Abstract
Sympathetic neurons synthesize and release tissue plasminogen activator (t-PA). We investigated whether t-PA modulates sympathetic activity. t-PA inhibition markedly reduced contraction of the guinea pig vas deferens to electrical field stimulation (EFS) and norepinephrine (NE) exocytosis from cardiac synaptosomes. Recombinant t-PA (rt-PA) induced exocytotic and carrier-mediated NE release from cardiac synaptosomes and cultured neuroblastoma cells; this was a plasmin-independent effect but was potentiated by a fibrinogen cleavage product. Notably, hearts from t-PA–null mice released much less NE upon EFS than their wild-type (WT) controls (i.e., a 76.5% decrease; P < 0.01), whereas hearts from plasminogen activator inhibitor-1 (PAI-1)–null mice released much more NE (i.e., a 275% increase; P < 0.05). Furthermore, vasa deferentia from t-PA–null mice were hyporesponsive to EFS (P < 0.0001) but were normalized by the addition of rt-PA. In contrast, vasa from PAI-1–null mice were much more responsive (P < 0.05). Coronary NE overflow from hearts subjected to ischemia/reperfusion was much smaller in t-PA–null than in WT control mice (P < 0.01). Furthermore, reperfusion arrhythmias were significantly reduced (P < 0.05) in t-PA–null hearts. Thus, t-PA enhances NE release from sympathetic nerves and contributes to cardiac arrhythmias in ischemia/reperfusion. Because the risk of arrhythmias and sudden cardiac death is increased in hyperadrenergic conditions, targeting the NE-releasing effect of t-PA may have valuable therapeutic potential.
Tissue plasminogen activator (t-PA) is a key component of the cardiovascular fibrinolytic system. In basal conditions, t-PA is constitutively released from endothelial cells. Upon appropriate stimulation, substantial amounts of t-PA can be rapidly released, resulting in a marked increase in fibrinolysis (1–4). The local presence of fibrin increases the rather slow catalytic activity of t-PA by up to three orders of magnitude, leading to immediate plasminogen activation (5–7). The activity of t-PA in plasma is regulated by specific inhibitors. Of these, plasminogen activator inhibitor-1 (PAI-1) is considered to be the main inhibitor of t-PA in the vascular compartment (8). Additionally, α2-antiplasmin inhibits plasmin (the principle proteolytic enzyme) as long as plasmin is unoccupied by fibrin, thereby counteracting overwhelming systemic fibrinolytic activity (9).
The acute release of t-PA is crucial for the prevention of intravascular fibrin deposits. Until recently, t-PA was thought to originate solely from endothelial cells (2), but it has now been established that vascular sympathetic neurons can synthesize, transport, store, and release t-PA (3, 10–12). Vascular t-PA expression is known to vary regionally and according to vessel size. In general, dense sympathetic innervation seems to correlate with high t-PA expression as judged by immunohistochemistry (4, 11), and the importance of neuronal t-PA is suggested by a 70% reduction in blood t-PA activity after chemical sympathectomy (10). Moreover, sympathetic activation (e.g., physical and mental stress) is known to provoke an acute release of t-PA into the circulation (13, 14). Recently, electrical sympathetic stimulation of the sympathetic cervical ganglion (15) as well as coronary ligation (16) were shown to induce a dramatic increase in coronary t-PA release.
t-PA is abundantly expressed in adrenal chromaffin and pheochromocytoma PC-12 cells (17–21). In PC-12 cells, t-PA antigen is mainly localized in catecholamine storage vesicles (30-fold enrichment; references 19, 20). Stimulation of PC-12 cells and primary bovine adrenal chromaffin cells with nicotine, KCl, and BaCl2 results in a prominent corelease of catecholamines and t-PA (19, 20). Accordingly, sympathoadrenal activation may be an important physiologic mechanism for the rapid release of t-PA.
Other than fibrinolysis, the functional significance of the release of t-PA is presently not fully understood, but an involvement in extracellular matrix degradation, angiogenesis, vascular remodeling, and even plaque rupture has been proposed (22–24). In the brain, this serine protease has been shown to play diverse roles in addition to its thrombolytic activity and participate in long-term potentiation, excitotoxicity, and postischemic neurodegeneration (25).
