Abstract
The innate immune system protects against infection and tissue injury through the specialized organs of the reticuloendothelial system, including the lungs, liver, and spleen. The central nervous system regulates innate immune responses via the vagus nerve, a mechanism termed the cholinergic antiinflammatory pathway. Vagus nerve stimulation inhibits proinflammatory cytokine production by signaling through the α7 nicotinic acetylcholine receptor subunit. Previously, the functional relationship between the cholinergic antiinflammatory pathway and the reticuloendothelial system was unknown. Here we show that vagus nerve stimulation fails to inhibit tumor necrosis factor (TNF) production in splenectomized animals during lethal endotoxemia. Selective lesioning of the common celiac nerve abolishes TNF suppression by vagus nerve stimulation, suggesting that the cholinergic pathway is functionally hard wired to the spleen via this branch of the vagus nerve. Administration of nicotine, an α7 agonist that mimics vagus nerve stimulation, increases proinflammatory cytokine production and lethality from polymicrobial sepsis in splenectomized mice, indicating that the spleen is critical to the protective response of the cholinergic pathway. These results reveal a specific, physiological connection between the nervous and innate immune systems that may be exploited through either electrical vagus nerve stimulation or administration of α7 agonists to inhibit proinflammatory cytokine production during infection and tissue injury.
Severe sepsis is the leading cause of death in intensive care units and accounts for 9% of deaths in the United States annually (1). Innate immune responses are critical for protection against lethal infection and tissue injury, but the uncontrolled production of proinflammatory cytokines, including TNF, IL-1, and high mobility group box 1 (HMGB1), causes the development of severe sepsis (2–3). Counterregulatory antiinflammatory mediators, such as glucocorticoids and IL-10, normally suppress proinflammatory cytokine production to prevent excessive inflammatory responses (4, 5). We recently discovered that the central nervous system also regulates proinflammatory cytokine production through the efferent vagus nerve (5–9). Termed the “cholinergic antiinflammatory pathway” because acetylcholine is the principal vagus neurotransmitter, activation of this mechanism via vagus nerve stimulation can control the production of proinflammatory cytokines in experimental models of systemic inflammation, including lethal endotoxemia, hemorrhagic shock, and ischemia-reperfusion injury (6–10).
Acetylcholine inhibits the production of proinflammatory cytokines from endotoxin-stimulated macrophages through a mechanism dependent on the α7 nicotinic acetylcholine receptor subunit (α7nAChR) (8, 11, 12). Electrical vagus nerve stimulation fails to reduce serum TNF levels in α7nAChR-deficient mice, and macrophages derived from these knockout mice are insensitive to the cytokine-inhibiting effects of cholinergic agonists, indicating that the α7nAChR is required for the antiinflammatory effects of the vagus nerve (8). α7 agonists can inhibit activation of the transcriptional factor NF-κB, prevent secretion of HMGB1 and TNF, and improve survival during experimental polymicrobial sepsis (11, 12). Collectively, these and other studies indicate that the cholinergic antiinflammatory pathway has a critical role in modulating the immune response to infection and injury (13, 14).
The reticuloendothelial system consists of macrophages and monocytes that target foreign pathogens in the lungs, liver, spleen, and other organs (15). These immune cells were originally grouped together because they engulfed vital dyes from the blood. It has since become clear that these cells are essential to the immediate, early response to circulating microbes and LPS, the bacterial endotoxin that stimulates tissue macrophages to secrete lethal quantities of proinflammatory cytokines (16). Bacteria and endotoxin localize to macrophages primarily in the spleen and liver, which in turn become activated to produce proinflammatory cytokines (17). Because the cholinergic antiinflammatory pathway inhibits early proinflammatory cytokine production during endotoxemia, we reasoned that the principal physiological components of this pathway must reside there.
Accordingly, here we examined the effects of vagus nerve stimulation and administration of α7nAChR agonists on proinflammatory cytokine production in organs of the reticuloendothelial system during lethal endotoxemia and polymicrobial sepsis. The results indicate that splenectomy and selective abdominal vagotomy inactivate the antiinflammatory effects of either vagus nerve stimulation or administration of α7nAChR agonists, and reveal that the spleen is a specific and essential target of the cholinergic antiinflammatory pathway.
