Abstract
Despite many efforts, the nature of thymic immigrants that give rise to T cells has remained obscure, especially since it became known that extrathymic lineage-negative, Sca-1–positive, c-kit high progenitor cells differ from intrathymic early T cell progenitors (ETPs) by functional potential and dependence on Notch signaling. After our observation that intrathymic T cell precursors expressing a human CD25 reporter under control of pre-TCRα regulatory elements almost exclusively have the ETP phenotype, we have analyzed the phenotypic changes of reporter-expressing common lymphoid progenitor (CLP) cells in the bone marrow when cultured on Delta-like 1–expressing stromal cells. We note that these quickly adopt the phenotype of double negative (DN)2 thymocytes with little display of the ETP phenotype. Our data suggest that common lymphoid progenitor (CLP) cells could be responsible for the rapid reconstitution of thymus function after bone marrow transplantation since CLP cells in the blood have the capacity to rapidly enter the thymus and become DN2 thymocytes.
Self-renewing hematopoietic stem cells reside in the fetal liver or adult BM and have the ability to generate all blood lineages. These cells serve as a source of progressively committed precursors, such as common lymphoid progenitor (CLP) cells (1) and common myeloid progenitors (2). The most primitive T cell progenitors in the thymus are found in the heterogenous double negative (DN)1 thymocyte subset (CD44+CD25−) and were originally characterized as CD4loc-kit+ (3, 4). These cells were shown to have the potential to generate T, B, NK, and lymphoid dendritic cells, although no clonogenic assay was performed to directly show pluripotentiality. More detailed analysis of the DN1 subset led to the identification of early T lineage progenitors (ETPs) as lineage-negative, Sca-1 positive, c-kit high (LSK) and IL-7Rα−/lo (5). These cells were shown to have high T but only limited B and myeloid potential. Another study using subfractionation of DN1 cells according to c-kit and heat stable antigen (HSA) expression showed that although most DN1 subsets are able to generate T cells, canonical T cell progenitors were localized in the DN1a and b subsets that express high levels of c-kit and are HSA negative/low or HSA positive, respectively, and presumably have a precursor–product relationship (6). Recently, it has been shown that ETPs are equivalent to the DN1a+b fractions and can be further subdivided according to their expression levels of the Fms-like tyrosine kinase receptor 3 (Flt3) (7). ETPs expressing Flt3 were shown to constitute an immature subset, and loss of Flt3 expression correlated with loss of B cell potential. Similar results were obtained using a mouse expressing a CC chemokine receptor 9 (CCR9)–eGFP reporter gene (8). Further developmental progression of T cell progenitors is characterized by expression of CD25 (DN2 stage) and loss of CD44 and c-kit expression (DN3 stage).
ETPs are phenotypically similar to BM LSK cells that constitute a heterogenous population of hematopoietic stem cells with long-term or short-term self-renewing potential. Flt3 expression on BM LSK cells correlates with loss of self-renewal ability and with loss of erythroid and megakaryocyte potential (9, 10). Flt3 expression has also been shown to be essential during early B cell development (11). Recently, LSK cells with T cell potential have been identified in the blood stream (8, 12). The phenotypic similarity of ETPs and BM LSK cells and the occurrence of LSK cells circulating in blood have led to the hypothesis that LSK cells or a subfraction of LSK cells constitute the thymus seeding T cell precursor. However, immigration of these cells into the thymus has not been directly demonstrated even though it has been shown that intrathymic DN1a/b cells can reenter the thymus when injected intravenously (6). Furthermore, inhibition of Notch signaling has been shown to eliminate the ETP population while not touching the LSK population. These studies refute the identity of LSK and ETPs and raise serious questions concerning their precursor–product relationship.
Using transgenic reporter mice that express human CD25 (hCD25) under control of the Ptcra promoter and enhancer, our laboratory has previously identified two populations of BM cells: lin−c-kit+IL-7-Rα+, representing CLP cells, and lin−c-kit−/loB220+, designated CLP-2, which originates from CLP cells in vitro (13, 14). CLP-2 cells efficiently enter the thymus upon intravenous transfer and give rise to a single wave of T cells indicating a limited self-renewal capacity (14, 15). It has also been observed by others that early thymic immigrants after BM transfer are mostly c-kit− and enriched for B220+ (16). Thus, most BM-derived progenitor populations shown to directly seed the thymus are phenotypically different from ETPs. In this context, it is of interest that Pax5-deficient pro-B cells that express B220 but are unable to further progress toward the B lineage were recently shown to rapidly acquire expression of c-kit under conditions that foster T cell differentiation in vitro (17).
