Abstract
Immune mechanisms have been implicated in placental dysfunction in patients with recurrent miscarriages and intrauterine growth restriction (IUGR), but the mediators are undefined. Here we show that complement activation, particularly C5a, is a required intermediary event in the pathogenesis of placental and fetal injury in an antibody-independent mouse model of spontaneous miscarriage and IUGR, and that complement activation causes dysregulation of the angiogenic factors required for normal placental development. Pregnancies complicated by miscarriage or growth restriction were characterized by inflammatory infiltrates in placentas, functional deficiency of free vascular endothelial growth factor (VEGF), elevated levels of soluble VEGF receptor 1 (sVEGFR-1, also known as sFlt-1; a potent anti-angiogenic molecule), and defective placental development. Inhibition of complement activation in vivo blocked the increase in sVEGFR-1 and rescued pregnancies. In vitro stimulation of monocytes with products of the complement cascade directly triggered release of sVEGFR-1, which sequesters VEGF. These studies provide the first evidence linking the complement system to angiogenic factor imbalance associated with placental dysfunction, and identify a new effector of immune-triggered pregnancy complications.
It has been estimated that 50–70% of human conceptions fail and that 1–3% of women in the United States suffer recurrent miscarriages (1). Moreover, intrauterine growth restriction (IUGR) occurs in up to 10% of infants born in the United States (2), and growth-restricted fetuses have higher mortality and morbidity rates than fetuses with weights greater than the 10th percentile. Although the insult to the fetus occurs in utero, the deleterious influence of IUGR contributes to long-term developmental delay, and a suboptimal intrauterine environment has been linked to metabolic disorders during adult life, including coronary artery disease, hypertension, hyperlipidemia, and insulin resistance (3).
Although the causes of recurrent miscarriages and IUGR are poorly understood, an immune mechanism involving inappropriate and subsequently injurious recognition of the conceptus by the mother's immune system has been proposed. This misdirected immune response to fetus, placenta, or both could yield a wide range of phenotypes, including miscarriages early in pregnancy and IUGR later in pregnancy. Pregnancy constitutes a major challenge to the maternal immune system because it requires tolerance of fetal alloantigens encoded by paternal genes. Local factors at the maternal–fetal interface are required to maintain such tolerance and assure normal development of semiallogenic concepti (4). The fetus is protected from maternal immune responses through mechanisms such as expression of HLA-G (5), inhibitory T cell costimulatory molecules (6), and complement regulatory proteins by trophoblasts (7), and by local maternal regulatory T cells (8) and production of the immunosuppressive enzyme indoleamine 2,3 dioxygenase (9).
A growing body of evidence now indicates that the adaptive immune response is regulated by the innate immune system; yet once engaged, adaptive immune responses can commandeer innate effectors to induce injury. Indeed, some perturbations of innate immune responses are associated with “spontaneous” abortion. Complement activation, in particular, has emerged as a common event in recurrent pregnancy loss (10), but whether it also contributes to abnormal placental development is unknown. We have previously identified complement as a critical early effector in antiphospholipid antibody-induced pregnancy loss and IUGR (11, 12). Although activation of complement and recruitment of inflammatory cells within decidual tissue are necessary intermediary steps in antibody-mediated pregnancy complications, the downstream pathogenic mediators of placental and fetal damage have not been defined. It is likely that neutrophils and monocytes stimulated by complement activation products directly damage the developing embryo. Alternatively, these armed effector cells may release factors that cause placental dysfunction and compromise fetal growth, a mechanism that we investigated and report on in this study.
Satisfactory development of the fetomaternal vasculature is required for successful embryonic growth, and insufficient placental vascularization has been associated with early embryonic mortality, preeclampsia, and IUGR (13). Normal placental development requires coordinated expression of angiogenic growth factors, vascular endothelial growth factor (VEGF) and placenta growth factor, as well as expression of their respective receptors on invasive trophoblasts (14). VEGF promotes placental development and invasiveness primarily through interaction with the high-affinity type III receptor tyrosine kinases: VEGF receptor 1 (VEGFR-1, also known as Flt-1) and VEGFR-2 (15). Alternative splicing of VEGFR-1 results in production of the secreted protein, soluble VEGFR-1 (sVEGFR-1, also known as sFlt-1), which lacks the cytoplasmic and transmembrane domains but retains the ligand-binding domain (16). The production of sVEGFR-1, a potent anti-angiogenic molecule that sequesters circulating VEGF and placenta growth factor and prevents their interaction with endogenous receptors (17), may be regulated independently of cell-bound VEGFR-1 (18, 19). Placental trophoblasts exposed to stress, such as hypoxia, release large amounts of sVEGFR-1 into the maternal circulation (20). Excess sVEGFR-1 has been shown to inhibit placental cytotrophoblast differentiation and invasion (21) and is thought to play a direct role in the pathogenesis of abnormal placentation associated with preeclampsia and IUGR (14, 22, 23). Indeed, sVEGFR-1 levels have been shown to be increased in the placenta and blood of women with preeclampsia (for review see reference 14). Although considerable evidence indicates that proteins and receptors in the VEGF family are involved in the pathogenesis of placental disorders, the relationship between inflammation and dysregulation of angiogenic factors in pregnancy is unexplored.
A link between inflammation and angiogenesis has been established in the pathogenesis of other disease processes. Inflammatory mediators have been shown to stimulate resident cells to produce VEGF and promote angiogenesis and tissue damage: joint destruction in rheumatoid arthritis and choroidal neovascularization in age-related macular degeneration (24–26). Under certain conditions, however, inflammatory cells may interrupt or prevent VEGF-induced angiogenesis by secreting sVEGR-1, which sequesters VEGF (18, 27). Given that functional VEGF deficiency leads to abnormal placental development, we considered the possibility that inflammation at the maternal–fetal interface triggers sVEFGR-1 production, leading to angiogenic factor imbalance and poor pregnancy outcomes.
Here we show that complement activation is a required intermediary event in the pathogenesis of fetal injury in an antibody-independent model of spontaneous miscarriage and IUGR, these pregnancy complications are associated with elevated levels of the anti-angiogenic factor sVEGFR-1, and products of the complement cascade directly trigger release of sVEGFR-1 from monocytes. We provide the first evidence that complement activation alters the balance of angiogenic factors in pregnancy and identify a novel mechanism for immune-triggered pregnancy complications.
