Abstract
The inappropriate expansion and activation of autoreactive memory B cells and plasmablasts contributes to loss of self-tolerance in systemic lupus erythematosus (SLE). Defects in the inhibitory Fc receptor, FcγRIIB, have been shown to contribute to B cell activation and autoimmunity in several mouse models of SLE. In this paper, we demonstrate that expression of FcγRIIB is routinely up-regulated on memory B cells in the peripheral blood of healthy controls, whereas up-regulation of FcγRIIB is considerably decreased in memory B cells of SLE patients. This directly correlates with decreased FcγRIIB-mediated suppression of B cell receptor–induced calcium (Ca2+) response in those B cells. We also found substantial overrepresentation of African-American patients among those who failed to up-regulate FcγRIIB. These results suggest that the inhibitory receptor, FcγRIIB, may be impaired at a critical checkpoint in SLE in the regulation of memory B cells; thus, FcγRIIB represents a novel target for therapeutic interventions in this disease.
Several genetic studies in both mice and humans have suggested linkage between Fc γ receptors (FcγRs), clustered on chromosome 1q23-24, and SLE. There are three classes of FcγRs: FcγRI (CD64), FcγRII (CD32), and FγRIII (CD16) (for review see reference 1). CD64 is encoded by three genes in the human (IA, IB, and IC) with similar biological properties (2), whereas CD16 is encoded by two genes (IIIA and IIIB) with distinct biological properties. CD16A is an activating FcγR, whereas CD16B is a glycosyl-phosphatidylinositol–anchored protein expressed exclusively on neutrophils and is likely to function as a decoy receptor for immune complexes (3). CD32 comprises the most complex cluster of low-affinity FcγRs. It is encoded by three genes in humans (IIA, IIB, and IIC), all with considerably different biological properties. Although these molecules share >95% sequence identity in their extracellular domains, which bind IgG immune complexes with low affinity, their intracytoplasmic domains are diverse, transducing different signals on receptor cross-linking (3). FcγRIIA and -C are unique to humans and are single-chain activation receptors bearing an immunoreceptor tyrosine-based activation motif sequence in their intracytoplasmic domains. FcγRIIA is widely expressed, found on B cells, myeloid cells, granulocytes, and dendritic cells, whereas FcγRIIC is often found to contain in-frame termination codons, which suggests that it may be evolving into a pseudogene (4). In contrast, FcγRIIB, found on B cells, macrophages, dendritic cells, neutrophils, and mast cells, is conserved between mouse and human and contains an immunorecptor tyrosine-based inhibition motif in its intracytoplasmic domain, thereby transducing an inhibitory signal on coligation to the B cell receptor (BCR) (5–7). This inhibition is mediated through the recruitment of the inositol polyphosphate phosphatase, SHIP, to the tyrosine-phosphorylated immunorecptor tyrosine-based inhibition motif sequence, leading to the hydrolysis of PIP3 and the release of PH domain–containing proteins, with subsequent abrogation of immunorecptor tyrosine-based activation motif–initiated activation signals (7, 8).
Amino acid substitutions in the activating FcγR genes—arginine (R) for histidine (H) at position 131 in FcγRIIA and phenylalanine (F) for valine (V) at position 158 in FcγRIIIA—have resulted in functional polymorphisms of these FcγRs with decreased binding affinity for IgG immune complexes. Numerous clinical studies have investigated the association between these polymorphisms and susceptibility to SLE, as well as specific disease manifestations, and found strikingly disparate results. Studies in Brazilian, Korean, African-American, German, and Thai populations (9–13) have demonstrated substantial associations between the FcγRIIA R131H allele and disease susceptibility or nephritis, whereas studies in Dutch, British, Greek, African-Caribbean, Spanish, Korean, Hispanic, and Korean populations (10, 12, 14–20) found no associations. Four studies in Dutch, Korean, and Caucasian populations (14, 18–20) demonstrated clear associations between the FcγRIIIA F158V polymorphism and disease susceptibility or nephritis, whereas an equal number of studies in German, Korean, and African-American populations (10, 12, 21) found no such associations. The FcγRIIIB NA2 allele has been associated with susceptibility to SLE in a Thai population (13), but five studies in German (12), Chinese (22), Spanish (23), and Dutch (14, 18) populations have failed to demonstrate an association. Several polymorphisms in the FcγRIIB gene have been identified, and one in particular, the I232T polymorphism, has been shown to associate with disease susceptibility in three studies in Asian populations (13, 24, 25). One of these studies also demonstrated a clear correlation of the I232T allele with nephritis (2, 4). Two studies in African-American and Caucasian populations (26, 27) failed to find any association between disease or nephritis and the I232T allele. The collective results of these studies have failed to provide a clear role of FcR polymorphisms in SLE susceptibility, though they do suggest a strong association with ethnicity.
In contrast to these results, studies in mouse models of SLE have demonstrated a clear association between the inhibitory FcγRIIB and disease susceptibility (28–31). Mice on the nonautoimmune C67BL/6 background develop spontaneous lupus when the gene for FcγRIIB is deleted (28), and several studies have demonstrated reduced FcγRIIB expression on activated B cells in autoimmune-susceptible strains of mice, resulting from a promoter polymorphism found in these strains (32, 33). Restoration of the level of FcγRIIB on these cells by retroviral transduction reverts the lupus phenotype of these susceptible strains (34). These results suggest that levels of expression of FcγRIIB may play a substantial role in lupus susceptibility. Quantitative assessment of FcγRIIB expression on specific human cell populations has been limited by the lack of serological reagents capable of distinguishing among these highly homologous molecules. To address this issue, we have used a recently developed novel antibody to quantitate the level of FcγRIIB expression on B cells in normal and lupus populations. We also looked for clinical associations with FcγRIIB expression in SLE patients and used a calcium flux assay to determine functional consequences of alterations in FcγRIIB expression.
