Abstract
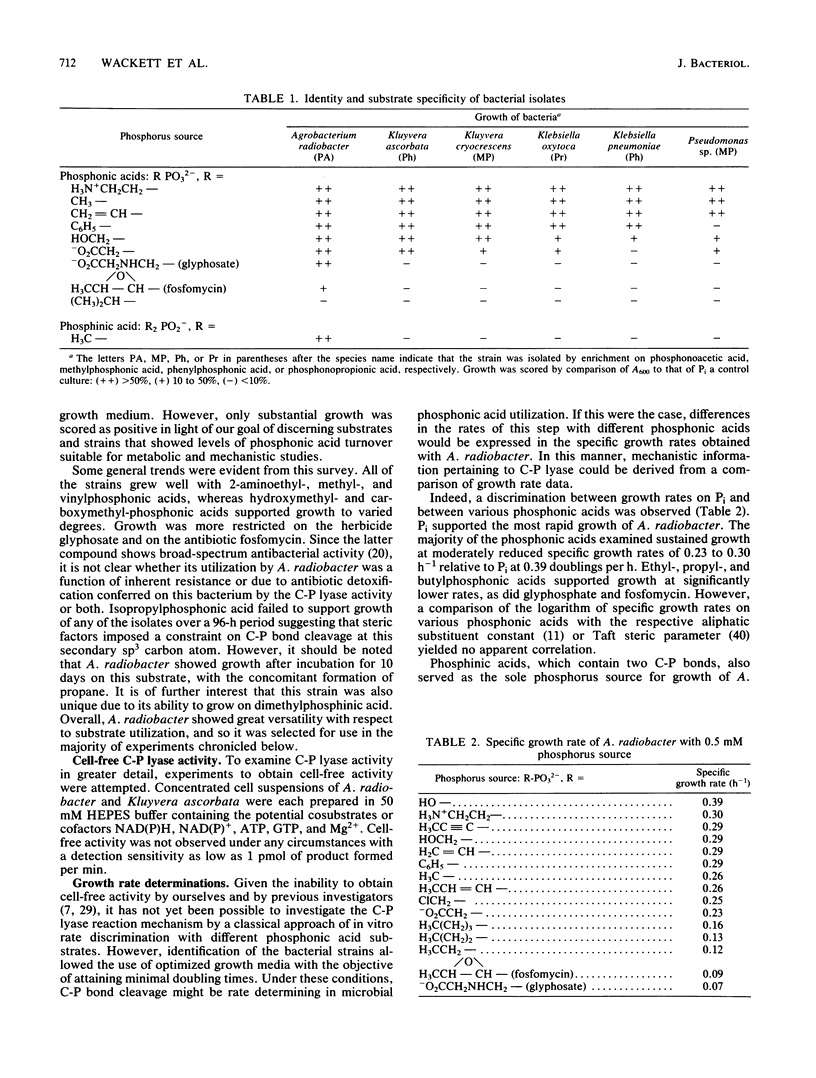
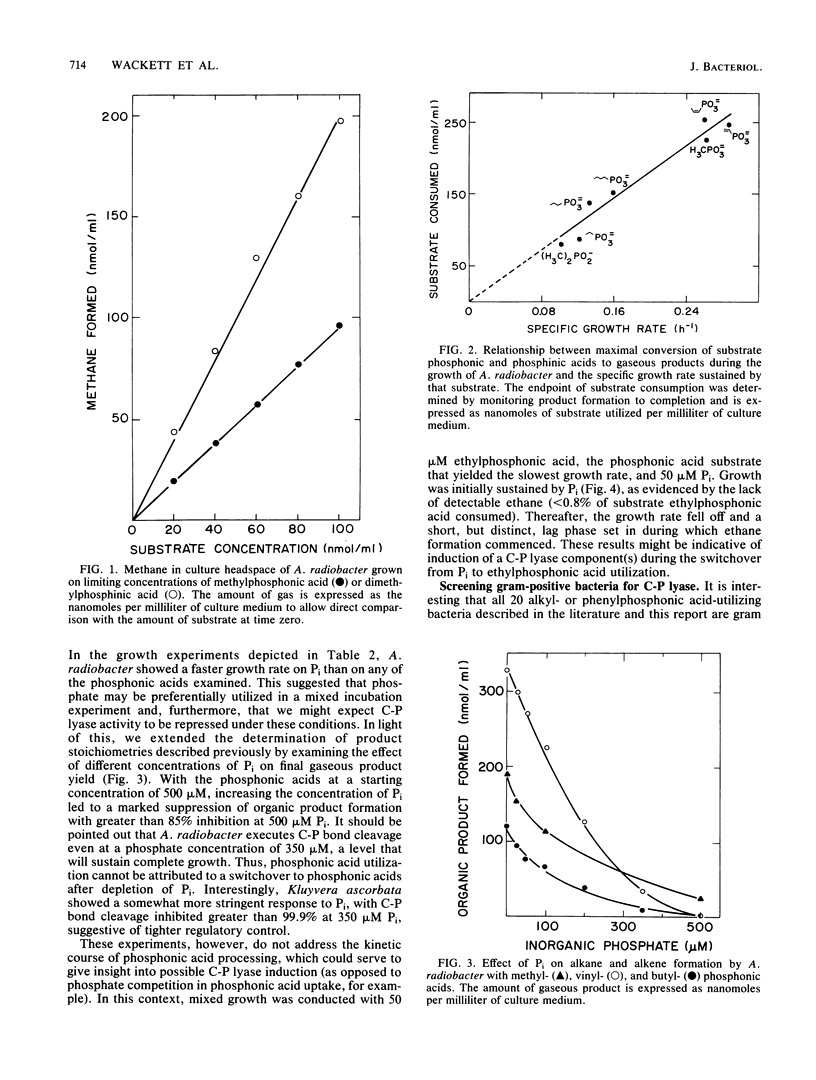
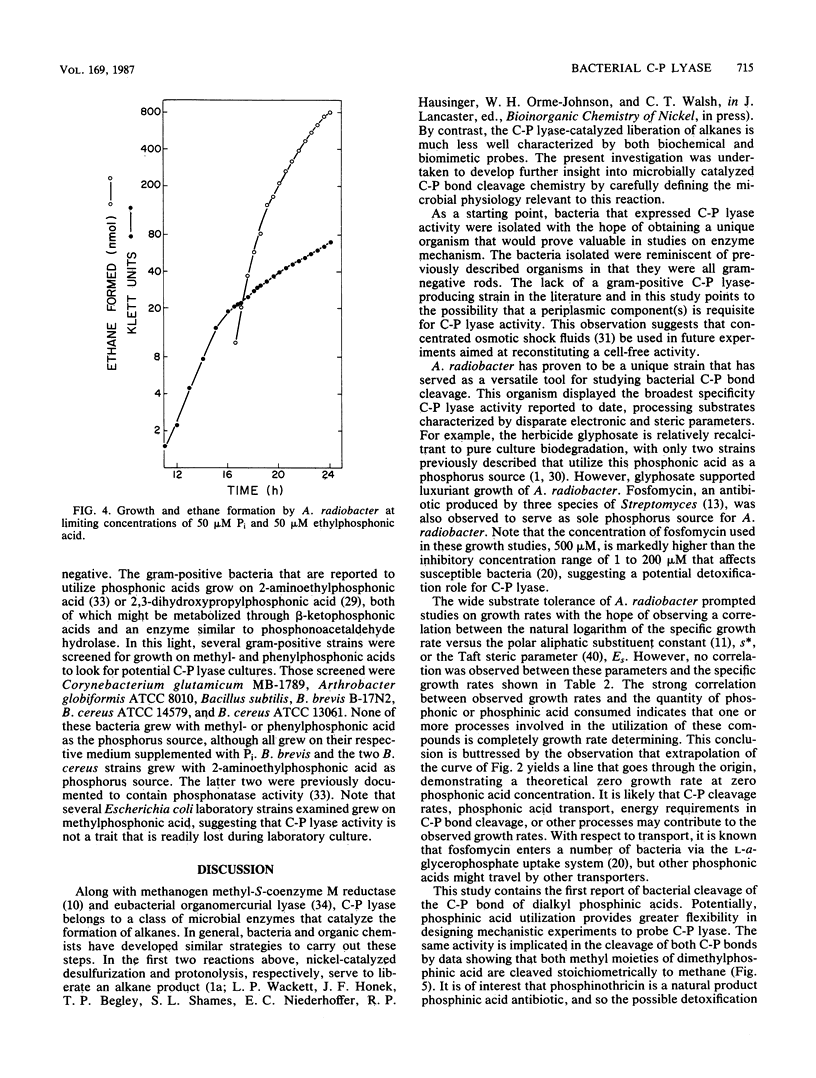
Carbon-phosphorus bond cleavage activity, found in bacteria that utilize alkyl- and phenylphosphonic acids, has not yet been obtained in a cell-free system. Given this constraint, a systematic examination of in vivo C-P lyase activity has been conducted to develop insight into the C-P cleavage reaction. Six bacterial strains were obtained by enrichment culture, identified, and characterized with respect to their phosphonic acid substrate specificity. One isolate, Agrobacterium radiobacter, was shown to cleave the carbon-phosphorus bond of a wide range of substrates, including fosfomycin, glyphosate, and dialkyl phosphinic acids. Furthermore, this organism processed vinyl-, propenyl-, and propynylphosphonic acids, a previously uninvestigated group, to ethylene, propene, and propyne, respectively. A determination of product stoichiometries revealed that both C-P bonds of dimethylphosphinic acid are cleaved quantitatively to methane and, furthermore, that the extent of C-P bond cleavage correlated linearly with the specific growth rate for a range of substrates. The broad substrate specificity of Agrobacterium C-P lyase and the comprehensive characterization of the in vivo activity make this an attractive system for further biochemical and mechanistic experiments. In addition, the failure to observe the activity in a group of gram-positive bacteria holds open the possibility that a periplasmic component may be required for in vivo expression of C-P lyase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balthazor T. M., Hallas L. E. Glyphosate-degrading microorganisms from industrial activated sludge. Appl Environ Microbiol. 