Abstract
Plasmacytoid dendritic cells (pDCs) contribute to innate antiviral immune responses by producing type I interferons. Although human pDCs can induce T cell responses upon viral infection, it remains unclear if pDCs can present exogenous antigens. Here, we show that human pDCs exploit FcγRII (CD32) to internalize antigen–antibody complexes, resulting in the presentation of exogenous antigen to T cells. pDCs isolated from melanoma patients vaccinated with autologous monocyte-derived peptide- and keyhold limpet hemocyanin (KLH)–loaded dendritic cells, but not from nonvaccinated patients or patients that lack a humoral response against KLH, were able to stimulate KLH-specific T cell proliferation. Interestingly, we observed that internalization of KLH by pDCs depended on the presence of serum from vaccinated patients that developed an anti-KLH antibody response. Anti-CD32 antibodies inhibited antigen uptake and presentation, demonstrating that circulating anti-KLH antibodies binding to CD32 mediate KLH internalization. We conclude that CD32 is an antigen uptake receptor on pDCs and that antigen presentation by pDCs is of particular relevance when circulating antibodies are present. Antigen presentation by pDCs may thus modulate the strength and quality of the secondary phase of an immune response.
Plasmacytoid DCs (pDCs) comprise one of two major subsets of human DCs. The myeloid subset is characterized by the presence of CD11c, whereas pDCs correspond to a small subset of CD11c-negative circulating blood DCs (1). Human pDCs are CD4+ CD45RA+IL-3Rα+ (CD123) ILT3+ILT1− CD11c− lineage− cells (2). Two additional markers, BDCA-2 and BDCA-4 are expressed on human pDCs in peripheral blood and bone marrow (3).
In response to viral and bacterial stimuli, pDCs can mature and produce large amounts of type I IFNs (IFN-α/β) (4). Type I interferons activate NK cell cytolytic activity, but protect uninfected cells from NK cell–mediated lysis and affect T cell function by inducing Th1 differentiation (5). Moreover, type I interferons promote differentiation, maturation, and immunostimulatory functions of DCs.
Recent findings suggest that pDCs play an important role in the balance of immune responses. Although resting pDCs may induce regulatory responses, their activated counterparts have a stimulatory capacity (6). In patients with ovarian carcinoma, resting pDCs present at the tumor site may help to maintain an immunosuppressive environment (7). Alternately, activated pDCs induce expansion of antigen-specific memory CD8+ T cells and Th1 CD4+ T cell populations specific for endogenous antigens (8), influenza virus (9, 10), and the MART-1/melan A26-35 epitope (11). pDCs participate in innate immune responses against different types of viruses, eliciting a potent Th1 polarization. During influenza viral infection, pDCs are able to prime virus-specific primary and secondary CD4 and CD8 T cell immune responses in vitro and in vivo (12), but only when the pDCs are exposed to the replicative virus and not with nonreplicating, boiled, or ultraviolet-irradiated virus (9). This important observation suggests that intracellular virus protein expression is essential for pDCs to present antigens in MHC class II. The poor capacity of freshly isolated human and mouse pDCs to induce T cell proliferation is likely the result of their inefficient capturing of antigens, in contrast with classical DCs (1).
In vivo, pDCs accumulate at sites of inflammation, suggesting that pDCs contribute to the ongoing inflammatory response through the release of cytokines and chemokines and the activation of lymphocytes. Because of their capacity to secrete large amounts of type I interferons, it has been suggested that pDCs induce maturation of local myeloid DCs, facilitating cross-priming of endocytosed targets (13). The question whether pDCs themselves can exploit antigen uptake receptors and present exogenous antigens has spurred the current study.
How pDCs might endocytose exogenous antigens is virtually unknown. Uptake might occur via BDCA-2, a C-type lectin transmembrane glycoprotein (14) or via Fcγ receptors. pDCs express the low-affinity Fcγ receptor (CD32). More specifically, pDCs express FcγRIIa (CD32a), but they lack FcγRIIb (CD32b) (15,16). FcγRIIa has been described as a potent immune-activating receptor and it contains an ITAM motif, capable of mediating phagocytosis, ADCC, and initiation of inflammatory cytokine release (17).