RESULTS
Anti-FcγRIIB staining
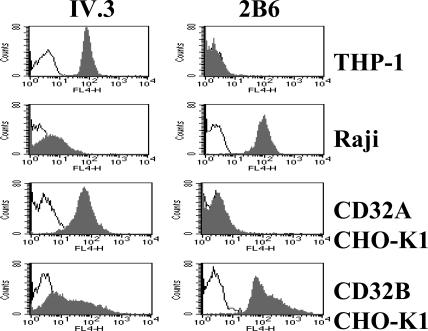
The monoclonal antibody 2B6 is specific for FcγRIIB and distinguishes between FcγRIIA and FcγRIIB on both native and transfected cells, as shown in Fig. 1. Chinese hamster ovary (CHO) cells transfected with either FcγRIIA or FcγRIIB (CD32A or CD32B, respectively) were analyzed by FACS using either 2B6 or the previously described IV.3 monoclonal antibody (35). The 2B6 antibody showed FcγIIB specificity and only background staining on FcγIIA transfectants. The B cell lymphoma line Raji was clearly stained by 2B6, whereas the monocytic line THP-1 did not display appreciable binding to 2B6. 2B6 thus represents an FcγRIIB-specific monoclonal antibody, capable of detecting the molecule on transfected cells as well as on B cell lines and native cells.
Figure 1.
Specificity of antibodies for the human Fc receptors FcγRIIA and FcγRIIB. The human myeloid (THP-1), B cell (Raji), or CHO-transfected cell lines (CD32A CHO-K1 and CD32B CHO-K1) were stained with the anti-RIIA monoclonal antibody IV.3 or the anti-RIIB monoclonal antibody 2B6. 2B6 detects only FcγRIIB on the surface of the Raji or CD32B CHO-K1 cell lines and is negative for THP-1 and CD32A CHO-K1, thus demonstrating its specificity. IV.3 preferentially detects IIA, although some cross-reactivity to IIB is seen on the transfected CD32B CHO-K1 cell line. Open graphs represent isotype control, and shaded graphs represent the specific antibody.
B cell expression of FcγRIIB in controls and SLE patients
B cells from 62 patients with SLE and 18 nonautoimmune individuals (control A; Tables I and II) were examined for expression of FcγRIIB. The control group was composed of healthy subjects of predominantly Asian and Caucasian descent. It has been shown in mouse studies that FcγRIIB expression changes with B cell maturation (32, 33); hence, naive, memory B cells and plasmablasts were all analyzed separately. There were no differences seen in either the percentage of B cells in the peripheral blood or in the distribution of naive and memory cells of the two groups, though the variability was greater for both measurements in the patients with SLE (unpublished data). As has been previously reported (36), patients with SLE had a significantly higher percentage of plasmablasts than nonautoimmune individuals (P < 0.01; unpublished data).
Table I.
Baseline characteristics of all SLE patients
| Characteristic | n = 62 (%) |
|---|---|
| Age (yr) | 39 ± 13 |
| Sex | |
| Male | 9 (14) |
| Female | 53 (86) |
| Race | |
| African American | 33 (53) |
| Othera | 29 (47) |
| Disease duration (yr) | 11 ± 9 |
| Mean prednisone dose (mg) | 13 ± 15 |
| On DMARDsb | 20 (32.3) |
| SLEDAI (mean) | 3.2 ± 3.4 |
| Baseline disease activity | |
| Renal | 9 (14.5) |
| Arthritis | 2 (3.2) |
| Mucocutaneous | 4 (6.5) |
| Alopecia | 7 (11.3) |
| CNS | (0) |
| Hemolytic anemia | (0) |
| Serositis | (0) |
90% were Hispanic.
DMARDs are disease modifying agents that include azathioprine, cyclophosphamide, cellcept, and methotrexate.
Table II.
Baseline characteristics of controls
| Characteristic | Control A n = 18 |
Control B n = 38 |
|---|---|---|
| Age | 34 ± 6.0 | 36 ± 10.9 |
| Sex | ||
| Male | 9 (50%) | 1 (3%) |
| Female | 9 (50%) | 37 (97%) |
| Race | ||
| African American | 1 (6%) | 19 (50%) |
| Hispanic | 1 (6%) | 16 (42%) |
| Asian | 10 (55%) | 1 (3%) |
| Caucasian | 6 (33%) | 2 (5%) |
The control B group was recruited to examine the association of ethnicity and FcγRIIB expression.
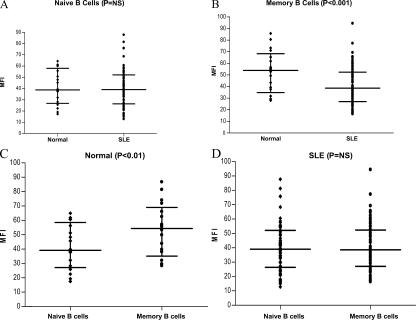
In the analysis of the SLE and control A groups, FcγRIIB was expressed on >80% of all B cell subsets in both groups. As seen in Fig. 2 A, the level of expression of FcγRIIB, as reflected by the level of mean fluorescent intensity (MFI) staining, was equivalent on the naive B cell population in both groups. In contrast, memory B cells from SLE patients had significantly lower surface expression of FcγRIIB than the normal controls (Fig. 2 B). This reduction in FcγRIIB expression results from a failure of SLE patients to up-regulate FcγRIIB expression as B cells become memory cells (Fig. 2). Nonautoimmune individuals displayed an increased level of FcγRIIB expression on memory cells (P < 0.01; Fig. 2 C), as has been demonstrated for mouse B cells. All of the control group demonstrated an increase in FcγRIIB expression as B cells progressed from the naive to the memory compartment, whereas only 34 out of 62 SLE patients displayed up-regulation of FcγRIIB on memory cells (Fig. 2, C and D), leading to a significant difference between FcγRIIB expression on memory cells of nonautoimmune individuals and SLE patients (P < 0.001).
