Abstract
Activation of naive T cells requires the integration of signals through the antigen receptor and CD28. Although there is agreement on the importance of CD28, there remains controversy on the mechanism by which CD28 regulates T cell function. We have generated a gene-targeted knockin mouse expressing a mutation in the C-terminal proline-rich region of the cytoplasmic tail of CD28. Our analysis conclusively showed that this motif is essential for CD28-dependent regulation of interleukin 2 secretion and proliferation. In vivo analysis revealed that mutation of this motif-dissociated CD28-dependent regulation of cellular and humoral responses in an allergic airway inflammation model. Furthermore, we find an important gene dosage effect on the phenotype of the mutation and provide a mechanistic explanation for the conflicting data on the significance of this motif in CD28 function.
Optimal T cell activation requires the delivery of costimulatory signals concomitant with T cell receptor engagement. CD28 is perhaps the most important and best-studied costimulatory receptor and is unique in its potency to augment IL-2 production. Ligation of CD28 increases cell proliferation and enhances cell survival as well as regulating production of multiple T cell–derived cytokines (1). Consistent with this, T cells from mice deficient in CD28 have impaired proliferative responses, make little IL-2, and have increased susceptibility to apoptosis (2–5). Although the importance of CD28 in T cell function is well established, many questions remain as to the mechanism by which CD28 mediates its biological effects.
CD28 is most critical in the activation of resting, naive T cells. Furthermore, the primary outcomes of CD28 signaling are changes in cell proliferation and survival. Previous work examining the structural motifs responsible for CD28 function have relied on either transformed cell lines, retroviral transduction of primary T cells, or reconstitution of CD28-deficient mice with transgenic constructs under the control of heterologous promoters. Although these have led to important insights, the results have often been conflicting and each has experimental limitations that make extrapolation to primary, naive T cells problematic.
CD28 possesses no intrinsic enzymatic activity; however, discrete regions within the cytoplasmic tail interact with intracellular adaptor proteins and enzymes to initiate signaling. Mutagenesis studies have identified two regions of the tail as being of particular importance. A tyrosine-based motif in the membrane proximal region of the cytoplasmic domain binds and activates PI-3 kinase, as well as interacting with the adaptor proteins Grb-2 and GADS (6–11). Less well characterized is a proline-based motif in the distal portion of the cytoplasmic domain. This region can interact with SH3 domain containing proteins including the Src family kinases Lck and Fyn to initiate signaling (7, 12). Recent studies have been conflicting as to the importance of this motif in CD28-dependent responses, with some suggesting that it is critical for enhancing proliferation and IL-2 secretion, whereas others indicate that it is dispensable for these functions but required for IL-4 production (13–16). Moreover, the requirement for this motif in the generation of a complex, in vivo immune response is unknown.
Given the importance of CD28 in the activation of naive T cells and that CD28 expression is itself regulated by cell activation, we generated a targeted knockin mouse expressing a mutation of the distal proline motif to determine its function. The CD28 P187,190A knockin (CD28-AYAA KI) mice thus express only the mutant form of CD28. In contrast with other experimental approaches, the genetic elements that regulate transcription are preserved and thus the mutant protein is expressed and regulated in a manner identical to the endogenous gene product. This strategy permits both in vitro and in vivo analysis of the functional consequences of the mutation with less concern that the results are the result of aberrant patterns of expression. In these studies, we find that the proline motif is critical for normal CD28-dependent regulation of IL-2 production and is absolutely required for antibody production and germinal center formation in vivo. Furthermore, we demonstrate that gene dosage is important in determining the phenotype of this mutation.
RESULTS
Generation of CD28 P187,190A knockin mice
To generate mice expressing mutant CD28 under endogenous regulatory control, we constructed a gene targeting vector containing exon IV of CD28 and flanking intronic sequences (Fig. 1 A). Specific mutations were introduced to substitute alanine for proline at residues 187 and 190, thus disrupting the motif responsible for interaction with SH3 domain–containing proteins (Fig. 1 B). This construct was transfected into murine embryonic stem cells and positive clones injected in C57BL/6 blastocysts. Germline transmission was verified by restriction mapping, Southern blotting, and sequencing (Fig. 1 C). To remove the Neomycin cassette, pups were crossed with mice carrying Cre-recombinase as a transgene under the control of the E2A promoter (initially generated by H. Westphal, National Institutes of Health (NIH) and provided to us in the C57BL/6 background by M. Bessler, Washington University School of Medicine). This strategy results in high expression of Cre-recombinase in the ovum, and results in efficient excision of the Neo cassette in the resultant zygote (17, 18). Loss of the Neo cassette was verified by Southern blotting of BglII-digested DNA using a 5′ external probe, as well as by PCR analysis using primers that flank the remaining introduced DNA (Fig. 1 C). Mice carrying the recombinant allele were then bred to CD28-deficient mice to generate mice heterozygous for the mutant CD28-AYAA KI allele (CD28 AYAA/−). These mice have a single copy of the mutant gene and express only the mutant CD28 protein. Additional breedings were established to generate offspring that are either CD28+/+, CD28+/−, CD28−/−, or homozygous mutant (CD28 AYAA/AYAA) in a similar mixed genetic background.
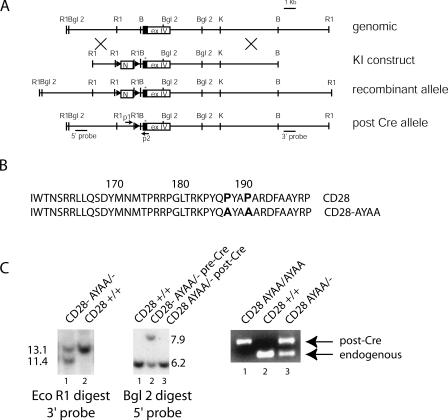
Figure 1.
Generation of CD28-AYAA knockin mouse. (A) A 14-kB genomic fragment containing exon IV and flanking intronic sequences was cloned from a murine 126/Sv library. The loxP-flanked neomycin resistance cassette was inserted into the 5′ intronic sequence. Point mutations, indicated by the asterisk, were introduced into exon IV at positions 187 and 190. The resultant recombinant allele contained two new EcoRI sites as well as the mutation. The shaded portion of exon IV represents the coding region. (B) Amino acid sequence of the cytoplasmic domain of CD28 coded for by the wild-type and mutant allele. (C) Southern blotting of wild-type and heterozygous mutant mice using a 3′ external probe on EcoRI-digested DNA and a 5′ external probe on BglII-digested DNA. Successful Cre-mediated excision of the Neo cassette was verified by loss of the 6.0-kB band on the BglII-digested DNA as well as using PCR strategy with primers p1 and p2 as indicated in A. The post-Cre recombinant allele is slightly larger than the endogenous allele as the result of remaining DNA introduced by the targeting vector.