The aim of this study was to investigate whether t-PA is involved in sympathetic neuronal function and transmitter release. We report that t-PA participates in peripheral sympathetic transmission by promoting norepinephrine (NE) release from sympathetic terminals and, in doing so, is likely to contribute to cardiac arrhythmias initiated by NE in ischemia/reperfusion.
RESULTS
t-PA inhibition attenuates the contractile response of guinea pig vas deferens to electrical field stimulation
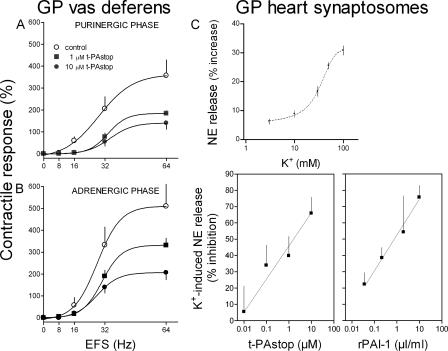
The isolated vas deferens, with its very dense sympathetic innervation, has been used extensively as a model to investigate drug effects at pre- and postsynaptic sites of the sympathetic junction (26, 27). As a first assessment of a possible role of t-PA in sympathetic transmission, we investigated whether the antagonism of t-PA with its synthetic inhibitor 2,7-Bis-(4-amidinobenzylidene)-cycloheptan-1-one dihydrochloride, t-PAstop (28), would affect the response of the vas deferens to sympathetic nerve stimulation. Sympathetic nerves were stimulated by electrical field stimulation (EFS; 0–64 Hz, supramaximal voltage; stimulation duration of 15 s at 5-min intervals, with pulses lasting 1 ms each). We first ascertained that the typical biphasic contractile response to EFS (i.e., purinergic and adrenergic phases) was completely blocked by 10 μM of the P2X antagonist PPADS (29) and by 1 μM of the α1-adrenoceptor antagonist prazosin (30). This excluded the possibility that EFS might directly elicit smooth muscle contraction rather than via the released neurotransmitters ATP and NE (unpublished data). EFS (8–64 Hz) induced a frequency-dependent increase in both purinergic and adrenergic twitch responses. t-PAstop inhibited each phase as a function of its concentration (1 and 10 μM; Fig. 1, A and B).
Figure 1.
t-PA inhibition attenuates sympathetic responses in the vas deferens and decreases NE exocytosis in cardiac synaptosomes. (A and B) Frequency response curves for the contractile responses of the isolated guinea pig (GP) vas deferens to electrical field stimulation (EFS; 0–64 Hz, supramaximal voltage; stimulation duration of 15 s at 5-min intervals, with pulses lasting 1 ms each). Typically, the contractile response of the vas deferens to EFS consists of two phases: an initial spike (A; purinergic) followed by a plateau (B; adrenergic). Peak response amplitudes (means ± SE [error bars]; n = 4) are expressed as percentages of the response to 40 mM K+. Responses were recorded either in the absence or presence of 1 and 10 μM tPAstop. (C, top) Release of endogenous NE from guinea pig heart synaptosomes by depolarization with 3–100 mM K+. Points are mean increases in NE release above basal level (± SE; n = 10; EC50 = 32.8 mM). (bottom) Concentration response curves for the inhibition of NE exocytosis (elicited by depolarization with 100 mM K+) by t-PAstop or rPAI-1. Equieffective inhibitory concentrations of t-PAstop and rPAI-1 were predetermined in a photometric assay of t-PA activity inhibition (IC50, 1.2 μM tPAstop and 9.2 nM rPAI-1; not depicted). Points (means ± SE; n = 4) are expressed as the percent inhibition of NE release by 100 mM K+.
t-PA inhibition attenuates NE exocytosis in guinea pig heart synaptosomes
Because the aforementioned experiments in the vas deferens suggested that t-PA inhibition might lead to a decrease in neurotransmitter release from sympathetic nerves, we tested this possibility in a model of isolated sympathetic nerve endings (i.e., cardiac synaptosomes; Fig. 1 C). We had previously shown that these synaptosomes release NE by exocytosis when depolarized with K+ (31). Increasing K+ concentrations (3–100 mM) progressively increased NE release from ∼5 to ∼30% above basal level. t-PAstop inhibited as a function of its concentration the NE release elicited by 100 mM K+. At concentrations of 0.01–10 μM, t-PAstop-induced inhibition increased from a minimum of ∼5% to a maximum of ∼65%. Furthermore, the t-PA inhibitor recombinant PAI-1 (rPAI-1) also inhibited as a function of its concentration the NE release elicited by 100 mM K+. At concentrations of 4 pM to 40 nM, the rPAI-1–induced inhibition increased from a minimum of ∼8% to a maximum of ∼75%.