Figure 2.
FcγRIIB expression on naive and memory B cells. PBMCs were stained with antibodies to CD19, CD27, and either antibody to FcγRIIB (2B6) or a control IgG antibody followed by antibody to mouse IgG1. Naive B cells (A) were identified as CD19+, CD27−; and memory B cells (B) were identified as CD19+, CD27+. The graph shows MFI of FcγRIIB after subtracting the MFI of the control antibody. The comparison is between B cells of nonautoimmune individuals (control A) and lupus patients. There is no difference in expression on naive cells, but there is decreased expression of FcγRIIB on memory cells of SLE patients. (C) The expression of FcγRIIB is increased on normal memory B cells, whereas in SLE patients (D) there is no increase in FcγRIIB expression on memory cells. The horizontal lines divide the data into quartiles.
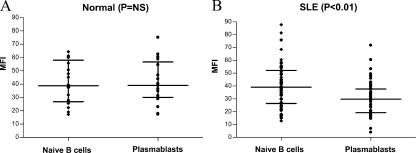
The level of FcγRIIB expression on plasmablasts from nonautoimmune individuals was similar to that found on naive B cells (Fig. 3 A). In contrast, SLE patients down-regulated FcγRIIB expression on plasmablasts to a level below that of naive B cells (Fig. 3 B). Thus, there was a significantly lower level of FcγRIIB expression on SLE plasmablasts than on nonautoimmune plasmablasts (P < 0.01).
Figure 3.
Plasmablasts of SLE patients have reduced expression of FcγRIIB. Naive B cells were identified as CD19+, CD27− and plasmablasts as CD19+, CD27++. Expression of FcγRIIB was determined by subtracting the MFI of the isotype control antibody from the MFI of the anti-FcγRIIB antibody. Nonautoimmune individuals (control A; A) have the same level of expression of FcγRIIB on naive B cells and plasmablasts, whereas lupus patients (B) have a decreased level of expression of FcγRIIB on plasmablasts. The horizontal lines divide the data into quartiles.
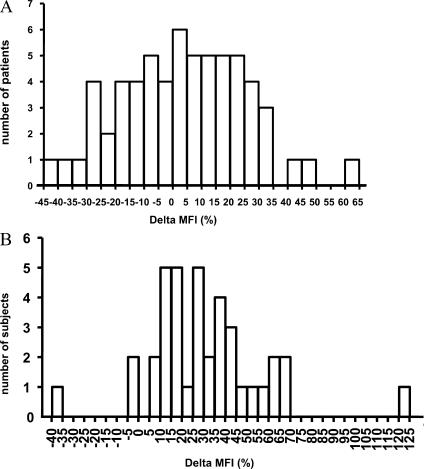
A second control group of 38 nonautoimmune individuals (control B; Table II), ethnically matched with the SLE patients, was recruited after the initial analysis as crossvalidation and to further explore the association between FcγRIIB and ethnicity. In this control group, 50% were African American and almost 50% were Hispanic. Similar to the previous control group, 92% of these healthy controls demonstrated increased expression of FcγRIIB on memory B cells. Thus, whereas 95% of all control subjects displayed increased expression of FcγRIIB on memory B cells, only 58% of the lupus patients displayed the same increased expression (P < 0.01; Fig. 4, A and B). 84% of the lupus patients showed decreased expression of FcγRIIB on plasmablasts compared with 23% of the controls (P < 0.01).
Figure 4.
Percent difference in MFI of FcγRIIB between naive and memory B cells. The percent change in MFI was calculated by subtracting the MFI of FcγRIIB on naive B cells from the MFI of FcγRIIB on memory B cells and dividing by the MFI of FcγRIIB on naive B cells × 100. (A) The percent change in MFI in SLE patients. 42% have a negative percent change in MFI of FcγRIIB as the naive B cells transition to memory B cells, which is consistent with down-regulation of this inhibitory receptor. In contrast, only 8% of ethnically matched healthy controls (control B; B) have a negative percent change in MFI as their B cells transition to memory B cells.
BCR-induced calcium (Ca2+) response in B cells with reduced FcγRIIB expression
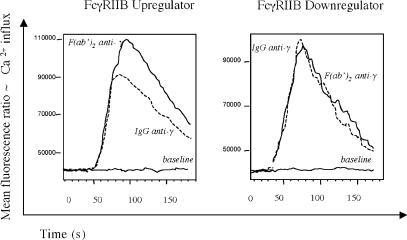
The important role of FcγRIIB in control of B cell activation through inhibition of BCR signaling is well established. We sought to investigate whether FcγRIIB-mediated suppression of BCR-induced Ca2+ response is impaired in B cells with decreased expression of FcγRIIB. To address this issue, we analyzed Ca2+ influx in memory B cells stimulated with either intact IgG or F(ab′) (2) fragments of anti-γ antibody. Cross-linking the BCR with whole Ig should elicit a lower Ca2+ response compared with cross-linking with F(ab′) (2), where there is no FcγRIIB ligation. Memory B cells comprise class-switched IgG expressing B cells; therefore, we chose to use an anti-γ antibody for B cell stimulation. Eight out of nine SLE patients with increased FcγRIIB expression on memory B cells (MFI increased by >15%) demonstrated the expected pattern of reduced Ca2+ influx in response to IgG stimulation compared with F(ab′) (2) fragment stimulation (Fig. 5), with a diminished flux when whole Ig was used. In contrast, no apparent difference between IgG- or F(ab′)-induced (2) Ca2+ influx was observed in six out of seven studied patients who failed to up-regulate FcγRIIB on memory B cells (MFI decreased by >15%; P = 0.008). These data demonstrate a reverse correlation between levels of FcγRIIB expression and BCR-mediated activation of memory B cells in SLE patients. Reduced FcγRIIB expression on the surface of memory B cells results in increased BCR-mediated B cell activation.