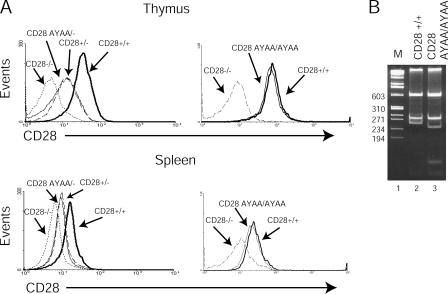
To determine whether T cells carrying the mutant CD28-AYAA allele expressed the protein at a level comparable to endogenous CD28, splenocytes and thymocytes were isolated from CD28 AYAA/−, CD28 AYAA/AYAA, CD28+/−, CD28+/+, and CD28−/− mice and stained with FITC-conjugated anti-CD4 and PE conjugated anti-CD28 and analyzed by flow cytometry (Fig. 2 A). The expression of mutant CD28 was comparable to the wild-type protein in both the heterozygous and homozygous states. After activation with anti-CD3 alone or anti-CD3 and anti-CD28, both wild-type and mutant alleles had similar expression patterns (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20052230/DC1). Thus, surface expression of the CD28-AYAA KI allele appears to be regulated during T cell activation in a manner similar to that of the endogenous alleles. To confirm that the expressed allele did in fact encode the desired mutation, RNA was isolated from wild-type or mutant thymocytes and the message for CD28 was amplified by RT-PCR. The mutation at position 187 introduces a new HaeIII site, which is apparent by the unique banding pattern seen in the sample prepared from the mutant and further confirmed by sequence analysis (Fig. 2 B and not depicted).
Figure 2.
Expression of CD28 on wild-type and mutant mice. (A) Thymocytes and splenocytes were isolated from CD28+/+, CD28+/−, CD28 AYAA/− CD28 AYAA/AYAA, and CD28−/− mice and stained for both CD4 and CD28. Shown are histograms of CD28 expression of each genotype after gating on CD4+ T cells. The expression of the mutant protein is equivalent to the endogenous protein. (B) Total RNA was isolated from thymocytes of CD28+/+ or CD28 AYAA/AYAA mice. RT-PCR was performed using primers that span exons I–IV. The PCR product was digested with HaeIII. The distinct banding pattern confirms that the transcribed RNA encodes for the desired mutation.
Impaired IL-2 production in T cells from CD28-AYAA KI mice
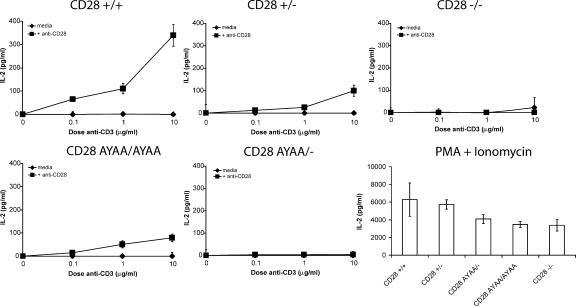
CD28 is unique among costimulatory receptors in the potency with which it regulates IL-2. We therefore directly assayed the amount of IL-2 present in the culture supernatants of stimulated T cells from wild-type and CD28-AYAA knockin mice (Fig. 3 and Table I). There was a marked reduction in IL-2 production from T cells isolated from CD28-AYAA mice. Consistent with previous reports, CD28+/− mice had an ∼50% reduction in IL-2 (2). In contrast, there was no detectable IL-2 in cultures from CD28 AYAA/− mice. Furthermore, the maximal IL-2 produced by CD28 AYAA/AYAA–expressing T cells was one third of that of CD28+/+ cells. IL-2 secretion was also absent in both the CD28 AYAA/− and CD28 AYAA/AYAA mice after stimulation with PMA and anti-CD28 (unpublished data). However, all genotypes produced significant amounts of IL-2 after stimulation with PMA and ionomycin (Fig. 3). Although there was a reduction in IL-2 secretion observed in both the CD28-deficient and CD28-AYAA mutant in response to PMA + ionomycin as compared with controls, this was not consistently observed. Importantly, these data demonstrate that there is not a generalized impairment in IL-2 production that would account for the profound deficiency observed after stimulation with anti-CD3 and anti-CD28. Thus, normal regulation of IL-2 production by CD28 requires signals initiated by the PYAP motif.
Figure 3.
Diminished IL-2 production in CD28 AYAA mice. Splenocytes were isolated from each of the indicated genotypes. Cultures were stimulated with graded doses of anti-CD3 alone or in the presence of 1.0 μg/ml anti-CD28 and IL-2 assayed in the culture supernatants after 48 h by ELISA. Representative data are shown from five independent experiments.
Table I.
Multiple cytokines are dependent on the proline motif of CD28
| CD28+/+
|
CD28 AYAA/AYAA
|
CD28−/−
|
||||
|---|---|---|---|---|---|---|
| CD3 | CD3/CD28 | CD3 | CD3/CD28 | CD3 | CD3/CD28 | |
| IL-2 | 22.63 | 503.92 | 19.30 | 30.00 | 3.69 | 5.03 |
| IL-4 | 1.46 | 22.15 | 2.78 | 11.47 | 0.45 | 0.60 |
| Il-5 | ND | 9.37 | 0.41 | 0.69 | ND | ND |
| IL-10 | 1.52 | 33.01 | 5.78 | 17.93 | 1.26 | 1.85 |
| IL-12 | 1.19 | 5.79 | 0.47 | 2.09 | 1.39 | 0.15 |
| GMCSF | 5.28 | 27.20 | 4.20 | 12.59 | ND | 1.97 |
| IFN-γ | 3.16 | 1,086.72 | 17.12 | 245.16 | 0.67 | 0.71 |
| TNF-α | 0.66 | 8.95 | 0.86 | 4.66 | 1.83 | 1.78 |
Splenocytes from the indicated genotypes were cultured with media alone, or activated with 1.0 μg/ml anti-CD3 alone or in combination with 1.0 μg/ml anti-CD28. Culture supernatants were harvested at 48 h and assayed for cytokine levels using the Th1/Th2 Bioplex multicytokine kit (BioRad Laboratories). All data are in pg/ml and the mean of triplicate samples presented.
To determine if the effect of the mutation was specific for IL-2 production or if it was representative of a more global defect in cytokine expression, we assayed a larger panel of T cell–derived cytokines (Table I). Mice expressing the mutant allele had a substantial reduction in the amount detected of several cytokines. These data suggest a more general requirement for the PYAP motif in CD28-dependent regulation of cytokine production.
Impaired proliferation in T cells from CD28-AYAA mice
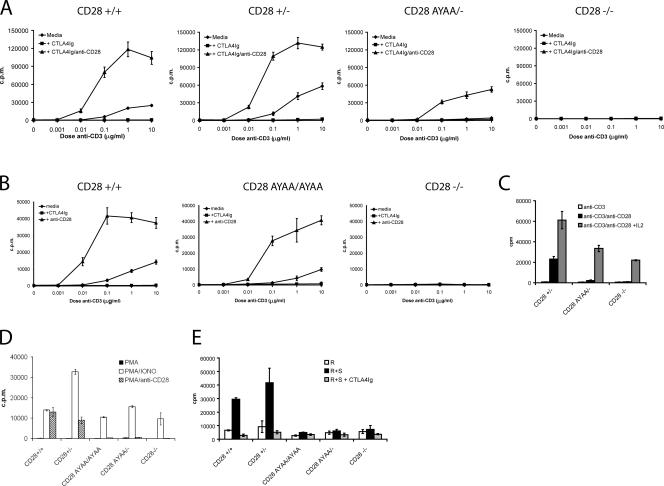
A fundamental property of CD28 costimulation is the augmentation of T cell proliferation, in particular to submitogenic levels of TCR engagement (19). To determine the effect of the P187,190A mutation on CD28-dependent proliferation, splenocytes from CD28+/+, CD28 AYAA/−, CD28+/− and CD28−/− mice were stimulated with increasing concentrations of anti-CD3 (0.01–10 μg/ml) alone or in combination with anti-CD28 mAb (1 μg/ml, clone PV-1) or CTLA4Ig (10 μg/ml) and proliferation was determined by [3H] thymidine incorporation (Fig. 4 A). T cells from CD28 AYAA/− mice had a marked defect in proliferative response to anti-CD3 alone and to the combination of anti-CD3 + anti-CD28. This was not the result of a reduction in expression as the CD28+/− and CD28-AYAA/− cells expressed comparable levels of CD28 (Fig. 2). In contrast with the results seen with cells from the CD28-AYAA/− heterozygous mice, proliferation of T cells from homozygous CD28-AYAA/AYAA mutant mice was near wild-type levels (Fig. 4 B). This reveals an important gene dosage effect, with a greater defect apparent at the lower expression levels seen in the heterozygous state.