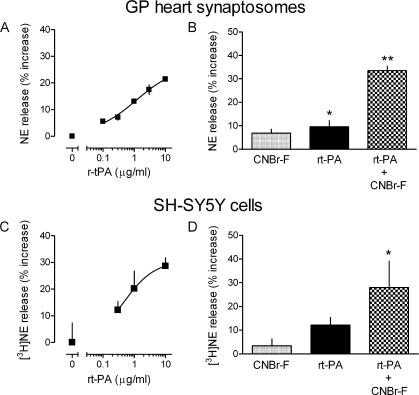
The administration of recombinant t-PA elicits NE release in guinea pig heart synaptosomes and neuroblastoma cells
Because the inhibition of t-PA activity was associated with a marked inhibition of sympathetic function and NE exocytosis (Fig. 1), it was possible that an increase in t-PA availability would yield the opposite effect. To address this possibility, we incubated guinea pig heart synaptosomes with 0.1–10 μg/ml of recombinant t-PA (rt-PA). rt-PA elicited a dose-dependent increase in the release of NE (EC50 = 1.17 ± 0.15 μg/ml; Fig. 2 A). In a second set of experiments, we preincubated cardiac synaptosomes with a well-known activator of t-PA, cyanogen bromide–digested fibrinogen (CNBr-F; reference 32). Preincubation with 200 μg/ml CNBr-F greatly enhanced t-PA–induced NE release in cardiac synaptosomes. The amount of NE released by r-tPA (1 μg/ml) increased ∼3.5-fold when synaptosomes were preincubated with CNBr-F (Fig. 2 B).
Figure 2.
rt-PA elicits NE release in guinea pig heart synaptosomes and neuroblastoma cells: potentiation by fibrinogen. (A and C) Concentration response curves for the NE-releasing effects of rt-PA in guinea pig heart synaptosomes (endogenous NE) and human SH-SY5Y neuroblastoma cells ([3H]NE). Points are means (± SE [error bars]; n = 8–14) of percent increases in NE release above baseline. (B and D) Coincubation of rt-PA with 200 μg/ml of the fibrinogen digest CNBr-F significantly potentiates the NE-releasing effect of 1 (B) and 0.3 μg/ml (D) rt-PA in guinea pig heart synaptosomes and SH-SY5Y neuroblastoma cells. Bars are means (± SE; n = 4–8) of increases in NE release above baseline. *, P < 0.05 versus baseline; **, P < 0.01 versus baseline.
We subsequently extended this investigation to the neuroblastoma cell line SH-SY5Y, which is an ideal model of the sympathetic neuron (33). rt-PA induced [3H]NE release in a concentration range similar to what was effective in cardiac synaptosomes (Fig. 2 C). Furthermore, CNBr-F potentiated the NE-releasing effect of t-PA in SH-SY5Y cells to an extent similar to that observed in cardiac synaptosomes (Fig. 2 D).
Mechanisms of rt-PA–induced NE release in guinea pig heart synaptosomes
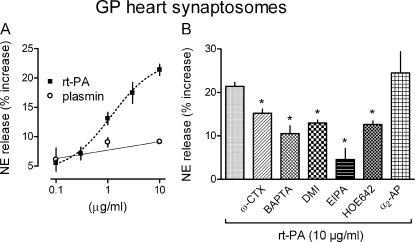
Next, we explored the mechanisms of t-PA–induced NE release. Initially, we questioned whether the release of NE by t-PA is a primary effect or secondary to plasmin formation. For this, we first determined whether plasmin has the capacity of releasing NE. At concentrations of 0.1–10 μg/ml, plasmin failed to elicit NE release from guinea pig heart synaptosomes, whereas in the same concentration range, rt-PA caused an ∼5–22% increase in NE release above basal level (i.e., 72% of the release obtained with 100 mM K+; Fig. 3 A). To further test whether the NE-releasing effect of t-PA is independent of the generation of plasmin, we preincubated cardiac synaptosomes with 0.2 μM of the plasmin inhibitor α2-antiplasmin (8) and exposed them to the highest effective dose of rt-PA (10 μg/ml). α2-Antiplasmin did not affect the rt-PA–induced NE release (Fig. 3 B).
