Abstract
T cells recognize protein antigens as short peptides processed and displayed by antigen-presenting cells. However, the mechanism of peptide selection is incompletely understood, and, consequently, the differences in the immunogenicity of protein antigens remain largely unpredictable and difficult to manipulate. In this paper we show that the susceptibility of protein antigens to lysosomal proteolysis plays an important role in determining immunogenicity in vivo. We compared the immunogenicity of proteins with the same sequence (same T cell epitopes) and structure (same B cell epitopes) but with different susceptibilities to lysosomal proteolysis. After immunizing mice with each of the proteins adsorbed onto aluminum hydroxide as adjuvant, we measured serum IgG responses as a physiological measure of the antigen's ability to be presented on major histocompatibility complex class II molecules and to prime CD4+ T cells in vivo. For two unrelated model antigens (RNase and horseradish peroxidase), we found that only the less digestible forms were immunogenic, inducing far more efficient T cell priming and antibody responses. These findings suggest that stability to lysosomal proteolysis may be an important factor in determining immunogenicity, with potential implications for vaccine design.
To be recognized by T lymphocytes, protein antigens must be converted into short peptides bound to MHC molecules, which are displayed on the surface of APCs. The ability of APCs to generate peptide–MHC complexes is, therefore, essential to the initiation of the immune response (1, 2). Although the interaction between peptides and MHC class II molecules and the ability of the T cell repertoire to generate antigen receptors of cognate specificity have been extensively studied, it remains difficult to predict or to manipulate the extraction of peptide ligands from protein antigens (3, 4). As a consequence, the differences in immunogenicity between protein antigens are poorly understood and, therefore, approaches to induce antigen-specific immunity remain largely empirical (5, 6).
Antigenic peptides are produced by lysosomal proteolysis, and, thus, efficient lysosomal degradation is often assumed to favor production of ligands for MHC class II molecules. This notion derives mostly from in vitro experiments. For instance, blocking lysosomal function with protease or acidification inhibitors decreased antigen presentation (7–11). Enhancing lysosomal proteolysis by the presence of protease-specific cleavage sites (12) or by destabilizing proteins also favored presentation to T cell hybridomas (13–15). However, these in vitro studies did not evaluate the role of lysosomal proteolysis on immunogenicity in vivo.
We decided to take a direct and physiological approach to investigating the relationship between antigen proteolysis and immunity in vivo. We chose not to use pharmacological or genetic approaches that could potentially have multiple effects on APCs. We studied instead the immunogenicity of proteins with the same sequence (same T cell epitopes) and structure (same B cell epitopes) but with different susceptibilities to lysosomal proteolysis. We found that less digestible forms of otherwise identical antigens are more immunogenic, inducing more efficient T cell priming and antibody responses.
RESULTS AND DISCUSSION
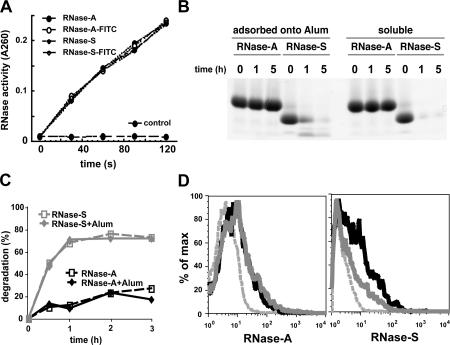
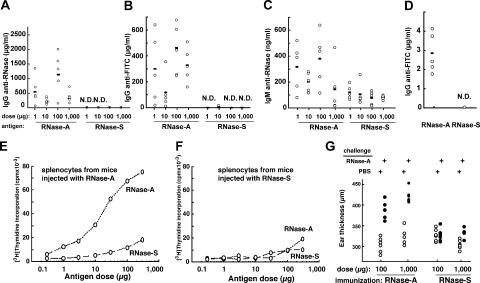
We began by comparing bovine pancreatic ribonuclease (RNase-A), a compact stable protein, with its variant RNase-S, in which a single peptide bond is cleaved (between Ala20 and Ser21) (16, 17). Although both RNase-A and RNase-S are otherwise structurally and enzymatically identical (16, 17) (Fig. 1 A), RNase-S was far more susceptible to lysosomal proteolysis both in vitro by lysosomal extracts and after internalization by bone marrow–derived DCs (BM-DCs); this difference was maintained after antigen adsorption onto an adjuvant such as aluminum hydroxide (Alum; Fig. 1, B and C). We next asked if the differential susceptibilities to proteolysis of these model antigens affected their capacity to induce IgG responses as a physiological in vivo measure of their ability to be presented on MHC class II molecules and to prime CD4+ T cells in vivo. After injecting each of the proteins adsorbed onto Alum into mice, the stable form of RNase (RNase-A) was found to induce much higher (>10,000-fold) IgG titers than did the unstable form (RNase-S; Fig. 2 A).