Figure 4.
Impaired proliferation in cells from CD28 AYAA mice. (A and B) Splenocytes were isolated from each of the indicated genotypes and stimulated with graded doses of anti-CD3 alone, with 10 μg/ml CTLA4Ig or with 1.0 μg/ml anti-CD28. (C) Splenocytes were stimulated with 0.01 μg/ml anti-CD3 alone, with 1.0 μg/ml anti-CD3 and anti-CD28, or with 100 U/ml anti-CD3, anti-CD28, and exogenous IL-2. Proliferation was measured after 48 h. (D) Replicate cultures were stimulated with either PMA alone, PMA + ionomycin, or PMA + anti-CD28. Proliferation was measured after an overnight pulse with tritiated thymidine initiated at 48 h. (E) Splenocytes of the indicated genotypes (R) were cocultured with irradiated allogeneic splenocytes (S) for 96 h and proliferation was determined by tritiated thymidine incorporation. Representative data are shown from a minimum of three independent experiments.
We reasoned that the profound proliferative defect observed in cells from the heterozygous mutant might reflect limiting IL-2 availability. Although addition of exogenous IL-2 increased the proliferation of cells from both CD28−/− and CD28 AYAA/− mice, it did not fully restore it to the level observed in the CD28+/− cultures (Fig. 4 C). This suggests that the proliferative defect in both the CD28-deficient and CD28-AYAA mutant is partially, but not solely, the result of limiting IL-2.
To determine the response to physiologic engagement of the TCR and CD28 with natural ligand, we stimulated cells of each genotype with alloantigen in a mixed lymphocyte culture (Fig. 4 E). Both homozygous and heterozygous mutant cells failed to proliferate, whereas cells expressing wild-type CD28 mounted a robust proliferative response. In addition, neither homozygous nor heterozygous CD28-AYAA mutant mice proliferated after activation with PMA and anti-CD28 (Fig. 4 D). Importantly, all genotypes proliferated in response to PMA and ionomycin and had similar expression of CD69 after activation (Fig. 4 D and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20052230/DC1). Thus, there is a relative requirement for the C-terminal proline residues after activation by anti-CD3 and anti-CD28 that is partially overcome by increased expression levels, whereas there is an absolute requirement for this motif for proliferation induced by alloantigen or by PMA and anti-CD28.
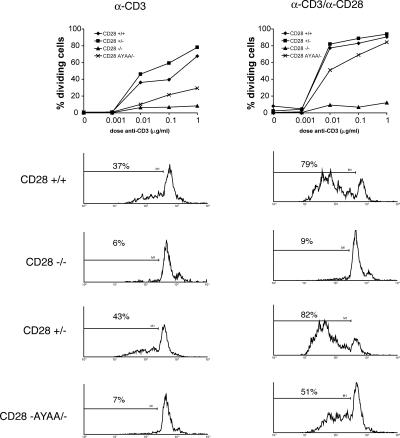
Proliferation was also determined by CFSE dye dilution. Splenocytes were loaded with CFSE and stimulated with anti-CD3 alone or in combination with anti-CD28. At 48, 72, and 96 h after stimulation, aliquots of cells were stained with anti-CD4–PE and analyzed by two-color flow cytometry. Presented in Fig. 5 is the percentage of CD4+ T cells that have undergone at least one cell division at 72 h. Representative histograms obtained from cultures stimulated with 0.01 μg/ml anti-CD3 alone (Fig. 5, left) or anti-CD3 + anti-CD28 (Fig. 5, right) are also shown. Markedly fewer cells expressing the mutant CD28 undergo cell division than cells expressing the wild-type allele, thus independently confirming the results obtained by thymidine incorporation. A similar pattern of results was observed at 48- and 96-h time points (unpublished data).
Figure 5.
Decreased cell division in T cells from CD28 AYAA mice. Splenocytes from the indicated genotypes were loaded with CFSE and stimulated with anti-CD3 alone or in combination with anti-CD28. 72 h after stimulation, the cells were stained with PE-conjugated anti-CD4 and analyzed by two-color flow cytometry. Shown is the percentage of cells having undergone at least one cell division. Representative CFSE histograms from cultures stimulated with 0.01 μg/ml anti-CD3. Shown is representative data from three independent experiments.
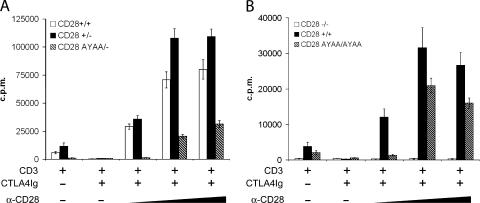
We consistently found that the homozygous CD28 AYAA mutant cells manifest a 1-log shift in the dose response to anti-CD3 as compared with control cultures (Fig. 4 B). In these experiments, endogenous CD80 and CD86 present on the APCs in the culture provided costimulation as indicated by the abrogation of proliferation after inclusion of CTLA4Ig. In addition, activation by alloantigen, which utilizes endogenous B7:CD28 interactions, was markedly impaired. This led us to hypothesize that differences in the level of receptor engagement between endogenous B7 and anti-CD28 mAb might be important in determining the consequence of the mutation. To investigate this, we stimulated splenocytes with anti-CD3 in the presence of CTLA4Ig to block endogenous costimulation and titrated in increasing amounts of anti-CD28 mAb (Fig. 6). In both the heterozygous and homozygous mutants, proliferation was markedly impaired at lower doses of anti-CD28. The effect was even more profound at submitogenic doses of anti-CD3 (unpublished data). Thus, at limiting levels of antigen receptor and CD28 engagement, signaling through the proline motif is critical for T cell proliferation.
Figure 6.
Diminished sensitivity to anti-CD28 in T cells from CD28 AYAA mutant mice. (A) Splenocytes from CD28+/+, CD28+/−, or CD28 AYAA/− mice were isolated and stimulated with 0.1 μg/ml of anti-CD3 alone, with the addition of 10 μg/ml CTLA4Ig to block endogenous CD80 or CD86, or with graded doses of anti-CD28 (0.03, 0.3, or 1.0 μg/ml). Proliferation was measured by tritiated thymidine incorporation after 48 h of culture. (B) Splenocytes from CD28+/+, CD28−/−, or homozygous mutant mice (CD28 AYAA/AYAA) were isolated and stimulated described in A. Representative data are shown from three independent experiments.