RESULTS
Complement activation and inflammation at the maternal–fetal interface in CBA/J × DBA/2 matings
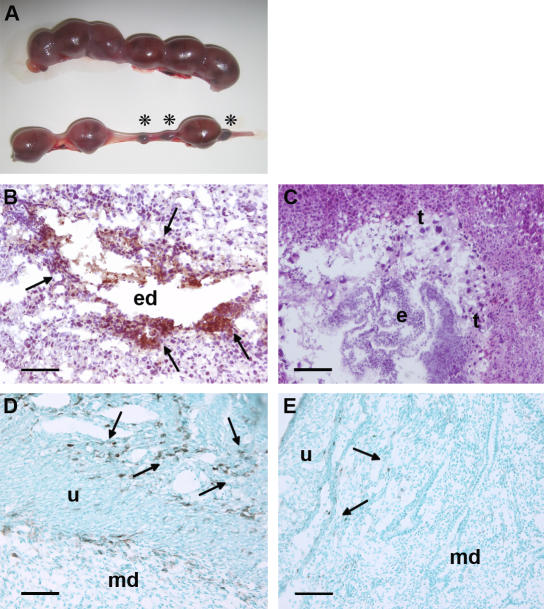
DBA/2-mated female CBA/J mice (CBA/J × DBA/2) are a well-studied model of immunologically mediated peri-implantation pregnancy loss that shares features with human recurrent miscarriage (28, 29). Embryos derived from mating CBA/J females with DBA/2 males showed an increased frequency of resorption (29.4 ± 6.5%), more than three times greater than that seen within these and other strains or strain combinations (CBA/J × CBA/J: 8.9 ± 5.1%; CBA/J × BALB/c: 8.2 ± 5.6%; DBA/2 × DBA/2: 8.5 ± 6.6%; n = 6–32 mice/group; CBA/J × DBA/2 vs. others, P < 0.01; Fig. 1 A). Embryonic lethality in DBA/2-mated female CBA/J mice is believed to represent rejection of the semiallogeneic placenta by maternal-derived activated effectors. Resorption is not universal, however, and surviving fetuses show consistent and significant growth restriction. The average weight of fetuses from CBA/J × DBA/2 matings was nearly 30% lower than that of fetuses from the BALB/c-mated female CBA/J mice (the control low abortion mating combination) at the same gestational age (212 ± 57 vs. 298 ± 42 mg, respectively; n = 80–120 fetuses/group, P < 0.005). The phenotype of IUGR in CBA/J × DBA/2 matings was homogenous: >95% of fetuses from DBA/2-mated CBA/J mice weighed less than the average CBA/J × BALB/c product.
Figure 1.
Resorption of embryos, deposition of complement C3, and infiltration of monocytes in decidual tissue of DBA/2-mated CBA mice. (A) Representative uteri from mice killed at day 15 of pregnancy are shown. The top panel, from a BALB/c-mated CBA/J mouse contains larger amnion sacs and no resorptions. The bottom panel, from a DBA/2-mated CBA/J mouse, shows six amnion sacs of varying sizes and three resorptions (asterisks). (B–E) Pregnant mice were killed on day 8, and sections were stained with anti–mouse C3 (B and C) or anti–mouse F4/80 (D and E) to detect monocytes. In CBA/J × DBA/2 mice, there was extensive C3 deposition (brown, arrows; B) and monocyte infiltration (arrows) in the deciduas (D). In contrast, the decidual tissue from CBA/J × BALB/c mice showed minimal staining for C3 (C) and monocyte infiltration (E). e, embryo; ed, embryonic debris; md, maternal deciduas; t, trophoblasts; u, uterine wall. Bars, 0.1 mm.
Given our findings that complement activation at the fetal–maternal interface presents a danger to the developing fetus and that complement inhibition is an absolute requirement for normal pregnancy (11, 12), we hypothesized that excessive activation of complement contributes to fetal loss in CBA/J × DBA/2 matings. We conducted immunohistological analyses of deciduas from days 5 to 15 of pregnancy. Staining with antibodies against mouse complement component C3 showed extensive complement deposition on trophoblast cells (Fig. 1 B), evident as early as day 6 in damaged embryos destined for resorption. Deposition of C3 in deciduas coincided with increased infiltration of inflammatory cells, which we identified as monocytes and neutrophils, and with the presence of necrosis and fetal debris (Fig. 1 D). In contrast, in embryos from the same mating but with normal morphology at day 6, we found that C3 staining was minimal and restricted to the ectoplacental cone, an area where C3 is present in normal pregnancies (7, 11). Similarly, in products of CBA/J × BALB/c matings examined from days 6 to 15, limited C3 staining was detectable, no inflammation was observed, and fetuses showed normal development (Fig. 1, C and E).
Trophoblasts and embryos from CBA/J × DBA/2 matings had normal levels of complement receptor 1–related gene/protein y (Crry), the complement regulatory protein essential for pregnancy survival in mice (7). Expression of Crry, as detected by Western blotting of lysates from embryos and deciduas of CBA/J × DBA/2 matings obtained from days 6 to 12, was comparable to that from CBA/J × BALB/c matings (ratio of arbitrary densitometry units: 1.03 ± 0.06, n = 12 pairs). Hence, relative deficiency of Crry does not explain increased fetal resorption. Although we do not know which factors initiate the complement cascade, we consistently found decidual complement deposition and extensive inflammation associated with embryonic death in products of CBA/J × DBA/2 pregnancies.
Inhibition of complement activation rescues pregnancies in CBA/J × DBA/2 matings
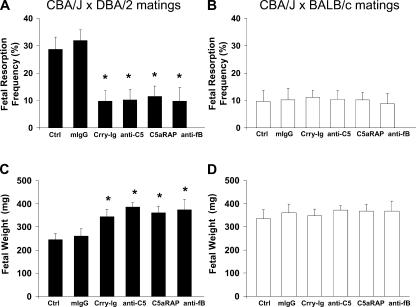
To provide direct evidence that complement activation is a critical mediator rather than a consequence of embryo injury in this model of spontaneous pregnancy loss, we attempted to rescue embryos of DBA/2-mated CBA/J mice with complement inhibitors. To block C3 activation by the alternative and classical pathways, and thereby prevent all downstream complement effector activity, we treated pregnant mice with an inhibitor of C3 convertase, Crry-Ig (30). We and others have shown that Crry-Ig blocks complement activation in vitro and in vivo (11). We treated CBA/J × DBA/2 and CBA/J × BALB/c mice with Crry-Ig or control mouse polyclonal IgG beginning at day 4 of pregnancy. That Crry-Ig prevented pregnancy losses in DBA/2-mated CBA/J mice (Fig. 2 A) is proof of concept that complement is required for fetal loss and that its inhibition could offer a target for therapy. Crry-Ig also protected surviving fetuses from IUGR (Fig. 2 C), indicating that complement activation also contributes to growth failure in developing embryos.
Figure 2.
Inhibition of complement prevents fetal death and growth restriction in CBA/J × DBA/2 matings. DBA/2- and BALB/c-mated CBA/J females were treated with recombinant Crry-Ig, anti-C5 mAb (mAb BB5.1), C5aR antagonist peptide (C5aRAP) (AcPhe[l-ornithine-Pro-d-cyclohexylalanine-Trp-Arg]), anti–factor B mAb (mAb 1379), or mouse polyclonal IgG (mIgG). Mice were killed on day 15 of pregnancy, uteri were dissected, embryos were weighed, and fetal resorption rates were calculated (number of resorptions/number of fetuses + number of resorptions). There were six to eight mice in each experimental group. (A and B) The frequency of fetal resorptions in treated and untreated CBA/J × DBA/2 and CBA/J × BALB/c pregnancies was determined (CBA/J × DBA/2 vs. CBA/J × BALB/c, P < 0.001; CBA/J × DBA/2 vs. CBA/J × DBA/2 plus complement inhibitors; *, P < 0.001). (C and D) Average fetal weights in treated and untreated CBA/J × DBA/2 and CBA/J × BALB/c pregnancies was determined (CBA/J × DBA/2 vs. CBA/J × BALB/c, P < 0.005; CBA/J × DBA/2 vs. CBA/J ×DBA/2 plus complement inhibitors; *, P < 0.005).
Once the complement cascade is triggered, any of several complement activation fragment-derived ligand–receptor interactions could mediate fetal injury and placental dysfunction. To define which elements of the complement pathway contribute to pregnancy complications, we focused on C5, a pivotal component of the cascade that generates two effector pathways: C5a, a potent anaphylatoxin and cell activator, and C5b, which initiates formation of the C5b-9 membrane attack complex. We treated DBA/2-mated CBA/J pregnant mice with an anti-C5 mAb that effectively prevents C5 activation in vitro and in vivo (31). Blockade of C5 cleavage with anti-C5 mAb averted pregnancy loss and fetal growth restriction in CBA/J × DBA/2 matings (Fig. 2, A and C).