Here, we show that human pDCs can indeed take up exogenous antigen through CD32/FcγRII and stimulate antigen-specific CD4+ T cells.
RESULTS AND DISCUSSION
Plasmacytoid DCs are able to take up exogenous antigen
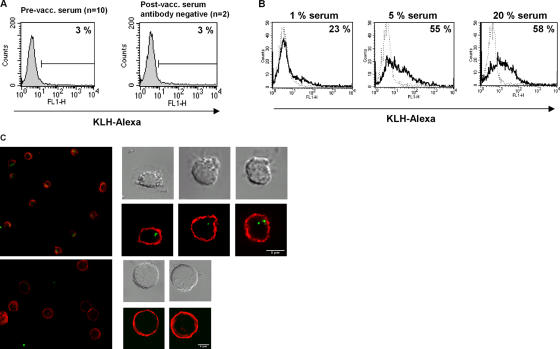
pDCs can induce alloresponses (18), drive Th1 polarization (19), and are able to prime specific CD4+ and CD8+ T cells when loaded with tumor peptides or upon viral infection (12). The previously reported poor ability of this subpopulation of human DCs to generate primary specific CD4+ T cell responses has been attributed to their apparent lack of an efficient machinery to internalize soluble proteins (2). Here, we investigated the capacity of highly purified pDCs (purity 96.5 ± 1.8%, Fig. 1 A) to internalize KLH conjugated to the fluorescent dye Alexa Fluor 488 (Figs. 1 and 2). No internalization of KLH was observed when pDCs were isolated from the blood of normal donors or of melanoma patients not vaccinated with KLH-loaded monocyte-derived DCs (Fig. 1 B). Surprisingly, we observed that KLH was efficiently taken up by freshly isolated pDCs from patients (n = 3; mean 45 ± 13% positive cells; Fig. 1 B) who were vaccinated with KLH-loaded monocyte-derived DCs. These patients all had high levels of specific antibodies to KLH of the IgG isotype (Fig. 1 C). In contrast, pDCs from patients (n = 2) vaccinated with KLH-loaded DCs, but who did not form IgG antibodies directed against KLH, did not take up this protein (Fig. 2 A). This further supports the notion that uptake is dependent on the presence of KLH antibodies.
Figure 1.
Internalization of Alexa Fluor 488–labeled KLH by freshly isolated human pDCs and the presence of anti-KLH antibodies in serum of vaccinated patients. (A) The scatter plot (side and forward scatter) of pDCs isolated by positive sorting using anti–BDCA-4–conjugated magnetic microbeads is shown. Purity was checked by double staining using BDCA-2 (CD302) and IL-3 receptor (CD123). (B) pDCs were isolated from blood of normal donors, nonvaccinated melanoma patients, and melanoma patients vaccinated with KLH-loaded monocyte-derived DCs. Only pDCs isolated from blood of vaccinated patients who developed a humoral response against KLH were able to take up KLH (right), whereas pDCs isolated from healthy donors or before vaccination (left and middle) were not able to take up exogenous protein. (C) Total IgG antibodies specific for KLH were measured by ELISA. (left) Serum obtained from one patient before (•) and 2 mo after (▪) the last inoculation of KLH-loaded monocyte-derived DCs. (right) The humoral response of individual patients after vaccination with KLH-loaded monocyte-derived DCs. Each square represents one patient (mean 1.7 ± 0.8). Data represent OD450 values.
Figure 2.
KLH uptake is dependent on the presence of anti-KLH antibodies. (A) Addition of serum from normal donors, melanoma patients before vaccination, and postvaccination serum from a patient who did not develop a humoral response to KLH did not mediate KLH uptake by pDCs. (B) pDCs from normal donors only take up KLH when incubated with serum containing antibodies against KLH (black line) (n = 12, mean 56 ± 16%) in a concentration-dependent manner. Serum from the same patient but now taken before vaccination and therefore negative for anti-KLH antibodies did not facilitate KLH internalization (negative control, dotted line). (C) Confocal images clearly show that KLH (green) is taken up by pDCs (MHC class II membrane staining in red) in the presence of postvaccination serum (top) and not in the presence of prevaccination serum (bottom).
