cells. PI3K is another essential kinase that amplifies BCR signaling by producing phosphatidylinositol-(3,4,5)-triphosphate. Phosphatidylinositol- (3,4,5)-triphosphate amplifies signaling by recruiting PH domain–containing proteins to the plasma membrane, including serine/threonine kinase Akt. Although we were unable to detect significant changes in p85 phosphorylation by Western blotting, we found that activation of Akt was dramatically increased and prolonged in IgβAA B cells in response to BCR cross-linking (Fig. 5 G and Fig. S2, which is available at http://www.jem.org/cgi/content/full/jem.20060221/DC1). Therefore, Igβ ITAM mutation enhances Akt activation in response to BCR signaling.
Mitogen-activated protein kinases (MAPKs) and NF-κB are nuclear effectors of BCR signaling. To determine whether MAPK and activation NF-κB were altered in IgβAA B cells, we measured phosphorylation of MAPK and NF-κB as well as degradation of NF-κB by Western blotting. We found that ERK phosphorylation was enhanced and prolonged, but p38, JNK, and IκBα phosphorylation as well as NF-κB degradation were not altered (Fig. 5, H and I, and not depicted). We conclude that there is a discrepancy between proximal and distal signaling responses in IgβAA B cells. Proximal signaling, as exemplified by Syk activation, is impaired, whereas phosphorylation of distal effectors, such as Akt and ERK, is enhanced by the same mutation.
Negative regulators of BCR signaling
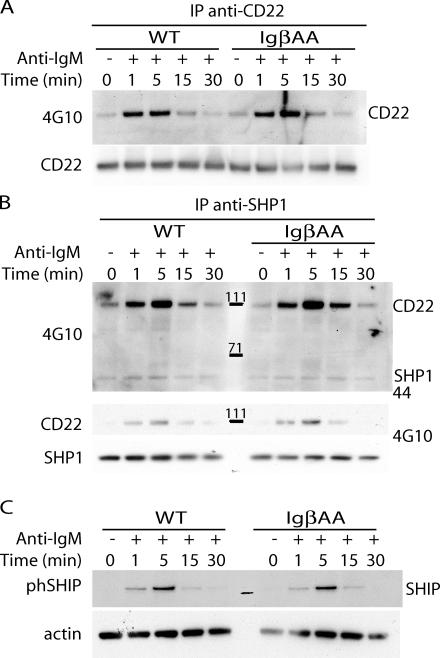
In addition to activators, BCR signaling is also controlled by several negative regulators, including CD72, CD45, CD5, FcγRIIb, and CD22 (38). We found no differences in the expression levels of the constitutive negative regulators CD72, CD45, and CD5 by flow cytometry (not depicted). FcγRIIb was ruled out because all of the signaling experiments were performed using F(ab′)2 antibodies and because BCR cross-linking with intact and Fab′2 antibody had similar effects on IgβAA B cells. CD22 becomes phosphorylated by the Lyn kinase in response to BCR cross-linking (39–42) and terminates BCR signaling by recruiting SH2-containing tyrosine phosphatase 1 (SHP-1) and SH2-containing inositol polyphosphate 5-phosphatase (SHIP) (43–45). We found normal levels of CD22 and SHP-1 phosphorylation after BCR cross-linking in IgβAA B cells (Fig. 6, A and B). In addition, association between SHP-1 and phosphorylated CD22 was unchanged in IgβAA B cells as measured by coimmunoprecipitation (Fig. 6 B). Finally, SHIP phosphorylation was normal in response to BCR cross-linking in IgβAA B cells (Fig. 6 C). Thus, neither CD22 nor SHIP phosphorylation nor absence of SHP-1 recruitment can account for prolonged and enhanced BCR signaling in IgβAA B cells.
Figure 6.
Negative regulators. IgβAA or wild-type splenic B cells were stimulated with 20 μg/ml F(ab′)2 anti-IgM at 37°C for the indicated times. (A) CD22 tyrosine phosphorylation analyzed by immunoblotting of anti-CD22 immunoprecipitates with 4G10. Anti-CD22 is shown as a loading control. (B) Recruitment of SHP-1 to CD22. Anti–SHP-1 immunoprecipitates were immunoblotted with 4G10 or anti-CD22 antibodies. The positions of SHP-1 and CD22 are indicated. Anti–SHP-1 immunoblot is shown as a loading control. (C) SHIP tyrosine phosphorylation analyzed in cellular lysates by immunoblotting with anti–phospho-SHIP antibodies. Anti-actin is shown as a loading control.