To distinguish the role of C5a and C5a receptor (C5aR) from that of membrane attack complex, we treated pregnant CBA/J × DBA/2 and control CBA/J × BALB/c matings with a highly specific cyclic peptide antagonist of C5aR, AcPhe[l-ornithine-Pro-d-cyclohexylalanine-Trp-Arg], which possesses potent in vivo antiinflammatory activity in mouse models of endotoxic shock and antiphospholipid syndrome (12, 32, 33). Administration of C5aR antagonist peptide prevented both fetal resorption and IUGR in CBA/J × DBA/2 matings (Fig. 2, A and C) but had no effect on the outcomes of CBA/J × BALB/c matings (Fig. 2, B and D). Protection conferred by the C5aR antagonist was comparable to that seen with anti-C5 mAb, suggesting that C5a–C5aR interactions are important mediators in pregnancy damage in CBA/J × DBA/2 matings. We cannot, however, exclude a role for C3a or C5b-9.
Immunohistological analysis of deciduas from CBA/J × DBA/2 mice treated with C5aR antagonist peptide showed minimal C3 deposition surrounding normal-appearing fetuses and no evidence of inflammation, raising the possibility that recruited inflammatory cells amplify complement activation on trophoblasts and within decidua. Previous evidence indicates that activated myeloid cells generate factor B and factor D (34). Indeed, cognate T cell–macrophage interactions are accompanied by alternative pathway activation (due to the production of C3, factor B, and factor D, as well as down-regulation of complement regulatory protein expression) leading to the generation of C5a and the creation of a local amplification loop (35). Given the extensive infiltration of monocytes and neutrophils observed in the deciduas of CBA/J × DBA/2 mice, we hypothesized that alternative pathway activation contributes to fetal injury. We treated CBA/J × DBA/2 and CBA/J × BALB/c mice with an anti–factor B mAb known to inhibit the alternative pathway in vitro and in vivo by blocking formation of the C3bBb complex (12, 36). Fetal rejection and growth failure in CBA/J × DBA/2 matings was prevented by anti–factor B mAb (Fig. 2, A and C), indicating that the alternative pathway plays a central role in initiating and/or amplifying injury.
In sum, our results show that factor B, C3, C5, and C5aR are required for pregnancy loss and growth restriction in an antibody-independent model of spontaneous miscarriage. We have previously shown that heparin inhibits complement activation in vivo and in vitro (37) and prevents pregnancy failure in antiphospholipid antibody-treated mice independent of its anticoagulant effects. Because some patients with recurrent miscarriage of undefined etiology are treated with low-dose heparin, we examined the efficacy of this therapy in our mouse model of spontaneous miscarriage. We treated DBA/2-mated CBA/J females with sub-anticoagulant doses of heparin (10 U s.c. twice daily) beginning at day 4. As predicted by the results of experiments with complement inhibitors, the administration of low-dose heparin was effective in preventing fetal resorptions (29.4 ± 6.5% vs. 7.9 ± 8.9%, P < 0.001). These results reemphasize the importance of complement in fetal damage and provide a framework for understanding how sub-anticoagulant doses of heparin exert beneficial effects in miscarriage-prone pregnancies.
TNF-α in CBA/J × DBA/2 matings
Although we have implicated abnormal complement activation as a causative event in fetal loss and growth restriction, we have not addressed the relative contributions of complement as compared with other mechanisms. TNF-α regulates placental architecture, hormone synthesis, protease expression, and embryonic development (38). It is released when infiltrating inflammatory cells are activated by complement split products (39). Therefore, we considered the possibility that TNF-α contributes to pregnancy complications in DBA/J-mated CBA/J mice. Elevated levels of TNF-α have been associated with miscarriage, placental oxidative stress, and preeclampsia (40) in human studies and with pregnancy failure in mice (39, 41). DBA/2-mated CBA/J mice showed intense TNF-α staining of decidual tissue at day 7 and increased plasma TNF-α levels compared with CBA/J × BALB/c mice. Systemic TNF-α levels increased steadily from day 4 to day 10 of pregnancy (day 10: 325 ± 17 pg/ml) and remained elevated until day 15 (340 ± 35 pg/ml), when mice were killed. In contrast, plasma levels of TNF-α remained low and did not change during pregnancy in CBA/J × BALB/c mice (average level on days 1–15: 73 ± 9 pg/ml; CBA/J × DBA/2 vs. CBA/J × BALB/c; n = 5–8 mice/group, P = 0.001). The increase in TNF-α observed in DBA/2-mated CBA/J females was prevented by inhibitors of complement activation, which we administered beginning on day 4. In CBA/J × DBA/2 mice treated with Crry-Ig or anti-C5 mAb, TNF-α levels did not increase (average levels on days 1–15: 94 ± 10 and 88 ± 7 pg/ml, respectively; n = 6–7 mice/group) and were similar to those of CBA/J × BALB/c matings, suggesting that the trigger for TNF-α production is downstream of complement activation.
Although elevated TNF-α levels induce trophoblast apoptosis and restrict trophoblast invasiveness (38, 42), phenotypes consistent with pregnancy failure we observed in CBA/J × DBA/2 mice, blockade of TNF-α with polyethylene glycol–conjugated soluble TNF-α receptor type I (PEG sTNFRI) (39) did not rescue pregnancies (sTNFRI vs. control: 29.4 ± 6.5% fetal resorptions vs. 30.7 ± 7.8%). These findings indicate that although TNF-α may contribute to semiallogenic fetal rejection, it is not the exclusive effector.
Angiogenic factors in CBA/J × DBA/2 matings
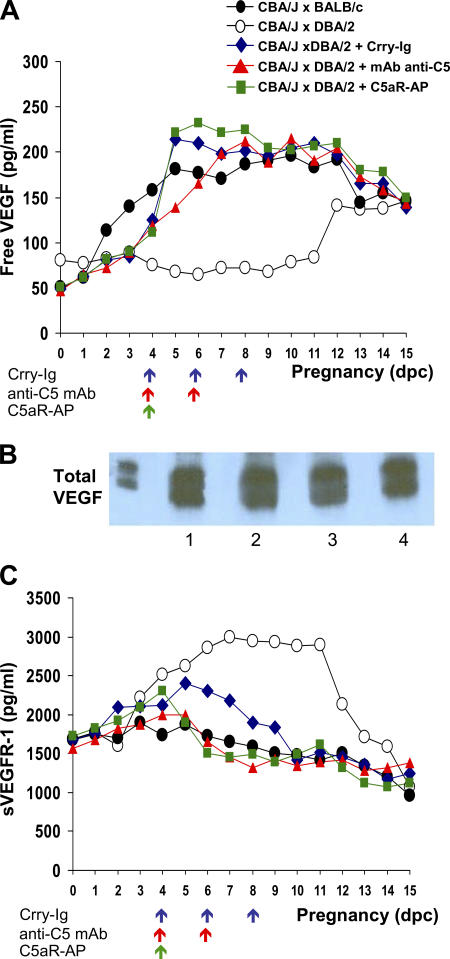
Given that proteins and receptors of the VEGF family are important for adequate placental development and that fetuses from DBA/2-mated CBA/J mice die or show inadequate growth in utero, we considered the possibility that inflammatory mediators, and specifically complement proteins, trigger dysregulation of angiogenic factors and produce placental dysfunction. We hypothesized that complement activation prevents VEGF signaling through cell-bound VEGFR and thereby leads to pregnancy complications in CBA/J × DBA/2 matings. To test this hypothesis, we measured free VEGF levels in plasma from days 1 through 15 of pregnancy. In CBA/J × BALB/c matings, free VEGF levels increased from days 2 to 5 and remained elevated throughout pregnancy (P < 0.001), whereas in DBA/2-mated CBA/J pregnancies, free VEGF did not increase above pre-pregnancy levels until day 12 (Fig. 3 A).