RESULTS AND DISCUSSION
Spleen response to antiinflammatory effects of vagus nerve stimulation
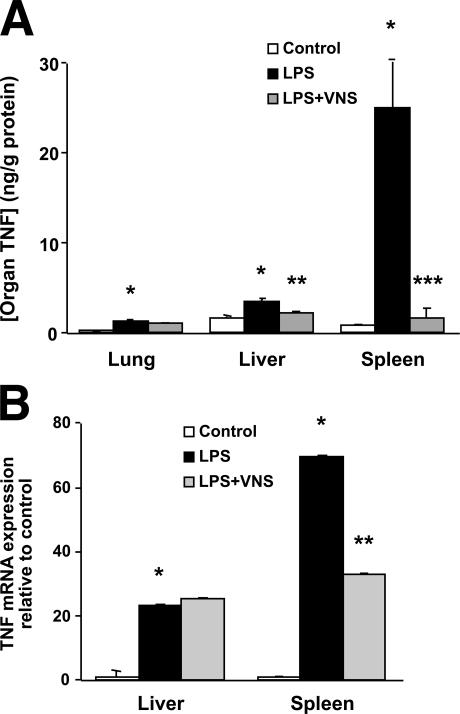
To explore the relationship between the reticuloendothelial system and the cholinergic antiinflammatory pathway, we first measured individual organ TNF concentrations during lethal endotoxemia (Fig. 1 A). Endotoxin administration significantly increases TNF production in the spleen by a factor of 30 as compared with six- and twofold increases in the lung and liver, respectively. Vagus nerve stimulation significantly reduces TNF levels in the spleen (94%) and liver (40%), but not in the lung (20%) (Fig. 1 A). Endotoxin significantly increases TNF mRNA levels in the spleen and liver by 70- and 23-fold, respectively. Vagus nerve stimulation significantly decreases TNF mRNA levels in the spleen, but it does not reduce TNF mRNA levels in the liver (Fig. 1 B). The reduction in splenic TNF mRNA levels mediated by vagus nerve stimulation was confirmed using the RNase protection assay (not depicted). Collectively, these findings reveal that the spleen is an important source of TNF production that is regulated by the antiinflammatory effects of vagus nerve stimulation.
Figure 1.
Effect of vagus nerve stimulation on organ-specific TNF production in lethal endotoxemia. (A) Lewis rats received electrical vagus nerve stimulation or sham stimulation 10 min before and 10 min after an LD50 dose of endotoxin (15 mg/kg, i.v.). Organs were collected after 90 min, and TNF was normalized to the amount of protein per organ (n = 4–8 per group). *, P < 0.01 versus control; **, P < 0.05 versus LPS; ***, P < 0.01 versus LPS. (B) Effect of vagus nerve stimulation on hepatic and splenic TNF mRNA levels in lethal endotoxemia. Lewis rats received electrical vagus nerve stimulation or sham stimulation 10 min before and 10 min after endotoxin injection (15 mg/kg, i.v.). Organs were collected after 60 min, and TNF mRNA expression was determined by quantitative RT-PCR (n = 4–8 per group). *, P < 0.01 versus control; **, P < 0.05 versus LPS.
Vagus nerve stimulation fails to inhibit systemic TNF production in splenectomized animals
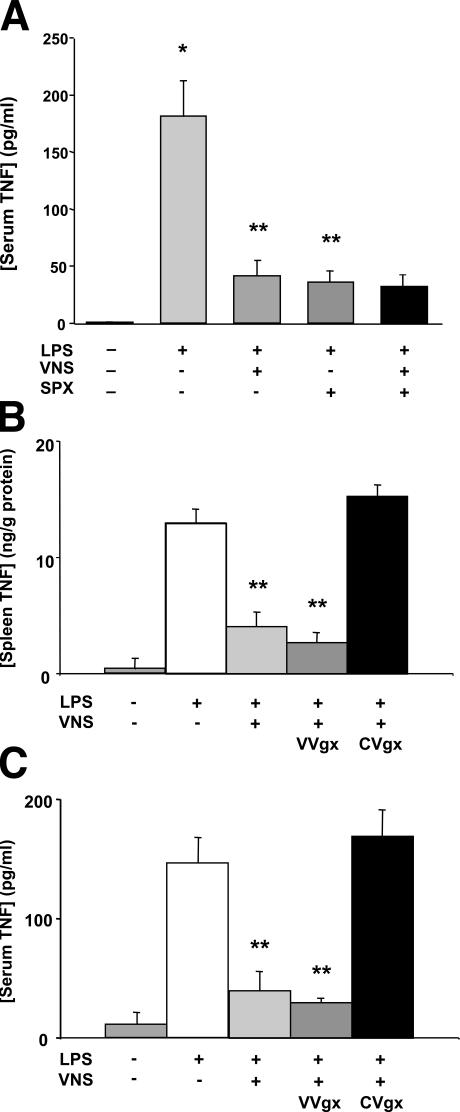
To determine the role of the spleen in the cholinergic antiinflammatory pathway, we subjected rats to splenectomy or sham surgery before lethal endotoxemia (Fig. 2 A). Splenectomy significantly reduces serum TNF levels by 80% as compared with sham controls, which match the 77% reduction in serum TNF levels after vagus nerve stimulation (Fig. 2 A). In contrast to the antiinflammatory effects of vagus nerve stimulation in intact