Figure 1.
Differential susceptibility of RNase-A and RNase-S to lysosomal proteolysis. (A) RNase-A, RNase-S, and their FITC derivatives have the same ribonuclease activity, indicating that attachment of FITC and subtilisin cleavage had no major effect on their three-dimensional structures. (B) SDS-PAGE analysis of the degradation of RNase-A and RNase-S, soluble or adsorbed onto Alum, by lysosomal extracts of BM-DCs at 37°C for the indicated times. (C) BM-DCs were pulsed with 0.5 mg/ml FITC–RNase-A or FITC–RNase-S (soluble or adsorbed onto Alum) for 1 h at 37°C, washed, and then further cultured for the indicated times. The percentage of degradation of antigen represents the percentage of FITC+ CD11c+ cells at the indicated time subtracted to the percentage FITC+ CD11c+ cells at 0 h. (D) Persistence of the stable form (RNase-A) in lymph node DCs. Mice were co-injected intradermally with 20 μg of Alexa 488–RNase-A and 20 μg of Alexa 647–RNase-S. Single cell suspensions were prepared from the draining lymph nodes, removed 2.5 or 16 h after injection, stained with anti-CD11c–PE, and analyzed by FACS. The histograms depict CD11c+ populations scored for their content of Alexa 488–RNase-A or Alexa 647–RNase-S 2.5 (black line) and 16 (gray line) h after injection. The dashed gray line depicts CD11c+ cells from noninjected control mice.
Figure 2.
Differential susceptibility of RNase-A and RNase-S to lysosomal proteolysis affects their immunogenicity. (A–D) The stable form (RNase-A) induces stronger IgG responses than the unstable form (RNase-S). Mice were immunized by intraperitoneal injection of the indicated doses of a mixture of RNase-A and FITC–RNase-A or RNase-S and FITC–RNase-S adsorbed onto Alum twice at 2-wk intervals. 10 d after the last injection, the sera were collected and IgG anti–RNase-A (A), IgG anti-FITC (B), and IgM anti–RNase-A (C) were titrated by ELISA. (D) C57BL/6 mice were immunized by a single injection in the footpad of BM-DCs loaded with FITC–RNase-A or FITC–RNase-S. 10 d later, the sera were collected, and IgG anti-FITC was titrated by ELISA. The horizontal lines represent the mean value of each group. N.D., none detected. (E and F) The protease-resistant form (RNase-A) induces a stronger priming of cellular immune responses than the form more readily degraded (RNase-S). Splenocytes from mice previously immunized with RNase-A (E) or RNase-S (F) were incubated with the indicated doses of antigens. T cell proliferation was estimated by [3H]thymidine incorporation 2 d later. (G) Delayed-type hypersensitivity response in mice immunized with the stable form (RNase-A) is higher than in mice immunized with the unstable form (RNase-S). Immunized mice were challenged by injection of 1 μg of RNase-A into one ear, while the other ear received PBS. The ear thickness was measured 24 h later.
We next examined antigen uptake by APCs in vivo by FACS analysis. DCs in the draining lymph nodes contained comparable amounts of RNase-A and RNase-S 2.5 h after intradermal injection (Fig. 1 D). Moreover, differential antibody responses were also observed when BM-DCs loaded ex vivo with the same amount of RNase-A or RNase-S were adoptively transferred into naive recipient mice (Fig. 2 D). Collectively these results rule out that the differences in immunogenicity between the two proteins could result from differential access to APCs.