Up-regulation of BclXL expression
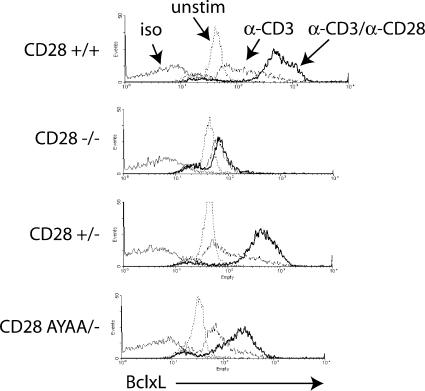
In addition to regulating cell proliferation and cytokine production, CD28 costimulation enhances cell survival. One mechanism by which this occurs is up-regulation of the antiapoptotic protein BclXL (20). Previous work has determined this to be downstream of PI-3 kinase activation and requires the tyrosine-based motif in the proximal portion of the cytoplasmic domain of CD28 (21–24). We had previously demonstrated that the expression pattern of Bcl-XL was preserved in CD28-deficient T cells retrovirally transduced with the CD28-AYAA mutation (14). However, in that system, the T cells had been previously activated to facilitate gene transduction. We therefore tested whether CD28-dependent regulation of Bcl-XL expression was intact in naive T cells isolated from the CD28-AYAA KI mice. As expected, CD28-deficient T cells failed to increase BCL-XL expression in response to anti-CD28 costimulation. Both CD28+/− and CD28 AYAA/− T cells up-regulated BCL-XL, although the increase in the AYAA mutant cells was less than that of the CD28+/− cells (Fig. 7). Thus, consistent with our previous findings and work demonstrating a strict requirement for the upstream tyrosine-based motif in regulation of Bcl-XL, up-regulation was not absolutely dependent on the proline-based motif (14, 23). However, there does appear to be some contribution to Bcl-XL expression mediated by signaling initiated by the proline motif. Nonetheless, these data do demonstrate that mutation of the C-terminal proline motif does not globally impair CD28 function.
Figure 7.
CD28-dependent up-regulation of Bcl-XL. Splenocytes from the indicated genotypes were activated with 1.0 μg/ml anti-CD3 alone or with 1.0 μg/ml anti-CD28 for 48 h. The cells were stained with FITC-conjugated anti-CD4, fixed, permeabilized, and stained with anti-BclXL antibody followed by a PE-conjugated secondary. Shown are representative histograms of Bcl-XL expression after gating on CD4+ T cells. Representative data are shown from three independent experiments.
CD28-AYAA KI mice manifest impaired humoral immunity in vivo
The aforementioned experiments demonstrate the importance of the PYAP motif in the regulation of T cell proliferation and IL-2 production in vitro. However, these findings may not accurately recapitulate events in vivo. The CD28-AYAA knockin mice provide a powerful tool to determine the role of signaling pathways initiated by the PYAP motif during an in vivo T cell–dependent immune response.
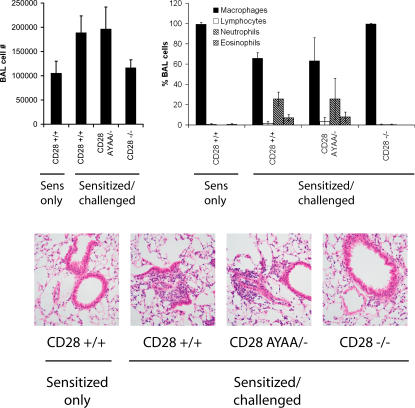
Experimental allergic airway inflammation is a model of a Th2 immune response characterized by an eosinophilic inflammatory cell infiltrate in the lung (25). Mice treated with soluble inhibitors of B7 or CD28-deficient mice develop no inflammatory or antibody response (26–28). To determine if the PYAP motif of CD28 was required, CD28-AYAA KI or control mice were sensitized and challenged with OVA. Despite the aforementioned profound in vitro defects, mice heterozygous for the mutant allele developed similar airway inflammation as wild-type control mice, as assessed by bronchoalveolar lavage and histology (Fig. 8).
Figure 8.
Allergic airway inflammation is intact in CD28-AYAA mice. Mice of each genotype were systemically sensitized to OVA and given an inhaled challenge. 72 h after challenge, the BAL and lung tissue were collected for analysis. There was no difference in cell recovery or differential analysis of the BAL fluid obtained from the wild-type CD28 AYAA mice. Similarly, histologic examination revealed inflammation in both wild-type and CD28 AYAA mutant mice, but not CD28−/− mice. Representative data are shown from three independent experiments.
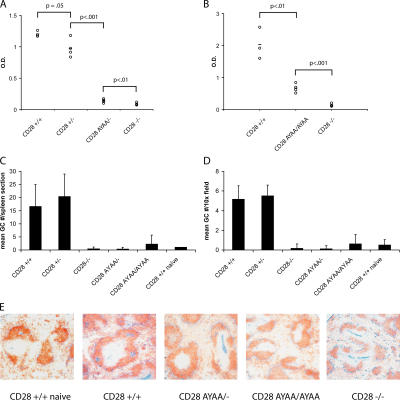
In contrast with the normal tissue inflammation observed in the mutant mice, there was a marked decrease in OVA-specific antibody relative to wild-type controls (Fig. 9, A and B). This was accompanied by a failure of the CD28-AYAA KI mice to develop germinal centers (GCs) in the spleen as determined by staining with PNA and anti-IgD (Fig. 9, C–E). Mice expressing mutant CD28 had a 5-fold reduction in GCs per 10× field or a >20-fold reduction in total GCs per section as compared with mice expressing wild-type CD28. This was evident in both heterozygous and homozygous CD28-AYAA KI mice. Thus, there is an absolute requirement for the PYAP motif in the development of OVA-specific antibodies and GC formation in vivo. Furthermore, this mutation dissociates specific CD28-dependent elements of the immune response, as the CD28-AYAA were able to mount a normal inflammatory response in the tissue, yet failed to produce a humoral response, whereas wild-type mice have both elements and CD28-deficient mice have neither.
Figure 9.
Impaired IgG and germinal center formation in vivo. (A and B) Serum was collected from mice (n = 3–5 mice/group) that had been systemically immunized with OVA/alum as described for Fig. 8. OVA-specific IgG1 was assayed by ELISA. p-values were calculated by two-tailed Student's t test. (C and D) Whole spleens were collected from OVA-immunized mice and frozen sections were stained with IgD and PNA to detect germinal centers. Only mice expressing wild-type CD28 had detectable germinal center formation after immunization. (E) Representative sections of spleen from each genotype stained with anti-IgD and PNA. Representative data are shown from three independent experiments.
DISCUSSION
CD28 was first described in studies reporting that ligation of a 44-kD protein, Tp44, on the surface of human T cells augmented proliferation induced by PHA or alloantigen and IL-2 production (19, 29). Numerous papers have been subsequently published characterizing the profound effect of CD28 on T cell function in particular and its importance in regulating IL-2 expression and proliferation. Later work identified CD28 as a determinant of cell survival and metabolism through regulation of the antiapoptotic protein Bcl-XL and promotion of glucose uptake and utilization, respectively (20, 30, 31). Thus, CD28 directly regulates several processes critical for the growth, survival, and effector function of T cells.
Although it is clear that CD28 activates multiple signaling pathways, the contribution of each to the downstream effector functions of CD28 remains controversial. Much of the conflicting data may arise from the nature and differences in the experimental systems that have been used. Many of these studies have used transformed cell lines that have intrinsic abnormalities in proliferation and survival, making extrapolation to primary cells difficult. To circumvent this problem, several groups have used retroviral gene transfer into primary cells; however, this requires activation of the T cell to facilitate infection. Therefore, the cells are no longer naive (13, 14). Given that CD28 is most important in the initial activation of resting naive T cells, this approach is also problematic. In addition, expression levels of the retrovirally transduced gene are variable and not regulated in a manner analogous to the endogenous gene product. Although reconstitution of the CD28-deficient mice with specific transgenes avoids the problem of cell activation and therefore allows for the study of naive cells, the use of heterologous promoters alters the regulation of expression. Thus, none of these experimental approaches faithfully recapitulate endogenous CD28. In contrast, the knockin mouse we have described allows the study of CD28 in naive T cells under conditions in which expression is correctly regulated by endogenous genetic elements.