BCR internalization decreases signaling
Others have shown that BCR internalization can regulate the magnitude and duration of signaling as measured by total tyrosine phosphorylation and ERK activation in B cell lines (46, 47). To determine whether blocking BCR internalization enhances signaling in primary B cells, we partially blocked BCR internalization by inhibiting actin polymerization with cytochalasin D (Fig. S3 A, available at http://www.jem.org/cgi/content/full/jem.20060221/DC1). Signaling was measured by immunoblotting with anti-phospho ERK antibodies after receptor cross-linking. We found that 10 μM cytochalasin D inhibited ligand-mediated BCR internalization and enhanced ERK phoshorylation, though to a lesser extent than IgβAA mutation (Fig. S3 B). Thus, our findings are consistent with the idea that normal termination of BCR signal transduction appears to require BCR internalization (46, 47).
Activation-induced ubiquitylation
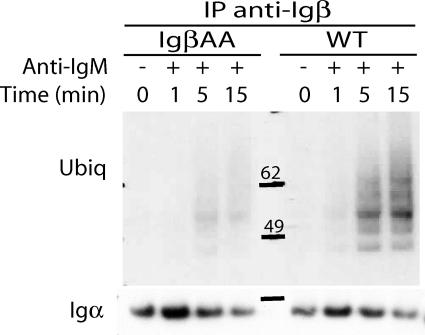
Ubiquitylation regulates the internalization of many cell surface receptors, including the TCR (for review see references 48 and 49). To determine whether BCR ligation results in its ubiquitylation and whether Igβ regulates ubiquitylation, we performed Western blotting experiments with anti-ubiquitin antibodies. Wild-type and IgβAA B cells were stimulated with F(ab′)2 anti-IgM antibodies, and ubiquitylation was measured on BCRs purified by immunoprecipitation with anti-Igβ or anti-Igα. We found inducible BCR ubiquitylation, and consistent with decreased c-Cbl phosphorylation and reduced IgβAA BCR internalization, we found decreased ubiquitylation of Igα or Igβ precipitates from IgβAA B cells (Fig. 7 and not depicted). We conclude that BCR-associated proteins are ubiquitilated upon receptor ligation and that the Igβ ITAM tyrosines are required for normal BCR ubiquitylation.
Figure 7.
BCR ubiquitylation upon receptor ligation. Wild-type and IgβAA splenic B cells were stimulated with F(ab′)2 anti-IgM at 37°C for the indicated times, and extracts were immunoprecipiated with anti-Igβ antibodies. Ubiquitylation was analyzed by immunoblotting with anti-ubiquitin antibodies. Igα immunoblotting was a loading control.
DISCUSSION
To evaluate how Igβ might regulate BCR signaling, we mutated Igβ ITAM tyrosine residues to alanine. We selected the alanine substitution because it destroys phosphorylation and internalization motifs, whereas phenylalanine substitution might only interfere with phosphorylation (50). In contrast to Igα or Igβ cytoplasmic domain deletion, which altered B cell maturation in the periphery (16, 27), B cell development in IgαFF and IgβAA mice was nearly indistinguishable from wild-type (15). Non-ITAM residues must account for these phenotypic differences and may do so by stabilizing or facilitating BCR signaling (51–54).
Although IgβAA B cells showed normal distribution of most cell surface antigens, they expressed higher levels of surface BCRs that were defective in steady-state and ligand-mediated endocytosis. The role of Igα and Igβ ITAM tyrosine residues in endocytosis was previously studied in cell lines using chimeric FcγRIIB receptors (55). In those experiments, Igα was able to mediate endocytosis by a mechanism that required an intact ITAM. Consistent with those observations, we found decreased ligand-mediated receptor internalization in IgαFF mice (Fig. 3) and enhanced internalization in Igαα mice whose BCRs carry two Igα cytoplasmic domains (56). In contrast to Igα, Igβ was inactive in endocytosis in transfection experiments (55). Thus, the observation that the IgβAA mutant BCR was profoundly defective in endocytosis in B cells in vivo reveals a novel and essential function for the Igβ ITAM. In addition, the finding that IgαFF is nearly as defective in steady-state endocytosis as IgβAA suggests that constitutive ITAM phosphorylation is an important regulator of BCR internalization.
The role of Igα and Igβ ITAM tyrosine residues in ligand-mediated BCR internalization was originally studied by measuring antigen presentation by B cell lines expressing chimeric Ig, Fc, and PDGF receptors by several groups (11, 55, 57–59). In all cases, both Igα and Igβ were active in ligand-mediated internalization (11, 55, 57–59). The role of the ITAM phosphorylation varied with the experimental system and was required in some (59) but not in others (56, 60). Our results with IgαFF and IgβAA mice are consistent with a requirement for phosphorylation in ligand-mediated receptor internalization in vivo. The difference in the internalization phenotype between the two mutants could be due to non-ITAM residues (51, 52) or to partial preservation of the internalization signal by the phenylalanine substitution in IgαFF (50). In addition, we show that Igβ regulates activation-induced ubiquitylation, which may control receptor levels by enhancing endocytosis or by targeting surface BCR to the degradative lysosomal compartment (48, 49).