1986 Feb;51(2):432–434. doi: 10.1128/aem.51.2.432-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley T. P., Walts A. E., Walsh C. T. Bacterial organomercurial lyase: overproduction, isolation, and characterization. Biochemistry. 1986 Nov 4;25(22):7186–7192. doi: 10.1021/bi00370a063. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Benzene from bacterial cleavage of the carbon-phosphorus bond of phenylphosphonates. Biochem J. 1979 Nov 15;184(2):453–455. doi: 10.1042/bj1840453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Phosphonate utilization by bacteria. J Bacteriol. 1978 Jan;133(1):85–90. doi: 10.1128/jb.133.1.85-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. G., Cook A. M., Alexander M. Phosphate and soil binding: factors limiting bacterial degradation of ionic phosphorus-containing pesticide metabolites. Appl Environ Microbiol. 1979 Mar;37(3):605–609. doi: 10.1128/aem.37.3.605-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIGUCHI M., KANDATSU M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature. 1959 Sep 19;184(Suppl 12):901–902. doi: 10.1038/184901b0. [DOI] [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- Kennedy K. E., Thompson G. A., Jr Phosphonolipids: localization in surface membranes of Tetrahymena. Science. 1970 May 22;168(3934):989–991. doi: 10.1126/science.168.3934.989. [DOI] [PubMed] [Google Scholar]