The C-terminal proline motif can bind SH3 domain containing proteins including the Src family kinases Lck and Fyn, the Tec kinase Itk, and the adaptor protein Grb2 (7, 11, 12, 15, 32–34). Knockin mice expressing a single copy of CD28 containing a mutation of this region had a profound defect in IL-2 production and proliferation after activation with anti-CD3 and anti-CD28, demonstrating that this motif is critical in the regulation of these functions. Higher expression levels present in homozygous mutant mice restored proliferation to near wild-type levels, but IL-2 secretion remained severely impaired. In contrast, activation by alloantigen in which both the TCR and CD28 are engaged by endogenous ligand failed to induce proliferation in cells from either homozygous or heterozygous mutant mice. Thus, under activation conditions that more closely model physiologic engagement of CD28, this motif is critical regardless of expression level. Similarly, there was an absolute defect in proliferation and IL-2 secretion after activation by PMA + anti-CD28 in both heterozygous and homozygous mutant mice, confirming a requirement for this motif in some CD28-dependent signaling pathways.
The importance of this region is consistent with early studies in cell lines in which deletion of this region abrogated CD28-dependent IL-2 production (35–37), and are supportive of some studies using retroviral gene transfer (14, 15). However, recent publications examining this have been conflicting. Andres et al. transduced bone marrow cells from CD28-deficient mice with a series of mutant CD28 constructs and demonstrated that no single motif was absolutely required for proliferation and IL-2 production; however, IL-4 production did require the C-terminal proline residues (13). Tai et al. addressed this question by reconstituting CD28-deficient mice with mutant CD28 transgenic constructs controlled by the CD2 promoter (16). These studies demonstrated that the proline motif is required for CD28-dependent IL-2 production after stimulation with PMA and anti-CD28, as well as for the generation of regulatory T cells in vivo independent of the effect on IL-2. Our data are in agreement with a requirement for the C-terminal proline residues for the normal regulation of IL-2 production and proliferation by CD28. Importantly, our data provide an explanation for the reported differences in these datasets. Our data clearly demonstrate that higher expression levels, which might be achieved in some retrovirally transduced cells, under some experimental conditions may mask the requirement for this motif. Furthermore, we demonstrate that there is an absolute requirement for an intact PYAP motif when the cells are activated by PMA and anti-CD28 or by alloantigen, whereas activation by anti-CD3 and anti-CD28 is less dependent on the distal proline residues. Thus, differences in both expression levels and activation conditions account for the reported discrepancies in the requirement for this region in the regulation of proliferation and IL-2.
Given the defects in proliferation and cytokine secretion, we examined signaling pathways activated after T cell activation. We saw no difference in either total protein tyrosine phosphorylation or ERK phosphorylation after stimulation with anti-CD3 and anti-CD28 antibody in primary T cells from either wild-type or mutant mice (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20052230/DC1). Although other reports have demonstrated CD28-dependent effects in these assays, we failed to reproduce these observations (34, 38). It is possible that differences in experimental systems may account for this discrepancy.
In vivo, CD28 is engaged by CD80 or CD86 on the surface of an APC. Recent studies suggest that the B7:CD28 interaction may be monovalent and therefore may not induce a high degree of receptor cross-linking (39, 40). In contrast, saturating doses of anti-CD28 mAb are likely to lead to extensive cross-linking and may activate signaling pathways that would not be activated under more physiologic conditions. Consistent with this hypothesis, we found that the proliferative defect in the CD28-AYAA mutant cells was more profound under conditions of endogenous costimulation or limiting amounts of mAb.
A large body of work has focused on the proximal motif centered around the tyrosine at position 170 (Y170), which is critical for PI-3 kinase activation as well as binding the adaptor proteins Grb2 and GADS through their SH2 domain (8, 9, 11, 41). Controversy exists as to the precise role of CD28-dependent activation of PI-3 kinase in the regulation of proliferation and IL-2 secretion. Recent studies have provided some resolution, with several confirming the importance of this pathway in induction of Bcl-XL (14, 23). More recently, it has been demonstrated that mutation of this motif abrogated CD28-dependent regulation of IL-2 transcription, yet leaves the posttranscriptional regulation of IL-2 mRNA stability intact (42). Thus, it would appear that distinct signaling cascades are responsible for the coordinated regulation of IL-2 secretion by CD28.
In contrast with the marked defect in proliferation and cytokine production, expression of Bcl-XL was less profoundly altered in the CD28-AYAA mutant cells. In studies performed by either retroviral gene transfer or transgenic mice, a point mutation of Y170 abolished Bcl-XL expression, yet left IL-2 intact (14, 23). Similarly, using chimeric constructs in human T cells, Parry et al. demonstrated that the membrane proximal domain of CD28 was required for Bcl-XL induction, although both proximal and distal regions were necessary for normal IL-2 production (24). The relative preservation of Bcl-XL expression in the CD28-AYAA knockin mutant demonstrates that the mutation has not globally impaired CD28 function and is consistent with a requirement for the proximal tyrosine based motif as the major pathway by which CD28 regulated Bcl-XL expression.
In contrast with reconstitution by retroviral gene transfer or transgenic constructs under the control of a heterologous promoter, the knockin mouse we have generated allows for determination of the effect of the mutation in vivo, with less likelihood that that aberrant expression patterns influence the result. Given previous data suggesting that this motif was critical in the generation of a Th2 response, we examined their response in a model of allergic airway inflammation. This model is CD28-dependent and leads to a Th2-type inflammatory response with recruitment of eosinophils to the lung and the generation of OVA-specific IgG1 and IgE (26, 27, 43). Despite the proliferative and cytokine defects observed in vitro, the inflammatory response of the mutant mice was indistinguishable from wild type as assessed by histology and analysis of the cellular composition of the BAL fluid. The ability to recruit inflammatory cells to the lung after the inhaled antigen challenge suggests that the initial priming and clonal expansion of antigen-specific T cells remained intact in the mutant mice. Nonetheless, it remains possible that Th2 development was impaired despite the presence of eosinophils in the airways (44, 45).
Although the cellular immune response appeared grossly intact, the humoral response was significantly impaired in homozygous and heterozygous CD28-AYAA mutant mice. There was a drastic reduction in OVA-specific IgG1 in both homozygous and heterozygous mutant mice. This was accompanied by a failure of the mutant mice to form germinal centers in the spleen. Thus, these aspects of CD28 function are absolutely dependent on an intact PYAP motif. A requirement for B7:CD28 in humoral responses was first noted in the characterization of mice expressing a soluble CTLA4Ig transgene as well as in the characterization of CD28-deficient mice (46, 47). This may be secondary to the loss of specific cognate interactions between T and B cells, as well as defective cytokine and/or chemokine production by the mutant T cells. Although a relative deficiency in IL-4 secretion might account for the lack of Th2 Ig isotypes, it is unlikely to lead to a complete failure to develop germinal centers. However, there is an intimate relationship between B7:CD28 and CD40:CD40L interactions (48, 49). A failure to up-regulate CD40L on the mutant T cells could account for the decreased antibody and GC formation, particularly in combination with a relative deficiency in IL-4 (50). Recent data suggests that CD28 regulates the expression of OX40 on T cells and that the combined action of CD28 and OX40 is important in the expression of CXCR5 on T cells (51, 52). This in turn may regulate the migration of T cells into the B cell follicle to support the GC reaction. Some investigators have proposed that the T cells supporting the GC reaction may in fact be a novel class of T helper cells (53). The requirement for costimulation in the development and function of these cells is only beginning to be examined.