Here we have analyzed the DN1 thymic subset of hCD25 transgenic mice and found that most reporter gene–positive cells were localized in the DN1a and b subfraction, thus corresponding to ETPs. This prompted us to investigate the relationship of BM-derived hCD25+ CLP-1 and CLP-2 cells with ETPs. We found that CLP-1 and CLP-2 cells progress toward the T lineage with kinetics similar to thymic ETPs and undergo rapid phenotypic changes in terms of c-kit, B220, IL-7Rα, and Flt3 expression, suggesting a high degree of phenotypic plasticity. Importantly, these changes coincided with the acquisition of CD25 expression, indicating a rapid transition to the DN2 stage. In addition, we show that CLP-1 and CLP-2 cells have differential requirements for Flt3 signaling early during developmental progression.
RESULTS
hCD25+ DN1 cells are enriched in the DN1a and DN1b subfractions
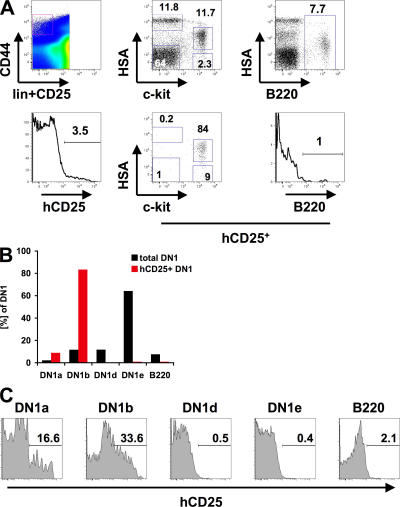
Using a human CD25 (hCD25) reporter gene under control of the Ptcra promoter and enhancer, our laboratory has previously identified c-kit−/loB220+ CLP-2 cells as T cell progenitors from BM that are able to efficiently seed the thymus after intravenous transfer (14, 15). Recently, subfractionation of the DN1 subset of thymocytes according to expression of HSA (CD24) and c-kit revealed that the earliest T cell progenitors are likely to reside in the c-kithiHSA− fraction (DN1a) that then gives rise to c-kithiHSA+ DN1b cells (6). To test whether reporter gene–positive DN1 cells are part of these early T cell progenitor populations, we have analyzed DN1 populations of hCD25 reporter mice using 6-color flow cytometry. Approximately 4.4 ± 0.2% of all DN1 cells expressed the hCD25 marker, which is consistent with previous results (Fig. 1 A and reference 14). Analysis of HSA and c-kit expression revealed that most of the hCD25+ DN1 cells expressed high levels of c-kit. Of these cells the majority was HSA+ and thus part of the DN1b population. About 10% of all hCD25+ DN1 cells were c-kithiHSA− and thus DN1a cells. Only a minor fraction of hCD25+ DN1 cells expressed B220 (∼1%). Compared with the total pool of DN1 cells, these data show an enrichment of hCD25+ cells in both DN1a and DN1b fractions (Fig. 1 B), and only a small subset of hCD25+ DN1 cells expressing B220. The enrichment of hCD25+ cells in the DN1a and DN1b subsets is also reflected by the frequency of reporter-positive cells in each DN1 subpopulation (Fig. 1 C). DN1a cells contained >15% hCD25+ cells, and 34% of DN1b cells were hCD25+, representing a fourfold and eightfold enrichment, respectively, compared with the total DN1 population (Fig. 1 A). DN1d and DN1e cells were almost completely hCD25 negative, and the B220+ subset contained 2% hCD25+ cells. These data indicate that reporter-expressing cells ultimately enter the canonical pathway of T cell development.
Figure 1.
hCD25+ DN1 cells are enriched in the DN1a and DN1b subfractions. (A) Lineage-depleted thymocytes from hCD25 reporter mice were stained for lineage markers, CD25, CD44, c-kit, HSA, B220, and hCD25. The top panels indicate the location of the DN1 gate and total DN1 cells. The bottom panels indicate expression of hCD25 within the DN1 subset and the distribution of hCD25+ cells with respect to their expression of HSA, c-kit, and B220. Numbers indicate the percentage of cells within gates. (B) Distribution of hCD25+ DN1 cells compared with total DN1 cells within the DN1 subsets a–e. (C) Frequency of hCD25+ cells within different DN1 subsets gated as in A.