That both forms of RNase indeed share the same B cell epitopes was further emphasized by the fact that the small amount of anti-RNase IgG elicited after injection of high doses of RNase-S also reacted with RNase-A, and vice versa (unpublished data). Moreover, both forms of RNase induced comparable soluble IgM responses (2–4-fold difference, as opposed to >10,000-fold difference in IgG responses; Fig. 2, A and C). This indicated that, despite being recognized similarly by B cells, RNase-S was not adequately presented to CD4+ T cells.
To further show that the rapid lysosomal degradation of RNase-S limited MHC class II presentation and T cell priming in vivo, we analyzed the antibody responses to defined B cell epitopes introduced on RNase-A or RNase-S. The IgG responses to haptens such as FITC and DNP were strong only when they were coupled to the stable protein carrier (RNase-A; Fig. 2 B and not depicted). When low doses of antigens were injected with stronger adjuvants (incomplete or complete Freund's), RNase-S was still less immunogenic than RNase-A (unpublished data). The differences in immunogenicity of the model antigens were maintained over a broad dose range (1–1,000 μg) and after multiple injections of antigen (unpublished data), emphasizing that the different immunogenicity of RNase-A and RNase-S most likely reflects their efficiency of presentation on MHC class II molecules.
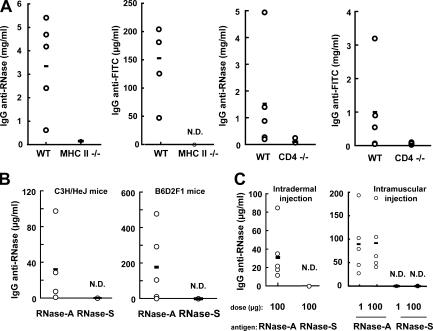
Indeed, cellular immune responses were also stronger to the antigen more resistant to lysosomal proteolysis (RNase-A). Primarily, immunization with RNase-A but not RNase-S elicited robust T cell recall responses in vitro (Fig. 2, E and F). Also, the delayed-type hypersensitivity response in mice immunized with the stable form (RNase-A) was substantial, whereas it was undetectable in mice immunized with RNase-S (Fig. 2 G). In addition, the IgG responses to RNase-A and to FITC coupled to RNase-A exhibited the hallmarks of conventional CD4-dependent T cell responses, as no anti-RNase or anti-FITC IgGs were detected in CD4- or MHC class II–deficient mice (Fig. 3 A). The same difference in immunogenicity between RNase-A and RNase-S was observed in different mouse strains (Fig. 3 B), indicating that the increased immunogenicity of RNase-A was not associated with individual MHC class II haplotypes. Similarly, the differential immunogenicity of RNase-A and RNase-S was independent of the route of injection (intraperitoneal, intradermal, or intramuscular; Fig. 2 A and Fig. 3 C) and, consequently, of the population of APCs that initially encounter the antigens.
Figure 3.
The IgG responses to RNase are CD4+ T cell dependent, and the differential immunogenicity between RNase-A and RNase-S is independent of the mouse strain and the route of injection. CD4−/− and MHC class II−/− mice on the C57BL/6 background (A) and mice from the C3H/HeJ and B6D2F1 strains (B) were immunized by intraperitoneal injection of a mixture of RNase-A and FITC–RNase-A or RNase-S and FITC–RNase-S (1 μg each) adsorbed onto Alum twice at a 2-wk interval. 10 d after the last injection, the sera were collected, and IgG anti-RNase and anti-FITC were titrated by ELISA. (C) Experiments shown as in A and B, except that C57BL/6 mice were immunized by intradermal or intramuscular injection of antigens. The horizontal lines represent the mean value of each group. N.D., none detected.
