Abstract
Light and dark have immediate effects on sleep and wakefulness in mammals, but the neural mechanisms underlying these effects are poorly understood. Lesions of the visual cortex or the superior colliculus–pretectal area were performed in albino rats to determine retinorecipient areas that mediate the effects of light on behavior, including rapid eye movement sleep triggering by lights-off and redistribution of non-rapid eye movement sleep in short light–dark cycles. Acute responses to changes in light conditions were virtually eliminated by superior colliculus-pretectal area lesions but not by visual cortex lesions. Circadian entrainment was evident in both groups with lesions and in normal controls. Thus, acute light-dark effects on sleep and wakefulness appear to be mediated independently from cortical vision or circadian rhythms.
Bright light has immediate alerting effects in diurnal mammals such as humans whereas darkness promotes sleep (1); nocturnal animals show the opposite effects, with bright light inducing sleep and darkness increasing wakefulness (2). Acute responses to changes in lighting conditions differ from circadian entrainment by light in that they occur immediately in response to the lighting change and do not persist in the absence of stimulation. Such responses sometimes are referred to masking in the circadian literature, meaning that the light stimulus may be acting on sleep and wakefulness centers directly without necessarily inducing changes in the circadian pacemaker. At sufficient light intensities, acute changes in lighting have been demonstrated to exert significant effects on rest–activity or sleep–waking patterns in rats (2–4), primates (5), and humans (1).
The purpose of the present study was to determine the component(s) of the visual system involved in mediating the effects of acute changes in lighting on sleep patterns. We used albino rats as a model because they show distinctive sleep–wakefulness responses following light–dark transitions that have been well documented. First, light–dark shifts have significant effects on rapid eye movement (REM) sleep expression in albino rats. They exhibit large increases in REM sleep immediately after lights-off, first described by Lisk and Sawyer in 1966 (6). REM sleep triggering has been observed in albino rats exposed to either short light–dark cycles (4) or short periods of darkness occurring intermittently during the light period of a normal 12-h light/12-h dark cycle (6, 7). Pigmented rats, including those heterozygous for albinism or congenic to albino rats, also show effects of light and dark on REM sleep expression, albeit in the opposite direction, with greater REM sleep expression occurring in light rather than dark periods (8, 9).
Another behavioral response of nocturnal rodents to changes in lighting conditions consists of increased amounts of non-REM (NREM) sleep and total sleep after lights-on and increased wakefulness following lights-off (4). None of the light-induced behaviors (i.e., REM sleep, NREM sleep, or waking responses to lighting changes) appears to be under primary circadian control: the behaviors are not eliminated by destruction of the suprachiasmatic nucleus (24) and can be elicited at any circadian time, although the circadian clock may influence the magnitude of the responses (2, 4).
The mechanism whereby light affects sleep–waking behaviors is not understood fully but involves the visual system, because it is the only known direct neural route by which light information is transmitted to the central nervous system in adult mammals, and orbital enucleation eliminates the responses (10). The major retinofugal pathways include projections to the lateral geniculate nucleus, the pretectum (PT), the superior colliculus (SC), and the suprachiasmatic nucleus. The pathway through the lateral geniculate nucleus to primary visual cortex mediates conscious visual perception. Visual perception without awareness (“blindsight”) can persist after damage to visual cortex (VC) (11) and may be mediated by the midbrain tectum (12). The PT is involved with pupillary constriction and the generation of eye movements (13). The SC is immediately caudal to and heavily interconnected with the PT (14) and is associated with visual attention, orientation, and eye movements (15). The suprachiasmatic nucleus is the endogenous circadian pacemaker and regulates the timing of various behaviors and neuroendocrine functions (16).
We have hypothesized that components of the visual system affected by albinism (17) are involved in REM sleep triggering by lights-off because REM sleep responses to changes in lighting conditions vary between albino and pigmented rats (8, 9). Furthermore, we sought to determine which retinorecipient area(s) mediate changes in NREM sleep and/or wakefulness patterns after light–dark transitions. We examined the roles of two retinorecipient areas affected by the albino visual system anomaly, the VC and the SC-PT, in behavioral state responses to acute changes in lighting.
MATERIALS AND METHODS
Materials.
Dexamethasone (2 mg/ml) and atropine (0.54 mg/ml) were obtained from Phoenix Pharmaceuticals (St. Joseph, MO), Metofane was obtained from Mallinckrodt, and Nembutal (50 mg/ml) was obtained from Abbott. Miniature pin connectors used for sleep recording were made by Continental Connectors (Bloomfield, NJ). Male albino Fisher 344 rats, aged 3–4 months (210–310 g), were purchased from Charles River Breeding Laboratories.