Figure 3.
Dysregulation of angiogenic factors in CBA/J × DBA/2 matings is prevented by inhibiting complement. Free VEGF, total VEGF, and sVEGFR-1 were assessed. Free VEGF (A) and total sVEGFR-1 (C) levels in plasma were measured in CBA/J × DBA/2 (◯) and CBA/J × BALB/c (•) mice from days 1 through 15 of pregnancy. Some DBA/2-mated CBA/J mice were treated with Crry-IgG (♦), anti-C5 mAb (▴), and C5aR-AP (▪). The arrows indicate the days when the complement inhibitors were administered. Values represent means from three to five mice/time point/group (free VEGF: CBA/J × DBA/2 vs. CBA/J × BALB/c, P < 0.001; CBA/J × DBA/2 vs. CBA/J × DBA/2 plus complement inhibitors, P < 0.001; sVEGFR-1: CBA/J × DBA/2 vs. CBA/J × BALB/c, P < 0.001; CBA/J × DBA/2 vs. CBA/J × DBA/2 plus complement inhibitors, P < 0.005). (B) Total VEGF levels were compared in CBA/J × BALB/c and CBA/J × DBA/2 matings. Plasma samples from early pregnancy (days 5–8) and late pregnancy (days 9–12) were pooled into two groups for detection of total plasma VEGF (bound to sVEGFR-1 and unbound) by Western blotting. Samples were immunoprecipitated with rabbit anti–mouse VEGF and analyzed with anti-VEGF mAb. Lane 1, CBA/J × BALB/c days 5–8; lane 2, CBA/J × BALB/c days 9–12; lane 3, CBA/J × DBA/2 days 5–8; lane 4, CBA/J × DBA/2 days 9–12.
To determine whether production of VEGF in CBA/J × DBA/2 matings was deficient compared with CBA/J × BALB/c matings or whether VEGF was sequestered (and undetectable by ELISA) in CBA/J × DBA/2 matings, we analyzed total VEGF levels (unbound and sVEGFR-1–bound VEGF) in mouse plasma by immunoprecipitation with rabbit anti–mouse VEGF and Western blot. In contrast to free VEGF, total VEGF levels were comparable in abortion-prone and control matings, suggesting that circulating VEFG was bound in DBA/2-mated CBA/J mice and that the decrease in functional (unbound) VEGF is not due to changes in VEGF production (Fig. 3 B). Indeed, as early as day 4, plasma levels of the VEGF antagonist sVEGFR-1 were higher in CBA/J × DBA/2 matings than in controls (Fig. 3 C). Levels of sVEGFR-1 were significantly higher in CBA/J × DBA/2 matings until day 12 (P < 0.001). These findings demonstrate early dysregulation of angiogenic factors in abortion-prone matings. Of note, the ELISA assay for sVEGFR-1 measured bound and free sVEGFR-1, and increased VEGF was only detectable after day 12 of pregnancy, when sVEGFR-1 levels had fallen (Fig. 3). At this time, resorption of injured fetuses was complete and growth of surviving fetuses was impaired.
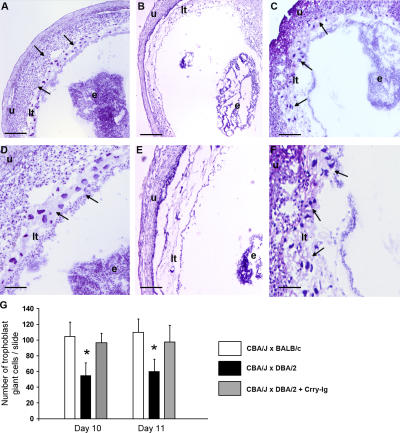
To determine whether the anti-angiogenic state characterized by excess sVEGFR-1 and deficient-free VEGF was associated with defective placental development, we examined placentas from viable fetuses on day 15 of pregnancy. The average weight of placentas from CBA/J × DBA/2 matings was 24% lower than that from CBA/J × BALB/c pairs (84 ± 14 mg vs. 110 ± 17; n = 80–120 placentas/group, P < 0.001), consistent with abnormal development. Because defects in trophoblast differentiation and placental development limit embryonic growth, we performed histologic analyses of midtrimester placentas from abortion-prone and control matings. We focused specifically on trophoblast giant cells because they mediate implantation and invasion of the conceptus into maternal decidua of the uterus, and reduction in their number or abnormalities in their differentiation can cause placental defects and compromised pregnancies (43, 44). Placentas from CBA/J × DBA/2 matings showed a relative deficiency of trophoblast giant cells (Fig. 4, B, E and G) compared with those from CBA/J × BALB/c matings (Fig. 4, A and D), indicating abnormal trophoblast differentiation. We also observed impaired placental vascularization in CBA/J × DBA/2 matings with fewer fetal vessels invading the labyrinthine layer (not depicted). In contrast, placentas from CBA/J × DBA/2 matings treated with the complement inhibitor Crry-Ig showed no decrease in giant cells (Fig. 4, C, F and G); the histology was similar to that of CBA/J x BALB/c matings.
Figure 4.
Decreased trophoblast giant cells in placentas from CBA/J × DBA/2 matings. (A–F) Histologic analysis of sections of uteri from CBA/J × BALB/c matings (A and D), CBA/J × DBA/2 (B and E) matings, and (C and F) CBA/J × DBA/2 matings treated with Crry-Ig killed at day 10 of pregnancy. Trophoblast giant cells (arrows) were reduced in midtrimester placentas from DBA/2-mated CBA/J pregnancies (B and E) compared with CBA/2 × BALB/c pregnancies, and this was prevented by treatment with Crry-Ig. Sections were stained with hematoxylin and eosin. e, embryo; lt, labyrinthine trophoblast u, uterine wall. Scale bars, 0.1 mm (A–C); 0.025 mm (D–F). (G) Number of trophoblast giant cells in placentas from days 10 and 11 of pregnancy was counted by light microscopy. Data are expressed as the mean of four sections counted by two readers blinded to experimental conditions. *, P < 0.01.
Although deposition of C3 and infiltration of inflammatory cells in placental tissue of viable, growth-restricted fetuses was only minimal, it is possible that elevated levels of sVEGFR-1 produced at sites of fetal resorption altered trophoblast function in neighboring (otherwise normal) concepti. Furthermore, abnormal placental development in such “innocent bystanders” could cause hypoxic stress in trophoblasts, stimulate release of more sVEGFR-1, and worsen the outcome of developing placentas and fetuses. The consistent decrease in placental and fetal weights in these surviving fetuses of CBA/J × DBA/2 matings supports our concept that both a circulating inhibitor of placental development and local inflammatory injury cause placental failure.