Figure 3.
Mechanisms of rt-PA–induced NE release in guinea pig heart synaptosomes. (A) Concentration response curves for the NE-releasing effect of rt-PA and plasmin (each at 0.1–10 μg/ml) in guinea pig (GP) heart synaptosomes. As opposed to rt-PA, plasmin did not elicit NE release. Points are means (± SE [error bars]; n = 10 for r-tPA and n = 4 for plasmin). (B) Effects of various inhibitors on the NE-releasing effect of 10 μg/ml r-tPA. Preincubation with the inhibitors reduced the rt-PA–induced NE release from guinea pig heart synaptosomes. 100 nM Ω-conotoxin (ω-CTX), 10 μM BAPTA-AM, 300 nM desipramine (DMI), 30 μM 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), and 1 μM cariporide (HOE642) each significantly inhibited rt-PA–induced NE release. In contrast, 0.2 μM α2-antiplasmin (α2-AP) did not affect the NE-releasing effect of rt-PA. Bars are means (± SE; n = 3–12) of percent increases in NE release above basal level. *, P < 0.05 versus rt-PA alone.
We next investigated whether the NE-releasing effect of t-PA is a Ca2+-dependent exocytotic process. Guinea pig heart synaptosomes were incubated with 100 nM of the N-type Ca2+ channel blocker ω-conotoxin (34) and were challenged with 10 μg/ml rt-PA. ω-Conotoxin inhibited rt-PA–induced NE release by ∼30% (Fig. 3 B). Pretreatment of cardiac synaptosomes with 10 μM of the intracellular Ca2+ chelator BAPTA-AM (35) also markedly reduced (by ∼50%) the rt-PA–induced NE release. This suggested that t-PA may release NE by an exocytotic mechanism that is dependent both on the influx of extracellular Ca2+ and an increase in intracellular Ca2+. However, the NE-releasing effect of 10 μg/ml rt-PA was also attenuated (by ∼40%) when cardiac synaptosomes were preincubated with 300 nM of the NE transporter inhibitor desipramine (36) and with each of two Na+/H+ exchange inhibitors, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA; 30 μM; ∼80% inhibition) and cariporide (compound HOE642; 1 μM; ∼40% inhibition; reference 36). These findings suggested that in addition to eliciting NE exocytosis, t-PA is also likely to elicit NE release via the NE transporter operating in an outward direction (37).
t-PA gene deletion attenuates and PAI-1 gene deletion potentiates the contractile response of mouse vas deferens to EFS
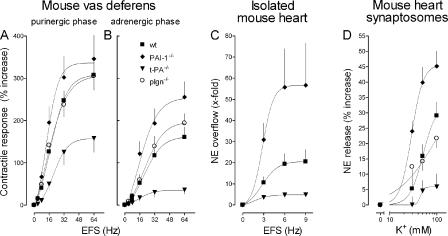
Because our findings in guinea pig preparations and the neuroblastoma cell line indicated a role for t-PA in sympathetic nerve activity, we further tested this possibility in mice lacking either the t-PA (t-PA−/−) or PAI-1 gene (PAI-1−/−). The contractile responses of vasa deferentia isolated from control mice (WT) to EFS had both purinergic and adrenergic phases that were characterized by a progressive increase in tension with increasing frequencies of stimulation (4–64 Hz). The contractile responses to EFS were greatly diminished in vasa isolated from t-PA−/− mice (P < 0.0001 vs. WT; purinergic and adrenergic phases); in contrast, there were greater increases in tension in response to EFS in vasa isolated from PAI-1−/− mice (P < 0.05 vs. WT; purinergic phase; Fig. 4, A and B). Notably, in vasa deferentia from plasminogen-null mice, the contractile responses to EFS were not different from WT controls (Fig. 4, A and B), supporting the notion that the modulatory function of t-PA at sympathetic junctions is independent of plasminogen availability.
Figure 4.