Lesion Protocol.
All procedures were in accordance with institutional guidelines for the care and use of laboratory animals. Rats were anesthetized for surgery with inhaled Metofane and Nembutal (50 mg/kg) and placed in a stereotaxic apparatus. Using aseptic precautions, the bone overlying the VC or SC was removed bilaterally by using stereotaxic coordinates obtained from the Paxinos and Watson atlas (18). The dura was reflected and brain tissue was aspirated under visual guidance by using a blunt 16-gauge cannula attached to a vacuum pump. The VC (areas 17, 18, and 18a) was removed by aspiration in six rats. The SC-PT was removed through visually guided aspiration after removing a small amount of overlying cortex in five rats. The surgical site was packed with saline-soaked Gelfoam (Upjohn), the skin was sutured, and the animals were allowed to recover from anesthesia before returning them to their home cages. Rats were treated with atropine (1 mg/kg) and dexamethasone (1 mg/kg) post-operatively.
Electrode Implantation.
Rats with lesions were allowed to recover for a minimum of 1 week before electrode implantation. In addition to the groups with SC-PT and VC lesions, six normal rats were implanted with electrodes for sleep recording. Rats were anesthetized with inhaled Metofane and Nembutal (50 mg/kg) and placed in a stereotaxic apparatus. Using aseptic precautions, six screw electrodes were implanted in the skull to record the electroencephalogram (EEG): two midline electrodes, one 3-mm caudal and one 3-mm rostral to bregma; two lateral electrodes immediately caudal to bregma; and two lateral electrodes immediately rostral to lambda. Flexible wire electrodes were sutured into temporalis and nuchal muscles to record the electromyogram (EMG). Electrodes were attached to a microminiature plug that was fixed to the skull with dental cement. Rats were allowed to recover before returning them to their home cages.
Sleep Recording.
At least 1 week after implantation surgery, rats were placed in individual recording cages and hooked up to a polygraph by a cable attached to a swivel commutator, which allowed freedom of movement. The animals were housed in the recording apparatus for the duration of the study and maintained at 23°C with food and water available ad libitum. Rats were adapted to the apparatus for at least 4 days before the start of the experimental protocol. Illuminance levels were 500 lux during light periods and 0 lux during dark periods as measured at the level of the floor of the cage. This illuminance level was chosen because it is approximately 10 times greater than the minimum level required to elicit acute responses to changes in light conditions in albino rats (8). Recording electrodes were implanted, and REM sleep triggering by lights-off was assessed by recording sleep continuously in rats exposed to 5 min of lights-off every 30 min during the light period of an overall 12-h light/12-h dark schedule (dark-pulse schedule). Sleep also was recorded in a 12-h light/12-h dark (baseline) and a 3-h light/3-h dark schedule. Rats with VC or SC-PT lesions and controls were recorded for 4 days in each condition.
EEG and EMG were recorded by using a Grass amplifier (Grass Astro-Med, West Warwick, RI) and Sandman [Nellcor Puritan Bennett (Melville), Ottawa] computerized collection system. Electrophysiological signals were integrated over 30-s epochs and classified as waking, REM sleep, or NREM sleep by using the PASS algorithm (19). Computer scoring was reviewed by an individual unaware of the light–dark schedule, and any obvious discrepancies with polygraphic records because of artifacts were corrected (on average fewer than 5% of epochs). Although lateral EEG and EMG signals appeared normal in rats with lesions, amplitude of the midline EEG during REM sleep (theta rhythm) was reduced in all rats with lesions (except for one rat with a VC lesion). This presumably was because of removal of underlying cortex and/or damage to the hippocampus. Therefore, sleep bouts scored as REM sleep in animals with either type of lesion probably included small amounts of low-amplitude NREM sleep (19), because the polygraphic parameters for these states are very similar under these conditions. To verify that behavioral REM sleep was present in the absence of high amplitude midline theta rhythm, samples of video records were obtained on rats with lesions and independently scored for W, NREM, and REM sleep. In addition to confirming the presence of behavioral REM sleep in all rats, behavioral scores were found to be consistent with computer-generated scores (>90% agreement).
Histology Protocol.
After completion of experiments, rats with lesions were overdosed with Nembutal and perfused through the heart with heparinized saline followed by 10% formal saline. Brains were embedded in paraffin, sectioned at 5 μm, and mounted on gelatin-coated slides. Every 40th section was stained for cell bodies. Lesions were reconstructed by using computerized drawings from the Swanson atlas of the rat brain (20).
