Abstract
Glucocorticoids (GCs), which are used in the treatment of immune-mediated inflammatory diseases, inhibit the expression of many inflammatory mediators. They can also induce the expression of dual specificity phosphatase 1 (DUSP1; otherwise known as mitogen-activated protein kinase [MAPK] phosphatase 1), which dephosphorylates and inactivates MAPKs. We investigated the role of DUSP1 in the antiinflammatory action of the GC dexamethasone (Dex). Dex-mediated inhibition of c-Jun N-terminal kinase and p38 MAPK was abrogated in DUSP1−/− mouse macrophages. Dex-mediated suppression of several proinflammatory genes (including tumor necrosis factor, cyclooxygenase 2, and interleukin 1α and 1β) was impaired in DUSP1−/− mouse macrophages, whereas other proinflammatory genes were inhibited by Dex in a DUSP1-independent manner. In vivo antiinflammatory effects of Dex on zymosan-induced inflammation were impaired in DUSP1−/− mice. Therefore, the expression of DUSP1 is required for the inhibition of proinflammatory signaling pathways by Dex in mouse macrophages. Furthermore, DUSP1 contributes to the antiinflammatory effects of Dex in vitro and in vivo.
Glucocorticoids (GCs) inhibit the expression of inflammatory mediators by macrophages and other cells and are used in the treatment of many immune-mediated inflammatory diseases (for review see reference 1). However, their long-term use may be limited by severe side effects. In addition, a proportion of patients treated with GCs do not display a strong antiinflammatory response. These patients can be difficult to treat effectively, but the molecular basis of GC insensitivity in inflammatory disease remains poorly understood (2).
GCs modulate gene expression via the GC receptor (GR), a member of the nuclear hormone receptor superfamily of transcription factors (for review see reference 1). When activated by a GC ligand, GR can dimerize, bind to palindromic GC response elements, and activate the transcription of target genes such as phosphoenol pyruvate carboxykinase. Side effects of GCs are commonly attributed to gene induction by ligand-activated GR, although few relevant GC-induced genes have been identified. GCs are also known to induce the expression of several antiinflammatory genes such as annexin 1, although the contributions of these genes to the antiinflammatory effects of GCs have been questioned (for review see reference 1).
Antiinflammatory actions of GCs are widely thought to be mediated by transrepression, in which the ligand-activated GR interferes with the capacity of NF-κB and activator protein 1 to induce the transcription of inflammatory mediators (for reviews see references 1, 3; 4). GR with a point mutation in the dimerization interface of the DNA-binding domain failed to activate GC response element–dependent reporter genes but transrepressed NF-κB and activator protein 1–dependent reporters (for review see reference 1). A knock-in mouse strain was generated harboring this GRdim mutation (5–7). In the GRdim mouse, GCs failed to induce phosphoenol pyruvate carboxykinase expression but exerted clear antiinflammatory effects. This and other observations have led to the hypothesis that the side effects and antiinflammatory properties of GCs can be uncoupled from one another. In other words, novel GR agonists that selectively induce the transrepression function of GR but do not efficiently activate transcription might have improved therapeutic indices, retaining antiinflammatory properties but causing fewer side effects (for reviews see references 1, 8). However, GCs can activate gene expression in a dimerization-independent manner (for review see reference 1; 9), making it unclear to what extent antiinflammatory effects are independent of gene induction or to what extent therapeutic effects can be dissociated from deleterious effects.
GCs have been shown to induce the rapid and sustained expression of dual specificity phosphatase 1 (DUSP1) in several cell types, including primary myeloid cells and myeloid cell lines (10; for review see reference 11). DUSP1, which is also known as mitogen-activated protein kinase (MAPK) phosphatase 1, is the founding member of a large family of phosphatases that can inactivate MAPKs (12). It is a particularly effective inhibitor of c-Jun N-terminal kinase (JNK) and p38 MAPK signaling pathways (10), which contribute to the expression of inflammatory mediators at both transcriptional and posttranscriptional levels (for reviews see references 13, 14). The overexpression of DUSP1 in macrophages dampened inflammatory responses to LPS (10, 15, 16). Responses to LPS were enhanced in DUSP1−/− macrophages, and susceptibility to lethal endotoxic shock was dramatically increased in a DUSP1−/− mouse strain (10, 17–21). Thus, DUSP1 is an important negative regulator of inflammatory responses, and the induction of DUSP1 gene expression is potentially a novel antiinflammatory mechanism of GCs. To date, no causal link has been proven to exist between DUSP1 gene induction, inhibition of MAPK signaling pathways, and antiinflammatory actions of GCs (for reviews see references 14, 22). In this study, we show that DUSP1 is necessary for the inhibition of JNK and p38 MAPK by GCs and that it contributes to antiinflammatory effects of GCs in vitro and in vivo.