IgβAA B cells resembled IgαFF B cells in several important ways, including the unexpected finding that both mutants showed enhanced Ca2+ and tyrosine phosphorylation responses to BCR cross-linking (15). The observation that IgαFF BCRs were hyperresponsive led to the suggestion that a major function of the Igα ITAM was to recruit negative regulators (15, 27, 28). This model predicts that Igβ ITAM mutation should produce an inactive BCR due to unopposed negative regulation by Igα. On the contrary, we found that IgβAA BCRs were also hyperactive in vitro and in vivo. IgβAA B cells produced enhanced T-independent antibody responses to NP-Ficoll. Thus, increased BCR signaling in IgαFF cannot be due to inability to recruit an Igα-specific negative regulator, but must be due to an abnormality in a pathway downstream of both Igα and Igβ ITAMs. The enhanced responses of IgβAA B cells to BCR cross-linking may account for the enhanced T-independent immune responses to NP-Ficoll and increased steady-state levels of serum Ig in these mice despite the decrease in B1 cells, which together with MZ B cells normally produce the majority of circulating antibodies in mice. Finally, decreased BCR internalization is also consistent with decreased T-dependent immune responses, which would be dependent on antigen internalization and processing for presentation to cognate T cells.
The BCR initiates signal transduction by activation of nonreceptor Src and Syk tyrosine kinases. Therefore, it was surprising to find that IgβAA B cells were hyperresponsive to BCR stimulation as measured by cellular tyrosine phosphorylation and Ca2+ flux, Akt, and ERK activation despite impaired Src and Syk family kinase activation. These kinases phosphorylate Igα and Igβ, which in turn recruit activated Syk by providing docking sites for its two SH2 domains (2–6). Our observation of decreased Syk activation and association with the IgβAA mutant BCR and decreased BLNK phosphorylation was consistent with the requirement for two functional ITAMs for maximal Syk activation (23). Thus, the IgβAA BCR is less functional than its wild-type counterpart in activating and recruiting proximal nonreceptor tyrosine kinases, but paradoxically, this leads to increased Ca2+ flux, Akt, and ERK activation, as well as total cellular tyrosine phosphorylation.
Enhanced signaling in the face of decreased Src and Syk activation could be due to decreased activation of negative regulators, such as CD45, CD72, CD5, FcR, and CD22 (38, 61). However, we found no abnormalities in any of these pathways in IgβAA B cells. CD45, CD72, and CD5 levels were all normal, and the involvement of FcγRIIb was ruled out by comparing F(ab′)2 and intact antibodies. Negative regulation by CD22 is initiated by BCR-mediated activation of lyn kinase, which phosphorylates CD22 leading to recruitment of the SHP-1 tyrosine phosphotase to the activated BCR (39–45). Although B cells deficient in any of the elements of the CD22 pathway show enhanced BCR signaling responses (38, 61), CD22 and SHP-1 phosphorylation and SHP-1 recruitment to CD22 were all normal in IgβAA B cells. In addition, activation of SHIP, which negatively regulates the BCR by interfering with accumulation of inositol phosphate second messengers, was also unaltered in IgβAA B cells (62–64). Finally, Cbl-b and c-Cbl are ubiquitin ligases that have been implicated as negative regulators of the BCR by targeting Syk for degradation by ubiquitinylation (65–67). Absence of Cbl-b in mice and overexpression of dominant-negative c-Cbl in B cell lines lead to enhanced Syk activation and signal transduction (66, 68). However, the role of Cbl-b and c-Cbl as negative regulators in B cells is debated, and they have also been implicated as positive regulators of BCR signaling in gene-targeting experiments (68, 69). Although there was decreased c-Cbl phosphorylation and a corresponding decrease in BCR-associated ubiquitylation in IgβAA B cells, we found no corresponding increase in Syk activation. Indeed, Syk phosphorylation was decreased in response to BCR ligation. Thus, we cannot attribute the increased BCR signaling in IgβAA mutant B cells to alterations in known negative regulators, but we cannot rule out effects of yet to be defined negative components.
Experiments with inhibitors of tyrosine kinases and phosphotases have implicated ligand-induced tyrosine phosphorylation in regulating BCR internalization (70, 71). How phosphorylation activates endocytosis of the BCR has not been determined, but several nonmutually exclusive endocytic pathways, including coated pits, rafts, actin, and ubiquitinylation, have been implicated. Blocking BCR endocytosis with pharmacologic agents or by deletion of the clathrin heavy chain in chicken B cell lines leads to enhanced signaling, suggesting that receptor internalization is an important mechanism for attenuating BCR signaling (46, 47). However, the mechanism by which BCR regulates its internalization was not determined. Our experiments establish a molecular link between Igβ, BCR internalization, and termination of signaling responses in vivo.
MATERIALS AND METHODS
Mice.