Statistical Analysis.
In all cases data for each rat and lighting condition were averaged and used in subsequent analyses. Comparisons among these data were made with SPSS General Linear Model (spss 7, SPSS, Chicago), using the Tukey Honestly Significant Difference (HSD) and an α = 0.05 for post-hoc comparisons.
RESULTS
Effects of Lesions and Light–Dark Schedules on Daily Amounts of Sleep and Wakefulness.
Total daily percentages of wakefulness, NREM sleep, and REM sleep for normal control rats, rats with VC lesions, and rats with SC-PT lesions for the three lighting schedules are shown in Table 1. Within each group of rats, daily amounts of sleep and waking did not differ significantly among the three schedules. There were minor variations in some sleep–waking percentages among groups. Comparisons of sleep and waking percentages among groups revealed that rats with SC-PT lesions spent slightly more time awake than rats with VC lesions (P < 0.05, Tukey HSD), although waking amounts in rats with SC-PT lesions or VC lesions did not differ significantly from the control group. There were no significant differences in daily NREM sleep percentages among experimental groups. All three groups differed significantly from each other in daily REM sleep percentages, however. Rats with VC lesions had significantly more REM sleep scored than either control rats or those with SC-PT lesions, and control rats had significantly less REM sleep scored than either of the groups with lesions (P < 0.05, Tukey HSD). Increased REM sleep amounts in rats with VC or SC-PT lesions were likely because REM sleep included small amounts of low-voltage NREM sleep in these animals (see sleep-recording methods above).
Table 1.
Daily sleep–waking percentages
| Group | n | LD schedule | W | NREM | REM |
|---|---|---|---|---|---|
| Control | 6 | 12/12 | 44.45% | 48.74% | 6.81% |
| ±2.25% | ±2.41% | ±0.41% | |||
| Dark pulse | 45.14% | 47.97% | 6.89% | ||
| ±1.52% | ±1.72% | ±0.37% | |||
| 3/3 | 43.43% | 49.34% | 7.23% | ||
| ±3.70% | ±3.84% | ±0.27% | |||
| SC-PT lesion | 5 | 12/12 | 46.20% | 45.89% | 7.91% |
| ±3.01% | ±1.55% | ±1.60% | |||
| Dark pulse | 45.83% | 45.70% | 8.47% | ||
| ±2.17% | ±2.19% | ±1.21% | |||
| 3/3 | 45.80% | 46.88% | 7.32% | ||
| ±2.28% | ±2.24% | ±1.00% | |||
| VC lesion | 6 | 12/12 | 42.29% | 48.47% | 9.24% |
| ±5.80% | ±4.68% | ±1.39% | |||
| Dark pulse | 41.58% | 48.98% | 9.44% | ||
| ±3.77% | ±2.74% | ±1.67% | |||
| 3/3 | 43.36% | 47.96% | 8.68% | ||
| ±4.07% | ±4.13% | ±0.40% |
Average daily percentages of wakefulness (W), NREM sleep, and REM sleep are reported for normal control rats, rats with SC-PT lesions, and rats with VC lesions exposed to three light/dark (LD) schedules: 12-h light/12-h dark (12/12); 12-h light/12-h dark with 5-min dark pulses occurring every 30 min during the 12-h light period (dark pulse); and 3-h light/3-h dark (3/3).
Effects of VC and SC-PT Lesions on REM Sleep Triggering.
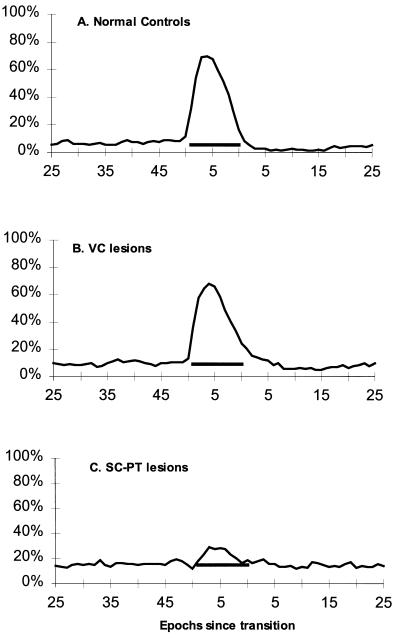
Normal control rats and rats with VC lesions showed large increases in REM sleep during 5-min lights-off periods (dark-pulse schedule), rising to an average of six times the levels seen during the immediately preceding 5-min light periods (Fig. 1). Rats with SC-PT lesions, however, showed greatly attenuated REM sleep triggering in a dark-pulse schedule (Fig. 1). All control rats and rats with VC lesions showed greater REM sleep increases than any rat with a lesion of the SC-PT region. The average increases in the REM sleep percentage of total sleep time during the 5-min dark periods (shaded bars) over the preceding 25-min light periods for control rats (42% ± 11%) and rats with VC lesions (40% ± 9%) were significantly greater than the increase for rats with SC-PT lesions (7% ± 7%; P < 0.05, Tukey HSD).