RESULTS AND DISCUSSION
DUSP1 is required for the inhibition of JNK and p38 MAPK by dexamethasone in mouse macrophages
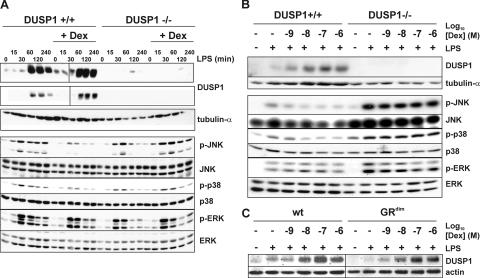
BM macrophages (BMMs) were generated from age- and sex-matched DUSP1+/+ and DUSP1−/− littermates and were stimulated with LPS for different times with or without pretreatment with 100 nM dexamethasone (Dex). In wild-type BMMs, LPS caused the transient induction of DUSP1 protein (Fig. 1 A, top). Pretreatment with Dex increased the basal expression of DUSP1 and augmented and prolonged its induction by LPS. Similar regulation of DUSP1 messenger RNA (mRNA) by LPS and Dex was observed (unpublished data). DUSP1 protein was not induced by LPS and/or Dex in DUSP1−/− cells (Fig. 1, A and B). The expression of DUSP4 (MAPK phosphatase 2) was transiently up-regulated by LPS at both mRNA and protein levels, did not differ between DUSP1+/+ and DUSP1−/− BMMs, and was unaffected by Dex (unpublished data). All three MAPK pathways were activated after LPS stimulation of mouse BMMs (Fig. 1 A, bottom). Both JNK and p38 MAPK pathways were inhibited by Dex pretreatment in DUSP1+/+ but not in DUSP1−/− BMMs. Extracellular signal-regulated kinase (ERK) activation did not differ between DUSP1+/+ and DUSP1−/− BMMs and was not altered by Dex pretreatment.
Figure 1.
Altered JNK and p38 MAPK signaling in DUSP1−/− BMMs. (A) 106 DUSP1+/+ or DUSP1−/− BMMs were pretreated with vehicle or 100 nM Dex for 4 h and stimulated with LPS for the indicated times. Lysates were blotted for DUSP1 or tubulin-α (top) and total or phosphorylated MAPKs (bottom). Two exposures of the same DUSP1 Western blot are shown to illustrate differences in basal and LPS-induced DUSP1 protein expression. (B) DUSP1+/+ or DUSP1−/− BMMs were pretreated with 1 nM to 1 μM Dex for 4 h and stimulated with LPS for 4 h. In two separate representative experiments, lysates were sequentially blotted for DUSP1 and tubulin-α (top) or for phosphorylated and total MAPKs (bottom). (C) Wild-type and GRdim BMMs were pretreated with 1 nM to 1 μM Dex for 4 h and stimulated with LPS for 4 h. Lysates were blotted for DUSP1 or actin.
Dex dose-response experiments were performed to confirm the relationship between DUSP1 expression and the inhibition of proinflammatory signaling (Fig. 1 B). DUSP1 protein was induced by LPS and dose-dependently increased by Dex with an apparent EC50 between 1 and 10 nM, which is in agreement with the reported Kd for the binding of Dex to GR. There was a corresponding dose-dependent inhibition of both JNK and p38 MAPK but no inhibition of the ERK pathway. In DUSP1−/− cells, there was no expression of DUSP1 protein or inhibition of MAPKs. The slight overexpression of JNK in DUSP1−/− BMMs was not consistently observed (Fig. 1 A). The expression of DUSP1 was induced by LPS alone and dose-dependently increased by the addition of Dex in both wild-type and GRdim BMMs (Fig. 1 C). Therefore, DUSP1 is required for the inhibition of JNK and p38 MAPK by GCs, and the regulation of DUSP1 gene expression is independent of GR dimerization. This is the first direct evidence of a causal link between DUSP1 induction and the inhibition of MAPK pathways by GC. DUSP1 is a member of a large family of MAPK phosphatases, several of which can inactivate JNK and p38 (12). In mouse BMMs, there is no redundancy in terms of DUSP-mediated effects of GC on MAPK signaling.