animals, we observed that vagus nerve stimulation fails to inhibit serum TNF levels in splenectomized animals (Fig. 2 A). To explore the functional anatomy of this vagal antiinflammatory pathway to the spleen, we performed selective abdominal vagotomies before vagus nerve stimulation (Fig. 2, B and C). Inferior to the rat diaphragm, the ventral vagus nerve trunk divides into gastric, hepatic, and celiac branches, and the dorsal vagus trunk divides into gastric and celiac branches (18, 19). Cervical vagus nerve stimulation attenuates splenic and serum TNF levels in animals subjected to ventral subdiaphragmatic vagotomy. Common celiac branch vagotomy, however, abrogates the TNF-suppressing effects of cervical vagus nerve stimulation in both the spleen and serum, indicating that intact vagal innervation via the common celiac branches are required for vagus nerve regulation of splenic and systemic TNF.
Figure 2.
Vagus nerve stimulation fails to inhibit systemic TNF production in splenectomized animals. (A) Lewis rats underwent splenectomy or sham surgery, and 3 d later they were injected with endotoxin (15 mg/kg, i.v.) and treated with vagus nerve stimulation or sham stimulation. Serum was collected after 90 min. Data are presented as mean ± SEM (n = 6 per group). *, P < 0.01 versus control; **, P < 0.05 versus LPS. (B and C) The common celiac vagus branches mediate the TNF-suppressing effect of vagus nerve stimulation. Selective subdiaphragmatic ventral vagotomy (VVgx) or common celiac branch vagotomy (CVgx) was performed before endotoxin administration (15 mg/kg, i.v.) and vagus nerve stimulation or sham stimulation. TNF concentrations in rat spleen and serum were determined after 90 min. Data are presented as mean ± SEM (n = 5 per group). *, P < 0.01 versus control; **, P < 0.05 versus LPS.
Vagus nerve regulation of TNF production in the spleen requires the α7nAChR
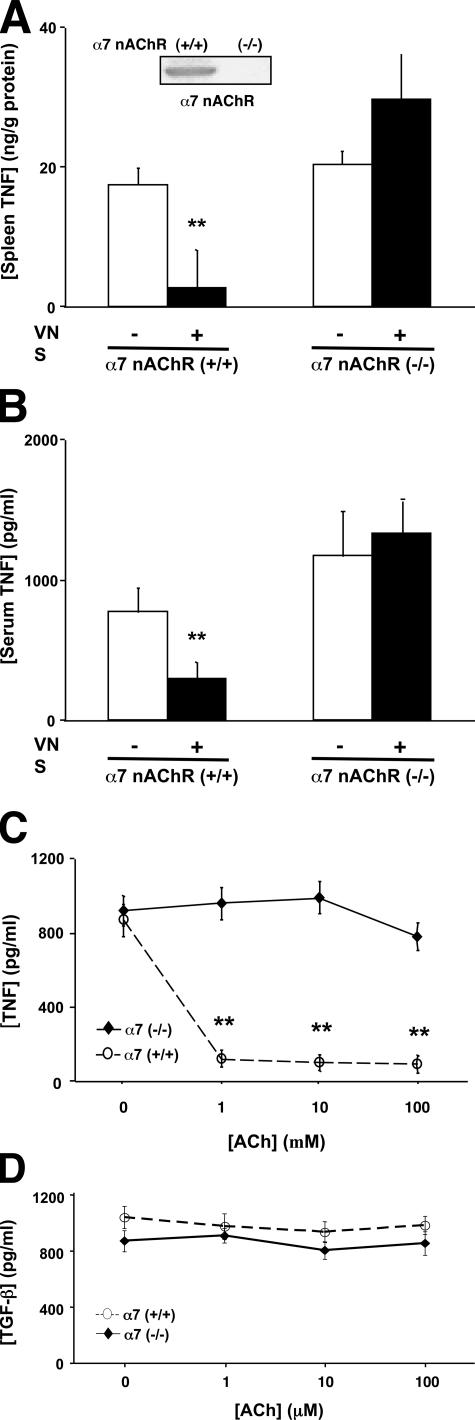
Regulation of serum TNF levels by vagus nerve stimulation is dependent on the α7nAChR (8, 11, 12). To determine the importance of the local α7nAChR antiinflammatory response in the spleen, we measured TNF levels in the spleen after vagus nerve stimulation of α7nAChR knockout mice (Fig. 3 A). Western blot and immunohistochemical analyses confirmed the presence of splenic α7nAChR expression in wild-type mice, but not in α7nAChR knockout mice (Fig. 3 A, inset). Vagus nerve stimulation in α7nAChR knockout mice fails to inhibit TNF production in the spleen. Consistent with our previous studies, vagus nerve stimulation in α7nAChR knockout mice also fails to inhibit serum TNF levels (Fig. 3 B).