Figure 1.
REM sleep triggering. Sleep was recorded continuously over 4 days on a 12-h light/12-h dark cycle with 5-min dark periods (0 lux) every half-hour during the light period (500 lux), yielding 96 REM sleep-triggering trials per rat. The percentage of time spent in REM sleep was determined by compiling trials and averaging state scores of corresponding epochs across trials for each rat. Shown is the average percentage of total sleep time spent in REM sleep plotted in 30-s epochs during the 5-min dark pulses and the intervening 25-min light periods for six control rats (A), six rats with VC lesions (B), and five rats with SC-PT lesions (C). Epochs are in sequence from the time of a transition from light to dark, or vice versa. Shaded bars represent the 5-min dark pulses and also indicate the average percentage of total sleep time spent in REM sleep for the 25-min light periods.
Effects of VC and SC-PT Lesions on Sleep and Waking Patterns in a 12-h Light/12-h Dark Cycle.
Sleep was recorded in the groups of normal albino rats and rats with either VC or SC-PT lesions exposed to a standard 12-h light/12-h dark cycle. All three groups of rats showed the expected sleep–waking pattern for nocturnal rodents: they slept more during the 12-h light period and were awake more during the 12-h dark period. No group differed significantly from any other with respect to the proportion of their NREM sleep obtained in the light. Rats with lesions of the SC-PT region did not differ from control rats in the average proportion of wakefulness during the 12-h dark periods. Rats with visual cortex lesions, however, had a greater proportion of their wakefulness in the dark (81% ± 3%; P < 0.05, Tukey HSD) when compared with either control rats (70% ± 4%) or those with SC-PT lesions (70% ± 3%).
Effects of VC and SC-PT Lesions on Sleep and Waking Patterns in a 3-h Light/3-h Dark Cycle.
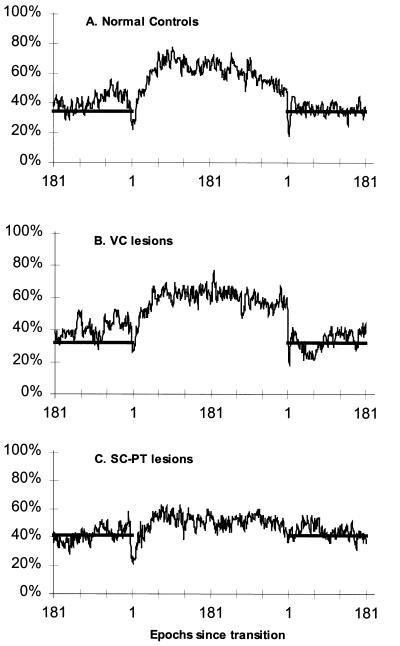
To accentuate the direct effects of light on sleep and wakefulness, the groups of rats also were exposed to a 3-h light/3-h dark cycle (Fig. 2), because this schedule is too short for circadian entrainment to occur in mammals. Normal rats and rats with VC lesions showed similar responses to short light–dark periods; more of their NREM sleep occurred during the light periods and more of their waking occurred during the dark periods (shaded bars). Rats with SC-PT lesions had a greater proportion of their NREM sleep in the dark (46% ± 2%; P < 0.05, Tukey HSD) in comparison to either control rats (39% ± 4%) or rats with VC lesions (39% ± 3%). Rats with SC-PT lesions also showed a significantly reduced proportion of waking during dark periods (55% ± 2%) in comparison to rats with VC lesions (63% ± 5%; P < 0.05, Tukey HSD); they showed a trend for the proportion of waking during the dark to be reduced in comparison to normal controls (61% ± 6%; P ≈ 0.12).
Figure 2.