Igβ cytoplasmic tyrosines 195 and 206 were replaced with alanine by gene targeting. The long arm of the targeting vector was 9-kb long with a 3′ end at the SpeI site located in the third intron of Igβ (Fig. 1 A). The short arm was a 2.5-kb fragment from the SpeI site in the third intron of Igβ to the NotI site located 1.8 kb downstream of the termination codon. TAT codons at positions 195 and 206 in the cytoplasmic tail were replaced with alanines by substitution with GCT. A LoxP-flanked neomycin-resistance gene was used for positive selection, and a diphtheria toxin gene was used for negative selection (72). The targeting construct was linearized with PacI and transfected into 129/Sv embryonic stem cells. 100 embryonic stem cell clones were screened, three positive clones were injected into C57B/6 blastocysts, and two produced chimeric mice that transmitted the mutation. The mutation was confirmed by sequencing mRNAs cloned from mutant B cells (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20060221/DC1). Heterozygous IgβAA mice were backcrossed to C57BL/6 for two generations after Cre deletion in the germline before intercrossing. All mice were maintained under specific pathogen-free conditions, and experiments were performed under Rockefeller University Institutional Animal Care and Use Committee–approved protocols.
Flow cytometry.
Single cell suspensions from bone marrow, spleen, and peritoneal cavity were stained with FITC, PE, APC, and biotin-conjugated monoclonal antibodies for 20 min on ice. Biotinylated antibodies were visualized with streptavidin PerCp. The following monoclonal antibodies were used: anti-CD43, anti-IgM, anti-B220, anti-CD25, anti-IgD, anti-CD19, anti-CD5, anti-CD21, anti-HSA, and anti-CD23 (BD Biosciences). Data were collected with a FACSCalibur and analyzed using CellQuest software (Becton Dickinson).
Immunizations and ELISA.
Age- and sex-matched 8–12-wk-old mice were injected intraperitoneally with either 12.5 g NP190-Ficoll or 50 g of alum-precipitated NP21-CGG (both from Biosearch Technologies) in 300 μl PBS. To measure serum antibody levels, goat anti–mouse Ig (H+L) was used for capture and horseradish peroxidase–conjugated goat anti–mouse isotype-specific antibodies (SouthernBiotech) was used for detection. Values were calculated by comparison with mouse Ig standards (SouthernBiotech). To measure NP-specific IgM and IgG antibody levels, we used 5 μg/ml NP2BSA (Biosearch Technologies) for capture and horseradish peroxidase–conjugated goat anti–mouse IgM or anti–mouse IgG for detection (Jackson ImmunoResearch Laboratories). Serial dilutions were performed for each sample, and readings were taken within the linear range for each sample and adjusted for dilution. Results reflect relative absorbance for each sample compared with the standard control. All plates were developed using a peroxidase substrate kit (Bio-Rad Laboratories), and absorbance was measured at 415 nm.
BCR internalization.
To measure endocytosis, splenocytes were preequilibrated at 4°C in RPMI 0.5% FBS and incubated with either biotin goat anti–mouse IgM Fab′ or goat anti–mouse IgM (Fab′)2. Cells were then either fixed in 0.5% paraformaldehyde in PBS (To) or incubated at 37°C for the indicated times (Tn) before termination with 0.5% paraformaldehyde in PBS. Fixed cells were stained with PE-B220 and streptavidin Red 670 (Invitrogen), and surface BCR expression was determined by flow cytometry. Percent internalization was calculated by the formula [% sIgM(T0) − % sIgM(Tn)]/% IgM(T0) × 100 (Fig. 3 A) or [MFI sIgM(T0) − MFI sIgM(Tn)]/MFI IgM(T0) × 100 (Fig. S3 A), and the results were comparable. For electron microscopy, B cells were incubated with 10 μg/ml rabbit anti–mouse F(ab′)2 (Jackson ImmunoResearch Laboratories) for 20 min on ice, washed twice with PBS 0.5% FBS, and incubated for an additional 30 min on ice with a 1:5 dilution of 10 nm gold-labeled goat anti–rabbit IgG (GE Healthcare). Excess reagent was removed by washing cells twice with PBS 0.5% FBS. Cells were then either fixed with 2.5% glutaraldehyde for time zero or incubated for 2, 5, or 10 min at 37°C before fixation and processing for electron microscopy. 20–30 grids were examined for each time point and labeled cells were photographed.
Ca2+ flux.
5 × 106 spleen cells were incubated with 2 μM Indo-1 AM (Invitrogen) in RPMI 2% FBS for 30 min at 37°C. The cells were washed and stained with anti–B220-FITC or PE–anti-B220 and Fab′ FITC goat anti–mouse IgM at room temperature (Jackson ImmunoResearch Laboratories) to determine gating. Fluorescence ratios of Indo-1 emission at 405/485 nm were measured on cells gated based on B220 and IgM expression by flow cytometry on a FACSVantage SE (Becton Dickinson). Data were acquired for 30 s without stimulation, and Ca2+ flux was induced by the addition of μ chain-specific F(ab′)2 goat anti–mouse IgM (Jackson ImmunoResearch Laboratories). Data were collected for 500 s and analyzed using FlowJo (TriStar) software.