Antigen uptake is postvaccination serum dependent
The findings presented in Fig. 2 provide further evidence that only in the presence of serum-containing IgG directed against KLH (Fig. 2 B, black line) pDCs were able to take up the exogenous KLH in a serum concentration dependent way. In the presence of serum obtained from patients prevaccination (Fig. 2 B, dotted line) or serum from healthy donors (Fig. 2 A), KLH was not internalized. Internalization was confirmed by confocal microscopy imaging (Fig. 2 C and Fig. S1 (available at http://www.jem.org/cgi/content/full/jem.20052364/DC1) in which the flow cytometric analysis of same sample is depicted; the presence of KLH-Alexa inside the cell was clearly demonstrated by a z-scan series (Video 1, available at http://www.jem.org/cgi/content/full/jem.20052364/DC1).
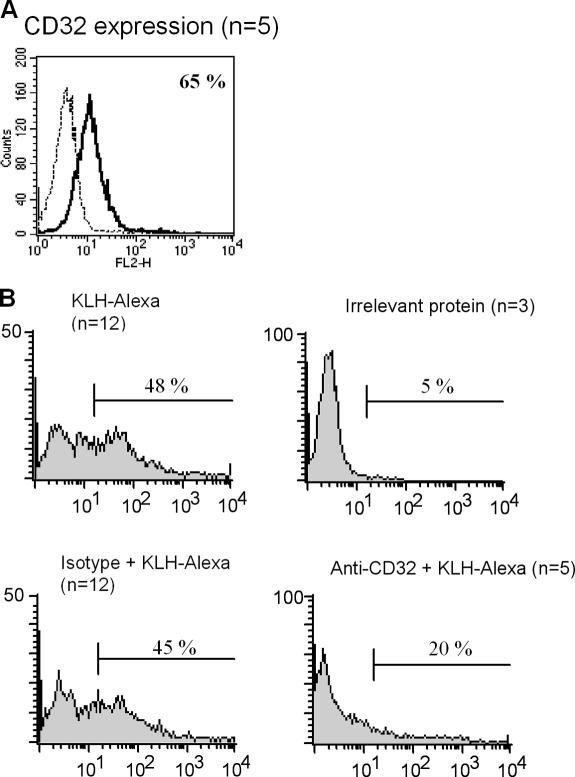
A predominant role for antibody-mediated internalization of proteins is described for the IgG receptor CD32 (20). Here, we confirm previous data on the expression of CD32 by pDCs (Fig. 3 A and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20052364/DC1) (15,16). The uptake of KLH was inhibited when antibodies specific for this receptor were added (Fig. 3 B). These data demonstrate that the uptake of KLH is mediated by antigen-specific IgG antibodies binding to CD32. The finding that pDCs freshly isolated from blood of vaccinated patients were able to internalize KLH without the addition of antibody-containing serum suggests the presence of antigen-specific antibodies on the surface of circulating pDCs in vivo.
Figure 3.
KLH uptake is mediated by FcγRII/CD32. (A) Flow cytometric analysis of FcγRII/CD32 surface expression on fresh pDCs (black line: FcγRII/CD32; dotted line: isotype control; n = 5 mean 67% ± 25). (B) The presence of anti-KLH antibody containing serum does facilitate the uptake of KLH (top left), but not of irrelevant protein (top right). This anti-KLH antibody–mediated uptake can be inhibited with anti-CD32 antibodies (bottom right), but not by isotype control antibodies (bottom left).
Fc receptors have previously been implicated in the uptake of foreign particles and macromolecules by myeloid and monocyte-derived DCs, as such reducing the concentration of antigen required for T cell activation. After proper routing and processing, the uptake of antigens via Fc receptors by monocyte-derived DC not only results in CD4-mediated T cell responses but ultimately in cross-presentation of exogenous antigen to CD8+ T cells (20).