To assess the effect of complement activation on levels of angiogenic factors, we measured free VEGF and sVEGFR-1 levels in CBA/J × DBA/2 matings treated with the complement inhibitors that protected pregnancies. If sVEGFR-1 is a critical effector of fetal injury acting downstream of complement activation, we would not expect a sustained elevation of plasma sVEGFR-1 in mice treated with blockers of complement. In fact, inhibition of complement activation at the levels of C3 (Crry-Ig), C5 (anti-C5 mAb), or C5a–C5aR interactions (C5aR antagonist) beginning on day 4 of pregnancy prevented the increase in sVEGFR-1 (CBA/J × DBA/2 vs. CBA/J × DBA/2 plus complement inhibitors; P < 0.005) and associated decrease in free VEGF (P < 0.001; Fig. 3). In contrast to the effects of complement inhibitors, blocking TNF-α with sTNFRI, which does not improve pregnancy outcomes, failed to prevent the decrease in free VEGF (not depicted). Collectively, our data indicate that blockade of complement activation prevents the increase in sVEGFR-1, permits plasma levels of free VEGF to rise appropriately, and averts the fetal resorptions and growth restriction characteristic of CBA/J × DBA/2 pregnancies.
Complement activation triggers release of sVEGFR-1 by monocytes in vitro
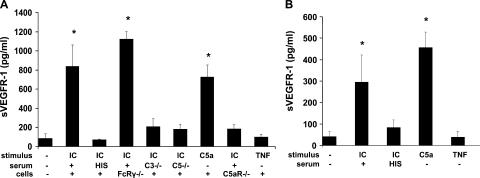
Because C3 deposition on decidual tissue and trophoblasts was associated with intense infiltration of monocytes (Fig. 1), because mononuclear phagocytes are essential effectors of damage in this model (28), and because peripheral blood mononuclear cells can express sVEGFR-1 (18, 27), we tested the hypothesis that complement activation products directly induce release of sVEGFR-1 by monocytes and thereby provide an additional (extra-placental) source of sVEGFR-1 to inhibit VEGF activity. To determine whether complement split products can trigger the production of sVEGFR-1, we incubated mouse splenic macrophages with immune complexes (ICs; heat-aggregated human IgG) in the presence of 10% mouse serum or heat-inactivated mouse serum. A heat-labile serum factor activated by ICs induced a 10-fold increase in secreted sVEGFR-1 (Fig. 5 A), consistent with a complement effect. IC-mediated sVEGFR-1 production was not inhibited in the absence of stimulatory Fcγ receptors on macrophages, whereas the absence of serum C3 blocked the increase in sVEGFR-1, emphasizing that complement is the primary effector. We could not detect free VEGF in supernatants from stimulated monocytes by ELISA, indicating that if VEGF is produced by IC-mediated activation of effector cells, it is bound by excess sVEGFR-1 and unavailable to VEGF receptors expressed on trophoblasts and endothelial cells. Indeed, using Western blotting, which detects total VEGF (unbound and sVEGFR-1–bound), we did detect increased VEGF in supernatants from complement-stimulated monocytes. Although complement activation products may stimulate VEGF release (25, 26), they also trigger monocytes to secrete sVEGFR-1, which sequesters VEGF.
Figure 5.
Complement activation triggers release of sVEGFR-1 by murine macrophages and human monocytes in vitro. (A) Splenic macrophages from CBA/J female mice were stimulated with heat-aggregated human IgG (IC) in the presence of 10% normal mouse serum, 10% heat-inactivated mouse serum (HIS), 10% serum from C3-deficient mice (C3−/−), or 10% serum from C5-deficient mice (C5−/−). Macrophages were also incubated with C5a, TNF-α, or control medium. Splenic macrophages from mice deficient in stimulatory Fcγ receptors (FcRγ−/−) or C5aR (C5aR−/−) were also treated with ICs. Culture supernatants were collected after 24 h and analyzed for sVEGFR-1 by ELISA (n = 3–9 experiments/group; *, P < 0.001 vs. control). (B) Adherent human mononuclear cells from healthy donors were stimulated with ICs plus 10% normal human serum and ICs plus 10% heat-inactivated human serum, C5a, or TNF-α. After 2 h, culture supernatants were collected and analyzed for sVEGFR-1 by ELISA (n = 4–5 experiments/group; *, P < 0.005 vs. control).
To determine whether complement activation at the level of C3 or C5 is necessary to trigger release of anti-angiogenic factors associated with adverse pregnancy outcomes, we performed experiments using serum deficient in C5. Production of sVEGFR-1 was similar in macrophages stimulated with IC in C3- or C5-deficient serum, indicating that C5 activation products, rather than C3a, were required. Indeed, stimulation with synthetic C5a induced sVEGFR-1 production, whereas C5aR-deficicent macrophages did not increase sVEGFR-1 release after stimulation with IC in the presence of serum (Fig. 5 A), arguing against a critical role for C3aR as a trigger for sVEGFR-1, although some contribution cannot be excluded. Furthermore, we found that C5a induced sVEGFR-1 release directly, rather than acting through TNF-α. A role for TNF-α was plausible because it is rapidly released by C5a-stimulated monocytes and is known to influence production of angiogenic factors (45). Incubation of monocytes with TNF-α at 2 ng/ml (a concentration sevenfold higher than peak levels measured in DBA/2-mated CBA/J females) did not stimulate increased sVEGFR-1 production (Fig. 5 A), consistent with our findings that TNF-α blockade did not prevent fetal loss in CBA/J × DBA/2 pregnancies, whereas complement blockade was protective. Collectively, these results support our in vivo findings that complement activation, and specifically C5a–C5aR interactions, induce infiltrating monocytes to produce sVEGFR-1, and that sVEGFR-1 then binds VEGF and inhibits its activity during placental development.
To determine whether similar mechanisms may be operative in patients, we incubated human peripheral monocytes with ICs in the presence of 10% human serum, heat-inactivated serum, or synthetic C5a. Similar to our findings in mouse macrophages, ICs induced a sixfold increase in sVEGFR-1 in supernatants, an effect that was blocked by heat inactivation of serum (Fig. 5 B). Here too, synthetic C5a was a potent stimulus for sVEGFR-1 production, whereas TNF-α had no effect.
DISCUSSION
The experiments we report in this study provide the first evidence linking the complement system to angiogenic factor imbalance associated with placental dysfunction. Our in vitro finding that complement activation products, and in particular C5a, stimulate monocytes to produce sVEGFR-1, which sequesters VEGF, confirms our in vivo experiments with complement inhibitors (Fig. 3). Collectively, these studies provide definitive proof that the complement system regulates VEGF activity and imply that deficiency of free VEGF induced by high levels of sVEGFR-1 leads to abnormal placental development (Fig. 4) and causes fetal growth restriction or death (Fig. 2).
Our findings that complement is activated at the maternal–fetal interface (Fig. 1) and that inhibition of complement activation rescues pregnancies (Fig. 2) and reverses angiogenic imbalance in CBA/J × DBA/2 matings (Fig. 3) demonstrate that complement has an essential and causative role in damage to the fetal–placental unit. Previous studies have implicated the complement pathway in pregnancy failure (6, 9–12), but this study is the first to show that local complement activation not only causes fetal death, but also leads to abnormal placental development. Although the triggers of immune dysregulation and complement activation in CBA/2-mated CBA/J mice remain unknown, the finding that factor B, C5, and C5aR are required to produce fetal injury in this autoantibody-independent disease model identifies specific elements in the complement pathway as potential targets for interventions to prevent recurrent pregnancy complications in patients.
Two features of the developing placenta provide an environment conducive to complement activation: relative hypoxia and the presence of externalized phosphatidylserine on the outer leaflets of trophoblasts (13, 46, 47). In this hypoxic milieu, natural antibodies that recognize neoepitopes and/or negatively charged phospholipids may initiate the complement cascade via the classical pathway, as has been described in experimental models of intestinal ischemia-reperfusion injury (48). The alternative pathway, activated independently of antibodies and the predominant initiator of damage in renal ischemia (36, 48), may also initiate the complement cascade and contribute to fetal damage in our model because anti–factor B mAb prevented fetal growth restriction and death in DBA/2-mated CBA/J mice. Our findings do not exclude a contribution of the lectin–complement pathway (49, 50).