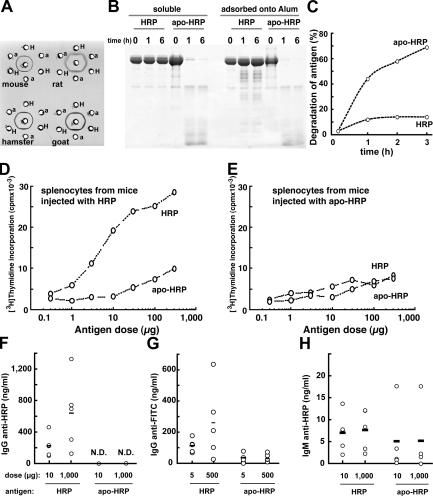
We extended the analysis to another antigen with a different structure and T cell epitopes. For this purpose, we compared the immunogenicity of horseradish peroxidase (HRP) and its variant apo-HRP, from which the calcium and heme group have been removed, leaving the intact but destabilized polypeptide chain of HRP. Both forms of HRP have the same amino acid sequence and were not covalently modified; therefore, they possess the same T cell epitopes. They also retain intact their four intramolecular disulfide bonds, maintaining similar three-dimensional structures and antigenicity, as reflected by the fact that polyclonal antibodies from several species recognized HRP and apo-HRP identically under native conditions (Fig. 4 A). However, the two forms of HRP, soluble or adsorbed onto Alum, differed markedly in their susceptibility to lysosomal proteolysis, with apo-HRP being more readily digested by DC lysosomal proteases in vitro and ex vivo after internalization by BM-DCs (Fig. 4, B and C).
Figure 4.
Limited lysosomal proteolysis of HRP results in enhanced immunogenicity. (A) HRP (H) and apo-HRP (a) give rise only to complete identity precipitation lines by gel immunodiffusion assay in the presence of anti-HRP sera (center well) raised in the indicated species, indicating that HRP and its derivative (apo-HRP) have the same antigenicity. (B) SDS-PAGE analysis of the degradation of HRP and apo-HRP, soluble or adsorbed onto Alum, by lysosomal extracts of BM-DCs at 37°C for the indicated times. (C) BM-DCs were pulsed with 0.5 mg/ml FITC-HRP or FITC–apo-HRP for 1 h at 37°C, washed, and then further cultured for the indicated times. The percentage of degradation of antigen represents the percentage of FITC+ CD11c+ cells at the indicated time, subtracted to the percentage FITC+ CD11c+ cells at 0 h. (D and E) The protease resistant form (HRP) is presented more efficiently to T cells than the form more readily degraded (apo-HRP). Splenocytes from mice previously immunized with HRP (D) or apo-HRP (E) were incubated with the indicated doses of antigens. After 2 d in culture, T cell proliferation was estimated by [3H]thymidine incorporation. (F–H) The stable form (HRP) induces stronger IgG responses than the unstable form (apo-HRP). Mice were immunized by intraperitoneal injection of the indicated doses of a mixture of HRP and FITC-HRP or apo-HRP and FITC–apo-HRP adsorbed onto Alum twice at a 2-wk interval. 10 d after the last injection, the sera were collected, and IgG anti-HRP (F), IgG anti-FITC (G), and IgM anti-HRP (H) were titrated by ELISA. The horizontal lines represent the mean value of each group. N.D., none detected.