KLH is processed and presented to antigen-specific T cells
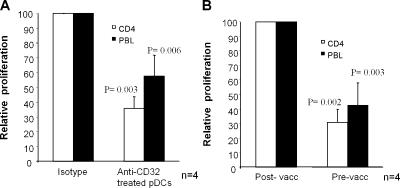
Here, we show that KLH is taken up by pDCs via KLH-specific antibodies binding to CD32. Fanger et al. was the first to show that circulating blood DCs can internalize antigens via FcγRII (21). Recently, Means et al. showed that FcγRIIa is implicated in the pathogenesis of lupus delivering exogenous immune complexes containing DNA to TLR9, revealing a novel signaling cascade in pDCs where a Fc receptors and TLRs interact functionally (22). Here, we confirm their data by really showing complexes of KLH–Alexa inside the pDCs by confocal microscopy (Fig. 2 C and Video 1). Furthermore, we extend their observations by showing that human pDCs not only take up immune complexes via FcγRII but also efficiently present internalized antigen to CD4+ T cells. We incubated PBLs from patients with a cellular response to KLH with autologous pDCs pulsed with KLH. pDCs from patients who lacked a humoral response against KLH were not able to induce KLH-specific proliferation (Fig. 4 A), corresponding to the lack of KLH uptake. This could not be attributed to the absence of specific T cells because monocyte-derived DCs loaded with KLH efficiently induced a proliferative response (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20052364/DC1). In contrast, pDCs from patients with a positive humoral response against KLH were able to induce a specific proliferative response of PBLs (Fig. 4 B) and purified CD4+ T cells (Fig. 4 C). Furthermore, we investigated whether pretreatment of pDCs with anti-CD32 mAb could also inhibit the KLH-specific proliferation. In Fig. 5 A, it is shown that anti-CD32 pretreatment with mAb significantly inhibits the specific KLH proliferation. In accordance with the incomplete inhibition by anti-CD32 mAb of the KLH uptake (Fig. 3 B), the proliferation was not completely abrogated (Fig. 5 A). These findings demonstrate that serum containing KLH-specific Ab appeared essential for the uptake of KLH. To further substantiate this finding to KLH presentation by pDCs to T cells, we incubated pDCs with pre- and postvaccination serum. As depicted in Fig. 5 B, only pDCs incubated with postvaccination serum mediated PBLs and CD4 T cell proliferation and division in response to KLH (Fig. 5 B and Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20052364/DC1). These findings indicate the necessity of antigen-specific antibodies for uptake and presentation of exogenous antigens.
Figure 4.
KLH-specific T cell responses induced by KLH-loaded pDCs. pDCs were isolated from vaccinated patients with (right) or without (left) an antibody response against KLH. pDCs were incubated overnight in the absence (white bars) or presence (black bars) of KLH. pDCs were washed and cocultured at several ratios with PBLs (A–C) or purified CD4+ T cells (C) in X-VIVO 15 supplemented with 2% pooled human serum. Freshly isolated pDCs were only able to induce a proliferative T cell response against KLH when anti-KLH antibodies were present in vivo (see Fig. 1 B for internalization). No increased proliferation was observed when irrelevant control protein was added (not depicted). Significant differences from control according to Student's t test: *, P < 0.05; **, P < 0.001.
Figure 5.
Role of anti-CD32 antibodies and anti-KLH antibody–containing sera in pDC-induced T cell proliferation. (A) pDCs isolated from patients before vaccination were incubated with anti-KLH antibody–containing serum in the presence of KLH. Anti-CD32 antibodies were added to investigate the role of this receptor. After overnight incubation, pDCs were washed and cocultured with PBLs (black bars) or CD4+ T cells (white bars) in X-VIVO 15 supplemented with 2% pooled human serum. Proliferation was measured after 48 h. For normalization, proliferation is depicted as relative proliferation. Maximum proliferation (T cell proliferation induced by KLH-loaded pDCs in the presence of isotype control antibody) of each experiment was set at 100 and relative proliferation (T cell proliferation induced by KLH-loaded pDCs in the presence of anti-CD32 antibody) was calculated per experiment. In the presence of anti-CD32 antibodies, a significant inhibition of the proliferation was observed (Student's t test). (B) pDCs isolated from patients before vaccination were incubated with either postvaccination serum (containing anti-KLH antibodies) or prevaccination serum (no antibodies against KLH) in the presence of KLH. After overnight incubation, pDCs were washed and cocultured with PBLs (black bars) or CD4+ T cells (white bars) in X-VIVO 15 supplemented with 2% pooled human serum. Proliferation was measured after 48 h. For normalization, proliferation is depicted as relative proliferation. Maximum proliferation (T cell proliferation induced by pDCs in the presence of postvaccination serum and KLH) of each experiment was set at 100 and relative proliferation (T cell proliferation induced by pDCs in the presence prevaccination serum and KLH) was calculated per experiment. Proliferation was predominantly observed in the presence of postvaccination serum (containing anti-KLH antibodies) and KLH. p-values indicate significant differences from maximum to Student's t test.