NREM sleep in a 3-h light/3-h dark cycle. Sleep was recorded continuously for 4 days in a 3-h light (500 lux)/3-h dark (0 lux) cycle, yielding 16 6-h trials per rat. The percentage of time spent in NREM sleep, REM sleep, and waking was determined by compiling trials and averaging state scores for corresponding epochs across trials for each rat. Data are plotted as the average percentage of time spent in NREM sleep determined in 30-s epochs, averaged across lighting periods and days in six control rats (A), six rats with VC lesions (B), and five rats with SC-PT lesions (C). Epochs are in sequence from the time of a transition from light to dark, or vice versa. Shaded bars represent lights-off and also indicate the average percentage of time spent in NREM sleep during the dark periods.
Analysis of REM sleep patterns in the 3-h light/3-h dark cycle also showed significant REM sleep increases at the beginnings of the 3-h dark periods in control rats and rats with VC lesions (not shown); these REM sleep triggering responses to lights-off were comparable to those seen in the dark pulse-paradigm (Fig. 1). Rats with SC lesions failed to show REM sleep increases after lights-off in the 3-h/3-h schedule.
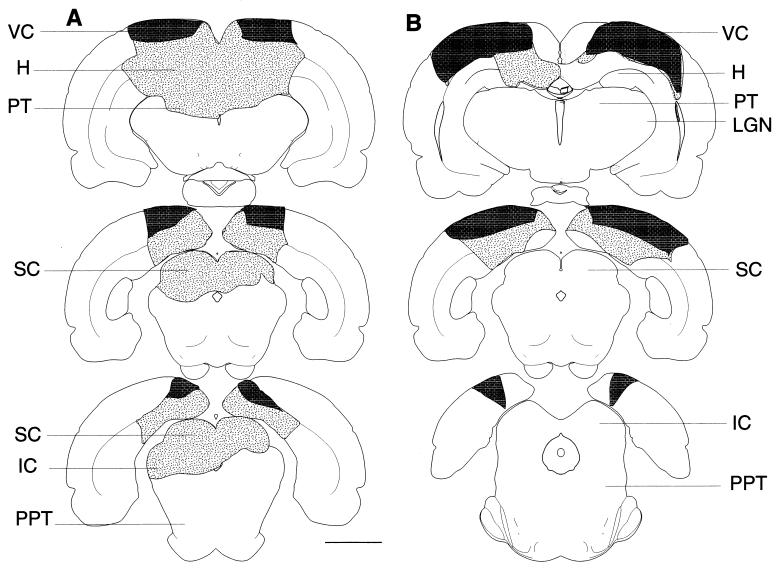
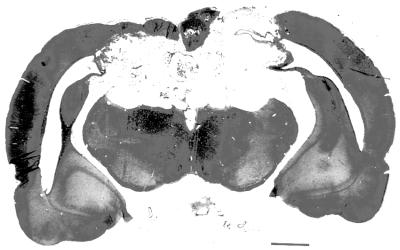
Histology.
Sample reconstructed lesions are depicted in Fig. 3, and a photomicrograph of an SC-PT lesion is shown in Fig. 4. Table 2 displays the extent of damage in rats with SC-PT and VC lesions. Approximately 85–100% of the SC was removed in rats with SC-PT lesions. PT damage ranged from minimal to virtually complete. Up to half of the VC was damaged in rats with SC-PT lesions, and the average loss of VC was about one-third. SC-PT lesions also resulted in variable amounts of damage to adjacent structures including hippocampus, caudal thalamus, periaqueductal gray, and inferior colliculus. Five of six rats with VC lesions had approximately three-quarters of the VC removed, including the medial, primary, and lateral areas, whereas one rat had a cortex lesion comparable to those seen in rats with SC-PT lesions, with close to one-third of the VC removed. Rats with VC lesions also sustained variable amounts of damage to the hippocampus and parietal cortex.
Figure 3.
Reconstruction of lesions. Shown are drawings of representative sections with superior colliculus–pretectum (SC-PT, A) and visual cortex (VC, B) aspiration lesions. Lesions are lightly stippled; darker stippling represents area of VC removed. (Bar = 3 mm.) H, hippocampus; IC, inferior colliculus; LGN, lateral geniculate nucleus of the thalamus; SC, superior colliculus; SCd, deep layers of the SC; SCs, superficial layers of the SC; PPT, pedunculopontine tegmentum; PT, pretectum; VC, visual cortex.
Figure 4.
Shown is a photomicrograph of the lesion depicted schematically in Fig. 1A. (Bar = 1.5 mm.)
Table 2.