Developmental progression of different BM precursor subpopulations in vitro
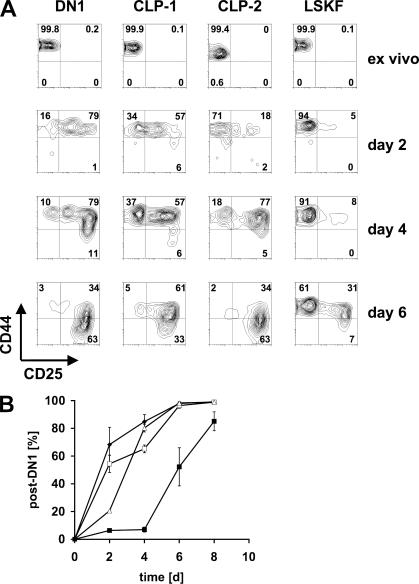
Two hCD25+ populations from BM have been previously identified: CLP-1 and CLP-2, the latter of which can efficiently seed the thymus upon intravenous transfer (14, 15). However, the surface phenotypes of CLP-2 (lin−c-kit−/loB220+) and hCD25+ DN1 cells (lin−c-kithi) differ considerably. In contrast, BM and blood-derived LSK cells expressing Flt3 (LSKF) are phenotypically similar to c-kithi DN1 cells. Analysis of the kinetics of developmental progression of different precursor populations can provide insight into precursor–progeny relationships irrespective of divergent surface phenotypes. We therefore compared the developmental progression of different BM-derived precursors and hCD25+ DN1 cells (predominantly representing ETPs) in OP9-DL1 coculture (18). At the beginning of the culture period, all precursor populations had a CD44+CD25−DN1 phenotype. Fig. 2 shows that hCD25+ CLP-1 and CLP-2 cells progress beyond the DN1 stage as indicated by the expression of CD25 with similar, but slightly delayed, kinetics compared with ETPs. The developmental progression of lin−Sca-1+c-kithiFlt3+ (LSKF) cells from BM, which contain the early lymphoid progenitor cell population, however, exhibits a delay of 2–4 d (Fig. 2, A and B). These data suggest that both CLP-1 and CLP-2 can more rapidly respond to Notch signals and thus to an environment that fosters T cell development compared with LSKF cells.
Figure 2.
Developmental progression of different precursor populations in vitro. (A) Sorted hCD25+ DN1 cells, BM hCD25+ CLP-1 and CLP-2, and BM LSKFlt3+ (LSKF) cells were cultured on OP9-DL1 cells in the presence of IL-7 (1 ng/ml) and Flt3L (5 ng/ml), and their developmental progression was assessed by surface staining for CD44 and CD25 and FACS analysis. Ex vivo indicates CD44 and CD25 expression of the starting population. Numbers in quadrants indicate the percentage of cells. (B) Quantitation of developmental progression beyond the DN1 stage. Populations described in A were cultured on OP9-DL1 cells in the presence of IL-7 (1 ng/ml) and Flt3L (5 ng/ml) and developmental progression along the T cell lineage was monitored by analysis of CD25 surface expression. Data are shown ± SEM.
Phenotypic plasticity of different precursor populations
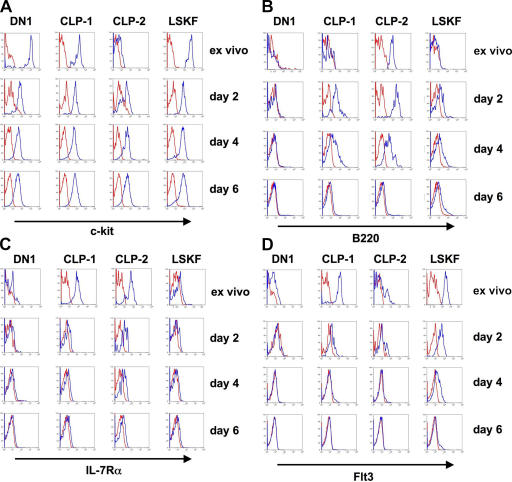
CLP-2 cells have been shown to have efficient thymic homing potential (14, 15). However, only a minute fraction of hCD25+ cells in the DN1 compartment expresses B220, whereas the majority of hCD25+ cells expresses high levels of c-kit, thus representing ETP (Fig. 1). We therefore addressed the possibility that different BM-derived precursor populations can undergo rapid phenotypic changes with respect to B220 and c-kit expression during short-term differentiation in an environment that fosters T cell development. As shown in Fig. 3 A, after 2 d of coculture on OP9-DL1 cells, DN1, CLP-1, and LSKF expressed similar levels of c-kit. CLP-2 cells displayed c-kit expression, albeit with slightly reduced amounts. After 4 and 6 d of culture, c-kit expression levels were similar in all four populations analyzed. Although B220 expression was absent on DN1 cells throughout the complete culture period of 6 d (Fig. 3 B), B220 remained high on CLP-2 cells after 2 d of culture, and both CLP-1 and LSKF cells acquired low levels of B220 expression. The acquisition of B220 by CLP-1 during short-term culture is consistent with the notion that CLP-1 cells are progenitors of CLP-2 cells (14). After 4 d of culture, both CLP-1 and CLP-2 cells exhibited reduced expression of B220 compared with day 2 of culture, whereas B220 remained basically constant on LSKF cells. After 6 d of culture, expression of B220 was absent in cultures of CLP-1 and CLP-2 cells, whereas residual levels could be detected in LSKF cultures.
Figure 3.
Phenotypic plasticity of different precursor populations. Sorted hCD25+ DN1 cells, BM hCD25+ CLP-1 and CLP-2, and BM LSKF cells were cultured on OP9-DL1 cells in the presence of IL-7 (1 ng/ml) and Flt3L (5 ng/ml) (A–C) or SCF (100 ng/ml) (D) for up to 6 d and analyzed for surface expression of (A) c-kit, (B) B220, (C) IL-7Rα, and (D) Flt3. Ex vivo indicates c-kit (A), B220 (B), IL-7Rα (C), and Flt3 (D) expression of the starting population. Red histograms represent controls, and blue histograms represent specific staining.