Collectively, our observations demonstrate that pDCs can take up, process, and present exogenous antigen, resulting in a memory T cell response. We propose that pDCs not only nonspecifically support innate immunity by the secretion of type I interferons but also support a secondary type of immune response. Based on the results shown in this study, pDCs may play a role in the presentation of exogenous antigens, inducing a memory type of response.
In vivo the immunological outcome of pDC-mediated T cell stimulation will much depend on the activation state of the pDC (6). Resting pDCs are thought to play an essential role in maintaining tolerance. In cancer patients, pDCs are found in the tumor tissue, but seem inactive despite the fact that circulating antibodies against tumor-associated proteins may be present (7, 23). Although resting pDCs express FcγRII and can therefore internalize immune complexes, they express only low amounts of MHC class II and do not secrete significant amounts of type I interferons. These last characteristics might favor tolerance rather than induction of immunostimulatory T cell responses (6). Therefore, the balance between immunity and tolerance (24) will critically depend on the activation state of the pDC.
Recent studies have shown that pDCs may also accumulate in peripheral tissues during certain noninfectious inflammatory diseases, including autoimmune disorders such as psoriasis. It has been indicated that pDCs initiate psoriasis through type I interferon production (25). Also, in systemic lupus erythematosus (SLE), another autoimmune disorder, circulating pDCs are decreased, but large numbers of activated pDCs infiltrate the skin lesions actively producing type I interferon in these patients (26, 27). Immune complexes, consisting of pathogenic autoantibodies and DNA derived from apoptotic cells, stimulate pDCs to produce cytokines and chemokines via a cooperative interaction between Toll-like receptor 9 and FcγRII (22, 28). Here, we hypothesize that the endogenous DNA-containing autoantibody complexes found in serum of patients with SLE not only lead to type I IFN production but also could play a role in the adaptive immune response by stimulating autoantigen-specific T cells in SLE patients.
Finally, our finding that FcγRII may serve as an important antigen uptake receptor for pDCs may result in the development of strategies to target antigens to pDCs in therapeutic settings. In autoimmune diseases, the role of pDCs is coming to the fore; for cancer or infectious diseases, antigen targeting to pDCs may prove a promising approach to modulate tolerance or immunostimulation.
MATERIALS AND METHODS
Cells.
pDCs were purified from PBLs by positive isolation using anti–BDCA-4–conjugated magnetic microbeads (Miltenyi Biotec) and adjusted to 106 cells/ml in X-VIVO-15 (Cambrex) supplemented with 10 ng/ml IL-3.
Flow cytometry.
CD32 staining and blocking were performed using anti-CD32 (clone AT-10; Serotec), mouse isotype control IgG1, and as secondary mAb PE-conjugated goat anti–mouse. Mean fluorescence intensity and percentage of positive cells were determined by flow cytometry.
Clinical vaccination protocol.
PBLs and monocyte-derived DCs were isolated from melanoma patients participating in ongoing DC vaccination trials (29). The study was approved by the local regulatory committee and written informed consent was obtained from all patients. Antigen-pulsed monocyte-derived DCs (gp100 peptides, tyrosinase peptide and KLH) were administered intravenously and intradermally.
Humoral responses to KLH.
Humoral responses to KLH were determined by ELISA (30).
KLH internalization assays.
Endotoxin-free protein KLH was purchased from Calbiochem and control protein carcinogenic embryonic antigen was obtained from Abcam. Protein binding and internalization by pDCs was assessed by direct labeling of protein with the Alexa Fluor 488 labeling kit (GE Healthcare). pDCs were incubated with 10 μg/ml Alexa-labeled protein overnight at 37°C. Subsequently, cells were washed and analyzed by flow cytometry.