In summary, our data demonstrates that the C-terminal proline motif is critical for normal regulation of proliferation and IL-2 production by CD28 in resting naive primary T cells. We also have shown that there is an important gene dosage effect in the mutant phenotype as a twofold change in the expression level of the mutant protein was sufficient to essentially normalize cell proliferation, but not IL-2 production. Furthermore, in vivo, this motif discriminates between specific elements of CD28 that regulate cellular and humoral aspects of the immune response in vivo, as it is essential for antibody production and GC formation, but not cell-mediated inflammation. This aspect, in particular, suggests that selective manipulation of this portion of the CD28 signal transduction pathway might be of potential therapeutic value by inhibiting humoral responses while preserving cellular immunity.
MATERIALS AND METHODS
Generation of CD28-AYAA knockin construct.
A 14-Kb EcoRI fragment containing exon IV of CD28 was cloned from a mouse genomic BAC library (Incyte Genomics). This was digested with BamH1 and a LoxP site–flanked Neomycin resistance cassette was inserted into the 5′ BamHI site. The 1.7-Kb short arm was generated by PCR spanning from the BamHI site to the EcoRI site upstream and cloned 5′ to the Neomycin cassette. The resultant construct contained two new EcoRI sites flanking the Neomycin cassette as indicated in Fig. 1. Oligonucleotide-directed site-specific mutagenesis was performed to generate specific base pair changes (CCC to GCC and CCT to GCT) resulting in a final sequence that substituted alanine for proline at positions 187 and 190. The sequence of the entire exon was verified by direct sequence analysis. The construct was transfected into 129/Sv embryonic stem cells (line RW4, provided by the Siteman Cancer Center, Washington University, St. Louis) and neomycin resistant clones were screened for homologous recombination by Southern blotting of BglII- and EcoRI-digested DNA using 5′ and 3′ external probes, respectively (Fig. 1 C). The mutation was confirmed by direct sequencing of exon IV after PCR amplification, as well as by restriction digestion with HaeIII, as the mutation results in the generation of a new HaeIII site (unpublished data). Germline transmission was verified by Southern blotting using both 5′ and 3′ external probes. In addition, the presence of the mutation was confirmed by both restriction digest and direct sequence analysis.
Mice.
C57BL/6 mice were purchased from The Jackson Laboratory. CD28-deficient mice on the C57BL/6 background were originally obtained from C. Thompson (University of Pennsylvania, Philadelphia, PA). All mice were bred and housed under specific pathogen-free conditions at Washington University School of Medicine. All animal experimentation has been approved by Institutional Animal Use and Care Committee at Washington University School of Medicine.
Antibodies.
Anti-CD3 (145-2c11 hamster IgG) and all fluorescently conjugated antibodies were purchased from BD Biosciences or eBioscience. For staining, fluorescently conjugated anti-CD28 (clone 37.5.1) was used. For stimulation, anti-CD28 clone PV-1 (Southern Biotechnology Associates, Inc.) was used. Anti-Bcl-XL clone 7B2.5 ascites (mouse IgG3) was provided by L. Boise (University of Miami, Miami, FL).
RT-PCR.
Total RNA was isolated from wild-type or mutant thymocytes using TRIzol reagent (Invitrogen). RT-PCR was performed using primers specific for exons I and IV of CD28. The product was purified, digested with HaeIII, and electrophoresed on a 15% polyacrylamide gel. The bands were visualized after staining with ethidium bromide.
[3H] incorporation assays.
Splenocytes were isolated by density gradient centrifugation, plated at 105 cells per well of a 96-well plate, and stimulated as indicated for each experiment. After 48 h of stimulation, the plates were pulsed with tritiated thymidine (1 μCi/well) overnight and harvested the following morning. For the mixed lymphocyte response, 5 × 105 splenocytes of each genotype were cocultured with an equal number of BALB/c splenocytes that had been γ-irradiated (2,000 rad). After 96 h of stimulation, the cultures were pulsed with tritiated thymidine and harvested the following morning. Incorporated thymidine was determined by liquid scintillation counting. All conditions were plated in quadruplicate and the mean ± the standard deviation presented. All experiments have been replicated a minimum of three times and representative data are presented.
CFSE assays.
Splenocytes were isolated and incubated at a concentration of 5.0 × 106 cells/ml with 5 mM CFSE in 1x PBS + 5% FCS for 5 min at room temperature. After washing three times with 1x PBS, the cells were resuspended in media at 2.0 × 106/ml and stimulated with anti-CD3, anti-CD28, and CTLA-4 Ig as described. Aliquots of cells were stained with PE-conjugated anti-CD4 mAb and the intensity of CFSE fluorescence of the CD4+ cells determined by two-color flow cytometry. Unstimulated cells were assayed simultaneously to determine the fluorescence intensity of undivided cells. All analysis was done using a FACSCalibur flow cytometer with CELLquest Software (Becton Dickinson Corporation).
Cytokines.
Splenocytes were isolated and stimulated as described for the proliferation assays. After 48 h, culture supernatants were collected and assayed for IL-2 using the Quantikine Murine IL-2 ELISA kit as per the manufacturer's protocol (R&D Systems). Additional cytokines were assayed using the Bioplex murine Th1/Th2 panel as per the manufacturer's directions (BioRad Laboratories).
Flow cytometry.
CD28 expression of thymocytes or splenocytes was determined by staining with anti-CD4–FITC and anti-CD28–PE or isotype controls and analyzed on a FACSCalibur flow cytometer using CELLquest software (Becton Dickinson). Similarly, CD69 expression was determined on resting or activated CD4+ T cells by two-color flow cytometric analysis using anti-CD4–FITC and PE-conjugated anti-CD69. Bcl-XL expression was determined by intracellular staining as previously described (14).
Induction of allergic airway inflammation.
Mice were immunized i.p. on days 0 and 7 with 8 μg OVA absorbed to 2 mg alum (Sigma-Aldrich) as previously described (25, 54). On day 14, the mice were intranasally challenged with 2% OVA and specimens collected on the third day after challenge. Bronchoalveolar lavage (BAL) was performed by intratracheal installation of 1% BSA in PBS. Cell differentials were performed on cytospin preparations stained with a modified Wright-Geimsa stain. For histology, the lungs were inflated with neutral buffered formalin to 25 cm of water pressure and fixed overnight. Samples were progressively dehydrated in ethanol and processed for sectioning and H&E staining. Serum was collected at the time the mice were killed and antigen-specific immunoglobulin titers were determined by specific ELISA as previously described (28). Spleens were also harvested and frozen in OCT compound on dry ice. Frozen sections were prepared, fixed in acetone, and stained with PNA-biotin and rat anti–mouse IgD followed by detection with AP-streptavidin and goat anti–rat IgG(H+L)-HRP to detect GCs. Multiple sections were examined and the number of GCs/10X field were determined as well as the total number of GCs per section counted.
Online supplemental material.
To determine whether the mutation altered the regulation of CD28 expression after T cell activation, cells were stimulated and CD28 expression was determined by flow cytometry (Fig. S1). Similarly, CD69 expression was assessed in wild-type and mutant T cells after activation (Fig. S2). Protein tyrosine phosphorylation and ERK phosphorylation was assessed in wild-type and mutant T cells by Western blotting (Fig. S3). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20052230/DC1.