Figure 5.
Calcium flux is altered in SLE patients who fail to up-regulate FCγRIIB. Representative intracytoplasmatic Ca2+ flux in memory B lymphocytes stimulated with whole IgG (dashed line) or F(ab′)2 fragments (continuous line) of anti-γ antibody gated on CD20 and CD27 double-positive cells that correspond to a memory B cell population. B cells with up-regulated FcγRIIB expression demonstrate significantly decreased Ca2+ flux in response to intact IgG compared with F(ab′)2 fragments, whereas both stimulants produce nearly identical Ca2+ signals in B cells with low FcγRIIB expression (P = 0.008). These data demonstrate impaired FcγRIIB-mediated suppression of BCR-induced Ca2+ response in memory B cells that failed to up-regulate FcγRIIB.
Clinical correlations
Patients were divided into groups based on the magnitude of the percent change in the MFI of FcγRIIB expression between naive and memory B cells. Down-regulators had a percent change in MFI ≤−15, and up-regulators had a percent change in MFI ≥15. The values of −15 and 15 were chosen arbitrarily based on the distribution of percent change in MFI values for the SLE patients (Fig. 4 A). There were no significant differences in terms of disease duration, disease activity as measured by the SLE disease activity index (SLEDAI), use of immunosuppressive agents, anti–double-stranded DNA (dsDNA) antibody titers, complement levels, or the presence of renal disease in patients that displayed normal or dysregulated FcγRIIB expression. Additionally, there were no significant differences in SLEDAI scores between the groups 3 mo after the time of the analysis of peripheral blood B cells; thus, down-regulation of the FcγRIIB receptor did not appear to be an early marker of disease flare.
There were statistically significant differences in racial distribution between the groups with high and low FcγRIIB expression (Table III). 69% of the patients that clearly down-regulated FcγRIIB were African American compared with 30% of the up-regulators (P = 0.037). Conversely, 48% of the non–African-American patients up-regulated FcγRIIB expression on memory B cells compared with 18% of the African-American patients (P = 0.037). 55% of the African-American patients down-regulated FcγRIIB expression on memory B cells compared with 27% of others (P = 0.032). The striking differences in racial distribution between those with high and low expression suggests that the down-regulation of FcγRIIB receptors on memory B cells may represent a genetic difference: either a polymorphism in the gene encoding FcγRIIB or in the expression of some factor involved in FcγRIIB regulation.
Table III.
Baseline characteristics of SLE patients grouped by extreme up- or down-regulation of the FcγRIIB receptors on memory B cells
|
Δ MFI < −15 (n = 13) |
Δ MFI > 15 (n = 20) |
p-value | |
|---|---|---|---|
| Race | |||
| African American | 9 (69%) | 6 (30%) | 0.037 |
| Other | 4 (31%) | 14 (70%) | 0.037 |
| Disease duration (yr) | 8.4 ± 6 | 12.8 ± 9.3 | 0.15 |
| SLEDAI (mean) | 3.7 ± 3.1 | 3.0 ± 3.7 | 0.552 |
| Mean prednisone dose (mg) | 19 ± 16 | 14.3 ± 16.5 | 0.422 |
| On DMARDs | 5 (39%) | 6 (30%) | 0.71 |
| Mean anti-dsDNA titer | 62.9 ± 82.6 | 57.6 ± 123 | 0.893 |
| Renal disease (baseline) | 3 (23%) | 3 (15%) | 0.659 |
Percent Δ MFI was calculated by subtracting the MFI of FcγRIIB on naive B cells from the MFI of FcγRIIB on memory B cells and dividing by the MFI on naive B cells × 100. A negative percent Δ MFI reflects down-regulation of the FcγRIIB receptors.
FcγRIIB expression on B cells was assessed a second time in six patients. Three of the patients that initially displayed increased FcγRIIB expression on memory B cells continued to display receptor up-regulation despite a maximum decrease in SLEDAI of six points and a decrease in prednisone dose of 20 mg. The other three patients were initially in the group with a small change in MFI between naive and memory cells and remained in that group with small increases in SLEDAI score (maximum increase of four) and no change in steroid doses.
DISCUSSION
We have demonstrated that FcγRIIB expression is up-regulated on memory B cells in nonautoimmune individuals. Increased expression of this inhibitory receptor that binds IgG immune complexes on activated B cells provides a feedback inhibitory loop whereby antibody homeostasis can be maintained. In addition, recent studies in mouse models expressing anti-DNA antibodies have indicated that this inhibitory pathway appears to regulate the progression of naive B cells to somatically mutated and class-switched memory and plasma cells (37). In the presence of sufficient FcγRIIB expression, the anti-DNA response is limited to nonpathogenic, IgM autoantibodies (37). Absence of FcγRIIB leads to the accumulation of IgG anti-DNA autoantibodies and immune complex–triggered glomerulonephritis (29). These studies demonstrate that FcγRIIB expression on activated B cells provides a peripheral checkpoint for the maintenance of self-tolerance and limits the progression of autoreactive B cells that escape central tolerance from becoming IgG-producing memory and plasma cells. Furthermore, a twofold increase in FcγRIIB expression can markedly reduce disease in spontaneously lupus-prone mice (34). Interestingly, our data demonstrated that nonautoimmune individuals had a mean expression of FcγRIIB on memory B cells that was ∼1.5-fold higher than that of SLE patients; the mean increase in FcγRIIB expression on memory B cells compared with naive B cells in nonautoimmune individuals was an ∼1.5-fold increase, whereas there was no mean increase in SLE patients.