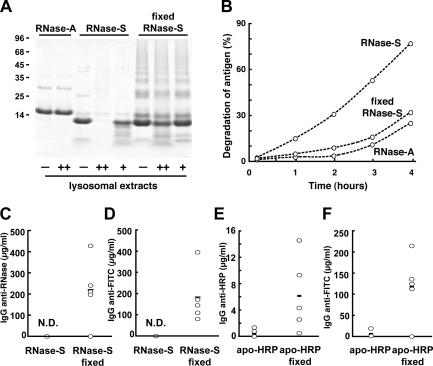
Immunization experiments in mice showed essentially the same pattern of immunogenicity as with stable and unstable forms of RNase: the stable form of HRP (intact HRP) induced stronger T cell priming (Fig. 4, D and E) and IgG responses (Fig. 4 F) than the unstable form (apo-HRP). Similarly, the IgG responses to a hapten (FITC) were more robust when it was presented on the protein backbone of the stable form (HRP; Fig. 4 G). As with RNase, these differences were maintained over a 1,000-fold dose range (unpublished data). Both forms of HRP also produced similar soluble IgM responses (Fig. 4 H), and all antisera raised against HRP fully recognized apo-HRP (unpublished data), indicating that both forms of HRP shared the same IgG epitopes, but that the rapidly degraded form was weakly immunogenic. If the poor priming of T cells by proteins that are rapidly degraded (RNase-S and apo-HRP) was primarily caused by enhanced susceptibility to lysosomal degradation, stabilizing them to proteolysis should enhance their capacity to induce IgG responses. To test this possibility, we generated inter- and intramolecular cross-linked forms of RNase-S or apo-HRP by fixation with aldehydes (18, 19), resulting in molecules that became more resistant to lysosomal proteolysis in vitro (Fig. 5 A and Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20052442/DC1). The stabilization to lysosomal proteolysis of RNase-S and apo-HRP by fixation was also observed in intact cells after internalization into BM-DCs (Fig. 5 B and Fig. S1 B). In both cases, aldehyde-mediated stabilization largely restored the ability of these otherwise poorly immunogenic proteins to induce IgG responses to RNase (Fig. 5 C) and HRP (Fig. 5 E), as well as to coupled haptens (FITC; Fig. 5, D and F).
Figure 5.
Stabilizing antigens to lysosomal proteolysis enhances their survival in vivo and their immunogenicity. (A) Fixation of RNase-S with aldehydes increases its resistance to lysosomal proteolysis. SDS-PAGE analysis of the degradation of RNase-A, RNase-S, and fixed RNase-S by 1 μg (++) or 0.25 μg (+) of lysosomal extracts of BM-DCs at 37°C for 16 h. (B) BM-DCs were pulsed with FITC–RNase-A, FITC–RNase-S, or fixed FITC–RNase-S (0.5 mg/ml each) for 1 h at 37°C, washed, and then further cultured for the indicated times. The percentage of degradation of antigen represents the percentage of FITC+ CD11c+ cells at the indicated times subtracted to the percentage FITC+ CD11c+ cells at 0 h. (C–F) The stabilization of RNase-S and apo-HRP to lysosomal degradation enhances their ability to induce IgG responses. Mice were immunized by intraperitoneal injection of a mixture of RNase-S and FITC–RNase-S (1μg each) or apo-HRP and FITC–apo-HRP (20 μg each) or their fixed forms adsorbed onto Alum twice at a 2-wk interval. 10 d after the last injection, serum IgG anti–RNase-A (C) or anti-HRP (E) and anti-FITC (D and F) were titrated by ELISA. The horizontal lines represent the mean value of each group. N.D., none detected.
One contributing factor to the increased ability of more stable antigens to elicit immune responses is that the restricted susceptibility to lysosomal proteolysis favored the production of peptide–MHC class II complexes by DCs, at least in vitro (Fig. 2 E and Fig. 4 D). In addition, and just as important in an in vivo setting, we found that the increased stability to lysosomal proteolysis also favored the retention of antigens captured by DCs to lymphoid organs. 16 h after a single intradermal injection, the stable forms of RNase (Alexa 488–RNase-A) could be detected in CD11c+ DCs in the draining lymph nodes (Fig. 1 D). In contrast, the rapidly degraded form (Alexa 647–RNase-S) was barely detectable under the same conditions (Fig. 1 D). Combined with the fact that differential immunogenicity was observed by adoptively transferring DCs containing either RNase-A or RNase-S (Fig. 2 D), these results strongly suggest that at least one effect of decreased susceptibility to proteolysis is to facilitate intracellular antigen survival in DCs, which would allow for a sustained provision of MHC–peptide complexes.
Our findings show that, in contrast to a prevailing view derived mostly from in vitro experiments, the immunogenicity of protein antigens in vivo can be enhanced by reducing their susceptibility to lysosomal proteolysis. This may therefore be an important factor contributing to the largely unexplained differences among proteins in their abilities to elicit immune responses. Although this feature may vary for different antigens, the fact that we have obtained identical results for two entirely unrelated proteins, and for haptens coupled to them, suggests that it represents a general principle. It is noteworthy that we did not find major differences in the presentation of defined epitopes from RNase-A and RNase-S to CD4+ T cell hybridomas in vitro (unpublished data), although such differences were observed in vivo and using primary T cells, emphasizing the importance of in vivo studies to examine protein immunogenicity. It would be interesting to examine the impact of protein digestibility on the activation of naive versus memory T cells.