Although characteristic for CLP cells, IL-7Rα expression is absent on LSKF cells and ETP. However, IL-7Rα expression has been reported for DN1e cells. We analyzed IL-7Rα during the course of developmental progression of different precursor populations. IL-7Rα expression was found to be very low on ETPs and LSKF cells over the entire culture period of 6 d. CLP-1 and CLP-2 rapidly lost IL-7Rα expression, CLP-2 with somewhat slower kinetics (Fig. 3 C).
Recently, it has been shown that ETPs can be subdivided according to their levels of Flt3 expression (7) in that loss of Flt3 expression correlates with progressive restriction toward the T lineage. It was shown that a fraction of BM- and blood-derived LSK cells (LSKF) and CLP-1 cells express Flt3 (10, 19). Levels of Flt3 expression by CLP-2 have not been investigated, and analysis of Flt3 expression on hCD25+ DN1 cells might provide additional insight into their developmental status. Flow cytometric analysis showed that both CLP-1 and LSKF cells expressed high levels of Flt3 as previously published, whereas only a minor fraction of CLP-2 cells was Flt3 positive (Fig. 3 D). hCD25+ DN1 cells expressed low levels of Flt3, suggesting that these constitute an early ETP subset. Notably, all three BM-derived precursor populations analyzed differed from hCD25+ DN1 cells in terms of Flt3 expression. After 2 d of coculture on OP9-DL1 cells, Flt3 expression was completely lost on hCD25+ DN1 cells, whereas low levels of Flt3 could still be detected on both CLP-1 and CLP-2 cells. BM LSKF cells retained high expression of Flt3 after 2 d (Fig. 3 D). After 4 d, residual levels of Flt3 on CLP-1 and CLP-2 cells and somewhat higher levels on LSKF cells could be detected. After 6 d of coculture, Flt3 expression was lost on DN1-, CLP-1–, and CLP-2–derived cells, but some Flt3 was still observed on LSKF cells. These data suggest that, although overall Flt3 expression is lost during developmental progression, CLP-2 cells initially acquire a more uniform expression of low levels of Flt3. In addition, both CLP-1 and CLP-2 show Flt3 expression levels after 2 d of culture comparable to ex vivo hCD25+ DN1 cells. In conclusion, these data indicate that all precursors analyzed show a high degree of phenotypic plasticity with respect to c-kit expression, rendering them virtually indistinguishable based on this surface marker after a short period of time. Interestingly, under these culture conditions all BM-derived precursors acquired B220 surface expression, whereas thymus-derived DN1 cells did not, suggesting that the latter cells have received signals in vivo before isolation that precludes expression of B220.
Differential requirement for Flt3 ligand of different precursor populations
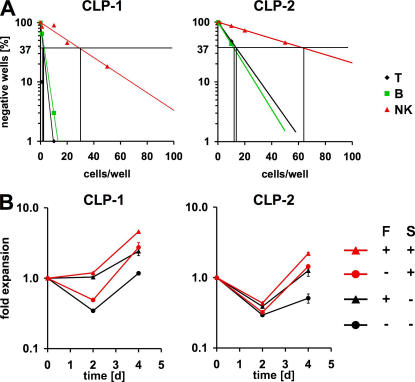
CLP-1 and CLP-2 cells rapidly acquire a similar surface phenotype during in vitro T cell differentiation. However, when freshly isolated they display drastic differences in surface receptor expression, especially expression of the receptor tyrosine kinases Flt3 and c-kit. To explore the consequences of these differences, we first quantitated T, B, and NK lineage potential of CLP-1 and CLP-2 cells. We sorted different amounts of precursor cells on OP9-DL1 and OP9-GFP cells and determined the frequency of lineage positive outgrowth by analysis of surface expression of Thy-1.1, CD19, and NK1.1, respectively. This analysis revealed that the potential of CLP-1 cells to generate both T and B cells is higher (∼1 in 2 and ∼1 in 3, respectively) than that of CLP-2 cells (∼1 in 14 and ∼1 in 12, respectively) (Fig. 4 A). The NK cell potential of the two populations was also different, though to a somewhat lesser extent (∼1 in 29 versus ∼1 in 63). These data could suggest that CLP-1 and CLP-2 cells differ in their requirements for survival factors during development. As shown in Fig. 3, CLP-1 (bearing both receptors) and CLP-2 differ in their expression of both Flt3 and c-kit. To functionally test the consequences of differential Flt3 and c-kit expression on CLP-1 and CLP-2 cells, these populations were cultured on OP9-DL1 cells in the presence or absence of Flt3 ligand (Flt3L) or stem cell factor (SCF) (Fig. 4 B). Whereas during the first 2 d of culture cell numbers in CLP-2 cultures declined by a factor of 2 to 3 in the presence of Flt3L, SCF, or both, numbers of CLP-1 cells did not decline in the presence of Flt3L. SCF had no effect on cell numbers during a 2-d culture period. During an additional 2 d of culture, Flt3L exerted only a minor effect on the expansion of CLP-1 cells, whereas a strong effect of SCF was observed. Interestingly, CLP-2 cells clearly expanded to a similar degree in the presence of either Flt3L or SCF (Fig. 4 B). After 6 d of culture, Flt3L and SCF had influenced expansion of both CLP-1– and CLP-2–derived cells similarly (not depicted), reflecting the nearly identical surface phenotype of both populations at this time point. The two cytokines had an additive effect after 6 d. Collectively, these data reflect both the observed changes in Flt3 surface receptor expression and the different cloning efficiencies in in vitro culture: whereas Flt3L exerts its effect mostly during the first 2 d of culture on CLP-1 cells, CLP-2 cells acquire responsiveness to Flt3L only after 2 d. Interestingly, SCF alone had no major effect on proliferation during the first 2 d of culture despite the fact that CLP-1 express c-kit, the ligand for SCF.