Inappropriate activation of the alternative pathway has also been implicated in mouse studies of pregnancy loss induced by antiphospholipid antibodies, activated maternal T cells, and deficient trophoblast expression of the complement inhibitor Crry (9, 12, 51), although the specific triggers that initiate the complement cascade are still unclear. Failure of mechanisms that normally protect the fetus from the maternal immune response, be they cellular or humoral, may lead to fetal injury, and the resulting damaged and dying (apoptotic and necrotic) cells can initiate the complement cascade. Potential mechanisms to amplify complement activation via the alternative pathway include increased synthesis of alternative pathway components by endogenous cells or by infiltrating inflammatory cells, and decreased expression of complement regulatory proteins. Further studies are necessary to identify the initiators of complement activation in complicated pregnancies and to establish whether any of these potential mechanisms are operative in patients.
In this study, we present the first evidence that monocytes exposed to complement activation products are stimulated to produce sVEGFR-1, a potent inhibitor of VEGF activity. Although trophoblasts and endothelial cells have been shown to produce sVEGFR-1, our work emphasizes the importance of infiltrating monocytes as proximate mediators of damage, findings consistent with evidence that depletion of monocytes or blockade of ICAM-1–LFA interactions prevents abortion in DBA/2-mated CBA/J mice (28, 29). That blockade of C5a–C5aR interactions prevents increased sVEGFR-1 release while murine trophoblasts lack C5a receptors (not depicted) also indicates that activated leukocytes are the source of excess sVEGFR-1.
Our findings stand in contrast with the recent reports of enhanced angiogenesis by complement components C3a and C5a. We found that monocytes recruited and activated by complement split products at the maternal–fetal interface release sufficient sVEGFR-1 to inhibit the increasing amounts of VEGF produced by the placenta, whereas in experimental models of arthritis and age-related macular degeneration, ICs and complement activation products induce release of VEGF and cause neovascularization (24–26). The primary source of VEGF in these models is resident synovial cells and retinal cells, respectively, although recruited leukocytes have also been shown to release VEGF in response to complement activation. It appears that under different conditions monocytes have different responses to complement activation products. Future studies can address whether the pathway of monocyte activation (Th1- vs. Th2-type cytokines; innate vs. acquired immune activation; reference 52) influences the balance between secretion of angiogenic and anti-angiogenic factors in response to a given stimulus.
By implicating inflammatory cells (recruited and activated by complement split products) as a source of inhibitors of angiogenesis, we have identified a new mechanism by which immune activation, long believed to be a contributor to pregnancy failure, may cause recurrent abortion and fetal growth restriction. Our findings emphasize the central role of complement proteins as key innate immune effectors that mediate poor pregnancy outcomes. The link between complement activation and the balance of angiogenic and anti-angiogenic factors, beyond its importance in pregnancy loss and IUGR, also has direct implications for understanding immune mechanisms in preeclampsia, ischemic injury, and tumor survival.
MATERIALS AND METHODS
Mice.
Inbred CBA/J (H-2k), DBA/2 (H-2d), C3−/−, and C5−/− male and female mice were obtained from The Jackson Laboratory. BALB/c males were obtained from Taconic Farms. FcRγ-deficient mice backcrossed to BALB/c mice were provided by J. Ravetch (The Rockefeller University, New York, NY; reference 53). C5aR-deficient mice on a BALB/c background were provided by C. Gerard (Harvard Medical School, Boston, MA; reference 54).
Mice matings and treatment protocols.
8–10-wk-old virgin female CBA/J mice were mated with 8–14-wk-old CBA/J, BALB/c, or DBA/2 males. Females were inspected daily for vaginal plugs, and presence of a vaginal plug was designated as day 0 of pregnancy. Pregnant females were killed at predetermined intervals (from days 6 to 15). The frequency of fetal resorption was calculated on day 15 (number of resorptions/total number of formed fetuses and resorptions). Resorption sites are easily identified and result from loss of a previously viable fetus. Weights of fetuses and placentas were also determined.
To inhibit C3 convertase, pregnant mice were injected i.p. on days 4, 6, and 8 of pregnancy with 3 mg of recombinant Crry-Ig, a soluble chimeric protein that contains the five extracytoplasmic short consensus repeat domains of Crry linked to the hinge and CH2 and CH3 domains of the noncomplement fixing mouse IgG1 isotype (30). To block C5 cleavage, mice were treated with anti-C5 mAb (1 mg i.p.; mAb BB5.1) on days 4 and 6 (31). To block C5a–C5aR interactions, mice received a C5aR cyclic antagonist peptide (50 μg i.p.; AcPhe[l-ornithine-Pro-d-cyclohexylalanine-Trp-Arg]) on day 4 (12, 32). Alternative pathway activation was inhibited by administering anti–factor B mAb (2 mg i.p.) on days 4–10 of pregnancy (36). For each complement inhibitor study, a group of mice treated with mouse IgG at the same doses and schedule of administration as those of the recombinant protein or mAbs served as the control. To study the effects of heparin, we treated mice with unfractionated heparin (10 U s.c., twice per day) from days 4 to 8. At this dose of heparin, we did not detect an increase in partial thromboplastin time values compared with untreated mice (32 ± 2 s vs. 29 ± 2 s). To inhibit TNF-α, pregnant mice were treated with PEG sTNFRI (5 mg/kg, i.p.; Amgen Inc.) on days 2, 4, and 6 of pregnancy.
Complement deposition and cellular infiltration were analyzed on decidual tissue at different points in pregnancy. Plasma samples were obtained from pregnant females from days 1 to 15 of pregnancy. TNF-α was measured using the OptEIA kit (BD Biosciences). VEGF and sVEGFR-1 levels were measured in plasma samples using ELISA methods (R&D Systems). The ELISA for VEGF detects only free (unbound) VEGF and that for sVEGFR-1 measures total sVEGFR-1 (VEGF-bound and unbound). Procedures that involved mice were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery and were conducted in strict accordance with guidelines for the care and use of laboratory research animals promulgated by the National Institutes of Health (NIH).
Immunohistochemistry.
Uteri removed at different points during gestation were frozen in O.C.T. compound, and 10-μm-thick sections of embryos and placentas were cut. Sections were incubated with goat anti–mouse C3 (Cappel), goat anti–mouse TNF-α (R&D Systems), or rat anti–mouse F4/80 (Serotec) mAbs, followed by incubation with specific secondary IgG antibodies conjugated with horseradish peroxidase (HRP; BD Biosciences). Bound IgG-HRP was detected with diaminobenzidine. Sections were counterstained with hematoxylin or methyl green. Sections of frozen tissue were also stained with hematoxylin and eosin. The number of trophoblast giant cells in midtrimester placentas was counted using light microscopy by two different observers blinded to the experimental conditions. Values presented represent means ± SD from eight different sections from the central portion of embryos for each condition and time.
Western blot analysis.
Uterine contents (fetal–placental unit) were removed from mice on days 7–12 of pregnancy and immediately frozen at −70°C. The tissue was homogenized and lysates were resolved by electrophoresis and transferred to a nitrocellulose membrane as described previously (12). Membranes were probed with HRP-conjugated goat anti–mouse Crry (Sigma-Aldrich) and visualized using a chemiluminescence detection kit (Amersham Life Science), and autoradiograms were analyzed by densitometry. To measure total VEGF (unbound and bound to sVEGFR-1) in mouse plasma, samples were immunoprecipitated with rabbit anti–mouse VEGF (Antigenix America), analyzed with goat anti–rat VEGF antibody (R&D Systems), and probed with rabbit anti–goat HRP (BD Biosciences).