Internalization of Alexa-labeled KLH was confirmed by confocal laser scanning microscopy. pDCs were incubated overnight with 10 μg/ml Alexa-labeled KLH in the presence of post- or prevaccination serum. Cells were fixed on poly-L-lysine coated glass slides, followed by staining with MHC class II mAb (clone Q5/13) or IgG2a isotype control as a secondary mAb goat anti–mouse Alexa 647 was used. Cells were imaged with a Bio-Rad MRC 1024 confocal system operating on a Nikon Optiphot microscope and a Nikon 60x planApo 1.4 oil immersion lens. Pictures were analyzed with Bio-Rad Lasersharp 2000 and Adobe Photoshop 7.0 (Adobe Systems) software.
Cellular responses to KLH.
pDCs were washed and cocultured with KLH-responsive PBLs or purified CD4+ T cells at 37°C. pDCs were incubated overnight with 5% pre- or postvaccination serum. Pretreatment with anti-CD32 antibody was performed for 20 min at 4°C followed by the overnight incubation with the appropriate serum. After 2–4 d of coculture, a tritiated thymidine incorporation assay was performed. Tritiated thymidine (1 μCi/well; MP Biomedicals) was added to the cell cultures and incorporation was measured after 16 h.
CFSE-labeled (1 μM) T cells.
CFSE-labeled cells (105) were cultured with the indicated stimulus for 2 d, and subsequently, CFSE fluorescence intensity was measured by flow cytometry.
Statistics.
All cultures were performed in triplicate and results are shown as the mean ± SD. Significant difference from control according to Student's t test.
Online supplemental material.
Fig. S1 shows the flow cytometric data concerning the uptake of KLH–Alexa of the same cells as used in Fig. 2 C. Fig. S2 shows CD32 expression by pDCs measured with different clones of antibodies recognizing CD32. Fig. S3 shows that the patients analyzed in Fig. 4 do have specific T cells. Fig. S4 shows the comparison of T cell division and T cell proliferation both induced by KLH-loaded pDCs. Video 1 shows a z-scan analysis of pDCs stained with MHC class II antibodies (red) and internalized KLH–Alexa (green). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20052364/DC1.
Supplemental Material
Acknowledgments
The authors thank Drs. P. Tacken and D. Schuurhuis for critical reading of the manuscript. B. Joosten is acknowledged for generating the confocal microscopy pictures. N. Scharenborg, M. Brouwer, A. de Boer, and M. van de Rakt are acknowledged for their technical assistance.
This work was supported by grant nos. 2004-3127 from the Dutch Cancer Society; EU grant nos. DC-VACC, DC-THERA, Cancerimmunotherapy, WO-MW 901-10-092, 9120-6030, 911-02-008; the TIL Foundation; and the NOTK.
The authors have no conflicting financial interests.
References
- 1.Grouard, G., M.C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y.J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colonna, M., G. Trinchieri, and Y.J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. [DOI] [PubMed] [Google Scholar]
- 3.Dzionek, A., Y. Inagaki, K. Okawa, J. Nagafune, J. Rock, Y. Sohma, G. Winkels, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2002. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum. Immunol. 63:1133–1148. [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadowaki, N., S. Antonenko, and Y.J. Liu. 2001. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c− type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J. Immunol. 166:2291–2295. [DOI] [PubMed] [Google Scholar]
- 6.Moseman, E.A., X. Liang, A.J. Dawson, A. Panoskaltsis-Mortari, A.M. Krieg, Y.J. Liu, B.R. Blazar, and W. Chen. 2004. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173:4433–4442. [DOI] [PubMed] [Google Scholar]
- 7.Wei, S., I. Kryczek, L. Zou, B. Daniel, P. Cheng, P. Mottram, T. Curiel, A. Lange, and W. Zou. 2005. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 65:5020–5026. [DOI] [PubMed] [Google Scholar]
- 8.Salio, M., M.J. Palmowski, A. Atzberger, I.F. Hermans, and V. Cerundolo. 2004. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 199:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonteneau, J.F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y.J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 101:3520–3526. [DOI] [PubMed] [Google Scholar]