Figure 3.
Vagus nerve regulation of TNF production in the spleen requires the α7nAChR. (A and B) Wild-type (+/+) and α7nAChR-deficient mice (−/−) received vagus nerve stimulation before endotoxin administration (7.5 mg/kg, i.p.). TNF concentrations were measured in the spleen and serum after 90 min. Data are presented as mean ± SEM (n = 10 per group). **, P < 0.05. (C) Regulation of splenocyte TNF production by acetylcholine requires the α7nAChR. Primary splenocyte cultures from wild-type (+/+) and α7nAChR-deficient mice (−/−) were treated with endotoxin in the presence of acetylcholine. Data are presented as mean ± SEM. **, P < 0.05. (D) Acetylcholine does not affect splenocyte TGF-β production. Primary splenocyte cultures from wild-type (+/+) and α7nAChR-deficient mice (−/−) were treated with endotoxin in the presence of acetylcholine. Data are presented as mean ± SEM.
The α7nAChR response in the spleen was studied using endotoxin-stimulated splenocytes isolated from wild-type and α7nAChR-deficient animals. Endotoxin significantly increases TNF levels in both wild-type and α7 knockout splenocytes. Acetylcholine significantly inhibits TNF release in endotoxin-stimulated splenocytes from wild-type mice, but not in splenocytes from α7nAChR-deficient animals (Fig. 3 C), indicating that like macrophages, splenocytes are unable to respond to cholinergic antiinflammatory signaling in the absence of the α7nAChR. To determine the cytokine specificity of splenic cholinergic signaling, we analyzed the expression of TGF-β, an antiinflammatory cytokine (20). Endotoxin stimulation increased TGF-β expression in splenocyte cultures from wild-type and α7nAChR-deficient animals. Acetylcholine did not significantly alter TGF-β expression in splenocyte cultures obtained from either wild-type or α7nAChR-deficient animals (Fig. 3 D), suggesting that the pathway is specific. These findings indicate that the α7nAChR response is a necessary molecular component of vagus nerve antiinflammatory signaling in the spleen.
Nicotine worsens survival in splenectomized animals with lethal polymicrobial sepsis
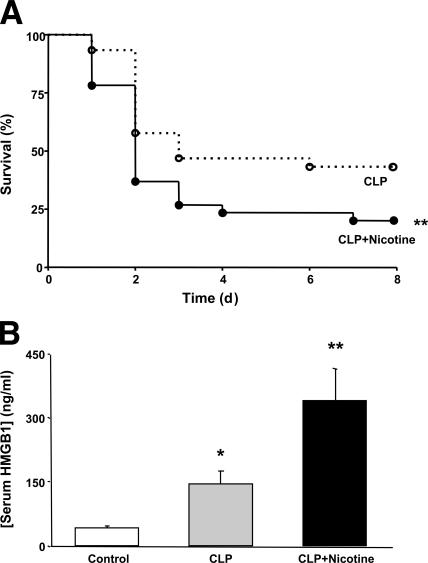
Administration of nicotine protects mice against the lethality of polymicrobial sepsis by inhibiting proinflammatory cytokine production via an α7nAChR-dependent mechanism (11). To determine the role of the spleen in this pathway, splenectomized mice subjected to cecal perforation were treated with nicotine. We observed that nicotine not only fails to protect against sepsis lethality in splenectomized mice, but it actually worsens survival (Fig. 4 A). Previously, the survival benefits of nicotine were found to correlate with reduced serum levels of HMGB1, a necessary and sufficient mediator of lethal systemic inflammation (11). Here we observed that nicotine not only fails to inhibit, but it actually significantly increases serum HMGB1 levels in splenectomized animals (Fig. 4 B). There were no differences in circulating TNF, IL-12, IFN-γ, or IL-10 levels between vehicle- or nicotine-treated animals (not depicted). These findings indicate that the protective effects of nicotine in lethal sepsis require the spleen, and suggest that splenectomy eliminates nicotine's ability to regulate circulating HMGB1 levels.