Production of sVEGFR-1 in vitro.
Splenocytes from female mice were incubated for 2 h in culture medium supplemented with 10% inactivated FCS. Nonadherent cells were removed, and adherent cells (>95% peroxidase-positive) were incubated in culture medium supplemented with 10% inactivated fetal bovine serum with the addition of the following stimuli: none (control); 200 μg/ml of heat-aggregated human IgG (ICs) plus 10% normal mouse serum; ICs plus 10% heat-inactivated mouse serum; 10 nM C5a; ICs plus 10% serum from C3-deficient mice; ICs plus 10% serum from C5-deficient mice; and 2 ng/ml TNF-α. Human peripheral blood monocytes purified by Ficoll density centrifugation and adherence (>95% peroxidase-positive) were stimulated with 200 μg/ml ICs plus 10% normal human serum and ICs plus 10% heat-inactivated human serum, 10 nM C5a, or 2 ng/ml TNF-α. After 2 h, culture supernatants were collected and analyzed for sVEGFR-1 by ELISA (R&D Systems).
Statistical analysis.
Data are expressed as mean ± SD. After it was determined that the data were normally distributed (Kolomogorov-Smirnov test of normalcy), the Student's t test (two-tailed) was used to compare fetal resorption frequencies and fetal weights between groups. Kruskal-Wallis ANOVA was used to compare patterns of angiogenic factor levels or TNF-α levels in different mating pairs and groups of mice receiving different treatments through pregnancy. A probability of <0.05 was used to reject the null hypothesis.
Acknowledgments
We are grateful to Drs. Susan Fisher and D. Michael Nelson for useful discussions, to Dr. Ulrich Feige (Amgen Inc) for generously providing PEG sTNFRI, to Dr. John D. Lambris for generously providing C5aR antagonist peptide, and to Marta Guerra for preparation of the manuscript.
This research was supported in part by NIH grants AI055007 (to J.E. Salmon), AI31105 (to V.M. Holers), DK64790 (to J.M. Thurman), and the Mary Kirkland Center for Lupus Research.
Dr. Holers is a consultant to and holds stock in Taligen Therapeutics, a company that has licensed technology from the University of Colorado at Denver and Health Sciences Center, and Drs. Salmon and Thurman hold stock in Taligen Therapeutics. All other authors have no conflicting financial interests.
Abbreviations used: Crry, complement receptor 1–related gene/protein y; HRP, horseradish peroxidase; IC, immune complex; IUGR, intrauterine growth restriction; PEG sTNFRI, polyethylene glycol–conjugated soluble TNF-α receptor type I; sVEGFR-1, soluble vascular endothelial growth factor receptor 1; VEGF, vascular endothelial growth factor; VEGFR-1, VEGF receptor 1.
References
- 1.Mills, J.L., J.L. Simpson, S.G. Driscoll, L. Jovanovic-Peterson, M. Van Allen, J.H. Aarons, B. Metzger, F.R. Bieber, R.H. Knopp, L.B. Holmes, et al. 1988. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N. Engl. J. Med. 319:1617–1623. [DOI] [PubMed] [Google Scholar]
- 2.Resnik, R., and R.K. Creasy. 2003. Intrauterine growth restriction. In Maternal-Fetal Medicine: Principles and Practice. J.D. Iams, editor. Saunders, Philadelphia. 495–512.
- 3.Godfrey, K.M., and D.J. Barker. 2000. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71:1344S–1352S. [DOI] [PubMed] [Google Scholar]
- 4.Mellor, A.L., and D.H. Munn. 2000. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu. Rev. Immunol. 18:367–391. [DOI] [PubMed] [Google Scholar]
- 5.Hunt, J.S., M.G. Petroff, R.H. McIntire, and C. Ober. 2005. HLA-G and immune tolerance in pregnancy. FASEB J. 19:681–693. [DOI] [PubMed] [Google Scholar]
- 6.Guleria, I., A. Khosroshahi, M.J. Ansari, A. Habicht, M. Azuma, H. Yagita, R.J. Noelle, A. Coyle, A.L. Mellor, S.J. Khoury, and M.H. Sayegh. 2005. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu, C., D. Mao, V.M. Holers, B. Palanca, A.M. Cheng, and H. Molina. 2000. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 287:498–501. [DOI] [PubMed] [Google Scholar]
- 8.Aluvihare, V.R., M. Kallikourdis, and A.G. Betz. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5:266–271. [DOI] [PubMed] [Google Scholar]
- 9.Mellor, A.L., J. Sivakumar, P. Chandler, K. Smith, H. Molina, D. Mao, and D.H. Munn. 2001. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat. Immunol. 2:64–68. [DOI] [PubMed] [Google Scholar]
- 10.Caucheteux, S.M., C. Kanellopoulos-Langevin, and D.M. Ojcius. 2003. At the innate frontiers between mother and fetus: linking abortion with complement activation. Immunity. 18:169–172. [DOI] [PubMed] [Google Scholar]
- 11.Holers, V.M., G. Girardi, L. Mo, J.M. Guthridge, H. Molina, S.S. Pierangeli, R. Espinola, L.E. Xiaowei, D. Mao, C.G. Vialpando, and J.E. Salmon. 2002. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 195:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardi, G., J. Berman, P. Redecha, L. Spruce, J.M. Thurman, D. Kraus, T.J. Hollmann, P. Casali, M.C. Caroll, R.A. Wetsel, et al. 2003. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 112:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Red-Horse, K., Y. Zhou, O. Genbacev, A. Prakobphol, R. Foulk, M. McMaster, and S.J. Fisher. 2004. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Invest. 114:744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam, C., K.H. Lim, and S.A. Karumanchi. 2005. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 46:1077–1085. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara, N., H.P. Gerber, and J. LeCouter. 2003. The biology of VEGF and its receptors. Nat. Med. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 16.Kendall, R.L., and K.A. Thomas. 1993. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA. 90:10705–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, Y., S.K. Smith, K.A. Day, D.E. Clark, D.R. Licence, and D.S. Charnock-Jones. 1999. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol. Endocrinol. 13:537–545. [DOI] [PubMed] [Google Scholar]
- 18.Eubank, T.D., R. Roberts, M. Galloway, Y. Wang, D.E. Cohn, and C.B. Marsh. 2004. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity. 21:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krysiak, O., A. Bretschneider, E. Zhong, J. Webb, H. Hopp, S. Verlohren, N. Fuhr, M. Lanowska, A. Nonnenmacher, R. Vetter, et al. 2005. Soluble vascular endothelial growth factor receptor-1 (sFLT-1) mediates downregulation of FLT-1 and prevents activated neutrophils from women with preeclampsia from additional migration by VEGF. Circ. Res. 97:1253–1261. [DOI] [PubMed] [Google Scholar]
- 20.Maynard, S.E., J.Y. Min, J. Merchan, K.H. Lim, J. Li, S. Mondal, T.A. Libermann, J.P. Morgan, F.W. Sellke, I.E. Stillman, et al. 2003. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charnock-Jones, D.S., and G.J. Burton. 2000. Placental vascular morphogenesis. Baillieres Best Pract. Res. Clin. Obstet. Gynaecol. 14:953–968. [DOI] [PubMed] [Google Scholar]