Western blotting and immunoprecipitation.
B cells were purified from the spleen by negative selection with anti-CD43 magnetic beads (MACS; Miltenyi Biotec). Cells were stimulated with 20 μg/ml of F(ab′)2 fragment goat anti–mouse IgM (Jackson ImmunoResearch Laboratories) at 37°C and lysed in 50 mM Tris HCl, pH 7.7, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM EGTA, 0.1% DOC, 10% glycerol, 1 mM PMSF, 0.2 mM Na3VO4, and protease inhibitors for Western, or 50 mM Tris HCl, pH 7.7, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.1% DOC, 1 mM PMSF, and 0.2 mM Na3VO4 for immunoprecipitation. Lysates were incubated for 15 min on ice, and cellular debris was sedimented at 14,000 rpm for 15 min at 4°C. Lysates or immunoprecipitates were resolved on a 4–12% NuPage gel (Invitrogen) and transferred onto Immobilon-P membranes (Millipore). The following antibodies were used in these experiments: anti-JNK1/2, anti-p38, and phosphorylation site-specific antibodies to ERK1/2, JNK1/2, p38, BLNK, c-Cbl, Akt, PLC-γ2, Src, IkB-α (112B2), and anti–phospho-IkB-α (Cell Signaling Technology); anti-ERK1/2 antibodies (Promega); anti-Syk (N-19), anti-phBLNK, anti-ubiquitin (P4D1), anti-Cbl-b (H-121), anti-Cbl (C-15), and anti-phospho pPI 3-kinase p85α (Tyr 508; Santa Cruz Biotechnology, Inc.); anti-phophotyrosine antibody 4G10 and anti-SHIP (Upstate Biotechnology); anti-phospho SHIP (StemCell Technologies Inc.); anti-actin (Sigma-Aldrich); SHP-1 and anti-CD22 for IP (BD Biosciences); and Igα, Igβ (10), and anti-CD22 (provided by H. Wortis, Tufts University, Boston, MA).
Online supplemented material.
Fig. S1 shows Igβ expression and phosphorylation in wild-type and mutant B cells. Fig. S2 shows PI3 kinase p85 and AKT phosphorylation in wild-type and mutant B cells. Fig. S3 shows internalization and ERK phosphorylation in cytochalasin D–treated cells. Fig. S4 shows Igβ cDNA sequence for wild-type and mutant B cells. Figs. S1–S4 are available at http://www.jem.org/cgi/content/full/jem.20060221/DC1.
Supplemental Material
Acknowledgments
We thank Dr. Henry Wortis for anti-CD22 antibody, Dr. Eva Besmer for her help with the manuscript, Thomas Eisenreich for his help with mice, and Klara Velinzon for flow cytometry.
This work was supported by National Institutes of Health grant AI051573 (to M.C. Nussenzweig) and the Howard Hughes Medical Institute (to M.C. Nussenzweig).
The authors have no conflicting financial interests.
Abbreviations used: BCR, B cell receptor; CGG, chicken γ globulin; ITAM, immune receptor tyrosine activation motif; MAPK, mitogen-activated protein kinase; NP, 4-hydroxy-3-nitrophenylacetyl; PLC-γ2, phospholipase C-γ2; SHIP, SH2-containing inositol polyphosphate 5-phosphatase; SHP-1, SH2-containing tyrosine phosphatase 1.
References
- 1.Meffre, E., R. Casellas, and M.C. Nussenzweig. 2000. Antibody regulation in B cell development. Nat. Immunol. 1:379–385. [DOI] [PubMed] [Google Scholar]
- 2.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 3.Reth, M., and J. Wienands. 1997. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15:453–479. [DOI] [PubMed] [Google Scholar]
- 4.Kurosaki, T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555–592. [DOI] [PubMed] [Google Scholar]
- 5.Gauld, S.B., J.M. Dal Porto, and J.C. Cambier. 2002. B cell receptor signaling: roles in cell development and disease. Science. 296:1641–1642. [DOI] [PubMed] [Google Scholar]
- 6.Craxton, A., K.L. Otipoby, A. Jiang, and E.A. Clark. 1999. Signal transduction pathways that regulate the fate of B lymphocytes. Adv. Immunol. 73:79–152. [DOI] [PubMed] [Google Scholar]
- 7.Flaswinkel, H., and M. Reth. 1994. Dual role of the tyrosine activation motif of the Ig-a protein during signal transduction via the B cell antigen receptor. EMBO J. 13:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, K.M., G. Alber, P. Weiser, and M. Reth. 1993. Differential signaling through the Ig-alpha and Ig-beta components of the B cell antigen receptor. Eur. J. Immunol. 23:911–916. [DOI] [PubMed] [Google Scholar]
- 9.Pao, L., S.J. Famiglietti, and J.C. Cambier. 1998. Asymmetrical phosphorylation and function of the immunoreceptor tyrosine-based activation motif tyrosines in B cell antigen receptor signal transduction. J. Immunol. 160:3305–3314. [PubMed] [Google Scholar]