Ablation of the DUSP1 gene impairs antiinflammatory actions of GC
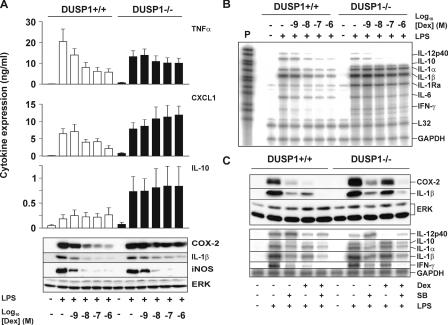
We next investigated the expression of various inflammatory mediators in DUSP1+/+ and DUSP1−/− BMMs (Fig. 2, A and B; and Fig. 3). LPS-induced TNF protein expression was dose-dependently inhibited by Dex in wild-type BMMs, whereas there was only weak inhibition of TNF by Dex in DUSP1−/− cells (Fig. 2 A). Chemokine (C-X-C motif) ligand 1 (CXCL1) was induced by LPS and dose-dependently inhibited by Dex in wild-type BMMs, whereas Dex caused a slight enhancement of gene expression in DUSP1−/− BMMs. The expression of IL-10 protein was close to the limits of detection in DUSP1+/+ BMMs, and although Dex caused a slight increase, this did not reach statistical significance. Consistent with previous studies, (17–20), IL-10 was more strongly expressed by DUSP1−/− BMMs (on average fivefold higher), and this response was not affected by Dex. Both cyclooxygenase 2 (COX-2) and IL-1β were strongly induced by LPS and dose-dependently inhibited by Dex in wild-type BMMs, whereas in DUSP1−/− BMMs, the inhibitory effects of Dex were impaired. Inducible nitric oxide synthase (iNOS) was up-regulated by LPS; however, the strong, dose-dependent inhibition by Dex did not differ between DUSP1+/+ and DUSP1−/− cells. This indicates that DUSP1−/− cells are not merely unresponsive to GCs. Rather, Dex inhibits the expression of some genes in a manner that is highly dependent on DUSP1, whereas other genes are suppressed in a more or less DUSP1-independent manner.
Figure 2.
Antiinflammatory actions of Dex are impaired in DUSP1−/− BMMs. (A) 106 DUSP1+/+ or DUSP1−/− BMMs were pretreated with 1 nM to 1 μM Dex for 4 h and stimulated with LPS for 4 h. Cytokines in supernatants were quantified by ELISA. Graphs indicate means ± SEM (error bars) from at least three independent experiments. Cell lysates were blotted for COX-2, IL-1β, iNOS, and ERK proteins. (B) Cells were treated as in A, and the expression of various mRNAs was examined by RPA. P, undigested probe (10% of input). (C) 106 DUSP1+/+ or DUSP1−/− BMMs were pretreated with 100 nM Dex and/or 10 μM SB202190 and stimulated with LPS for 4 h. Lysates were blotted for Cox-2, IL-1β, or ERK proteins (top), or the expression of various mRNAs was examined by RPA (bottom).
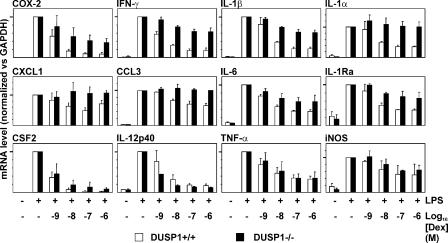
Figure 3.
Dose-dependent suppression of various inflammatory mediator mRNAs by Dex in DUSP1+/+ and DUSP1−/− BMMs. BMMs were treated as in Fig. 2 B. mRNAs were quantified using qPCR (CSF2, TNF, CXCL1, and COX-2) or RPA. mRNA levels were normalized against GAPDH and expressed as percentages of the level in cells treated with LPS alone. Graphs show mean values ± SEM (error bars) from three to six independent experiments. 100% values are identified by heavy tick marks on the y axes.