Vas deferens contraction and NE exocytosis in the heart are both attenuated in t-PA–null mice, potentiated in mice with PAI-1 gene deletion, and unaffected in plasminogen-deficient mice. (A and B) Frequency response curves for the contractile responses of the isolated mouse vas deferens to electrical field stimulation (EFS; 0–64 Hz, supramaximal voltage; every 1 ms for 15 s). Peak response amplitudes of both purinergic and adrenergic phases (means ± SE [error bars]; n = 6–9) are expressed as percentages of the response to 80 mM K+. Vasa deferentia isolated from t-PA−/− mice developed markedly less tension in response to EFS than vasa from WT control mice. In contrast, vasa from PAI-1−/− mice developed more tension than vasa from WT mice. Vasa from plasminogen−/− mice developed the same tension as vasa from WT mice. (C) Coronary NE overflow from isolated mouse hearts in response to EFS (0–9 Hz; 5 V for a duration of 60 s, with pulses of 2 ms each). Hearts were perfused with buffer containing 0.1 μM desipramine, 0.1 μM rauwolscine, 1 μM atropine, and 10 μM hydrocortisone. NE overflow was significantly smaller in hearts from t-PA−/− mice than in hearts from WT mice, whereas it was significantly greater in PAI-1−/− hearts. Points are means (± SE; n = 6–9) of x-fold increases in NE overflow above basal levels. (D) K+-induced NE exocytosis in mouse heart synaptosomes. Points are means (± SE; n = 12–16) of increases in NE release above basal levels. Synaptosomes isolated from hearts of t-PA−/− mice released significantly less NE in response to K+ than synaptosomes from WT hearts. In contrast, synaptosomes from PAI-1−/− hearts released greater amounts of NE than synaptosomes from WT hearts, whereas NE exocytosis in synaptosomes from plasminogen−/− mice was not different from that of synaptosomes from WT hearts.
t-PA gene deletion attenuates and PAI-1 gene deletion potentiates NE exocytosis in the mouse heart
In isolated Langendorff-perfused mouse hearts, EFS (3–9 Hz) elicited a frequency-dependent increase in NE overflow over baseline (Fig. 4 C). NE overflow was greatly diminished in hearts isolated from t-PA−/− mice as compared with WT control hearts (a 76.5% decrease; P < 0.01). In contrast, NE overflow was markedly increased in hearts isolated from PAI-1−/− mice (a 275% increase over WT; P < 0.05; Fig. 4 C).
Because the experiments with Langendorff-perfused mouse hearts suggested that the absence of t-PA leads to a decrease in NE exocytosis from sympathetic nerves, we extended our observations to the cardiac synaptosome model. Depolarization of mouse heart synaptosomes with increasing K+ concentrations (30, 50, and 100 mM) resulted in a concentration-dependent increase in NE release (Fig. 4 D). NE release was greatly reduced in synaptosomes isolated from t-PA−/− mouse hearts but were markedly enhanced in PAI-1−/− synaptosomes (Fig. 4 D). K+-induced NE release in synaptosomes isolated from plasminogen−/− mice was not different from that of their WT controls (Fig. 4 D).
Similar to NE, K+-induced (100 mM) ATP release (assessed with a luciferin-luciferase assay; reference 38) was 4.4-fold (P < 0.01) greater in synaptosomes from WT mice than in those from t-PA−/− mice. Furthermore, 10 μM t-PAstop markedly reduced (by 45.8%; P < 0.05) the K+-induced ATP release in synaptosomes from WT mice.
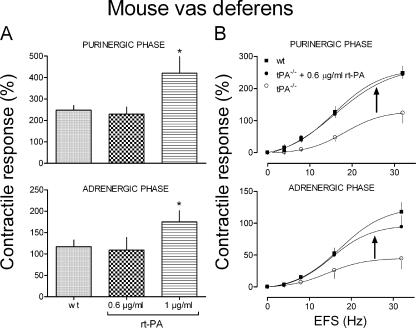
The administration of rt-PA restores the contractile response of vasa deferentia isolated from mice lacking t-PA
We questioned whether supplying exogenous t-PA to a t-PA–depleted system would restore functional responses to normality. Accordingly, we isolated vasa deferentia from t-PA−/− mice and incubated them with rt-PA. The contractile response of vasa deferentia isolated from WT mice and subjected to EFS (30 Hz) was measured in the absence or presence of 0.6 and 1 μg/ml rt-PA. In the presence of 1 μg/ml rt-PA, both purinergic and adrenergic responses to EFS were significantly increased (P < 0.05), whereas the 0.6-μg/ml concentration was without effect (Fig. 5 A). Notably, when this subthreshold concentration of 0.6 μg/ml rt-PA was added to vasa deferentia isolated from t-PA−/− mice, both purinergic and adrenergic contractile responses were restored to the levels of WT controls (Fig. 5 B).