- 10.Sanchez, M., Z. Misulovin, A.L. Burkhardt, S. Mahajan, T. Costa, R. Franke, J.B. Bolen, and M.C. Nussenzweig. 1993. Signal transduction by immunoglobulin is mediated through Igα and Igß. J. Exp. Med. 178:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams, G.T., C.J. Peaker, K.J. Patel, and M.S. Neuberger. 1994. The alpha/beta sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping of for serine/threonine-kinase recruitment. Proc. Natl. Acad. Sci. USA. 91:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papavasiliou, F., M. Jankovic, H. Suh, and M.C. Nussenzweig. 1995. The cytoplasmic domains of Igα and Igβ can independently induce the pre–B cell transition and allelic exclusion. J. Exp. Med. 182:1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teh, Y., and M.S. Neuberger. 1997. The immunoglobulin (Ig)α and Igβ cytoplasmic domains are independently sufficient to signal B cell maturation and activation in transgenic mice. J. Exp. Med. 185:1753–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres, R.M., H. Flaswinkel, M. Reth, and K. Rajewsky. 1996. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 272:1804–1808. [DOI] [PubMed] [Google Scholar]
- 15.Kraus, M., L. Pao, A. Reichlin, Y. Hu, B. Canono, J.C. Cambier, M.C. Nussenzweig, and K. Rajewsky. 2001. Interference with immunoglobulin (Ig)α immunoreceptor tyrosine activation motif (ITAM) phosphorylation modulates or blocks B cell development, depending on the availability of an Igβ cytoplasmic tail. J. Exp. Med. 194:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichlin, A., Y. Hu, E. Meffre, H. Nagaoka, S. Gong, M. Kraus, K. Rajewsky, and M.C. Nussenzweig. 2001. B cell development is arrested at the immature B cell stage in mice carrying a mutation in the cytoplasmic domain of immunoglobulin β. J. Exp. Med. 193:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus, M., M.B. Alimzhanov, N. Rajewsky, and K. Rajewsky. 2004. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 117:787–800. [DOI] [PubMed] [Google Scholar]
- 18.Hermanson, G.G., D. Eisenberg, P.W. Kincade, and R. Wall. 1988. B29: a member of the immunoglobulin gene superfamily exclusively expressed on B-lineage cells. Proc. Natl. Acad. Sci. USA. 85:6890–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hombach, J., T. Tsubata, L. Leclercq, H. Stappert, and M. Reth. 1990. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 343:760–762. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi, N., S. Kashiwamura, M. Kimoto, P. Thalmann, and F. Melchers. 1988. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 7:3457–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw, A.C., R.N. Mitchell, Y.K. Weaver, T.J. Campos, A.K. Abbas, and P. Leder. 1990. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 63:381–392. [DOI] [PubMed] [Google Scholar]
- 22.Reth, M. 1989. Antigen receptor tail clue. Nature. 338:383–384. [PubMed] [Google Scholar]
- 23.Kurosaki, T., S.A. Johnson, L. Pai, K. Sada, H. Yamamura, and J.C. Cambier. 1995. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J. Exp. Med. 182:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takata, M., H. Sabe, A. Hata, T. Inazu, Y. Homma, T. Nukada, H. Yamamura, and T. Kurosaki. 1994. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 13:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng, A.M., B. Rowley, W. Pao, A. Hayday, J.B. Bolen, and T. Pawson. 1995. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 378:303–306. [DOI] [PubMed] [Google Scholar]
- 26.Turner, M., P.J. Mee, P.S. Costello, O. Williams, A.A. Price, L.P. Duddy, M.T. Furlong, R.L. Geahlen, and V.L. Tybulewicz. 1995. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 378:298–302. [DOI] [PubMed] [Google Scholar]
- 27.Kraus, M., K. Saijo, R.M. Torres, and K. Rajewsky. 1999. Ig-alpha cytoplasmic truncation renders immature B cells more sensitive to antigen contact. Immunity. 11:537–545. [DOI] [PubMed] [Google Scholar]