Previous studies of FcγRIIB in human subjects with SLE have focused on the presence of specific transmembrane and promoter polymorphisms of FcγRIIB (232T,187T, and -386C-120A) (13, 24–27). The strong disease association between the 232T allele and SLE reported in Japanese (24), Thai (13), and Chinese (25) populations has been followed by an elegant study showing that the 232T allele does not partition to lipid rafts and therefore lacks functional inhibitory activity (38). In this study, the 232T polymorphism did not alter membrane expression of FcγRIIB. It has also been reported that SLE B cells demonstrate an increased intracellular Ca2+ influx in response to BCR/FcγRIIB cross-linking compared with normal and disease controls (39). This presumably reflects inadequate inhibitory signaling in the SLE B cells. We now show that reduced expression of FcγRIIB contributes to decreased FcγRIIB-mediated suppression of BCR-induced Ca2+ response in memory B cells of SLE patients. Paradoxically, Su et al. have reported the strong disease association in Caucasians of a “gain of function” polymorphism in the promoter region of FcγRIIB presumed to result in the increased expression of FcγRIIB on B lymphocytes and monocytes, although this was not directly assayed (40). Although the role of this polymorphism is not clear, it is of interest that a presumed increase in expression of FcγRIIB is present in non–African-Americans patients, consistent with the data we report in this paper. Further studies are needed to investigate whether the down-regulation of FcγRIIB on memory B cells and plasmablasts observed in our cohort is the result of one of the previously described genetic polymorphisms.
Autoimmune diseases reflect a breakdown of multiple checkpoints, both centrally and in the periphery, that normally prevent the emergence, expansion, and activation of autoreactive cells. In SLE, multiple susceptibility genes act in a cumulative, threshold-driven manner to breach these checkpoints, which results in clinical disease. Understanding the molecular mechanisms that control these checkpoints is of primary importance in the design and implementation of rational strategies for therapeutic intervention. Evidence from mouse studies has established the role of the inhibitory FcγRIIB receptor in maintaining the tolerogenic state; perturbations in the expression of this gene can result in autoimmunity and a lupus-like syndrome in susceptible genetic backgrounds. Conversely, restoring correct expression of FcγRIIB on B cells in lupus-prone mice has recently been demonstrated to prevent the expansion and accumulation of autoantibody-producing plasma cells (34). We now demonstrate that SLE patients show a similar dysregulation of FcγRIIB expression on activated B cells that correlates with increased Ca2+ influx after BCR cross-linking by intact IgG, thereby validating the observations made in mouse models.
It has been previously reported that B cells from SLE patients are hyperresponsive to antigen. Recent experiments by Nussenzweig et al. (41) have demonstrated a defect in central tolerance in the selection of immature and transitional B cells in SLE patients, which is most consistent with decreased responsiveness to antigen. Indeed, in estradiol-treated mice and in CD100-deficient mice, reduced BCR signaling leads to the survival and activation of autoreactive B cells (42, 43). In this study, we demonstrate a defect in SLE patients in the expression of an inhibitor receptor known to play a role in peripheral tolerance in antigen-activated B cells. Together, these observations suggest a model in which B cell hyporesponsiveness early in B cell development permits the maturation of autoreactive B cells to immunocompetence. Subsequently, B cell hyperresponsiveness, mediated by inadequate expression of FcγRIIB, permits the unregulated expansion and differentiation of activated autoreactive B cells and the production of pathogenic autoantibodies. This model suggests that novel treatment strategies might focus on peripheral checkpoints and FcγRIIB expression.
MATERIALS AND METHODS
Study subjects
The patient population was composed of 62 patients followed at the Montefiore and Jacobi Medical Centers in New York, all of whom met the American College of Rheumatology 1982 revised criteria for SLE (44). The study was approved by the Committee for Clinical Investigations, and all subjects were provided written informed consent. Anticoagulated peripheral blood was drawn once in most subjects and repeated in 6 out of the 62 subjects. Demographic data, including age, sex, ethnicity, and disease duration, were collected from the medical record at the time of the blood draw. Data regarding medication use and disease activity (assessed using the SLEDAI), as well as measurements of hematological and immunological parameters including anti-DNA antibodies, C3, C4, and urinalyses, were obtained at the time of the blood draw and 3 mo later. Normal control subjects were recruited from healthy patients attending a gynecology clinic and employees in the clinics and laboratory. All control subjects also provided written informed consent. There are two control groups. Control group A consisted of 18 healthy controls recruited from employees in the laboratory. 55% of this group was Asian, 33% was Caucasian, 6% was African American, and 6% was Hispanic. After the association between FcγRIIB down-regulation and African-American ethnicity was noted, control group B (with comparable ethnic backgrounds to the SLE patients) was recruited. One half of this group of 38 nonautoimmune controls was African American; most others were Hispanics. Subjects were excluded from the control groups if they had a first-degree relative with SLE or were taking corticosteroids or estrogens.
Mouse monoclonal antibodies directed against human FcγRIIB (CD32B)
The procedures used for characterizing the 2B6 anti-FcγRIIB antibody have been previously described completely (unpublished data). In brief, CD32A transgenic mice were immunized with a purified soluble protein containing the complete extracellular domain of CD32B. Splenocytes from immunized mice were fused with a myeloma line, and the hybridomas were selected by ELISA for their differential ability to bind the inhibitory and activating Fc receptors. 2B6 was one out of seven hybridomas that reacted with CD32B at high titers with no or marginal reactivity with CD32A.