Figure 5.
The administration of rt-PA restores the contractile response of vasa deferentia isolated from mice lacking t-PA. (A) Contractile responses (both purinergic and adrenergic) of vasa deferentia isolated from WT mice to EFS (30 Hz, supramaximal voltage; duration of 15 s at 5-min intervals, with pulses of 1 ms) either in the absence or presence of 0.6 and 1 μg/ml rt-PA (15-min incubation). The 0.6-μg/ml concentration was ineffective, whereas at 1 μg/ml, rt-PA potentiated both the purinergic and adrenergic responses by ∼70 and ∼50%, respectively (*, P < 0.05). Bars represent the mean contractile response amplitude, which is expressed as a percentage of the response to 80 mM K+ (± SE [error bars]; n = 4–8). (B) Frequency response curves for the contractile response of vasa deferentia to EFS (0–32 Hz, supramaximal voltage; for 1 ms every 15 s). Vasa were isolated from t-PA−/− mice and from their WT controls. Incubation of vasa from t-PA−/− mice with rt-PA (at the subthreshold concentration of 0.6 μg/ml for 15 min) restored the depressed contractile response to EFS to the same magnitude as in vasa from control mice. Points are means (± SE; n = 8 and 4 for WT and t-PA−/−, respectively) of maximal contractile amplitudes expressed as percentages of the response to 80 mM K+. Arrows indicate the upward shifts elicited by the administration of rt-PA in vasa deferentia isolated from animals deprived of t-PA.
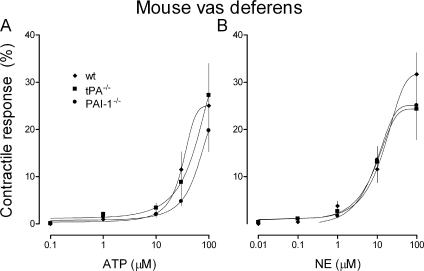
The potentiation of sympathetic responses by t-PA is not caused by an action at postjunctional sites
Because the contractile response of the vas deferens to EFS is a composite of pre- and postjunctional events (i.e., neurotransmitter release and postsynaptic effects), we questioned whether t-PA might potentiate sympathetic responses in part by an action at postjunctional sites. Therefore, we assessed whether the postsynaptic effects of sympathetic neurotransmitters (i.e., ATP and NE) would be affected by changes in endogenous t-PA availability. Mouse vasa deferentia were incubated in increasing concentrations of exogenous ATP or NE, and the contractile response was measured. The concentration response curves for the effects of ATP and NE in vasa from t-PA−/− and PAI-1−/− mice were superimposable on the curve obtained from vasa of WT control mice (Fig. 6, A and B). These observations indicated that t-PA modulates sympathetic responses by acting solely at prejunctional sites.
Figure 6.
t-PA potentiates sympathetic responses by an action at prejunctional sites. (A) Noncumulative concentration response curves for the contractile response of mouse vas deferens to the administration of exogenous ATP. Vasa deferentia were isolated from t-PA−/−, PAI-1−/−, and their WT control mice. The maximum contractile response for each increment in ATP concentration occurred within 30 s; ATP was quickly washed out thereafter to prevent receptor desensitization. The following higher ATP concentration was added after 30 min of reequilibration. (B) Cumulative concentration response curves for the contractile response of mouse vas deferens to the administration of exogenous NE. Vasa deferentia were isolated from t-PA−/−, PAI-1−/−, and their WT control mice. The finding that the concentration response curves obtained from vasa deferentia of gene-deleted mice were superimposable on the curves obtained from their WT controls indicates that the lack of t-PA or PAI-1 does not influence postsynaptic responses at sympathetic junctions and that the action of t-PA on sympathetic neurons is limited to presynaptic sites. (A and B) Points are means (± SE [error bars]; n = 7–10) of maximal contractile responses to ATP (A) and NE (B) expressed as percentages of the response to 80 mM K+.