- 28.Torres, R.M., and K. Hafen. 1999. A negative regulatory role for Ig-alpha during B cell development. Immunity. 11:527–536. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt, A., T. Costa, Z. Misulovin, B. Stealy, J.B. Bolen, and M.C. Nussenzweig. 1994. Ig alpha and Ig beta are functionally homologous to the signaling proteins of the T cell receptor. Mol. Cell. Biol. 14:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa, T., R.R. Franke, M. Sanchez, Z. Misulovin, and M.C. Nussenzweig. 1992. Functional reconstitution of an immunoglobulin antigen receptor in T cells. J. Exp. Med. 175:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato, S., A.S. Miller, M. Inaoki, C.B. Bock, P.J. Jansen, M.L.K. Tang, and T.F. Tedder. 1996. CD22 is both a positive and a negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 5:551–562. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa, K., R.R. Hardy, and L.A. Herzenberg. 1986. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur. J. Immunol. 16:450–456. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, R., F.W. Alt, L. Davidson, S.H. Orkin, and W. Swat. 1995. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 374:470–473. [DOI] [PubMed] [Google Scholar]
- 34.Sidman, C.L., L.D. Shultz, R.R. Hardy, K. Hayakawa, and L.A. Herzenberg. 1986. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 232:1423–1425. [DOI] [PubMed] [Google Scholar]
- 35.Rickert, R.C., K. Rajewsky, and J. Roes. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 376:352–355. [DOI] [PubMed] [Google Scholar]
- 36.Ahearn, J.M., and D.T. Fearon. 1989. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv. Immunol. 46:183–219. [DOI] [PubMed] [Google Scholar]
- 37.Wienands, J., F. Freuler, and G. Baumann. 1995. Tyrosine-phosphorylated forms of Ig beta, CD22, TCR zeta and HOSS are major ligands for tandem SH2 domains of Syk. Int. Immunol. 7:1701–1708. [DOI] [PubMed] [Google Scholar]
- 38.Ravetch, J.V., and L.L. Lanier. 2000. Immune inhibitory receptors. Science. 290:84–89. [DOI] [PubMed] [Google Scholar]
- 39.Leprince, C., K.E. Draves, R.L. Geahlen, J.A. Ledbetter, and E.A. Clark. 1993. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc. Natl. Acad. Sci. USA. 90:3236–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulte, R.J., M.A. Campbell, W.H. Fischer, and B.M. Sefton. 1992. Tyrosine phosphorylation of CD22 during B cell activation. Science. 258:1001–1004. [DOI] [PubMed] [Google Scholar]
- 41.Chan, V.W., F. Meng, P. Soriano, A.L. DeFranco, and C.A. Lowell. 1997. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 7:69–81. [DOI] [PubMed] [Google Scholar]
- 42.Smith, K.G., D.M. Tarlinton, G.M. Doody, M.L. Hibbs, and D.T. Fearon. 1998. Inhibition of the B cell by CD22: a requirement for Lyn. J. Exp. Med. 187:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doody, G.M., L.B. Justement, C.C. Delibrias, R.J. Matthews, J. Lin, M.L. Thomas, and D.T. Fearon. 1995. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 269:242–244. [DOI] [PubMed] [Google Scholar]
- 44.Poe, J.C., M. Fujimoto, P.J. Jansen, A.S. Miller, and T.F. Tedder. 2000. CD22 forms a quaternary complex with SHIP, Grb2, and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J. Biol. Chem. 275:17420–17427. [DOI] [PubMed] [Google Scholar]
- 45.Chen, J., P.A. McLean, B.G. Neel, G. Okunade, G.E. Shull, and H.H. Wortis. 2004. CD22 attenuates calcium signaling by potentiating plasma membrane calcium-ATPase activity. Nat. Immunol. 5:651–657. [DOI] [PubMed] [Google Scholar]
- 46.Brown, B.K., and W. Song. 2001. The actin cytoskeleton is required for the trafficking of the B cell antigen receptor to the late endosomes. Traffic. 2:414–427. [DOI] [PubMed] [Google Scholar]
- 47.Stoddart, A., P. Jackson, and F. Brodsky. 2005. Plasticity of B cell receptor internalization upon conditional deletion of clathrin. Mol. Biol. Cell. 16:2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hicke, L. 1999. Gettin' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 9:107–112. [DOI] [PubMed] [Google Scholar]
- 49.Bonifacino, J.S., and A.M. Weissman. 1998. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol. 14:19–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575–625. [DOI] [PubMed] [Google Scholar]
- 51.Cassard, S., D. Choquet, W.H. Fridman, and C. Bonnerot. 1996. Regulation of ITAM signaling by specific sequences in Ig-beta B cell antigen receptor subunit. J. Biol. Chem. 271:23786–23791. [DOI] [PubMed] [Google Scholar]
- 52.Muller, R., J. Wienands, and M. Reth. 2000. The serine and threonine residues in the Ig-alpha cytoplasmic tail negatively regulate immunoreceptor tyrosine-based activation motif-mediated signal transduction. Proc. Natl. Acad. Sci. USA. 97:8451–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamanashi, Y., T. Kakiuchi, J. Mizuguchi, T. Yamamoto, and K. Toyoshima. 1991. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 251:192–194. [DOI] [PubMed] [Google Scholar]