Quantitative PCR (qPCR) and ribonuclease protection assays (RPAs) were used to quantify a number of mRNAs involved in the inflammatory response. Fig. 2 B shows a representative RPA, whereas Fig. 3 graphically illustrates the results of several independent qPCR and RPA experiments, and Table I presents statistical analysis of the effects of 10 nM Dex on proinflammatory mRNAs and proteins in DUSP1+/+ and DUSP1−/− BMMs. This dose was selected for detailed statistical analysis because it is close to both the Kd for binding of Dex to GR and the estimated EC50 for induction of DUSP1 gene expression by Dex.
Table I.
Inhibition of certain proinflammatory targets by 10 nM Dex in DUSP1+/+ and DUSP1−/− BMMs
| Percent inhibition by 10 nM Dex
|
|||
|---|---|---|---|
| Target | DUSP1+/+ | DUSP1−/− | p-value |
| TNF protein | 58.5 ± 7.9 | 26.0 ± 13.9 | 0.007 |
| TNF mRNA | 53.2 ± 14.8 | 47.3 ± 22.7 | 0.60 (NS) |
| COX-2 protein | 80.1 ± 9.2 | 36.7 ± 10.0 | <0.0001 |
| COX-2 mRNA | 84.3 ± 5.2 | 48.3 ± 21.8 | 0.003 |
| CXCL1 protein | 36.0 ± 8.4 | −32.0 ± 21.0 | 0.007 |
| CXCL1 mRNA | 46.8 ± 11.0 | −11.4 ± 17.9 | 0.0003 |
| IL-1α mRNA | 50.4 ± 3.3 | −8.7 ± 20.8 | 0.001 |
| IL-1β mRNA | 62.3 ± 1.6 | 25.5 ± 2.0 | <0.0001 |
| IL-6 mRNA | 53.6 ± 4.3 | 32.9 ± 11.3 | 0.014 |
| IFN-γ mRNA | 69.5 ± 2.2 | 25.1 ± 3.7 | <0.0001 |
| IL-12p40 mRNA | 68.3 ± 10.7 | 83.1 ± 1.8 | 0.034 |
| CSF2 mRNA | 92.6 ± 4.6 | 81.7 ± 11.5 | 0.13 (NS) |
In all cases, n > 3, and each measurement represents an independent experiment with BMMs derived from single age- and sex-matched DUSP1+/+ and DUSP1−/− mice. mRNAs were quantified by qPCR or RPA. COX-2 protein levels were estimated by scanning densitometry of Western blots.
Dex inhibited the expression of LPS-induced genes between 43 (chemokine (C-C motif) ligand 3 [CCL3]) and 98% (CSF2). Three distinct groups of genes could be recognized. COX-2, IFN-γ, IL-1α, and IL-1β were all strongly inhibited by Dex, and inhibition was severely impaired in DUSP1−/− BMMs (Fig. 3, top). The expression of the COX-2 protein closely mirrored that of COX-2 mRNA (Table I). In the cases of CXCL1, CCL3, IL-6, and IL-1Ra, the inhibitory effects of Dex were less strong yet were substantially impaired in DUSP1−/− BMMs (Fig. 3, middle). The expression of CXCL1 protein mirrored that of CXCL1 mRNA (Fig. 2 B and Table I). In other cases, inhibitory effects of Dex ranged from weak (iNOS) to powerful (CSF2 and IL-12p40) but were not impaired in DUSP1−/− BMMs (Fig. 3, bottom). IL-10 mRNA was unaffected by Dex in DUSP1−/− BMMs (Fig. 2 B); however, in DUSP1+/+ BMMs, IL-10 mRNA levels were close to the limit of detection, and the effects of Dex could not be determined.