Figure 4.
Nicotine worsens survival in splenectomized animals with lethal polymicrobial sepsis. (A) BALB/c mice were subjected to splenectomy before cecal ligation and puncture (CLP). Nicotine (400 μg/kg, i.p.) or vehicle treatment began 24 h later and was administered twice a day for 3 d. Data are shown as percent of animals surviving (n > 20 per group). *, P < 0.05 versus control; **, P < 0.05 versus CLP. (B) Nicotine increases serum HMGB1 levels in splenectomized animals. BALB/c mice were subjected to splenectomy before cecal ligation and puncture, and nicotine (400 μg/kg, i.p.) or vehicle treatment began 24 h after surgery. Blood was collected 44 h after surgery, and serum HMGB1 concentration was determined by Western blot and densitometric analysis. Data are presented as mean ± SEM (n > 8 per group). **, P < 0.05.
Cholinergic antiinflammatory pathway innervation of the spleen
The innate immune system coordinates the early and rapid defense against invasive pathogens and toxins (21–22). The afferent, sensory arm of the vagus nerve is critical to the central nervous system's response to toxins in the peripheral immune environment (23–24). The cholinergic antiinflammatory pathway is the efferent arm of this vagus nerve–mediated, brain-to- immune pathway that inhibits systemic proinflammatory cytokine production by signaling through the α7nAChR (5–12). We studied the functional relationship between the innate immune system and the cholinergic antiinflammatory pathway and found a specific, α7nAChR-dependent vagus nerve pathway to the spleen that inhibits proinflammatory cytokine production during lethal endotoxemia and polymicrobial sepsis. Interruption of this pathway via splenectomy or selective abdominal vagotomy completely abolishes the protective, antiinflammatory effects of the cholinergic antiinflammatory pathway.
Vagus nerve stimulation significantly reduces TNF protein levels in the spleen and liver, and specifically reduces TNF mRNA levels in the spleen, but not liver. These findings indicate that the vagus nerve is an early and local modulator of proinflammatory cytokine production in the spleen and suggest that this specific regulation of TNF production occurs at the transcriptional level. Splenectomy reduces systemic TNF production by 80% during lethal endotoxemia, suggesting that the spleen is a major contributor to systemic TNF production in this experimental model. Vagus nerve stimulation fails to inhibit proinflammatory cytokine production after splenectomy or selective interruption of the abdominal vagus nerve at the common celiac branch. Thus, signals originating in the vagus nerve traverse the common celiac branch to control proinflammatory cytokine production in the spleen.
Vagus nerve stimulation fails to regulate splenic TNF production in α7nAChR-deficient mice, and splenocytes from α7 knockout mice do not respond to in vitro stimulation with acetylcholine. Administration of the α7 nicotinic nicotine to splenectomized animals significantly increases mortality and serum HMGB1 levels, whereas we previously demonstrated that nicotine significantly improves survival and attenuates circulating HMGB1 levels in nonsplenectomized mice by acting through the α7nAChR (11, 12). These results indicate that the spleen is essential to the α7nAChR-dependent protective antiinflammatory response. It is possible that the worsened survival after nicotine treatment is related to nicotinic suppression of normal immune responses in the absence of the spleen. In agreement with this hypothesis, previous studies indicate that nicotine can significantly impair antimicrobial activity in macrophages lacking the α7nAChR via its action on other non-α7 acetylcholine receptor subtypes (25).
Collectively, these findings have important implications for the development of therapeutic, selective α7nAChR agonists. It may be necessary to develop methods that specifically target the critical, α7nAChR antiinflammatory response peripherally in the spleen as well as to consider methods to stratify patients who may or may not benefit from activation of the cholinergic antiinflammatory pathway. Vagus nerve modulation of the spleen may represent the functionally, hard-wired counterpart to humoral antiinflammatory responses, such as the hypothalamic–pituitary–adrenal axis (26). The neural connection between the vagus nerve and the spleen can allow for rapid and precise control of systemic cytokine production. It may also control the trafficking of inflammatory cells to distant sites (27). Vagus nerve stimulation is clinically approved for the treatment of medically refractory seizures, and more than 25,000 individuals have undergone this safe and well-tolerated therapy worldwide (28, 29). Future studies are needed to explore the protective effects of vagus nerve stimulation and α7nAChR agonists in the treatment of systemic inflammation and sepsis.