Histology of superior colliculus–pretectal and visual cortex lesions
| Structure/case | SCPT1 | SCPT2 | SCPT3 | SCPT4 | SCPT5 | VC1 | VC2 | VC3 | VC4 | VC5 | VC6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parietal cortex | +/− | −/− | +/+ | −/− | −/− | +/− | −/− | +/+ | +/+ | −/+ | +/+ |
| Visual cortex | ++/+ | +/+ | ++/++ | ++/+ | +/+ | +++/+++ | ++/+ | +++/+++ | +++/+++ | ++/+++ | +++/+++ |
| Primary visual cortex | ++/+ | ++/+ | ++/++ | +++/+ | +/+ | +++/+++ | ++/++ | +++/+++ | +++/+++ | ++/+++ | +++/+++ |
| Retrosplenial cortex | ++/++ | ++/++ | ++/++ | ++/+ | ++/++ | ++/++ | +/− | +/+ | +/− | +/+ | +/+ |
| Hippocampus | ++/+ | +/+ | +++/++ | ++/++ | +/+ | +/+ | +/+ | +/+ | −/− | +/+ | +/+ |
| Lateral geniculate nucleus | −/− | +/− | +/++ | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Pretectum | ++/++ | ++/+ | +++/+++ | +++/+++ | +/+ | −/− | −/− | −/− | −/− | −/− | −/− |
| Superior colliculus superficial | +++/+++ | +++/+++ | +++/+++ | +++/+++ | +++/+++ | −/− | −/− | −/− | −/− | −/− | −/− |
| Superior colliculus deep | +++/+++ | +++/+++ | +++/+++ | +++/+++ | +++/+++ | −/− | −/− | −/− | −/− | −/− | −/− |
| Periaqueductal gray | ++ | + | ++ | ++ | ++ | −/− | −/− | −/− | −/− | −/− | −/− |
| Inferior colliculus | −/− | ++/+ | +/++ | ++/++ | +++/− | −/− | −/− | −/− | −/− | −/− | −/− |
+++, Damage to greater than two-thirds of structure; ++, damage to one-third to two-thirds of structure; +, damage to less than one-third of structure. Results show left brain/right brain.
DISCUSSION
Ablation of the SC-PT but not the VC attenuated acute sleep–waking responses to changes in lighting conditions in albino rats, including triggering of REM sleep after lights-off stimulation as well as redistribution of NREM sleep to the light periods of short light–dark cycles, in comparison to normal control rats. The increases in REM sleep after lights-off as well as the redistribution of NREM sleep in short light–dark cycles seen in rats with lesions of the VC were comparable to those seen in the normal control rats as well as to values reported for various strains of albino rats (8, 9, 21).
The present results suggest that the VC is not sufficient for mediation of light–dark effects on sleep-wakefulness. Furthermore, preliminary data from our laboratory indicate that the VC may not be necessary for these responses. Ibotenic acid lesions of areas within the SC-PT area that completely spared the VC also greatly reduced the behavioral responses to changes in lighting conditions (unpublished data). It is also unlikely that structures adjacent to the SC-PT region mediate the behaviors, because damage to these structures (e.g., hippocampus, caudal thalamus, periaqueductal gray, and inferior colliculus) was variable, and none of the structures is retinorecipient, making it unlikely that they are involved in mediating light effects on sleep.
The VC, however, is known to have significant modulatory input to the SC and PT (22). The delicate and complex balance that exists between the VC and the SC first was established by Sprague (23), who showed that VC lesions in cat resulted in a profound contralateral visual neglect. Subsequent removal of the SC opposite to the VC lesion restored the animal’s ability to orient. In the present experiments, it is possible that ablation of the VC resulted in increased excitability of the SC-PT. Rats with VC lesions showed a trend for larger differences in waking amounts between light and dark periods in comparison to normal controls, indicating that the VC lesion had opposite, if any, effects on light–dark regulation of sleep and wakefulness compared with the SC-PT lesion. Nevertheless, the marked attenuation of the responses by removal of the SC-PT (and overlying visual cortex) suggests that the SC-PT mediates sleep and wakefulness responses to changes in lighting conditions.
Earlier efforts to define areas of the brain involved in REM sleep induction by lights-off in albino rats were unsuccessful; ablation of the pituitary, the pineal, the suprachiasmatic nucleus, or accessory optic nuclei all left the behavior intact (10, 24, 25).
It has long been known that light-responsive centers in the midbrain are involved in reflexive responses to visual stimuli, such as moving the eyes to fixate on an object of interest and accommodation of the pupils to varying levels of light. In addition, the midbrain tectum has been shown to be involved in a variety of behaviors, including attention, arousal, search, and escape (15, 26). The data reported in this paper suggest an even broader role for the midbrain in response to changes in light conditions. Just as a novel visual stimulus generates a cascade of responses originating from the midbrain tectum, including the initiation of eye, head, pinnae, and neck movements, changes in light levels also initiate a complex series of responses including changes in pupil size, arousal, sleep-wakefulness, and, in some cases, mood.