Supplemental Material
Acknowledgments
The generation of the targeting vector, screening of the ES cells, and mice and RT-PCR analysis of CD28 mRNA was performed by D. Shah. Germinal center staining and analysis was performed by C. Deppong. T. Juehne, T. Bricker, and C, Rose aided in the allergic airway inflammation experiments and breeding of the mice. J, Lin performed the biochemical assays. The remaining assays were performed by L, Friend. The authors would like to thank R. Arch, A. Shaw, and T. Ley for help throughout this project. We also thank R. McCarthy and M. White for skilled ES cell injection work. We thank L. Boise for anti-Bcl-XL antibody and M. Bessler for E2A-Cre mice on the C57BL/6 background. We would like to thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Embryonic Stem Cell Core that provides transfection and ES cell culture service.
This research is supported in part by grants from the NIH (HL062683 to J.M. Green). C. Deppong is supported by NIH training grant no. T32 HL07317. L.D. Friend is supported through a fellowship by the Ford Foundation. The Siteman Cancer Center is supported in part by NCI Cancer Center Support grant no. P30 CA91842.
The authors have no conflicting financial interests.
Abbreviation used: GC, germinal center.
L.D. Friend and D.D. Shah contributed equally to this work.
References
- 1.Sharpe, A.H., and G.J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116–126. [DOI] [PubMed] [Google Scholar]
- 2.Shahinian, A., K. Pfeffer, K.P. Lee, T.M. Kündig, K. Kishihara, A. Wakeham, K. Kawai, P.S. Ohashi, C.B. Thompson, and T.W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 261:609–612. [DOI] [PubMed] [Google Scholar]
- 3.Green, J.M., P.J. Noel, A.I. Sperling, T.L. Walunas, G.S. Gray, J.A. Bluestone, and C.B. Thompson. 1994. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1:501–508. [DOI] [PubMed] [Google Scholar]
- 4.Noel, P.J., L.H. Boise, J.M. Green, and C.B. Thompson. 1996. CD28 costimulation prevents cell death during primary T cell activation. J. Immunol. 157:636–642. [PubMed] [Google Scholar]
- 5.Sperling, A.I., J.A. Auger, B.D. Ehst, I.C. Rulifson, C.B. Thompson, and J.A. Bluestone. 1996. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J. Immunol. 157:3909–3917. [PubMed] [Google Scholar]
- 6.Schneider, H., Y.C. Cai, K.V. Prasad, S.E. Shoelson, and C.E. Rudd. 1995. T cell antigen CD28 binds to the GRB-2/SOS complex, regulators of p21ras. Eur. J. Immunol. 25:1044–1050. [DOI] [PubMed] [Google Scholar]
- 7.Raab, M., Y.C. Cai, S.C. Bunnell, S.D. Heyeck, L.J. Berg, and C.E. Rudd. 1995. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc. Natl. Acad. Sci. USA. 92:8891–8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagès, F., M. Ragueneau, R. Rottapel, A. Truneh, J. Nunes, J. Imbert, and D. Olive. 1994. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 369:327–329. [DOI] [PubMed] [Google Scholar]
- 9.Pagès, F., M. Ragueneau, S. Klasen, M. Battifora, D. Couez, R. Sweet, A. Truneh, S.G. Ward, and D. Olive. 1996. Two distinct intracytoplasmic regions of the T-cell adhesion molecule CD28 participate in phosphatidylinositol 3-kinase association. J. Biol. Chem. 271:9403–9409. [DOI] [PubMed] [Google Scholar]
- 10.Stein, P.H., J.D. Fraser, and A. Weiss. 1994. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol. Cell. Biol. 14:3392–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, H.-H., M. Tharayil, and C.E. Rudd. 1998. Growth factor receptor-boud protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J. Biol. Chem. 273:296–301. [DOI] [PubMed] [Google Scholar]
- 12.zur Hausen, J.D., P. Burn, and K.E. Amrein. 1997. Co-localization of Fyn with CD3 complex, CD45 or CD28 depends on different mechanisms. Eur. J. Immunol. 27:2643–2649. [DOI] [PubMed] [Google Scholar]
- 13.Andres, P.G., K.C. Howland, A. Nirula, L.P. Kane, L. Barron, D. Dresnek, A. Sadra, J. Imboden, A. Weiss, and A.K. Abbas. 2004. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat. Immunol. 5:435–442. [DOI] [PubMed] [Google Scholar]
- 14.Burr, J.S., N.D.L. Savage, G.E. Messah, S.L. Kimzey, A.S. Shaw, R.H. Arch, and J.M. Green. 2001. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 166:5331–5335. [DOI] [PubMed] [Google Scholar]
- 15.Holdorf, A.D., J.M. Green, S.D. Levin, M.F. Denny, D.B. Straus, V. Link, P.S. Changelian, P.M. Allen, and A.S. Shaw. 1999. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 190:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai, X., M. Cowan, L. Feigenbaum, and A. Singer. 2005. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 6:152–162. [DOI] [PubMed] [Google Scholar]
- 17.Tremml, G., C. Dominguez, V. Rosti, Z. Zhang, P.P. Pandolfi, P. Keller, and M. Bessler. 1999. Increased sensitivity to complement and a decreased red blood cell life span in mice mosaic for a nonfunctional Piga gene. Blood. 94:2945–2954. [PubMed] [Google Scholar]
- 18.Lakso, M., J.G. Pichel, J.R. Gorman, B. Sauer, Y. Okamoto, E. Lee, F.W. Alt, and H. Westphal. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA. 93:5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gmünder, H., and W. Lesslauer. 1984. A 45-kDa human T-cell membrane glycoprotein functions in the regulation of cell proliferative responses. Eur. J. Biochem. 142:153–160. [DOI] [PubMed] [Google Scholar]
- 20.Boise, L.H., A.J. Minn, P.J. Noel, C.H. June, M.A. Accavitti, T. Lindsten, and C.B. Thompson. 1995. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 3:87–98. [DOI] [PubMed] [Google Scholar]
- 21.Dahl, A.M., C. Klein, P.G. Andres, C.A. London, M.P. Lodge, R.C. Mulligan, and A.K. Abbas. 2000. Expression of Bcl-XL restores cell survival, but not proliferation and effector differentiation, in CD28-deficient T lymphocytes. J. Exp. Med. 191:2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, R.G., M. Parsons, M. Bonnard, V.S. Chan, W.C. Yeh, J.R. Woodgett, and P.S. Ohashi. 2000. Protein kinase B regulates T lymphocyte survival, nuclear factor κB activation, and Bcl-X(L) levels in vivo. J. Exp. Med. 191:1721–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okkenhaug, K., L. Wu, K.M. Garza, J. La Rose, W. Khoo, B. Odermatt, T.W. Mak, P.S. Ohashi, and R. Rottapel. 2001. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2:325–332. [DOI] [PubMed] [Google Scholar]
- 24.Parry, R.V., C.A. Rumbley, L.H. Vandenberghe, C.H. June, and J.L. Riley. 2003. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J. Immunol. 171:166–174. [DOI] [PubMed] [Google Scholar]