The susceptibility to lysosomal proteolysis may not only enhance the preservation of CD4+ T cell epitopes themselves but also clearly increases the persistence of the proteins from which those epitopes are extracted. DCs can take many hours or days to traverse from the periphery to lymphoid organs where they engage their cognate T cells to initiate immune responses (20); therefore, antigen persistence provides a source of antigen for sustained processing and presentation by DCs within (or en route to) secondary lymphoid organs (21, 22) and, potentially, presentation of intact antigens to B cells (23, 24). Although the production of peptide–MHC class II complexes from a given antigen may not always be favored by restricting digestion, the enhanced dissemination and persistence of stable antigens (or antigens in APCs of restricted lysosomal proteolysis) should consistently contribute to immunogenicity. It is interesting to speculate that, as part of their mechanism of action, some adjuvants and carrier proteins could act at least in part by protecting the antigens they carry against lysosomal destruction. For example, coupling peptides to large, poorly digestible carriers would in essence convert labile peptides into relatively stable proteins.
The susceptibility of exogenous antigens to lysosomal proteolysis may also affect their ability to elicit CD8+ T cell responses during cross-presentation. Less digestible antigens may have a greater chance of surviving the digestive environment inside lysosomes to gain access to the cystosol for presentation on MHC class I molecules. This in fact may contribute to the enhancement of antigen cross-presentation observed in the presence of chloroquine (25). In addition, the comparative efficiency of DCs relative to macrophages in cross presentation (26) may at least in part reflect the relative inefficiency with which DCs degrade endocytosed antigens (27).
Our results provide direct support for the concept that the limited proteolytic capacity of DCs plays an important role in vivo in augmenting their efficiency as APCs by enhancing not only peptide–MHC class II production but also antigen dissemination and persistence in vivo (27). This may have implications for vaccine design, particularly for the elicitation of MHC class II–dependent antibody responses. Chemical modifications or the use of carriers that enhance antigen resistance to lysosomal proteolysis may enhance antigen immunogenicity. This approach would capitalize on one of the key biological properties of DCs to further enhance their antigen-presenting functions.
MATERIALS AND METHODS
Mice and cells.
C57BL/6, C3H/HeJ, and B6D2F1 mice were purchased from The Jackson Laboratory. CD4−/− and MHC class II−/− mice on the C57BL/6 background were purchased from Taconic. All mice were males and were used at 6–12 wk of age. The Institutional Animal Care and Use Committee at Yale University approved all animal protocols. BM-DCs were grown as previously described (28). Lysosomal extracts of DCs were prepared as previously described (27).
Model antigens.
RNase-A and RNase-S (Sigma-Aldrich) were characterized as previously described (29). Apo-HRP was prepared as previously described (30). FITC (Sigma-Aldrich), Alexa 488, and Alexa 647 (Invitrogen) derivatives were prepared according to the manufacturer's recommendations. For fixation with aldehydes, 2 mg/ml of antigens were incubated in PBS in the presence of 10 mM paraformaldehyde and 2 mM glutaraldehyde on ice for 30 min. Reactions were stopped by addition of 50 mM glycine, followed by a 10-min centrifugation at 10,000 g to remove aggregates and a subsequent desalting into PBS. Recognition of model antigens by polyclonal antibodies was conducted by ELISA or by immunodiffusion in gels.
Immunizations.
Mice were immunized by intraperitoneal, intradermal, or intramuscular injection of 1–1,000 μg of the different antigens adsorbed onto Alum adjuvant (Imject Alum; Pierce Chemical Co.) twice at a 2-wk interval. Alternatively, mice were immunized by a single injection with 300,000 CD11c+ BM-DCs (loaded with 0.5 mg/ml of antigens for 2 h) in the footpad. Sera were collected before immunization and 10 d after the last injection.
ELISA.