- 22.Levine, R.J., S.E. Maynard, C. Qian, K.H. Lim, L.J. England, K.F. Yu, E.F. Schisterman, R. Thadhani, B.P. Sachs, F.H. Epstein, et al. 2004. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350:672–683. [DOI] [PubMed] [Google Scholar]
- 23.Zhou, Y., M. McMaster, K. Woo, M. Janatpour, J. Perry, T. Karpanen, K. Alitalo, C. Damsky, and S.J. Fisher. 2002. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am. J. Pathol. 160:1405–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Bandt, M., M.H. Ben Mahdi, V. Ollivier, M. Grossin, M. Dupuis, M. Gaudry, P. Bohlen, K.E. Lipson, A. Rice, Y. Wu, et al. 2003. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J. Immunol. 171:4853–4859. [DOI] [PubMed] [Google Scholar]
- 25.Nozaki, M., B.J. Raisler, E. Sakurai, J.V. Sarma, S.R. Barnum, J.D. Lambris, Y. Chen, K. Zhang, B.K. Ambati, J.Z. Baffi, and J. Ambati. 2006. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA. 103:2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bora, P.S., J.H. Sohn, J.M. Cruz, P. Jha, H. Nishihori, Y. Wang, S. Kaliappan, H.J. Kaplan, and N.S. Bora. 2005. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 174:491–497. [DOI] [PubMed] [Google Scholar]
- 27.Rajakumar, A., H.M. Michael, P.A. Rajakumar, E. Shibata, C.A. Hubel, S.A. Karumanchi, R. Thadhani, M. Wolf, G. Harger, and N. Markovic. 2005. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 26:563–573. [DOI] [PubMed] [Google Scholar]
- 28.Clark, D.A., G. Chaouat, P.C. Arck, H.W. Mittruecker, and G.A. Levy. 1998. Cytokine-dependent abortion in CBA x DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase. J. Immunol. 160:545–549. [PubMed] [Google Scholar]
- 29.Blois, S., M. Tometten, J. Kandil, E. Hagen, B.F. Klapp, R.A. Margni, and P.C. Arck. 2005. Intercellular adhesion molecule-1/LFA-1 cross talk is a proximate mediator capable of disrupting immune integration and tolerance mechanism at the feto-maternal interface in murine pregnancies. J. Immunol. 174:1820–1829. [DOI] [PubMed] [Google Scholar]
- 30.Kim, Y.U., T. Kinoshita, H. Molina, D. Hourcade, T. Seya, L.M. Wagner, and V.M. Holers. 1995. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J. Exp. Med. 181:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., S.A. Rollins, J.A. Madri, and L.A. Matis. 1995. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl. Acad. Sci. USA. 92:8955–8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strachan, A.J., T.M. Woodruff, G. Haaima, D.P. Fairlie, and S.M. Taylor. 2000. A new small molecule C5a receptor antagonist inhibits the reverse-passive Arthus reaction and endotoxic shock in rats. J. Immunol. 164:6560–6565. [DOI] [PubMed] [Google Scholar]
- 33.Higginbottom, A., S.A. Cain, T.M. Woodruff, L.M. Proctor, P.K. Madala, J.D. Tyndall, S.M. Taylor, D.P. Fairlie, and P.N. Monk. 2005. Comparative agonist/antagonist responses in mutant human C5a receptors define the ligand binding site. J. Biol. Chem. 280:17831–17840. [DOI] [PubMed] [Google Scholar]
- 34.Sundsmo, J.S., J.R. Chin, R.A. Papin, D.S. Fair, and Z. Werb. 1985. Factor B, the complement alternative pathway serine proteinase, is a major constitutive protein synthesized and secreted by resident and elicited mouse macrophages. J. Exp. Med. 161:306–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heeger, P.S., P.N. Lalli, F. Lin, A. Valujskikh, J. Liu, N. Muqim, Y. Xu, and M.E. Medof. 2005. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 201:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurman, J.M., D.M. Kraus, G. Girardi, D. Hourcade, H.J. Kang, P.A. Royer, L.M. Mitchell, P.C. Giclas, J. Salmon, G. Gilkeson, and V.M. Holers. 2005. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol. Immunol. 42:87–97. [DOI] [PubMed] [Google Scholar]
- 37.Girardi, G., P. Redecha, and J.E. Salmon. 2004. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat. Med. 10:1222–1226. [DOI] [PubMed] [Google Scholar]
- 38.Hunt, J.S., H.L. Chen, and L. Miller. 1996. Tumor necrosis factors: pivotal components of pregnancy? Biol. Reprod. 54:554–562. [DOI] [PubMed] [Google Scholar]
- 39.Berman, J., G. Girardi, and J.E. Salmon. 2005. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J. Immunol. 174:485–490. [DOI] [PubMed] [Google Scholar]
- 40.Hung, T.H., D.S. Charnock-Jones, J.N. Skepper, and G.J. Burton. 2004. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am. J. Pathol. 164:1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arck, P.C., A.B. Troutt, and D.A. Clark. 1997. Soluble receptors neutralizing TNF-alpha and IL-1 block stress-triggered murine abortion. Am. J. Reprod. Immunol. 37:262–266. [DOI] [PubMed] [Google Scholar]
- 42.Knofler, M., B. Mosl, S. Bauer, G. Griesinger, and P. Husslein. 2000. TNF-alpha/TNFRI in primary and immortalized first trimester cytotrophoblasts. Placenta. 21:525–535. [DOI] [PubMed] [Google Scholar]
- 43.Kraut, N., L. Snider, C.M. Chen, S.J. Tapscott, and M. Groudine. 1998. Requirement of the mouse I-mfa gene for placental development and skeletal patterning. EMBO J. 17:6276–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross, J.C. 2005. How to make a placenta: mechanisms of trophoblast cell differentiation in mice–a review. Placenta. 26:S3–S9. [DOI] [PubMed] [Google Scholar]
- 45.Patterson, C., M.A. Perrella, W.O. Endege, M. Yoshizumi, M.E. Lee, and E. Haber. 1996. Downregulation of vascular endothelial growth factor receptors by tumor necrosis factor-alpha in cultured human vascular endothelial cells. J. Clin. Invest. 98:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rote, N.S., E. Vogt, G. DeVere, A.R. Obringer, and A.K. Ng. 1998. The role of placental trophoblast in the pathophysiology of the antiphospholipid antibody syndrome. Am. J. Reprod. Immunol. 39:125–136. [DOI] [PubMed] [Google Scholar]
- 47.Rodesch, F., P. Simon, C. Donner, and E. Jauniaux. 1992. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 80:283–285. [PubMed] [Google Scholar]
- 48.Carroll, M.C., and V.M. Holers. 2005. Innate autoimmunity. Adv. Immunol. 86:137–157. [DOI] [PubMed] [Google Scholar]
- 49.Fujita, T., M. Matsushita, and Y. Endo. 2004. The lectin-complement pathway–its role in innate immunity and evolution. Immunol. Rev. 198:185–202. [DOI] [PubMed] [Google Scholar]
- 50.Jordan, J.E., M.C. Montalto, and G.L. Stahl. 2001. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 104:1413–1418. [DOI] [PubMed] [Google Scholar]
- 51.Mao, D., X. Wu, C. Deppong, L.D. Friend, G. Dolecki, D.M. Nelson, and H. Molina. 2003. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 19:813–822. [DOI] [PubMed] [Google Scholar]
- 52.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35. [DOI] [PubMed] [Google Scholar]
- 53.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J.V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 76:519–529. [DOI] [PubMed] [Google Scholar]
- 54.Hopken, U.E., B. Lu, N.P. Gerard, and C. Gerard. 1996. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 383:86–89. [DOI] [PubMed] [Google Scholar]