- 25.Kung, T.T., H. Jones, G.K. Adams III, S.P. Umland, W. Kreutner, R.W. Egan, R.W. Chapman, and A.S. Watnick. 1994. Characterization of a murine model of allergic pulmonary inflammation. Int. Arch. Allergy Immunol. 105:83–90. [DOI] [PubMed] [Google Scholar]
- 26.Harris, N., C. Campbell, G. Le Gros, and F. Ronchese. 1997. Blockade of CD28/B7 co-stimulation by mCTLA4-Hγ1 inhibits antigen-induced lung eosinophilia but not Th2 cell development or recruitment in the lung. Eur. J. Immunol. 27:155–161. [DOI] [PubMed] [Google Scholar]
- 27.Keane-Myers, A., W.C. Gause, P.S. Linsley, S.J. Chen, and M. Wills-Karp. 1997. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J. Immunol. 158:2042–2049. [PubMed] [Google Scholar]
- 28.Burr, J.S., S.L. Kimzey, D.R. Randolph, and J.M. Green. 2001. CD28 and CTLA4 coordinately regulate airway inflammatory cell recruitment and T-helper cell differentiation after inhaled allergen. Am. J. Respir. Cell Mol. Biol. 24:563–568. [DOI] [PubMed] [Google Scholar]
- 29.Moretta, A., G. Pantaleo, M. Lopez-Botet, and L. Moretta. 1985. Involvement of T44 molecules in an antigen-independent pathway of T cell activation. Analysis of the correlations to the T cell antigen–receptor complex. J. Exp. Med. 162:823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frauwirth, K.A., J.L. Riley, M.H. Harris, R.V. Parry, J.C. Rathmell, D.R. Plas, R.L. Elstrom, C.H. June, and C.B. Thompson. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity. 16:769–777. [DOI] [PubMed] [Google Scholar]
- 31.Boise, L.H., P.J. Noel, and C.B. Thompson. 1995. CD28 and apoptosis. Curr. Opin. Immunol. 7:620–625. [DOI] [PubMed] [Google Scholar]
- 32.Marengere, L.E., K. Okkenhaug, A. Clavreul, D. Couez, S. Gibson, G.B. Mills, T.W. Mak, and R. Rottapel. 1997. The SH3 domain of Itk/Emt binds to proline-rich sequences in the cytoplasmic domain of the T cell costimulatory receptor CD28. J. Immunol. 159:3220–3229. [PubMed] [Google Scholar]
- 33.Okkenhaug, K., and R. Rottapel. 1998. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J. Biol. Chem. 273:21194–21202. [DOI] [PubMed] [Google Scholar]
- 34.Carey, K.D., T.J. Dillon, J.M. Schmitt, A.M. Baird, A.D. Holdorf, D.B. Straus, A.S. Shaw, and P.J. Stork. 2000. CD28 and the tyrosine kinase lck stimulate mitogen-activated protein kinase activity in T cells via inhibition of the small G protein Rap1. Mol. Cell. Biol. 20:8409–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barz, C., T. Nagel, K.E. Truitt, and J.B. Imboden. 1998. Mutational analysis of CD28-mediated costimulation of Jun-N-terminal kinase and IL-2 production. J. Immunol. 161:5366–5372. [PubMed] [Google Scholar]
- 36.Nagel, T., J.R. Kalden, and B. Manger. 2000. Co-stimulation of IL-2 production by CD28 is independent of tyrosine-based signaling motifs in a murine T cell hybridoma. Eur. J. Immunol. 30:1632–1637. [DOI] [PubMed] [Google Scholar]
- 37.Truitt, K.E., T. Nagel, L.F. Suen, and J.B. Imboden. 1996. Structural requirements for CD28-mediated costimulation of IL-2 production in Jurkat T cells. J. Immunol. 156:4539–4541. [PubMed] [Google Scholar]
- 38.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 283:680–682. [DOI] [PubMed] [Google Scholar]
- 39.Collins, A.V., D.W. Brodie, R.J. Gilbert, A. Iaboni, R. Manso-Sancho, B. Walse, D.I. Stuart, P.A. van der Merwe, and S.J. Davis. 2002. The interaction properties of costimulatory molecules revisited. Immunity. 17:201–210. [DOI] [PubMed] [Google Scholar]
- 40.Evans, E.J., R.M. Esnouf, R. Manso-Sancho, R.J. Gilbert, J.R. James, C. Yu, J.A. Fennelly, C. Vowles, T. Hanke, B. Walse, et al. 2005. Crystal structure of a soluble CD28-Fab complex. Nat. Immunol. 6:271–279. [DOI] [PubMed] [Google Scholar]
- 41.King, P.D., A. Sadra, J.M. Teng, R.L. Xiao, A. Han, A. Selvakumar, A. August, and B. Dupont. 1997. Analysis of CD28 cytoplasmic tail tyrosine residues as regulators and substrates for the protein tyrosine kinases, EMT and LCK. J. Immunol. 158:580–590. [PubMed] [Google Scholar]
- 42.Sanchez-Lockhart, M., E. Marin, B. Graf, R. Abe, Y. Harada, C.E. Sedwick, and J. Miller. 2004. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J. Immunol. 173:7120–7124. [DOI] [PubMed] [Google Scholar]
- 43.Harris, N., R. Peach, J. Naemura, P.S. Linsley, G. Le Gros, and F. Ronchese. 1997. CD80 costimulation is essential for the induction of airway eosinophilia. J. Exp. Med. 185:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogan, S.P., A. Mould, H. Kikutani, A.J. Ramsay, and P.S. Foster. 1997. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J. Clin. Invest. 99:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopf, M., G. Le Gros, M. Bachmann, M.C. Lamers, H. Bluethmann, and G. Kohler. 1993. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 362:245–248. [DOI] [PubMed] [Google Scholar]
- 46.Pascual, M., G. Steiger, S. Sadallah, J.P. Paccaud, J.L. Carpentier, R. James, and J.A. Schifferli. 1994. Identification of membrane-bound CR1 (CD35) in human urine: evidence for its release by glomerular podocytes. J. Exp. Med. 179:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson, S.E., S. Han, G. Kelsoe, and C.B. Thompson. 1996. CD28 is required for germinal center formation. J. Immunol. 156:4576–4581. [PubMed] [Google Scholar]
- 48.Ding, L., J.M. Green, C.B. Thompson, and E.M. Shevach. 1995. B7/CD28-dependent and -independent induction of CD40 ligand expression. J. Immunol. 155:5124–5132. [PubMed] [Google Scholar]
- 49.Roy, M., A. Aruffo, J. Ledbetter, P. Linsley, M. Kehry, and R. Noelle. 1995. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 25:596–603. [DOI] [PubMed] [Google Scholar]
- 50.Reiter, R., and K. Pfeffer. 2002. Impaired germinal centre formation and humoral immune response in the absence of CD28 and interleukin-4. Immunology. 106:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker, L.S., A. Gulbranson-Judge, S. Flynn, T. Brocker, C. Raykundalia, M. Goodall, R. Forster, M. Lipp, and P. Lane. 1999. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker, L.S., A. Gulbranson-Judge, S. Flynn, T. Brocker, and P.J. Lane. 2000. Co-stimulation and selection for T-cell help for germinal centres: the role of CD28 and OX40. Immunol. Today. 21:333–337. [DOI] [PubMed] [Google Scholar]
- 53.Kim, C.H., L.S. Rott, I. Clark-Lewis, D.J. Campbell, L. Wu, and E.C. Butcher. 2001. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center–localized subset of CXCR5+ T cells. J. Exp. Med. 193:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimzey, S.L., P. Liu, and J.M. Green. 2004. Requirement for CD28 in the effector phase of allergic airway inflammation. J. Immunol. 173:632–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.