Sera were titrated by using plates (Maxisorp; Nunc) coated with RNase-A, HRP, or FITC-BSA (5 μg/ml each). Antibodies were detected using alkaline phosphatase–conjugated donkey antibodies against mouse IgM or IgG (Jackson ImmunoResearch Laboratories), as well as with 4-methylumbelliferyl phosphate (Sigma-Aldrich).
Delayed-type hypersensitivity response.
After immunization, the mice were challenged by injection of 1 μg of antigen into one ear, while the other ear received PBS. The ear thickness was measured 24 h later using a micrometer (Ultra-Mic; Fowler).
In vitro degradation assays.
Antigen degradation assays were done as previously described (27).
Ex vivo degradation assays.
BM-DCs were loaded with 0.5 mg/ml of antigens for 1 h and incubated at 37°C for the times indicated in the figures. The presence of the proteins and cell surface expression of CD11c were monitored by FACS.
In vivo degradation assays.
Alexa 488–RNase-A and Alexa 647–RNase-S (20 μg each) were simultaneously injected intradermally. At the times indicated in the figures, the draining lymph nodes were removed, and cells were dissociated by treatment with Blendzyme 2 (Roche). The presence of the proteins and cell surface expression of CD11c were monitored by FACS.
Antigen processing and presentation assays.
Splenocytes from immunized mice were incubated with the doses of antigens indicated in the figures for 48 h. T cell responses were evaluated by measuring T cell proliferation, as estimated by [3H]thymidine incorporation.
Online supplemental material.
Fig. S1 shows that fixation of apo-HRP with aldehydes increases its resistance to lysosomal proteolysis in vitro and enhances its survival ex vivo. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20052442/DC1.
Supplemental Material
Acknowledgments
We are grateful to Tracy Ferguson and Craig Burton for expert help.
This work was supported by the National Institutes of Health (I. Mellman), the American Heart Association (E.S. Trombetta), and the Ludwig Institute for Cancer Research (I. Mellman and E.S. Trombetta).
The authors have no conflicting financial interests.
References
- 1.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Germain, R.N. 1994. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 76:287–299. [DOI] [PubMed] [Google Scholar]
- 3.Garcia, K.C., and E.J. Adams. 2005. How the T cell receptor sees antigen – a structural view. Cell. 122:333–336. [DOI] [PubMed] [Google Scholar]
- 4.Lovitch, S.B., and E.R. Unanue. 2005. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol. Rev. 207:293–313. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin, D.C., J.A. Berzofsky, I.J. East, F.R. Gurd, C. Hannum, S.J. Leach, E. Margoliash, J.G. Michael, A. Miller, E.M. Prager, et al. 1984. The antigenic structure of proteins: a reappraisal. Annu. Rev. Immunol. 2:67–101. [DOI] [PubMed] [Google Scholar]
- 6.Sela, M., and I. Pecht. 1996. The nature of the antigen. Adv. Protein Chem. 49:289–328. [DOI] [PubMed] [Google Scholar]
- 7.Vidard, L., K.L. Rock, and B. Benacerraf. 1991. The generation of immunogenic peptides can be selectively increased or decreased by proteolytic enzyme inhibitors. J. Immunol. 147:1786–1791. [PubMed] [Google Scholar]
- 8.Watts, C., C.X. Moss, D. Mazzeo, M.A. West, S.P. Matthews, D.N. Li, and B. Manoury. 2003. Creation versus destruction of T cell epitopes in the class II MHC pathway. Ann. NY Acad. Sci. 987:9–14. [DOI] [PubMed] [Google Scholar]
- 9.Manoury-Schwartz, B., G. Chiocchia, V. Lotteau, and C. Fournier. 1997. Selective increased presentation of type II collagen by leupeptin. Int. Immunol. 9:581–589. [DOI] [PubMed] [Google Scholar]
- 10.Villadangos, J.A., and H.L. Ploegh. 2000. Proteolysis in MHC class II antigen presentation: who's in charge? Immunity. 12:233–239. [DOI] [PubMed] [Google Scholar]