MATERIALS AND METHODS
Animals.
Adult male Lewis rats (280–300 g; Charles River Laboratories), male 8–12-wk-old BALB/c mice (25–30 g; Taconic), and male and female 8–12-wk-old α7nAChR-deficient mice (C57BL/6 background) as well as wild-type littermates (The Jackson Laboratory) were housed at 25°C on a 12-h light/dark cycle. Standard animal chow and water were freely available. All experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research, North Shore-LIJ Health System.
Endotoxemia.
Endotoxin (Escherichia coli LPS 0111:B4; Sigma-Aldrich) was dissolved in sterile pyrogen-free PBS and sonicated for 30 min immediately before use. Animals received an LD50–75 dose of LPS (7.5–15 mg/kg). Blood was collected after 90–120 min, allowed to clot for 2 h at room temperature, and centrifuged for 15 min at 2,000 g. TNF concentrations were measured by ELISA (R&D Systems).
Vagus nerve stimulation.
Animals were anesthetized i.m. with 100 mg/kg ketamine and 10 mg/kg xylazine, and subjected to sham surgery (sham) or left cervical vagotomy with electrical stimulation of the distal nerve trunk. In sham-operated animals, the vagal trunk was exposed but not divided. The nerve trunk was placed across a bipolar platinum electrode (Plastics One) connected to a stimulation module (STM100A) controlled by AcqKnowledge software (Biopac Systems). Stimulation (1 V, 2 ms, 5 Hz) was applied for 10 min before and after endotoxin administration.
Subdiaphragmatic vagotomy.
Animals were anesthetized with ketamine and xylazine. After an upper midline laparotomy, the stomach was retracted inferiorly to access the common celiac and ventral vagus nerve trunks, and vagotomy was performed by ligating and excising at least 1 cm of either the ventral vagus nerve or both common celiac branches. For sham controls, the vagal trunks were exposed but not ligated.
Cecal ligation and puncture.
Cecal ligation and puncture were performed on BALB/c mice as described previously (11). Animals received antibiotic (0.5 mg/kg Primaxin, s.c.) and 0.9% normal saline (20 ml/kg body weight, s.c.) immediately after surgery. i.p. administration of 400 μg/kg nicotine or vehicle injection (1× PBS) began 24 h after surgery, and thereafter twice a day for 3 d. Animals were killed after 44 h for serum cytokine analysis. Serum HMGB1 levels were determined by Western blot as we described previously (11). HMGB1 concentrations were calculated against standard curves of purified recombinant HMGB1, which was purified as described previously (11). Serum TNF, IL-12, IFN-γ, and IL-10 levels were measured using Cytometric Bead Array (Becton Dickinson) according to the manufacturer's instructions.
Splenectomy.
Mice were anesthetized with ketamine and xylazine. The spleen was identified after a midline laparotomy incision and removed after appropriate blood vessel ligation. Sham animals underwent laparotomy without splenectomy.
Splenocyte culture.
Spleens were mechanically disrupted with a 100-μm pore cell strainer (BD Falcon), erythrocytes were lysed in solution (Gentra Systems) for 10 min, and intact cells were washed twice in PBS. Cells were resuspended in medium (RPMI, FBS 10%, 2 mM l-glutamine, 100 U/ml penicillin/100μg/ml streptomycin) and plated at a final density of 5 × 105 cells/well. Splenocytes were incubated for 10 h with 50 ng/ml LPS (E. coli 0111:B4; Sigma-Aldrich) plus or minus acetylcholine (0, 1, 10, and 100 μM; Sigma-Aldrich) combined with 1 mM pyridostigmine bromide (Sigma-Aldrich). Supernatant cytokine levels were measured by ELISA (R&D Systems).
Quantitative RT-PCR.