B cell phenotyping
Anticoagulated blood was obtained in tubes containing anticoagulant-citrate-dextrose. Red blood cells were lysed with Pharm Lyse (Becton Dickinson). Cells were incubated with the following antibodies: CD19-APC, CD27-PE (Caltag), anti-FcRIIB (as described in the previous paragraph), mouse IgG1 control antibody, and goat anti–mouse IgG1 (BD Biosciences). Flow cytometry for the SLE subjects and the control A group was performed with a FACSCalibur (Becton Dickinson) and analyzed with software (FlowJo version 4.5.8; Tree Star, Inc.) at the Albert Einstein College of Medicine. Flow cytometry for the control B group was performed with a FACSCalibur and analyzed with FlowJo software at Columbia University Medical Center.
Analysis of Ca2+ influx
Cell preparation.
PBMCs were isolated by density gradient centrifugation using Ficoll (GE Healthcare). PBMCs were resuspended as 107 cells/ml in phosphate-buffered saline containing 10% FBS (PBS/FBS 10%) and incubated with Ca2+-binding dye 1-indo, AM (Invitrogen) at a final concentration of 2.5 μM for 40 min at 37° in the dark. Surface staining with anti-CD20 FITC (BD Biosciences) and anti-CD27 PE (Caltag) monoclonal antibodies was used for identification of memory B cells. Stained PBMCs were resuspended in DMEM containing 10% FBS and stored at room temperature before analysis by flow cytometry.
Measurement of Ca2+ influx by flow cytometry.
After 10 min rest at 37°C, 106 PBMCs in 200 μl complete DMEM were analyzed using a flow cytometer (LSRII; BD Biosciences). A 355-nm, 20-mW UV laser (XCYTE CY-355-020; LightWave Electronics) was used for excitation of indo-1. After the baseline intracellular Ca2+ level was recorded for 30 s, either whole IgG or F(ab)2 fragments of anti-γ antibody (Rockland Immunochemicals) were added in equimolar concentration (40 μg/ml and 27 μg/ml, respectively). An increase in binding of cytosolic Ca2+ to indo-1 results in a change of the emission spectrum of indo-1 from ∼510 nm (free form) to ∼420 nm (bound to Ca2+ form). The mean fluorescence ratio of these two wavelengths represents calcium influx and was recorded for a total of 3 min. Data was analyzed using software (FlowJo version 6.4; Tree Star, Inc.).
Statistical analysis
The principle analyses for comparisons of MFI staining of FcγRIIB on B cells were the two-tailed Mann-Whitney test for comparisons between controls and SLE patients and the two-tailed Wilcoxon signed rank test for comparisons within the control and SLE groups. p-values for race were calculated using a two-sided Pearson's χ2 test, and p-values for renal disease and DMARD use were calculated using a two-sided Fisher's exact test. Because the data were not normally distributed, p-values for disease duration, SLEDAI scores, mean prednisone dose, and mean anti-dsDNA titers were obtained using the two-tailed Mann-Whitney test. Correlation of FcγRIIB expression on memory B cells with calcium influx on stimulation was analyzed using a two-sided Fisher's exact test.
Acknowledgments
We would like to thank Kristie Gordon for her assistance with flow cytometry and Kevin Rochford for help with patient enrollment.
This study was supported by grants from the National Institutes of Health (to B. Diamond and J. Ravetch) and the Alliance for Lupus Research (to J. Ravetch).
Jeffrey Ravetch and Betty Diamond have financial interests in MacroGenics Inc., and those financial interests have been managed by institutional Conflict of Interest Committees. The authors have no other conflicting financial interests.
Abbreviations used: BCR, B cell receptor; CHO, Chinese hamster ovary; dsDNA, double-stranded DNA; FcγR, Fc γ receptor; MFI, mean fluorescence intensity; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
References
- 1.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 2.Ernst, L.K., A.M. Duchemin, K.L. Miller, and O.L. Anderson. 1998. Molecular characterization of six variant Fc gamma receptor class I (CD64) transcripts. Mol. Immunol. 35:943–954. [DOI] [PubMed] [Google Scholar]
- 3.Ravetch, J.V. 2003. Fc receptors. In Fundamental Immunology. Fifth edition. William E. Paul, editor. Lippincott Williams & Wilkins, Philadelphia. 685–700.