The SC has projections to many brain regions, including periaqueductal gray, thalamus, cortex, brainstem, and spinal cord (27). Thus, the midbrain tectum is in a pivotal position to initiate behavioral responses to sensory stimuli. The large projection from the deep layers of the SC to the pedunculopontine tegmentum (PPT) (28) and the projection from the PT to the laterodorsal tegmentum (LDT) (29) are possible candidates for mediation of lights-off stimulation and/or lights-on suppression of REM sleep generation, because the LDT/PPT region is known to mediate REM sleep (30, 31). Further studies are necessary to determine which projections from the SC-PT to putative sleep–wakefulness regulatory areas mediate the various effects of light–dark transitions on sleep behavior.
Although circadian rhythms were not assessed specifically in this study, diurnal patterns of waking, NREM, and REM sleep in a 12-h light/12-h dark cycle were consistent with circadian entrainment. Both the SC-PT and the suprachiasmatic nucleus appear to regulate the timing of sleep and wakefulness in changing light–dark conditions, although neither appears to have a primary role in determining overall amount of sleep. The circadian timing system aids an organism in anticipating the regular, daily cycles of day and night and is relatively nonresponsive to rapid changes in illuminance levels (32, 33). In contrast, the SC-PT determines behavioral responses to unpredictable and/or short-lived changes in lighting conditions.
It is likely that these two systems interact. For example, the magnitude of the acute response to light depends on the time of day at which the stimulus is presented (4), suggesting circadian modulation. It also seems reasonable that acute responses feed back to the circadian clock, possibly via the small projection from the PT to the suprachiasmatic nucleus (34). A second, more indirect route could involve projections from the PT and/or SC to the lateral geniculate nucleus of the thalamus or the intergeniculate leaflet (35, 36), which has been shown to have profound modulatory effects on the circadian clock (37).
In summary, our results demonstrate that acute changes in sleep patterns in response to light and dark in albino rats are mediated by the SC-PT region and are regulated independently from cortical vision and circadian rhythms. The SC-PT region may be important in determining the onset of NREM sleep, REM sleep, and wakefulness in mammals. These findings also may have implications for understanding the effect of light on humans. Bright light is used clinically for the treatment of winter depression or seasonal affective disorder, and light effects on sleep and mood have been observed within the first days of treatment (38). Alerting and activating effects during bright-light exposure have been demonstrated in humans as well (1). The mechanism for the therapeutic efficacy of bright light in depression is unclear, but may involve the SC-PT.
Acknowledgments
We thank R. Kalil and A. E. Kelley for assistance in lesion surgery and histology and R. J. Davidson, R. Guillery, R. Kalil, N. Kalin, and A. Rechtschaffen for helpful discussions. Research was supported by National Research Service Award GM07507 to A.M.M., National Institute of Mental Health Grants MH01224 and MH52226 to R.M.B, and funds from Meriter Hospital.
ABBREVIATIONS
- VC
visual cortex
- SC-PT
superior colliculus–pretectum
- REM
rapid eye movement
- NREM
non-rapid eye movement
- HSD
honestly significant difference
References
- 1.Campbell S S, Dijk D-J, Boulos Z, Eastman C I, Lewy A J, Terman M. J Biol Rhythms. 1995;10:129–132. doi: 10.1177/074873049501000205. [DOI] [PubMed] [Google Scholar]
- 2.Borbely A A. Brain Res. 1976;114:305–317. doi: 10.1016/0006-8993(76)90673-9. [DOI] [PubMed] [Google Scholar]
- 3.Goff R, Finger F W. Science. 1966;154:1346–1348. doi: 10.1126/science.154.3754.1346. [DOI] [PubMed] [Google Scholar]
- 4.Borbely A A. Prog Neurobiol. 1978;10:1–31. doi: 10.1016/0301-0082(78)90018-7. [DOI] [PubMed] [Google Scholar]