TNF mRNA was equally inhibited by Dex in DUSP1+/+ and DUSP1−/− BMMs at the 4-h time point (Fig. 3 and Table I) as well as at earlier time points (not depicted). This contrasts with the impaired inhibition of TNF protein levels in DUSP1−/− BMMs (Fig. 2 B). The uncoupling of TNF mRNA and protein levels reflects the fact that TNF biosynthesis is strongly regulated at the translational level. Dex reportedly blocks TNF translation by inhibiting JNK (23), and p38 MAPK is also known to regulate TNF translation (for review see reference 14). Interestingly, several other genes strongly affected by the DUSP1 knockout are positively regulated by p38 MAPK via mRNA stabilization and are negatively regulated by GCs via mRNA destabilization (for review see reference 14; 24–26). However, few of these studies were performed in mouse macrophages.
To investigate the involvement of p38 MAPK signaling, DUSP1+/+ and DUSP1−/− BMMs were stimulated with LPS in the presence of Dex and/or a p38 MAPK inhibitor, SB202190. Inhibition of p38 MAPK reduced the expression of COX-2 and IL-1β proteins (Fig. 2 C, top), IL-1α, IL-1β, and IFN-γ mRNAs (Fig. 2 C, bottom) in both DUSP1+/+ and DUSP1−/− BMMs. Dex impaired the expression of these genes strongly in DUSP1+/+ but weakly in DUSP1−/− BMMs. IL-10 mRNA was undetectable in DUSP1+/+ BMMs but was reduced by SB202190 in DUSP1−/− BMMs. The expression of IL-12p40 mRNA was increased by the inhibition of p38 MAPK in both types of BMMs, although the effect was particularly clear in DUSP1−/− BMMs (in which basal expression was lower). These observations suggest that Dex inhibits the expression of COX-2, IL-1α, IL-1β, and IFN-γ in large part by inhibiting p38 MAPK. In contrast, genes like IL-12p40 are not dependent on p38 MAPK and are inhibited by Dex in the absence or presence of DUSP1. In fact, IL-12p40 appears to be negatively regulated by the p38 MAPK pathway, although it is not clear whether this is direct or indirect (e.g., mediated by IL-10). We also confirm that IL-10 expression in mouse macrophages is p38 MAPK dependent (20).
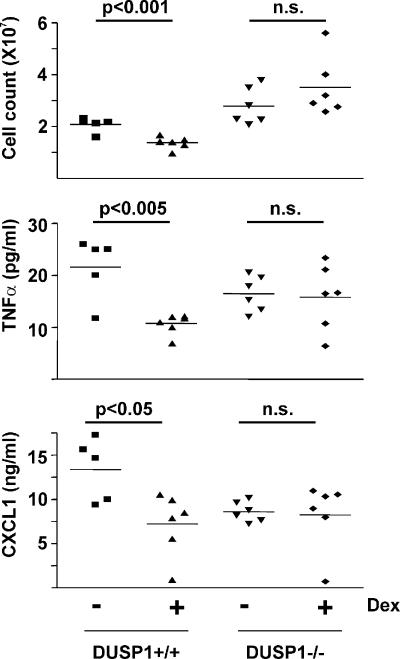
We next tested the effects of DUSP1 gene knockout on the antiinflammatory function of GC in vivo using the cutaneous air pouch model (27). Zymosan-induced infiltration of leukocytes into a preformed dorsal cavity is macrophage driven, GC sensitive, and dependent on proinflammatory cytokines and chemokines, including TNF and CXCL1. In wild-type mice (Fig. 4), 1 mg/kg Dex (administered orally) significantly decreased the concentration of both TNF (P < 0.005) and CXCL1 (P < 0.05) in the air pouch inflammatory exudate and the number of infiltrating leukocytes (P < 0.001). In DUSP1−/− mice, Dex did not significantly inhibit the expression of TNF, CXCL1, or the infiltration of leukocytes to the air pouch (P > 0.05). Thus, antiinflammatory effects of Dex in this model are dependent on the expression of DUSP1. Collagen-induced arthritis, a well-established mouse model of chronic inflammatory disease, is exacerbated in DUSP1−/− mice (19). It will be of interest to investigate whether the DUSP1 knockout also impairs the antiinflammatory actions of GCs in this and other models of immune-mediated inflammatory disease.
Figure 4.