- La Nauze J. M., Coggins J. R., Dixon H. B. Aldolase-like imine formation in the mechanism of action of phosphonoacetaldehyde hydrolase. Biochem J. 1977 Aug 1;165(2):409–411. doi: 10.1042/bj1650409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Nauze J. M., Rosenberg H., Shaw D. C. The enzymic cleavage of the carbon-phosphorus bond: purification and properties of phosphonatase. Biochim Biophys Acta. 1970 Aug 15;212(2):332–350. doi: 10.1016/0005-2744(70)90214-7. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- MASTALERZ P., WIECZOREK Z., KOCHMAN M. UTILIZATION OF CARBON-BOUND PHOSPHORUS BY MICROORGANISMS. Acta Biochim Pol. 1965;12:151–156. [PubMed] [Google Scholar]
- Moore J. K., Braymer H. D., Larson A. D. Isolation of a Pseudomonas sp. Which Utilizes the Phosphonate Herbicide Glyphosate. Appl Environ Microbiol. 1983 Aug;46(2):316–320. doi: 10.1128/aem.46.2.316-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Rosenberg H., La Nauze J. M. The metabolism of phosphonates by microorganisms. The transport of aminoethylphosphonic acid in Bacillus cereus. Biochim Biophys Acta. 1967 Jun 13;141(1):79–90. doi: 10.1016/0304-4165(67)90247-4. [DOI] [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Shinabarger D. L., Schmitt E. K., Braymer H. D., Larson A. D. Phosphonate Utilization by the Glyphosate-Degrading Pseudomonas sp. Strain PG2982. Appl Environ Microbiol. 1984 Nov;48(5):1049–1050. doi: 10.1128/aem.48.5.1049-1050.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- ZELEZNICK L. D., MYERS T. C., TITCHENER E. B. GROWTH OF ESCHERICHIA COLI ON METHYL- AND ETHYLPHOSPHONIC ACIDS. Biochim Biophys Acta. 1963 Nov 15;78:546–547. doi: 10.1016/0006-3002(63)90921-1. [DOI] [PubMed] [Google Scholar]