Figure 4.
Developmental potential in vitro and differential requirement for Flt3L of different precursor populations. (A) Limiting dilution analysis of T, B, and NK potential of BM-derived CLP-1 and CLP-2 cells. 1, 5, 10, 20, or 50 cells were directly sorted onto OP9-DL1 or OP9-GFP cells and analyzed by FACS after 18 d. (B) 103 sorted BM CLP-1 and CLP-2 cells were cultured on OP9-DL1 cells for 4 d in the presence or absence of Flt3L (5 ng/ml) or SCF (100 ng/ml) or both. Cell numbers were assessed during FACS analysis and are shown ± SEM.
DISCUSSION
At present there is little information on how extrathymic precursors of T cells are connected to intrathymic progenitors, i.e., the nature of cells that immigrate into the thymus most efficiently and give rise to T cells has remained elusive. An ETP was originally discovered by Wu et al. (3) and reported to have a c-kit+CD4lo phenotype. Even though it was not assayed under clonal conditions, this precursor was endowed with the potential to generate T, B, and dendritic cells under appropriate conditions. This subset of cells was then further characterized as a c-kithi, IL-7Rα− ETP population (5) and shown to reside in the DN1a and b subsets of thymocytes characterized by high expression of c-kit, lack of IL-7Rα expression, and low or high expression of HSA (6). The fact that the ETP apparently still had some myeloid potential and resembled in surface phenotype hematopoietic precursors in blood and BM that were lineage marker negative, c-kit high, and Sca-1 positive (LSK) was taken as evidence that LSK cells were direct progenitors of ETP (12). In this view, so-called common lymphoid progenitor cells, which have T, B, and dendritic cell potential, but no myeloid potential, were excluded as candidates for thymic immigrants, because cells with this phenotype (c-kitloSca-1+IL-7Rα+) were not found in blood. However, the functional characterization of LSK and ETP and the dependence on Notch signaling have introduced a severe caveat to a direct LSK-ETP precursor–progeny relationship: ETPs contained in the DN1a and b fraction have very little B cell precursor potential compared with LSK cells from BM and blood (6). Furthermore, inhibition of Notch-dependent transcriptional regulation by two distinct approaches showed that the ETPs were almost entirely dependent on Notch, whereas LSK were not at all affected by the lack of Notch-dependent transcription (7, 20). These results therefore failed to establish a direct precursor–product relationship between LSK and ETP and stressed the need to better define immigrants that may give rise to ETP.
Our laboratory has previously used a human CD25 reporter under control of Ptcra regulatory elements to search for T cell precursors inside and outside the thymus (13, 14). These studies have been continued here to place the reporter-expressing precursors within the context of recently described canonical T cell precursors. Here we show that within the thymus the reporter-positive cells within the DN1 population represent almost exclusively ETPs in the DN1a+b subsets. These results prompted us to analyze the changes in surface phenotype of reporter-expressing BM-derived precursors by using the in vitro culture system employing Delta-like 1–expressing OP9 stromal cells.