Frozen spleen and liver samples were homogenized before total RNA isolation using an RNeasy kit and QIAshredder mini-spin columns (QIAGEN) and treated with DNase to remove genomic contamination (QIAGEN). The relative expression of TNF mRNA was determined by quantitative real-time PCR using TaqMan technology using the Eurogentec's RTqPCR mastermix and the ABI PRISM 7700 Sequence Detection System. Optimal concentrations of primers, probes, and the RNA were standardized. TNF primer (forward: GCTGCCCCGACTATGTGCT, reverse: GACTTTCTCCTGGTATGAAATGGC) was added at a final concentration of 900 nM. Mouse GAPDH was used as an internal control gene, and mouse GAPDH primers (forward: TGTGTCCGTCGTGGATCTGA, reverse: CCTGCTTCACCACCTTCTTGA) and the GAPDH TaqMan probe (CCGCCTGGAG-AAACCTGCCA) were added at final concentrations of 500 nM and 100 nM, respectively. The thermal cycler conditions were 35 cycles of 94°C for 20 s, 55°C for 20 s, and 72°C for 30 s. Data were analyzed using Sequence Detection System software version 1.9.1. Relative expression of the TNF gene in the spleen and liver obtained from mice treated with endotoxin or endotoxin plus vagus nerve stimulation was calculated in comparison to vehicle-treated control samples using the delta delta Ct method (User Bulletin no. 2; Applied Biosystems). All samples were run in duplicate.
RNase protection assay.
Total RNA was extracted using the RNeasy Mini kit (catalog no. 74104; QIAGEN) according to the manufacturer's instructions (Tel-Test B Inc.). Purity and integrity of the preparation were verified by electrophoresis on 1.2% agarose/17% formaldehyde gels and by RT-PCR for TNF mRNA. Extraction efficiency was measured by absorbance to normalized concentrations of total RNA. TNF and actin mRNA in the spleen and liver were measured by RNase protection assay using the RPA III kit (catalog no. 1415; Ambion). The antisense RNA probe was labeled with 800 Ci/mmol α-32P-UTP (GE Healthcare) using T7 RNA polymerase. Molecular weight markers were prepared using RNA Century marker template set (catalog no. 7780; Ambion) transcribed using a Maxiscript kit (catalog no. 1308-1326; Ambion) with 800 Ci/mmol α-32P-dUTP (GE Healthcare). mRNA levels were measured with an InstantImager (Packard Instrument Co.).
Statistical analysis.
All data in the figures and text are expressed as mean ± SEM. One-way ANOVA using the Bonferroni correction was used to compare mean values between multiple groups. The Student's t test was used to compare mean values between two experimental groups. Differences in survival were determined using the log-rank test. p-values <0.05 were considered significant.
Acknowledgments
The authors would like to thank C.N. Metz and B. Sherry for their thoughtful insights.
This work was supported by the General Clinical Research Center of The Feinstein Institute for Medical Research (M01-RR018535), the National Institute of General Medical Sciences, the Defense Advanced Research Projects Agency, and a Feinstein Institute Faculty Award.
K.J. Tracey and J.M. Huston are inventors on patents related to vagus nerve stimulation and cholinergic agonists as antiinflammatory agents, and K.J. Tracey is a consultant to Critical Therapeutics, Inc. All other authors have no conflicting financial interests.
References
- 1.Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analyses of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Nathan, C. 2002. Points of control in inflammation. Nature. 420:846–852. [DOI] [PubMed] [Google Scholar]
- 3.Ulloa, L., and K.J. Tracey. 2005. The “cytokine profile”: a code for sepsis. Trends Mol. Med. 11:56–63. [DOI] [PubMed] [Google Scholar]
- 4.Tracey, K.J. 2005. Fatal Sequence: The Killer Within. Dana Press, Washington, DC. 225 pp.
- 5.Tracey, K.J. 2002. The inflammatory reflex. Nature. 420:853–859. [DOI] [PubMed] [Google Scholar]
- 6.Borovikova, L.V., S. Ivanova, M. Zhang, H. Yang, G.I. Botchkina, L.R. Watkins, H. Wang, N. Abumrad, J.W. Eaton, and K.J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–462. [DOI] [PubMed] [Google Scholar]
- 7.Bernik, T.R., S.G. Friedman, M. Ochani, R. DiRaimo, L. Ulloa, H. Yang, S. Sudan, C.J. Czura, S.M. Ivanova, and K.J. Tracey. 2002. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, H., M. Yu, M. Ochani, C.A. Amella, M. Tanovic, S. Susarla, J.H. Li, H. Wang, H. Yang, L. Ulloa, et al. 2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 421:384–387. [DOI] [PubMed] [Google Scholar]
- 9.Bernik, T.R., S.G. Friedman, M. Ochani, R. DiRaimo, S. Susarla, C.J. Czura, and K.J. Tracey. 2002. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg. 36:1231–1236. [DOI] [PubMed] [Google Scholar]
- 10.Guarini, S., D. Altavilla, M.M. Cainazzo, D. Giuliani, A. Bigiani, H. Marini, G. Squadrito, L. Minutoli, A. Bertolini, R. Marini, et al. 2003. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 107:1189–1194. [DOI] [PubMed] [Google Scholar]
- 11.Wang, H., H. Liao, M. Ochani, M. Justiniani, X. Lin, L. Yang, Y. Al-Abed, H. Wang, C. Metz, E.J. Miller, et al. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10:1216–1221. [DOI] [PubMed] [Google Scholar]
- 12.Ulloa, L. 2005. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov. 4:673–683. [DOI] [PubMed] [Google Scholar]
- 13.Libert, C. 2003. A nervous connection. Nature. 421:328–329. [DOI] [PubMed] [Google Scholar]
- 14.Matthay, M.A., and L.B. Ware. 2004. Can nicotine treat sepsis? Nat. Med. 10:1161–1162. [DOI] [PubMed] [Google Scholar]
- 15.Guyton, A.C., and J.E. Hall. 2000. Textbook of Medical Physiology. Saunders, Philadelphia. 1064 pp.