Impaired antiinflammatory action of Dex in DUSP1−/− mice. Dorsal air pouches were created in DUSP1+/+ and DUSP1−/− mice. Mice were orally dosed with 1 mg/kg Dex or vehicle. After 1 h, zymosan was injected into the air pouches. After a further 4 h, mice were killed, and leukocyte numbers and cytokine concentrations in exudates were quantified. Horizontal bars above graphs represent statistical analysis of differences between control and Dex-treated animals (Student's t test).
Our observations suggest the existence of both DUSP1-dependent and -independent mechanisms of the antiinflammatory action of GCs in a single cell type. Inhibitory effects of Dex on individual genes may be strongly DUSP1 dependent (e.g., IL-1α), independent of DUSP1 (e.g., CSF2), or partially dependent on DUSP1 (e.g., TNF, COX-2, and several others). An important conclusion is that antiinflammatory effects of GCs involve the induction of gene expression via a noncanonical mechanism that does not require GR dimerization. To understand and predict the actions of novel dissociated GR agonists, it may be important to determine whether they are capable of inducing DUSP1 expression. As described previously (20), the consequences of ablating the DUSP1 gene are complex, involving the dysregulated expression of both pro- and antiinflammatory cytokines that are likely to exert secondary effects upon signaling pathways. Both in vitro and in vivo, the net outcome is the expression of inflammatory mediators that is (to a greater or lesser extent) insensitive to GCs. Thus, the phenotype of the DUSP1 knockout superficially resembles GC insensitivity. We note that GC insensitivity in asthma and inflammatory bowel disease has been linked to elevated JNK and p38 MAPK activities, which failed to be suppressed by GCs (28, 29). This raises the interesting possibility that in some instances, GC insensitivity in human inflammatory diseases may be related to defects in the expression or activity of DUSP1 (for review see reference 22).
MATERIALS AND METHODS
Reagents.
Reagents were purchased from Sigma-Aldrich unless otherwise stated. Antibodies against phosphorylated JNK, ERK, and p38 MAPK were obtained from Cell Signaling. Antibodies against DUSP1, DUSP4, and iNOS were obtained from Santa Cruz Biotechnology, Inc. Antibodies against tubulin-α and COX-2 were purchased from Sigma-Aldrich and Cayman Chemical, respectively. A rabbit polyclonal antiserum was raised against a C-terminal peptide of ERK1 and detected both ERK1 and 2 in Western blots. DUSP1−/− mice were originally generated as described previously (21) and were rederived at the Charles River Laboratories by implantation of DUSP1−/− blastocysts into pseudopregnant C57BL/6 females.
Mice, genotyping, cells, and in vitro and in vivo treatments.
All animal procedures were performed under United Kingdom Home Office regulations and with local Ethical Review Committee approval. A DUSP1+/− colony was maintained on an ad libitum expanded rodent SDS RM3 diet and fresh water in a specific pathogen-free animal facility (Federation of European Laboratory Animal Science Associations). Heterozygotes were bred to generate DUSP1+/+ and DUSP1−/− littermates, which were identified by a PCR-based screen of genomic DNA from tail snips. GRdim mice were identified as described previously (5). Age- and sex-matched animals were used to generate BMMs by differentiation from BM haemopoeitic stem cells for 5–7 d in Dulbecco's modified Eagle's medium supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 ng/ml CSF1 (PeproTech). In vitro experiments were performed using the same medium without CSF1. BMMs were stimulated with 10 ng/ml Salmonella typhimurium LPS with or without pretreatment for 4 h with Dex or vehicle (0.1% ethanol).
For the air pouch acute inflammation model, mice were subjected to light anesthesia using halothane. A localized cavity on the dorsal surface of the mice was created by injecting 3 ml of air subcutaneously. 4 d later, a further 1.5 ml of air was injected. 1 wk after the initial injection, the mice were gavaged with 1 mg/kg Dex or PBS. 1 h later, 1 mg zymosan in PBS was injected into the air pouch. 4 h later, the mice were culled by asphyxiation in CO2. The pouches were injected with 1 ml PBS/EDTA, massaged, were carefully dissected, and the exudates were collected. The exudate was analyzed for cytokine content by ELISA, and a cell count was performed by trypan blue staining and hemocytometry.
Detection and measurement of proteins.