- 4.Brooks, D.G., W.Q. Qiu, A.D. Luster, and J.W. Ravetch. 1989. Structure and expression of human IgG FcRII (CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J. Exp. Med. 170:1369–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daeron, M., S. Latour, O. Malbec, E. Espinoza, P. Pina, S. Pasmans, and W.H. Fridman. 1995. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 3:635–646. [DOI] [PubMed] [Google Scholar]
- 6.Malbec, O., D.C. Fong, M. Turner, V.L. Tybulewicz, J.C. Cambier, W.H. Fridman, and M. Daeron. 1998. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J. Immunol. 160:1647–1658. [PubMed] [Google Scholar]
- 7.Ono, M., S. Bolland, P. Tempst, and J.V. Ravetch. 1996. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the Fc gamma RIIB. Nature. 383:263–266. [DOI] [PubMed] [Google Scholar]
- 8.Bolland, S., R.N. Pearse, T. Kurosaki, and J.V. Ravetch. 1998. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 8:509–516. [DOI] [PubMed] [Google Scholar]
- 9.Bazilio, A.P., V.S.T. Viana, R. Toledo, V. Woronik, E. Bonfa, and R.C. Monteiro. 2004. FcGRIIa polymorphism: a susceptibility factor for immune complex-mediated lupus nephritis in Brazilian patients. Nephrol. Dial. Transplant. 19:1427–1431. [DOI] [PubMed] [Google Scholar]
- 10.Lee, H.S., Y.H. Chung, T.G. Kim, T.H. Kim, J.B. Jun, S. Jung, S.C. Bae, and D.H. Yoo. 2003. Independent association of HLA-DR and Fc gamma receptor polymorphisms in Korean patients with systemic lupus erythematosus. Rheumatology (Oxford). 42:1501–1507. [DOI] [PubMed] [Google Scholar]
- 11.Salmon, J.E., S. Millard, L.A. Schachter, F.C. Arnett, E.M. Ginzler, M.F. Gourley, R. Ramsay-Goldman, M.G. Peterson, and R.P. Kimberly. 1996. FC gamma RIIA alleles are heritable risk factors for lupus nephritis in African Americans. J. Clin. Invest. 97:1348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manger, K., R. Repp, M. Jansen, M. Geisselbrecht, R. Wassmuth, N.A. Westerdaal, A. Pfahlberg, B. Manger, J.R. Kalden, and J.G. van de Winkel. 2002. FcG receptor IIa, IIIa, and IIIb polymorphisms in German patients with systemic lupus erythematosus: association with clinical symptoms. Ann. Rheum. Dis. 61:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siriboonrit, U., N. Tsuchiya, M. Sirikong, C. Kyogoku, S. Bejrachandra, P. Suthipinittharm, K. Luangtrakool, D. Srinak, R. Thongpradit, K. Fujiwara, et al. 2003. Association of FcG receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 61:374–383. [DOI] [PubMed] [Google Scholar]
- 14.Dijstelbloem, H.M., M. Bijl, R. Fijnheer, R.H. Scheepers, W.W. Oost, M.D. Jansen, W.J. Sluiter, P.C. Limburg, R.H. Derksen, J.G. van de Winkel, and C.G. Kallenberg. 2000. FcGR polymorphisms in systemic lupus erythematosus. Arthritis Rheum. 43:2793–2800. [DOI] [PubMed] [Google Scholar]
- 15.Smyth, L.J.C., N. Snowden, D. Carthy, C. Papasteriades, A. Hajeer, and W.E.R. Ollier. 1997. FcGRIIa polymorphism in systemic lupus erythematosus. Ann. Rheum. Dis. 56:744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botto, M., E. Theodoridis, E.M. Thompson, H.L. Beynon, D. Briggs, D.A. Isenberg, M.J. Walport, and K.A. Davies. 1996. FCGRIIa polymorphism in systemic lupus erythematosus (SLE): no association with disease. Clin. Exp. Immunol. 104:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villarreal, J., D. Crosdale, W. Ollier, A. Hajeer, W. Thomson, J. Ordi, E. Balada, M. Villardell, L.S. Teh, and K. Pouton. 2001. Mannose binding lectin and FcGRIIa (CD32) polymorphism in Spanish systemic lupus erythematosus patients. Rheumatology (Oxford). 40:1009–1012. [DOI] [PubMed] [Google Scholar]
- 18.Koene, H.R., M. Kleijer, A.J. Swaak, K.E. Sullivan, M. Bijl, M.A. Petri, C.G. Kallenberg, D. Roos, A.E. von dem Borne, and M. de Haas. 1998. FC gammaRIIIA-158F allele is a risk factor for systemic lupus erythematosus. Arthritis Rheum. 41:1813–18. [DOI] [PubMed] [Google Scholar]
- 19.Salmon, J.E., S. Ng, D.H. Yoo, T.H. Kim, S.Y. Kim, and G.G. Song. 1999. Altered distribution of FcG receptor IIIA alleles in a cohort of Korean patients with lupus nephritis. Arthritis Rheum. 42:818–823. [DOI] [PubMed] [Google Scholar]
- 20.Seligman, V.A., C. Suarez, R. Lum, S.E. Inda, D. Lin, H. Li, J.L. Olson, M.F. Seldin, and L.A. Criswell. 2001. The FCgamma receptor IIIA-158F allele is a major risk factor for the development of lupus nephritis among Caucasians but not non-Caucasians. Arthritis Rheum. 44:618–625. [DOI] [PubMed] [Google Scholar]
- 21.Oh, M., M.A. Petri, N.A. Kim, and K.E. Sullivan. 1999. Frequency of the Fc gamma R IIIA-158 F allele in African American patients with systemic lupus erythematosus. J. Rheumatol. 26:1486–1489. [PubMed] [Google Scholar]
- 22.Yap, S.N., M.E. Phipps, M. Manivasagar, and J.J. Bosco. 1999. Fc gamma receptor IIIB-NA gene frequencies in patients with systemic lupus erythematosus and healthy individuals of Malay and Chinese ethnicity. Immunol. Lett. 68:295–300. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Escribano, M.F., F. Aguilar, J. Sanchez-Roman, and A. Nunez-Roldan. 2002. FCgammaRIIA, FCgammaRIIIA and FCgammaRIIIB polymorphisms in Spanish patients with systemic lupus erythematosus. Eur. J. Immunogenet. 29:301–306. [DOI] [PubMed] [Google Scholar]