- 11.Trombetta, E.S., and I. Mellman. 2005. Cell biology of antigen processing in vivo and in vitro. Annu. Rev. Immunol. 23:975–1028. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou, A.N., S.L. Blackwood, D. Mazzeo, and C. Watts. 2000. Control of antigen presentation by a single protease cleavage site. Immunity. 12:391–398. [DOI] [PubMed] [Google Scholar]
- 13.Thai, R., G. Moine, M. Desmadril, D. Servent, J.L. Tarride, A. Menez, and M. Leonetti. 2004. Antigen stability controls antigen presentation. J. Biol. Chem. 279:50257–50266. [DOI] [PubMed] [Google Scholar]
- 14.So, T., H.O. Ito, T. Koga, S. Watanabe, T. Ueda, and T. Imoto. 1997. Depression of T-cell epitope generation by stabilizing hen lysozyme. J. Biol. Chem. 272:32136–32140. [DOI] [PubMed] [Google Scholar]
- 15.So, T., H. Ito, M. Hirata, T. Ueda, and T. Imoto. 2001. Contribution of conformational stability of hen lysozyme to induction of type 2 T-helper immune responses. Immunology. 104:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards, F.M., and W.W. Wickoff. 1971. Bovine pancreatic ribonucleases. In The Enzymes, vol. 4. P.D. Boyer, editor. Academic Press, New York. 647–806.
- 17.Kim, E.E., R. Varadarajan, H.W. Wyckoff, and F.M. Richards. 1992. Refinement of the crystal structure of ribonuclease S. Comparison with and between the various ribonuclease A structures. Biochemistry. 31:12304–12314. [DOI] [PubMed] [Google Scholar]
- 18.Rappuoli, R. 1994. Toxin inactivation and antigen stabilization: two different uses of formaldehyde. Vaccine. 12:579–581. [DOI] [PubMed] [Google Scholar]
- 19.Richards, F.M., and J.R. Knowles. 1968. Glutaraldehyde as a protein cross-linkage reagent. J. Mol. Biol. 37:231–233. [DOI] [PubMed] [Google Scholar]
- 20.Itano, A.A., and M.K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733–739. [DOI] [PubMed] [Google Scholar]
- 21.Itano, A.A., S.J. McSorley, R.L. Reinhardt, B.D. Ehst, E. Ingulli, A.Y. Rudensky, and M.K. Jenkins. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 19:47–57. [DOI] [PubMed] [Google Scholar]
- 22.Ludewig, B., K. McCoy, M. Pericin, A.F. Ochsenbein, T. Dumrese, B. Odermatt, R.E. Toes, C.J. Melief, H. Hengartner, and R.M. Zinkernagel. 2001. Rapid peptide turnover and inefficient presentation of exogenous antigen critically limit the activation of self-reactive CTL by dendritic cells. J. Immunol. 166:3678–3687. [DOI] [PubMed] [Google Scholar]
- 23.Wykes, M., A. Pombo, C. Jenkins, and G.G. MacPherson. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313–1319. [PubMed] [Google Scholar]
- 24.Qi, H., J.G. Egen, A.Y. Huang, and R.N. Germain. 2006. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 312:1672–1676. [DOI] [PubMed] [Google Scholar]
- 25.Accapezzato, D., V. Visco, V. Francavilla, C. Molette, T. Donato, M. Paroli, M.U. Mondelli, M. Doria, M.R. Torrisi, and V. Barnaba. 2005. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 202:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts, C., and S. Amigorena. 2001. Phagocytosis and antigen presentation. Semin. Immunol. 13:373–379. [DOI] [PubMed] [Google Scholar]
- 27.Delamarre, L., M. Pack, H. Chang, I. Mellman, and E.S. Trombetta. 2005. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 307:1630–1634. [DOI] [PubMed] [Google Scholar]
- 28.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony–stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards, F.M., and P.J. Vithayathil. 1959. The preparation of subtilisin-modified ribonuclease and the separation of the peptide and protein components. J. Biol. Chem. 234:1459–1465. [PubMed] [Google Scholar]
- 30.Teale, F.W. 1959. Cleavage of the haem-protein link by acid methylethylketone. Biochim. Biophys. Acta. 35:543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.