Previous studies have shown that a novel CLP cell subset with a c-kit−/loB220+IL-7Rα+ surface phenotype, designated CLP-2, expresses the reporter and can efficiently colonize the thymus within 24 h after intravenous injection (14–16). To analyze how these cells differentiate apart from producing a wave of bona fide CD4+CD8+ thymocytes after intravenous injection, they were cultured on OP9-DL1 feeder cells and compared with the CLP-1 population and LSKF cells. The results show that the CLP-2 cells under the influence of Notch signaling quickly down-regulate B220 and Flt3 and become mostly c-kit+CD25+ cells that resemble DN2 thymocytes. Thus, as described for B220+ cells from Pax5-deficient mice, these CLP cells are able to rapidly revert their surface phenotype, reiterating that c-kit and B220 expression do not represent stable markers in the differentiation of hematopoietic and lymphoid cells but can be up- and down-regulated under the influence of external factors that regulate differentiation (17). These changes in surface phenotype expression cannot be accounted for by the outgrowth of B220−c-kit+ cells since intermediates, i.e., c-kit+B220+ cells, are clearly visible in culture. When CLP-1 cells are cultured, some of the cells transiently express B220 but otherwise assume the DN2 phenotype, as do the CLP-2 cells. It was previously shown that CLP-2 can be derived from CLP-1 cells, and hence the transient B220 expression does not come as a surprise (14). Thus, the CLP cell populations under the influence of Notch signaling rapidly progress to a thymic DN2 phenotype without generating many cells that exhibit an ETP phenotype. The DN2 cells generated in culture exhibit reduced IL-7 receptor levels compared with ex vivo DN2 cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20060731/DC1), possibly because of elevated IL-7 levels in the culture medium. On the other hand, LSK cells developmentally progress in terms of acquisition of CD25 expression with some delay compared with the CLP cell populations, indicating that the CLP cells have already further differentiated toward the T cell lineage, an observation which is consistent with their expression of the Ptcra-controlled reporter, which is absent on LSK (13). In this context, it is of interest that it has been recently reported that upon BM transplantation into irradiated mice, thymocytes expressing CD25, thus resembling DN2/3 cells, are detectable in the thymus before the appearance of ETP (21). This is consistent with our finding (Figs. 2 and 3) that both CLP-1 and CLP-2 cells rapidly progress to a CD25+ stage upon in vitro differentiation with only a few cells exhibiting the ETP phenotype.
It was shown that in the BM transplantation model (21), most CD25+ cells in the spleen were derived from Flt3+LSK cells after 16 d, whereas CLP cells produced very few CD25+ cells. The authors interpret these results as an indication that LSK cells generate DN2-like cells that then migrate to the thymus to generate T cells, whereas CLP cells generate mostly B cells. However, the explanation that CLP cells quickly immigrate into the thymus and generate DN2 cells has not been ruled out by these studies. Thus, the source of intrathymic DN2 cells in these studies is largely obscure, and the majority of these cells may actually be derived from CLP cells that have been reported to have an excellent thymus-homing capacity (14, 16).
An observation similar to that for CLP cells has been made for Pax5−/− pro-B cells, which already express CD25 but display rapid further up-regulation when cultured on OP9-DL1 cells (17). Rapid up-regulation of CD25 might account for the fact that cells with CLP-1 and CLP-2 phenotype are virtually absent in the steady-state thymus.
It has been suggested that one critical effect of Notch signaling during early T cell development might be the up-regulation or maintenance of c-kit expression on precursor cells ultimately leading to similar c-kit surface expression (22), which is required for developmental progression along the T cell lineage (23). When comparing CLP-1 and CLP-2 cells with regard to c-kit and Flt3 ligands, we found that Flt3L had a greater impact than SCF on the survival and expansion of CLP-1 cells early during developmental progression, although both receptors, Flt3 and c-kit, were differentially expressed on CLP-1 and CLP-2 cells. These results are consistent with an earlier study showing that Flt3L is more potent than SCF with regard to the expansion of early thymic progenitors (24). Some progression and expansion was even observed in the absence of both cytokines, although we cannot exclude the presence of minute amounts of these cytokines in the culture media. In particular, it was shown previously that OP9 cells express SCF mRNA (25), whereas they appear negative for Flt3L message. This pattern of cytokine expression might account in part for the observations in our assay that Flt3L but not SCF enhanced expansion during the first days of culture (Fig. 4 B). However, exogenous SCF clearly had a beneficial effect on expansion of both populations between day 2 and 4 of culture, indicating that SCF produced by OP9 cells alone was not sufficient to promote optimal expansion of CLP-1 and CLP-2 cells.
In conclusion, our data showing that CLP-1 and CLP-2 cells rapidly acquire a phenotype corresponding to DN2 thymocytes provide a possible explanation for the fact that very few cells with CLP phenotype can be detected in the steady-state thymus. Moreover, our data suggest that CLP cells are responsible for the rapid reconstitution of thymus function after BM transplantation in the absence of considerable numbers of ETP (21).
MATERIALS AND METHODS
Mice.
hCD25 transgenic mice (FVB) have been described (13, 14). All mice were maintained in the specific pathogen–free animal facilities of the Dana-Farber Cancer Institute (DFCI). All animal procedures were done in compliance with the guidelines of the DFCI Animal Resources Facility, which operates under regulatory requirements of the U.S. Department of Agriculture and Association for Assessment and Accreditation of Laboratory Animal Care.
Cell lines and cell preparations.