Cell culture supernatants and air pouch exudates were analyzed for cytokine content using sandwich ELISA kits from R&D Systems. Cells were harvested by lysis in sample buffer (125 mM Tris-HCl, pH 6.8, 100 mM DTT, 2% SDS, 10% glycerol, and 0.1% bromophenol blue). Proteins were detected by immunoblotting using appropriate horseradish peroxidase–coupled secondary antibodies (DakoCytomation) and enhanced chemiluminescence reagents (GE Healthcare). COX-2 protein expression was estimated by scanning densitometry of Western blots using a calibrated imaging densitometer (GS-710; Bio-Rad Laboratories) and Phoretix ID software.
Measurement of mRNAs.
Total cellular RNA was isolated using the QIAamp RNA Blood kit (QIAGEN). Several mRNAs were quantified using Riboquant multiprobe RPA reagents (BD Biosciences) according to the manufacturer's instructions. Probes were synthesized using either the mCK2b template kit or a custom-made kit containing iNOS, IL-1β, LTβ, CXCL1, IL-6, CCL3, IL-18, L32, and GAPDH templates (both from BD Biosciences). Protected RNA fragments were detected and quantified by phosphorimaging (FLA2000; Fuji). Other transcripts were measured by quantitative real-time PCR using One-Step TaqMan RT-PCR reagents, prevalidated primer-probe sets, and a thermal cycler (Prism 7700; all from Applied Biosystems). Changes in mRNA abundance were assessed by the comparative threshold cycle (ΔCt) method and normalized against GAPDH (measured by the same method).
Statistical analysis.
The in vitro cytokine production of TNF and CXCL1 protein levels between DUSP1+/+ and DUSP1−/− macrophages was analyzed using one-way analysis of variance with the Bonferroni Post test for multiple comparisons. The in vivo cellular infiltrate and cytokine production levels were analyzed using the Student's t test for normally distributed data and the Mann-Whitney U test for nonparametric data. All tests were performed using Prism software version 4 (GraphPad). A P value < 0.05 was considered significant.
Acknowledgments
We thank Ewa Paleolog for help with statistical analyses, Andy Cato (Forschungszentrum Karlsruhe), and Bristol-Myers Squibb for the provision of DUSP1−/− mice.
This work was supported, in part, by a Wellcome Trust (United Kingdom) Clinical Training Fellowship to S.M. Abraham, Medical Research Council (United Kingdom) Programme grant G8623776 to J. Saklatvala and A.R. Clark, and Deutsche Forschungsgemeinschaft grant DFG TU 220/3-1 to J. Tuckermann.
The authors have no conflicting financial interests.
References
- 1.Newton, R. 2000. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 55:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adcock, I.M., and S.J. Lane. 2003. Corticosteroid-insensitive asthma: molecular mechanisms. J. Endocrinol. 178:347–355. [DOI] [PubMed] [Google Scholar]
- 3.Adcock, I.M., and G. Caramori. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 79:376–384. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa, S., J. Lozach, C. Benner, G. Pascual, R.K. Tangirala, S. Westin, A. Hoffmann, S. Subramaniam, M. David, M.G. Rosenfeld, and C.K. Glass. 2005. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 122:707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichardt, H.M., K.H. Kaestner, J. Tuckermann, O. Kretz, O. Wessely, R. Bock, P. Gass, W. Schmid, P. Herrlich, P. Angel, and G. Schutz. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 93:531–541. [DOI] [PubMed] [Google Scholar]
- 6.Reichardt, H.M., J.P. Tuckermann, M. Gottlicher, M. Vujic, F. Weih, P. Angel, P. Herrlich, and G. Schutz. 2001. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 20:7168–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuckermann, J.P., H.M. Reichardt, R. Arribas, K.H. Richter, G. Schutz, and P. Angel. 1999. The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J. Cell Biol. 147:1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schacke, H., H. Rehwinkel, and K. Asadullah. 2005. Dissociated glucocorticoid receptor ligands: compounds with an improved therapeutic index. Curr. Opin. Investig. Drugs. 6:503–507. [PubMed] [Google Scholar]