- 54.Burg, D.L., M.T. Furlong, M.L. Harrison, and R.L. Geahlen. 1994. Interactions of Lyn with the antigen receptor during B cell activation. J. Biol. Chem. 269:28136–28142. [PubMed] [Google Scholar]
- 55.Cassard, S., J. Salamero, D. Hanau, D. Spehner, J. Davoust, W.H. Fridman, and C. Bonnerot. 1998. A tyrosine-based signal present in Ig alpha mediates B cell receptor constitutive internalization. J. Immunol. 160:1767–1773. [PubMed] [Google Scholar]
- 56.Reichlin, A., A. Gazumyan, H. Nagaoka, K.H. Kirsch, M. Kraus, K. Rajewsky, and M.C. Nussenzweig. 2004. A B cell receptor with two Igα cytoplasmic domains supports development of mature but anergic B cells. J. Exp. Med. 199:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonnerot, C., D. Lankear, D. Hanau, D. Spehner, J. Davoust, J. Salamero, and W.H. Fridman. 1995. Role of B cell receptor Igα and Igß subunits in MHC class II-restricted antigen presentation. Immunity. 3:335–347. [DOI] [PubMed] [Google Scholar]
- 58.Patel, K., and M.S. Neuberger. 1993. Antigen presentation by the B cell antigen receptor is driven by the alpha/beta sheath and occurs independently of its cytoplasmic tyrosines. Cell. 74:939–946. [DOI] [PubMed] [Google Scholar]
- 59.Siemasko, K., B.J. Eisfelder, C. Stebbins, S. Kabak, A.J. Sant, W. Song, and M.R. Clark. 1999. Ig alpha and Ig beta are required for efficient trafficking to late endosomes and to enhance antigen presentation. J. Immunol. 162:6518–6525. [PubMed] [Google Scholar]
- 60.Weiser, P., R. Muller, U. Braun, and M. Reth. 1997. Endosomal targeting by the cytoplasmic tail of membrane immunoglobulin. Science. 276:407–409. [DOI] [PubMed] [Google Scholar]
- 61.Veillette, A., S. Latour, and D. Davidson. 2002. Negative regulation of immunoreceptor signaling. Annu. Rev. Immunol. 20:669–707. [DOI] [PubMed] [Google Scholar]
- 62.Ono, M., H. Okada, S. Bolland, S. Yanagi, T. Kurosaki, and J.V. Ravetch. 1997. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 90:293–301. [DOI] [PubMed] [Google Scholar]
- 63.Bolland, S., R.N. Pearse, T. Kurosaki, and J.V. Ravetch. 1998. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 8:509–516. [DOI] [PubMed] [Google Scholar]
- 64.Okada, H., S. Bolland, A. Hashimoto, M. Kurosaki, Y. Kabuyama, M. Iino, J.V. Ravetch, and T. Kurosaki. 1998. Role of the inositol phosphatase SHIP in B cell receptor-induced Ca2+ oscillatory response. J. Immunol. 161:5129–5132. [PubMed] [Google Scholar]
- 65.Rao, N., A.K. Ghosh, S. Ota, P. Zhou, A.L. Reddi, K. Hakezi, B.K. Druker, J. Wu, and H. Band. 2001. The non-receptor tyrosine kinase Syk is a target of Cbl-mediated ubiquitylation upon B-cell receptor stimulation. EMBO J. 20:7085–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sohn, H.W., H. Gu, and S.K. Pierce. 2003. Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J. Exp. Med. 197:1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ota, S., K. Hazeki, N. Rao, M.L. Lupher Jr., C.E. Andoniou, B. Druker, and H. Band. 2000. The RING finger domain of Cbl is essential for negative regulation of the Syk tyrosine kinase. J. Biol. Chem. 275:414–422. [DOI] [PubMed] [Google Scholar]
- 68.Shao, Y., C. Yang, C. Elly, and Y.C. Liu. 2004. Differential regulation of the B cell receptor-mediated signaling by the E3 ubiquitin ligase Cbl. J. Biol. Chem. 279:43646–43653. [DOI] [PubMed] [Google Scholar]
- 69.Yasuda, T., T. Tezuka, A. Maeda, T. Inazu, Y. Yamanashi, H. Gu, T. Kurosaki, and T. Yamamoto. 2002. Cbl-b positively regulates Btk-mediated activation of phospholipase C-γ2 in B cells. J. Exp. Med. 196:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pure, E., and L. Tardelli. 1992. Tyrosine phosphorylation is required for ligand-induced internalization of the antigen receptor on B lymphocytes. Proc. Natl. Acad. Sci. USA. 89:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salamero, J., M. Fougereau, and P. Seckinger. 1995. Internalization of B cell and pre-B cell receptors is regulated by tyrosine kinase and phosphatase activities. Eur. J. Immunol. 25:2757–2764. [DOI] [PubMed] [Google Scholar]
- 72.Yagi, T., Y. Ikawa, K. Yoshida, Y. Shigetani, N. Takeda, I. Mabuchi, T. Yamamoto, and S. Aizawa. 1990. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diptheria toxin A-fragment gene in negative selection. Proc. Natl. Acad. Sci. USA. 87:9918–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.