Abstract
In 1973, Ralph Steinman and Zanvil Cohn discovered an unusual looking population of cells with an unprecedented ability to activate naive T cells. Dubbed “dendritic cells,” these cells are now known as the primary instigators of adaptive immunity.
The clonal selection theory, proposed by Frank Macfarlane Burnet in 1957, posited that lymphocytes proliferate in response to antigens only if the antigen matches their receptor (1). But in the following decade, a big unknown remained: how was the antigen presented to initiate the response?
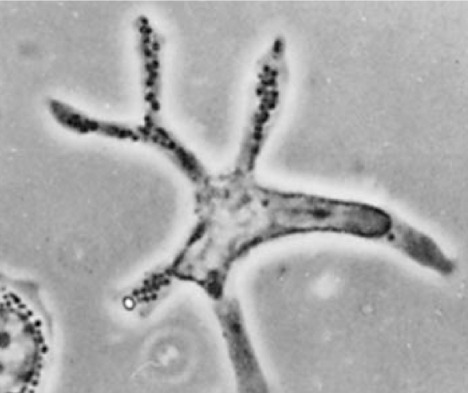
Figure 1.
Phase-contrast image of a dendritic cell (4).
During his medical training at Harvard University and the Massachusetts General Hospital, Steinman felt that the ability to understand and manipulate this initiation step was essential for developing new approaches to treating disease. “The theory at the time was that lymphocytes had receptors for antigens, which would respond on contact. But that clearly wasn't enough,” he says, as simply adding a specific antigen did not elicit a primary immune response.
A new cell type
In 1966, Robert Mishell and Richard Dutton (Scripps Research Institute) added sheep red blood cells to a suspension of mouse spleen cells, generating the first in vitro primary antibody response (2). Others soon reported that this response required the presence of a pool of splenic accessory cells, of which a large component was macrophages (3).
In 1970 Steinman began a post-doctoral fellowship at Rockefeller University with Zanvil Cohn, who was conducting pioneering studies on how macrophages catabolize microbes. Steinman decided to take a closer look at these splenic accessory cells. Using phase-contrast light microscopy, he found a small number of extensively branched, motile and mitochondria-rich cells mixed in with the expected macrophages.
“Dr. Cohn had probably more experience than anyone working with macrophages,” Steinman says. “We realized that these just didn't look or behave like any other cells.” Because of the cells' unique morphology, the pair dubbed them “dendritic cells” (DCs). Steinman published the phenotypic and functional characterization of DCs in two papers in the Journal of Experimental Medicine (4, 5).
From form to function
Steinman hypothesized that DCs were key immune response initiators, an idea that did not initially gain much support. “There was a lot of debate as to whether these cells were real or an artifact,” says Antonio Lanzavecchia (Institute for Research in Biomedicine, Bellinzona, Switzerland). “They seemed to be too rare to be relevant.”
Steinman and colleagues characterized the proteins expressed on the surface of the DCs, which gave hints about their function. One major clue was the high-level expression of major histocompatibility complex (MHC) proteins, such as Ia antigens (6), which later proved to be required for antigen presentation to T cells. Using the mixed leukocyte reaction, a well-known technique used to mimic T cell–mediated rejection of donor tissue during transplantation, Steinman showed that DCs could initiate the reaction and were 100 to 1,000 times more potent than bulk spleen cells (7).
Steinman's team, and later others, showed that DCs were equally potent at stimulating both T cell cytotoxicity and antibody responses (8, 9). Subsequent studies described DC maturation—the process by which immature DCs, which capture antigens in peripheral tissues, become efficient initiators of immunity (10, 11).
“Up till then [DCs were] a bit of an esoteric interest in the lab,” says Siamon Gordon (Oxford University), then a postgraduate fellow in Cohn's lab. But their potency suggested that only DCs could present antigens to naive lymphocytes. And many other researchers soon confirmed their function as the immune system's primary antigen-presenting cells.
A new field explodes
Steinman, Kayo Inaba (Kyoto University), Gerold Schuler (University of Erlangen), Jacques Banchereau (Baylor Institute for Immunology Research), Lanzavecchia, and others went on to develop techniques to grow DCs, eliminating the arduous task of purifying them from lymphoid organs. This work set the stage for current research on the regulation of DC function and the design of DC-based vaccines for HIV and cancers.
References
- 1.Burnet, F.M. 1957. Austr. J. Sci. 20:67–69. [Google Scholar]
- 2.Mishell, R.I., and R.W. Dutton. 1966. Science. 153:1004–1006. [DOI] [PubMed] [Google Scholar]
- 3.Mosier, D.E. 1967. Science. 158:1573–1575. [DOI] [PubMed] [Google Scholar]
- 4.Steinman, R.M., and Z.A. Cohn. 1973. J. Exp. Med. 137:1142–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman, R.M., and Z.A. Cohn. 1974. J. Exp. Med. 139:380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman, R.M., G. Kaplan, M.D. Witmer, and Z.A. Cohn. 1979. J. Exp. Med. 149:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman, R.M., and M.D. Witmer. 1978. Proc. Natl. Acad. Sci. USA. 75:5132–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nussenzweig, M.C., R.M. Steinman, B. Gutchinov, and Z.A. Cohn. 1980. J. Exp. Med. 152:1070–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba, K., R.M. Steinman, W.C. Van Voorhis, and S. Muramatsu. 1983. Proc. Natl. Acad. Sci. USA. 80:6041–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuler, G., and R.M. Steinman. 1985. J. Exp. Med. 161:526–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romani, N., S. Koide, M. Crowley, M. Witmer-Pack, A.M. Livingston, C.G. Fathman, K. Inaba, and R.M. Steinman. 1989. J. Exp. Med. 169:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]