- 10.Krug, A., R. Veeraswamy, A. Pekosz, O. Kanagawa, E.R. Unanue, M. Colonna, and M. Cella. 2003. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salio, M., M. Cella, W. Vermi, F. Facchetti, M.J. Palmowski, C.L. Smith, D. Shepherd, M. Colonna, and V. Cerundolo. 2003. Plasmacytoid dendritic cells prime IFN-γ-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur. J. Immunol. 33:1052–1062. [DOI] [PubMed] [Google Scholar]
- 12.Schlecht, G., S. Garcia, N. Escriou, A.A. Freitas, C. Leclerc, and G. Dadaglio. 2004. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 104:1808–1815. [DOI] [PubMed] [Google Scholar]
- 13.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D.F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015. [DOI] [PubMed] [Google Scholar]
- 14.Dzionek, A., Y. Sohma, J. Nagafune, M. Cella, M. Colonna, F. Facchetti, G. Gunther, I. Johnston, A. Lanzavecchia, T. Nagasaka, et al. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194:1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bave, U., M. Magnusson, M.L. Eloranta, A. Perers, G.V. Alm, and L. Ronnblom. 2003. Fcγ RIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J. Immunol. 171:3296–3302. [DOI] [PubMed] [Google Scholar]
- 16.Dhodapkar, K.M., J.L. Kaufman, M. Ehlers, D.K. Banerjee, E. Bonvini, S. Koenig, R.M. Steinman, J.V. Ravetch, and M.V. Dhodapkar. 2005. Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl. Acad. Sci. USA. 102:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amigorena, S., and C. Bonnerot. 1999. Fc receptor signaling and trafficking: a connection for antigen processing. Immunol. Rev. 172:279–284. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki, N., S. Antonenko, J.Y. Lau, and Y.J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- 20.Amigorena, S., and C. Bonnerot. 1999. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin. Immunol. 11:385–390. [DOI] [PubMed] [Google Scholar]
- 21.Fanger, N.A., K. Wardwell, L. Shen, T.F. Tedder, and P.M. Guyre. 1996. Type I (CD64) and type II (CD32) Fcγ receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 157:541–548. [PubMed] [Google Scholar]
- 22.Means, T.K., E. Latz, F. Hayashi, M.R. Murali, D.T. Golenbock, and A.D. Luster. 2005. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermi, W., R. Bonecchi, F. Facchetti, D. Bianchi, S. Sozzani, S. Festa, A. Berenzi, M. Cella, and M. Colonna. 2003. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J. Pathol. 200:255–268. [DOI] [PubMed] [Google Scholar]
- 24.Kuwana, M., J. Kaburaki, T.M. Wright, Y. Kawakami, and Y. Ikeda. 2001. Induction of antigen-specific human CD4(+) T cell anergy by peripheral blood DC2 precursors. Eur. J. Immunol. 31:2547–2557. [DOI] [PubMed] [Google Scholar]
- 25.Nestle, F.O., C. Conrad, A. Tun-Kyi, B. Homey, M. Gombert, O. Boyman, G. Burg, Y.J. Liu, and M. Gilliet. 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas, L., K. Beiske, F. Lund-Johansen, P. Brandtzaeg, and F.L. Jahnsen. 2001. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cederblad, B., S. Blomberg, H. Vallin, A. Perers, G.V. Alm, and L. Ronnblom. 1998. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-α- producing cells. J. Autoimmun. 11:465–470. [DOI] [PubMed] [Google Scholar]
- 28.Vallin, H., A. Perers, G.V. Alm, and L. Ronnblom. 1999. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-α inducer in systemic lupus erythematosus. J. Immunol. 163:6306–6313. [PubMed] [Google Scholar]
- 29.de Vries, I.J., W.J. Lesterhuis, N.M. Scharenborg, L.P. Engelen, D.J. Ruiter, M.J. Gerritsen, S. Croockewit, C.M. Britten, R. Torensma, G.J. Adema, et al. 2003. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin. Cancer Res. 9:5091–5100. [PubMed] [Google Scholar]
- 30.Holtl, L., C. Rieser, C. Papesh, R. Ramoner, M. Herold, H. Klocker, C. Radmayr, A. Stenzl, G. Bartsch, and M. Thurnher. 1999. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J. Urol. 161:777–782. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.