OP9 BM stromal cells expressing the Notch ligand delta-like ligand 1 (OP9-DL1) and OP9 control cells (OP9-GFP) provided by Juan Carlos Zúñiga-Pflücker (University of Toronto, Toronto, Canada) were maintained in αMEM supplemented with 55 μM 2-mercaptoethanol, 10 mM Hepes (pH 7.5), 1 mM sodium pyruvate, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 50 μg/ml gentamycine, and 20% heat-inactivated FBS and passaged as described (18). BM cells and thymocytes were obtained as described previously (14).
Flow cytometry and cell sorting.
mAbs specific for CD4 (RM4-5, GK1.5), CD8 (53–6.7), CD25 (PC61), CD44 (IM7), TCRβ (H57-597), TCRγδ (GL3), Gr-1 (RB6-8C5), erythroid cell marker (Ter-119), CD19 (1D3), CD11c (HL3), CD11b (M1/70), pan-NK (DX5), NK1.1 (PK136), CD45.1 (A20), CD45.2 (104), B220 (RA3-6B2), c-kit (2B8), Sca-1 (E13-161.7), HSA (M1/69), and human CD25 (M-A251) were purchased from BD Biosciences and were used as biotin, FITC, phycoerythrin (PE), peridinin chlorophyll protein (PerCP), PerCP-Cy5.5, PE-Cy7, allophycocyanin (APC), or APC-Cy7 conjugates. mAbs specific for Flt3 (A2F10) and IL-7Rα (A7R34) were purchased from eBioscience. PE-Texas red or PerCP-conjugated streptavidin was used to reveal staining with biotinylated mAb. Four-color flow cytometry was performed on a FACSCalibur (BD Biosciences). Six-color flow cytometry was performed on a FACSAria (BD Biosciences). Data were analyzed with FlowJo software (Treestar). For analysis, dead cells and debris were excluded by appropriate gating of forward and side scatter. Lineage-negative cells were isolated from total thymocytes or BM by staining cell suspensions with a biotinylated lineage-specific antibody cocktail, followed by incubation with streptavidin-conjugated magnetic beads (Dynal) and magnetic bead depletion of mature lineages. Enriched cell suspensions were surface stained with streptavidin-APC. Cells were sorted using a FACSAria (BD Biosciences). All populations were resorted; sorted cells were of ≥99% purity, as determined by post-sort analysis.
OP9 cocultures.
OP9 coculture assays were essentially performed as described (18). Precursors were plated at an initial density of 5 × 102–2.5 × 103 onto subconfluent OP9-GFP or OP9-DL1 monolayers at 5 × 104 cells/well in a 24-well plate. All cocultures were performed in the presence of 1 ng/ml IL-7 and 5 ng/ml Flt3L for OP9-DL1 T cell differentiation assays and 5 ng/ml IL-7 and 5 ng/ml Flt3L for OP9-GFP cocultures. In certain experiments, Flt3L was replaced by 100 ng/ml SCF as indicated. At day 4 of differentiation, the culture medium was exchanged. Contaminating OP9 cells were eliminated by filtering the harvested cocultured cells through a 70-μm cell strainer before flow cytometric analysis. For cultures of less than 102, precursors cells were plated directly onto 96-well plates containing 104 γ-irradiated OP9-GFP or OP9-DL1 cells (15 Gy) using a FACSAria cell sorter.
Online supplemental material.
Fig. S1 shows IL-7Rα surface expression levels on DN thymocyte populations ex vivo and is available at http://www.jem.org/cgi/content/full/jem.20060731/DC1.
Supplemental Material
Acknowledgments
We would like to thank Dr. Fotini Gounari and Dr. Meinrad Busslinger for helpful discussions and Dr. Juan Carlos Zúñiga-Pflücker for the gift of OP9-DL1 cells. The authors are grateful to Xiaoyan Li for expert technical assistance and to Linnea Benson for editorial help.
This work was supported by grants from the Lymphoma Research Foundation and the German Research Foundation (Emmy-Noether Fellowship, KR2320/1-1 to A. Krueger) and the National Institutes of Health (AI45846) (to H. von Boehmer).
The authors have no conflicting financial interests.
Abbreviations used: CCR9, CC chemokine receptor 9; CLP, common lymphoid progenitor; DN, double negative; ETP, early T cell progenitor; Flt3, Fms-like tyrosine kinase receptor 3; Flt3L, Flt3 ligand; HSA, heat stable antigen; LSK, lineage negative, Sca-1–positive, c-kit high cells; LSKF, LSK cells expressing Flt3; SCF, stem cell factor.