- 24.Kyogoku, C., H.M. Dijstelbloem, N. Tsuchiya, Y. Hatta, H. Kato, A. Yamaguchi, T. Fuzakawa, M.D. Jansen, H. Hashimoto, J.G. van de Winkel, et al. 2002. FcG receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCG2RB to genetic susceptibility. Arthritis Rheum. 46:1242–1254. [DOI] [PubMed] [Google Scholar]
- 25.Chu, Z.T., N. Tsuchiya, C. Kyogoku, J. Ohashi, Y.P. Qian, S.B. Xu, C.Z. Mao, J.Y. Chu, and K. Tokunaga. 2004. Associations of FcG receptor IIb polymorphism with susceptibility to systemic lupus erythematosus in Chinese: a common susceptibility gene in the Asian populations. Tissue Antigens. 63:21–27. [DOI] [PubMed] [Google Scholar]
- 26.Li, X., J. Wu, R.H. Carter, J.C. Edberg, K. Su, G.S. Cooper, and R.P. Kimberly. 2003. A novel polymorphism in the FCgamma receptor II B(CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. 48:3242–3252. [DOI] [PubMed] [Google Scholar]
- 27.Magnusson, V., R. Zunec, J. Odeberg, G. Sturfelt, L. Truedsson, I. Gunnarsson, and M.E. Alarcon-Riquelme. 2004. Polymorphisms of the FcG receptor type IIB gene are not associated with systemic lupus erythematosus in the Swedish population. Arthritis Rheum. 50:1348–1350. [DOI] [PubMed] [Google Scholar]
- 28.Bolland, S., and J.V. Ravetch. 2000. Spontaneous autoimmune disease in FcγRII deficient mice results from strain specific epistasis. Immunity. 13:277–285. [DOI] [PubMed] [Google Scholar]
- 29.Clynes, R., J.S. Maizes, R. Guinamard, M. Ono, T. Takai, and J.W. Ravetch. 1999. Modulation of immune complex–induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 189:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura, A., T. Yuasa, A. Ujike, M. Ono, T. Nukiwa, J.V. Ravetch, and T. Takai. 2000. Fcγ receptor IIB-deficient mice develop Goodpasture's syndrome upon immunization with type IV collagen: a novel murine model for autoimmune glomerular basement membrane disease. J. Exp. Med. 191:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuasa, T., S. Kubo, T. Yoshino, A. Ujike, K. Matsumura, M. Ono, J.V. Ravetch, and T. Takai. 1999. Deletion of Fcγreceptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J. Exp. Med. 189:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchard, N.R., and K.G. Smith. 2003. B cell inhibitory receptors and autoimmunity. Immunology. 108:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morel, L., B.P. Croker, K.R. Blenman, C. Mohan, G. Huang, G. Gilkeson, and E.K. Wakeland. 2000. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc. Natl. Acad. Sci. USA. 97:6670–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGaha, T., B. Sorrentino, and J.V. Ravetch. 2005. Restoration of tolerance in lupus by targeted receptor expression. Science. 307:590–593. [DOI] [PubMed] [Google Scholar]
- 35.Looney, R.J., G.N. Abraham, and C.L. Anderson. 1986. Human monocytes and U2937 cells bear two distinct Fc receptors for IgG. J. Immunol. 136:1641–1647. [PubMed] [Google Scholar]
- 36.Mockridge, C.I., A. Rahman, S. Buchan, T. Hamblin, D.A. Isenberg, F.K. Stenenson, and K.N. Potter. 2004. Common patterns of B cell perturbation and expanded V4-34 immunoglobulin gene usage in autoimmunity and infection. Autoimmunity. 37:9–15. [DOI] [PubMed] [Google Scholar]
- 37.Fukuyama, H., F. Nimmerjahn, and J.V. Ravetch. 2005. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G (+) anti-DNA plasma cells. Nat. Immunol. 6:99–106. [DOI] [PubMed] [Google Scholar]
- 38.Floto, R.A., M. Clatworthy, K.R. Heilbronn, D.R. Rosner, P.A. MacAry, A. Rankin, P.J. Lehner, W.H. Ouwehand, J.M. Allen, N.A. Watkins, and K.G. Smith. 2005. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 11:1056–1058. [DOI] [PubMed] [Google Scholar]
- 39.Enyedy, E., J. Mitchell, M.P. Nambiar, and G. Tsokos. 2001. Defective FcγRIIb1 signaling contributes to enhanced calcium response in B cells from patients with systemic lupus erythematosus. Clin. Immunol. 101:130–135. [DOI] [PubMed] [Google Scholar]
- 40.Su, K., J. Wu, J.C. Edberg, X. Li, P. Ferguson, G.S. Cooper, C.D. Langefeld, and R.P. Kimberly. 2004. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcγRIIb alters receptor expression and associates with autoimmunity. 1. Regulatory FcGRIIB polymorphisms and their association with systemic lupus erythematosus. J. Immunol. 172:7186–7191. [DOI] [PubMed] [Google Scholar]
- 41.Yurasov, S., H. Wardemann, J. Hammersen, M. Tsuiji, E. Meffre, V. Pascual, and M.C. Nussenzweig. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimaldi, C.M., R. Hicks, and B. Diamond. 2005. B cell selection and susceptibility to autoimmunity. J. Immunol. 174(4):1775–1781. [DOI] [PubMed]
- 43.Kumanogoh, A., T. Shikina, C. Watanabe, N. Takegahara, K. Suzuki, M. Yamamoto, H. Takamatsu, D.V. Prasad, M. Mizui, T. Toyofuku, et al. 2005. Requirement for CD100-CD72 interactions in fine-tuning of B-cell antigen receptor signaling and homeostatic maintenance of the B-cell compartment. Int. Immunol. 17:1277–1282. [DOI] [PubMed] [Google Scholar]
- 44.Tan, E.M., A.S. Cohen, J.F. Fries, A.T. Masi, D.J. McShane, N.F. Rothfield, J.G. Schaller, N. Talal, and R.J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271–1277. [DOI] [PubMed] [Google Scholar]