- 5.Gander P H, Moore-Ede M C. Am J Physiol. 1983;245:R927–R934. doi: 10.1152/ajpregu.1983.245.6.R927. [DOI] [PubMed] [Google Scholar]
- 6.Lisk R D, Sawyer C H. Proc Soc Exp Biol Med. 1966;123:664–667. doi: 10.3181/00379727-123-31571. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Dates R, Tobias M, Whitehead W E. Commun Behav Biol. 1969;3:93–99. [Google Scholar]
- 8.Benca R M, Bergmann B M, Leung C, Nummy D, Rechtschaffen A. Physiol Behav. 1991;49:83–87. doi: 10.1016/0031-9384(91)90235-g. [DOI] [PubMed] [Google Scholar]
- 9.Leung C, Bergmann B M, Rechtschaffen A, Benca R M. Physiol Behav. 1992;52:127–131. doi: 10.1016/0031-9384(92)90441-4. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J H, Adler N T, Sawyer C H. Exp Neurol. 1970;27:162–171. doi: 10.1016/0014-4886(70)90210-4. [DOI] [PubMed] [Google Scholar]
- 11.Weiskrantz L, Warrington E K, Sanders M D, Marshall J. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- 12.Fischman M W, Meikle T H. J Comp Physiol Psychol. 1965;59:193–201. doi: 10.1037/h0021811. [DOI] [PubMed] [Google Scholar]
- 13.Berman N. J Comp Neurol. 1977;174:227–254. doi: 10.1002/cne.901740204. [DOI] [PubMed] [Google Scholar]
- 14.Huerta M F, Harting J K. Trends Neurosci. 1984;17:286–289. [Google Scholar]
- 15.Dean P, Redgrave P, Westby G W. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- 16.Klein D C, Moore R Y, Reppert S M. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 17.Lund R D. Science. 1965;149:1506–1507. doi: 10.1126/science.149.3691.1506. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann B M, Winter J B, Rosenberg R S, Rechtschaffen A. Sleep. 1987;10:1–11. doi: 10.1093/sleep/10.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Swanson L W. Brain Maps: Structure of the Rat Brain. New York: Elsevier; 1992. [Google Scholar]
- 21.Benca R M, Overstreet D H, Gilliland M A, Russell D, Bergmann B M, Obermeyer W H. Neuropsychopharmacology. 1996;20:1–7. doi: 10.1016/0893-133X(95)00154-6. [DOI] [PubMed] [Google Scholar]
- 22.Ogasawara K, McHaffie J G, Stein B E. J Neurophysiol. 1984;52:1226–1245. doi: 10.1152/jn.1984.52.6.1226. [DOI] [PubMed] [Google Scholar]
- 23.Sprague J M. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- 24.Sisk C L, Stephan F K. Physiol Behav. 1982;29:231–239. doi: 10.1016/0031-9384(82)90009-9. [DOI] [PubMed] [Google Scholar]
- 25.Tobler I, Borbely A A. Behav Biol. 1978;23:395–398. doi: 10.1016/s0091-6773(78)91435-9. [DOI] [PubMed] [Google Scholar]
- 26.Sprague J M. Prog Brain Res. 1996;112:1–15. doi: 10.1016/s0079-6123(08)63317-8. [DOI] [PubMed] [Google Scholar]
- 27.Albers F J. Structure and Organization of the Superior Colliculus of the Rat. Amsterdam: Thesis Publishers; 1990. [Google Scholar]
- 28.Steininger T L, Rye D B, Wainer B H. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- 29.Cornwall J, Cooper J D, Phillipson O T. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 30.Steriade M, McCarley R W. Brainstem Control of Wakefulness and Sleep. New York: Plenum; 1990. [Google Scholar]
- 31.Siegel J M. In: Principles and Practice of Sleep Medicine. Kryger M H, Roth T, Dement W C, editors. Philadelphia: Saunders; 1994. pp. 125–144. [Google Scholar]
- 32.Moore R Y. Annu Rev Med. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- 33.Morin L P. Brain Res Brain Res Rev. 1994;19:102–127. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 34.Mikkelsen J D, Vrang N. Neuroscience. 1994;62:497–505. doi: 10.1016/0306-4522(94)90382-4. [DOI] [PubMed] [Google Scholar]
- 35.Morin L P, Blanchard J H. J Comp Neurol. 1998;396:288–309. [PubMed] [Google Scholar]
- 36.Sugita S, Otani K, Tokunaga A, Terasawa K. Neurosci Lett. 1983;43:143–147. doi: 10.1016/0304-3940(83)90178-7. [DOI] [PubMed] [Google Scholar]
- 37.Miller J D, Morin L P, Schwartz W J, Moore R Y. Sleep. 1996;19:641–667. doi: 10.1093/sleep/19.8.641. [DOI] [PubMed] [Google Scholar]
- 38.Rosenthal N E, Sack D A, Gillin J C, Lewy A J, Goodwin F K, Davenport Y, Mueller P S, Newsome D A, Wehr T A. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]