References
- 1.Kondo, M., I.L. Weissman, and K. Akashi. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 91:661–672. [DOI] [PubMed] [Google Scholar]
- 2.Akashi, K., D. Traver, T. Miyamoto, and I.L. Weissman. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 404:193–197. [DOI] [PubMed] [Google Scholar]
- 3.Wu, L., R. Scollay, M. Egerton, M. Pearse, G.J. Spangrude, and K. Shortman. 1991. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 349:71–74. [DOI] [PubMed] [Google Scholar]
- 4.Moore, T.A., and A. Zlotnik. 1995. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 86:1850–1860. [PubMed] [Google Scholar]
- 5.Allman, D., A. Sambandam, S. Kim, J.P. Miller, A. Pagan, D. Well, A. Meraz, and A. Bhandoola. 2003. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4:168–174. [DOI] [PubMed] [Google Scholar]
- 6.Porritt, H.E., L.L. Rumfelt, S. Tabrizifard, T.M. Schmitt, J.C. Zuniga-Pflucker, and H.T. Petrie. 2004. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 20:735–745. [DOI] [PubMed] [Google Scholar]
- 7.Sambandam, A., I. Maillard, V.P. Zediak, L. Xu, R.M. Gerstein, J.C. Aster, W.S. Pear, and A. Bhandoola. 2005. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat. Immunol. 6:663–670. [DOI] [PubMed] [Google Scholar]
- 8.Benz, C., and C.C. Bleul. 2005. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J. Exp. Med. 202:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adolfsson, J., R. Mansson, N. Buza-Vidas, A. Hultquist, K. Liuba, C.T. Jensen, D. Bryder, L. Yang, O.J. Borge, L.A. Thoren, et al. 2005. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 121:295–306. [DOI] [PubMed] [Google Scholar]
- 10.Adolfsson, J., O.J. Borge, D. Bryder, K. Theilgaard-Monch, I. Astrand-Grundstrom, E. Sitnicka, Y. Sasaki, and S.E. Jacobsen. 2001. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1 (+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 15:659–669. [DOI] [PubMed] [Google Scholar]
- 11.Mackarehtschian, K., J.D. Hardin, K.A. Moore, S. Boast, S.P. Goff, and I.R. Lemischka. 1995. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 3:147–161. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz, B.A., and A. Bhandoola. 2004. Circulating hematopoietic progenitors with T lineage potential. Nat. Immunol. 5:953–960. [DOI] [PubMed] [Google Scholar]
- 13.Gounari, F., I. Aifantis, C. Martin, H.J. Fehling, S. Hoeflinger, P. Leder, H. von Boehmer, and B. Reizis. 2002. Tracing lymphopoiesis with the aid of a pTalpha-controlled reporter gene. Nat. Immunol. 3:489–496. [DOI] [PubMed] [Google Scholar]
- 14.Martin, C.H., I. Aifantis, M.L. Scimone, U.H. von Andrian, B. Reizis, H. von Boehmer, and F. Gounari. 2003. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat. Immunol. 4:866–873. [DOI] [PubMed] [Google Scholar]
- 15.Scimone, M.L., I. Aifantis, I. Apostolou, H. von Boehmer, and U.H. von Andrian. 2006. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc. Natl. Acad. Sci. USA. 103:7006–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori, S., K. Shortman, and L. Wu. 2001. Characterization of thymus-seeding precursor cells from mouse bone marrow. Blood. 98:696–704. [DOI] [PubMed] [Google Scholar]
- 17.Höflinger, S., K. Kesavan, M. Fuxa, C. Hutter, B. Heavey, F. Radtke, and M. Busslinger. 2004. Analysis of Notch1 function by in vitro T cell differentiation of Pax5 mutant lymphoid progenitors. J. Immunol. 173:3935–3944. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt, T.M., and J.C. Zúñiga-Pflücker. 2002. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 17:749–756. [DOI] [PubMed] [Google Scholar]
- 19.Sitnicka, E., D. Bryder, K. Theilgaard-Monch, N. Buza-Vidas, J. Adolfsson, and S.E. Jacobsen. 2002. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 17:463–472. [DOI] [PubMed] [Google Scholar]
- 20.Tan, J.B., I. Visan, J.S. Yuan, and C.J. Guidos. 2005. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat. Immunol. 6:671–679. [DOI] [PubMed] [Google Scholar]
- 21.Maillard, I., B.A. Schwarz, A. Sambandam, T. Fang, O. Shestova, L. Xu, A. Bhandoola, and W.S. Pear. 2006. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 107:3511–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodewald, H.R. 2006. Making a Notch in the lymphocyte kit. Eur. J. Immunol. 36:508–511. [DOI] [PubMed] [Google Scholar]
- 23.Massa, S., G. Balciunaite, R. Ceredig, and A.G. Rolink. 2006. Critical role for c-kit (CD117) in T cell lineage commitment and early thymocyte development in vitro. Eur. J. Immunol. 36:526–532. [DOI] [PubMed] [Google Scholar]
- 24.Moore, T.A., and A. Zlotnik. 1997. Differential effects of Flk-2/ Flt-3 ligand and stem cell factor on murine thymic progenitor cells. J. Immunol. 158:4187–4192. [PubMed] [Google Scholar]
- 25.Cho, S.K., T.D. Webber, J.R. Carlyle, T. Nakano, S.M. Lewis, and J.C. Zuniga-Pflucker. 1999. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc. Natl. Acad. Sci. USA. 96:9797–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.