- 16.Hadjiminas, D.J., K.M. McMasters, J.C. Peyton, and W.G. Cheadle. 1994. Tissue tumor necrosis factor mRNA expression following cecal ligation and puncture or intraperitoneal injection of endotoxin. J. Surg. Res. 56:549–555. [DOI] [PubMed] [Google Scholar]
- 17.Ge, Y., R.M. Ezzell, B.D. Clark, P.M. Loiselle, S.F. Amato, and H.S. Warren. 1997. Relationship of tissue and cellular interleukin-1 and lipopolysaccharide after endotoxemia and bacteremia. J. Infect. Dis. 176:1313–1321. [DOI] [PubMed] [Google Scholar]
- 18.Dixon, K.D., F.E. Williams, R.L. Wiggins, J. Pavelka, J. Lucente, L.L. Bellinger, and D.W. Gietzen. 2000. Differential effects of selective vagotomy and tropisetron in aminoprivic feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R997–R1009. [DOI] [PubMed] [Google Scholar]
- 19.Simons, C.T., V.A. Kulchitsky, N. Sugimoto, L.D. Homer, M. Szekely, and A.A. Romanovsky. 1998. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am. J. Physiol. 275:R63–R68. [DOI] [PubMed] [Google Scholar]
- 20.Tsunawaki, S., M. Sporn, A. Ding, and C. Nathan. 1988. Deactivation of macrophages by transforming growth factor-beta. Nature. 334:260–262. [DOI] [PubMed] [Google Scholar]
- 21.Hornef, M.W., M.J. Wick, M. Rhen, and S. Normark. 2002. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 3:1033–1040. [DOI] [PubMed] [Google Scholar]
- 22.Heumann, D., and T. Roger. 2002. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim. Acta. 323:59–72. [DOI] [PubMed] [Google Scholar]
- 23.Fleshner, M., L.E. Goehler, B.A. Schwartz, M. McGorry, D. Martin, S.F. Maier, and L.R. Watkins. 1998. Thermogenic and corticosterone responses to intravenous cytokines (IL-1beta and TNF-alpha) are attenuated by subdiaphragmatic vagotomy. J. Neuroimmunol. 86:134–141. [DOI] [PubMed] [Google Scholar]
- 24.Goehler, L.E., R.P. Gaykema, K.T. Nguyen, J.E. Lee, F.J. Tilders, S.F. Maier, and L.R. Watkins. 1999. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 19:2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunaga, K., T.W. Klein, H. Friedman, and Y. Yamamoto. 2001. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J. Immunol. 167:6518–6524. [DOI] [PubMed] [Google Scholar]
- 26.Webster, J.I., L. Tonelli, and E.M. Sternberg. 2002. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 20:125–163. [DOI] [PubMed] [Google Scholar]
- 27.Saeed, R.W., S. Varma, T. Peng-Nemeroff, B. Sherry, D. Balakhaneh, J. Huston, K.J. Tracey, Y. Al-Abed, and C.N. Metz. 2005. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 201:1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guberman, A. 2004. Vagus nerve stimulation in the treatment of epilepsy. CMAJ. 171:1165–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spanaki, M.V., L.S. Allen, W.M. Mueller, and G.L. Morris III. 2004. Vagus nerve stimulation therapy: 5-year or greater outcome at a university-based epilepsy center. Seizure. 13:587–590. [DOI] [PubMed] [Google Scholar]