- 9.Rogatsky, I., J.C. Wang, M.K. Derynck, D.F. Nonaka, D.B. Khodabakhsh, C.M. Haqq, B.D. Darimont, M.J. Garabedian, and K.R. Yamamoto. 2003. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA. 100:13845–13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao, Q., E.G. Shepherd, M.E. Manson, L.D. Nelin, A. Sorokin, and Y. Liu. 2005. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J. Biol. Chem. 280:8101–8108. [DOI] [PubMed] [Google Scholar]
- 11.Clark, A.R., and M. Lasa. 2003. Crosstalk between glucocorticoids and mitogen-activated protein kinase signalling pathways. Curr. Opin. Pharmacol. 3:404–411. [DOI] [PubMed] [Google Scholar]
- 12.Camps, M., A. Nichols, and S. Arkinstall. 2000. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 14:6–16. [PubMed] [Google Scholar]
- 13.Kracht, M., and J. Saklatvala. 2002. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 20:91–106. [DOI] [PubMed] [Google Scholar]
- 14.Clark, A.R., J.L. Dean, and J. Saklatvala. 2003. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 546:37–44. [DOI] [PubMed] [Google Scholar]
- 15.Nimah, M., B. Zhao, A.G. Denenberg, O. Bueno, J. Molkentin, H.R. Wong, and T.P. Shanley. 2005. Contribution of MKP-1 regulation of p38 to endotoxin tolerance. Shock. 23:80–87. [DOI] [PubMed] [Google Scholar]
- 16.Chen, P., J. Li, J. Barnes, G.C. Kokkonen, J.C. Lee, and Y. Liu. 2002. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 169:6408–6416. [DOI] [PubMed] [Google Scholar]
- 17.Hammer, M., J. Mages, H. Dietrich, A. Servatius, N. Howells, A.C. Cato, and R. Lang. 2006. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J. Exp. Med. 203:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao, Q., X. Wang, L.D. Nelin, Y. Yao, R. Matta, M.E. Manson, R.S. Baliga, X. Meng, C.V. Smith, J.A. Bauer, et al. 2006. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J. Exp. Med. 203:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salojin, K.V., I.B. Owusu, K.A. Millerchip, M. Potter, K.A. Platt, and T. Oravecz. 2006. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J. Immunol. 176:1899–1907. [DOI] [PubMed] [Google Scholar]
- 20.Chi, H., S.P. Barry, R.J. Roth, J.J. Wu, E.A. Jones, A.M. Bennett, and R.A. Flavell. 2006. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. USA. 103:2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorfman, K., D. Carrasco, M. Gruda, C. Ryan, S.A. Lira, and R. Bravo. 1996. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 13:925–931. [PubMed] [Google Scholar]
- 22.Clark, A.R. 2003. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J. Endocrinol. 178:5–12. [DOI] [PubMed] [Google Scholar]
- 23.Swantek, J.L., M.H. Cobb, and T.D. Geppert. 1997. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor α (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 17:6274–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai, Y., S. Datta, M. Novotny, and T.A. Hamilton. 2003. TGFβ inhibits LPS-induced chemokine mRNA stabilization. Blood. 102:1178–1185. [DOI] [PubMed] [Google Scholar]
- 25.Mavropoulos, A., G. Sully, A.P. Cope, and A.R. Clark. 2005. Stabilization of IFN-gamma mRNA by MAPK p38 in IL-12- and IL-18-stimulated human NK cells. Blood. 105:282–288. [DOI] [PubMed] [Google Scholar]
- 26.Lasa, M., M. Brook, J. Saklatvala, and A.R. Clark. 2001. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol. 21:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence, T., D.W. Gilroy, P.R. Colville-Nash, and D.A. Willoughby. 2001. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 7:1291–1297. [DOI] [PubMed] [Google Scholar]
- 28.Sousa, A.R., S.J. Lane, C. Soh, and T.H. Lee. 1999. In vivo resistance to corticosteroids in bronchial asthma is associated with enhanced phosphorylation of JUN N-terminal kinase and failure of prednisolone to inhibit JUN N-terminal kinase phosphorylation. J. Allergy Clin. Immunol. 104:565–574. [DOI] [PubMed] [Google Scholar]
- 29.Bantel, H., M.L. Schmitz, A. Raible, M. Gregor, and K. Schulze-Osthoff. 2002. Critical role of NF-kappaB and stress-activated protein kinases in steroid unresponsiveness. FASEB J. 16:1832–1834. [DOI] [PubMed] [Google Scholar]