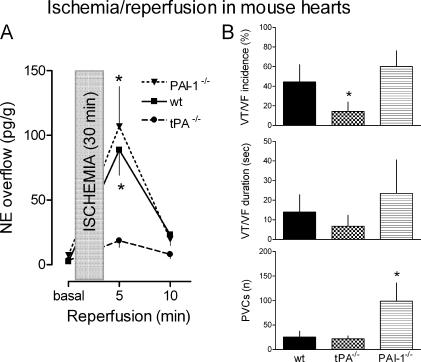
t-PA promotes NE release and associated arrhythmias in myocardial ischemia/reperfusion
Langendorff-perfused mouse hearts were subjected ex vivo to 30-min stop-flow global ischemia followed by 30-min reperfusion. Hearts isolated from WT mice released ∼90 pg NE/gram of tissue in the first 5 min of reperfusion (Fig. 7 A). NE overflow subsided in the following 5 min. In hearts isolated from PAI-1−/− mice, NE overflow was slightly but not significantly greater than that of control hearts. In contrast, in hearts isolated from t-PA−/− mice, NE overflow during reperfusion was approximately fivefold lower than that of WT control and PAI-1−/− mouse hearts. Preischemia NE overflow was comparable in all hearts. Reperfusion was also associated with the development of ventricular arrhythmias such as ventricular tachycardia (VT), ventricular fibrillation (VF), and premature ventricular contractions (PVCs). The lowest incidence and shortest duration of rhythm disturbances was observed in hearts from t-PA−/− mice (Fig. 7 B). In contrast, PAI-1−/− mouse hearts had the highest number of PVCs. These findings suggested that t-PA may contribute to NE-initiated arrhythmias in ischemia and reperfusion.
Figure 7.
t-PA promotes NE release and associated arrhythmias in myocardial ischemia/reperfusion. (A) Coronary NE overflow before ischemia (basal) and during 10-min reperfusion in hearts isolated from t-PA−/−and PAI-1−/− mice and their WT controls. Global stop-flow ischemia was applied for 30 min after an initial stabilization period of 30 min. NE overflow was individually adjusted for coronary flow and heart weight. Points are means (± SE [error bars]; n = 9–11). Asterisks indicate significant differences from 5-min reperfusion levels in t-PA−/− hearts (*, P < 0.05). (B) Analysis of ventricular arrhythmias during reperfusion of the same hearts as in A. The incidence of high-grade ventricular arrhythmias (i.e., ventricular tachycardia [VT] and ventricular fibrillation [VF]) is expressed as percentages of the total number of hearts used in each of the three groups (± SE). The duration of VT and VF represents the cumulative duration of arrhythmia during the 30-min reperfusion. The occurrence of premature ventricular contractions (PVCs) is expressed as the total number (n) of PVCs counted during the 30-min reperfusion. Bars are means (± SE; n = 9–11). Asterisks indicate significant differences from WT and PAI-1−/− hearts (incidence of VT/VF) and from WT and t-PA−/− hearts (number of PVCs).
DISCUSSION
Our findings clearly demonstrate that t-PA plays an important role in peripheral sympathetic responses by promoting neurotransmitter release from sympathetic neurons via an action restricted to presynaptic terminals. As a result, in hyperadrenergic states such as myocardial ischemia/reperfusion, in which excessive NE release is a primary arrhythmogenic factor (39, 40), t-PA participates with NE in arrhythmic cardiac dysfunction.
Although endothelial cells have traditionally been viewed as a major source of t-PA (41, 42), we were intrigued by the report that the stimulation of cardiac sympathetic nerves caused the release of t-PA into the coronary circulation (15) and by the prospect that this t-PA might derive from sympathetic neurons, which can both synthesize it and release it (11, 12). It had also been shown that chemical sympathectomy decreases t-PA release from blood vessels (10) and that t-PA is stored in the same vesicular pool with NE in adrenal chromaffin and PC12 cells, from where it could be released together with NE upon depolarization (19, 20). As all of these studies favored the notion of a dual neuronal and endothelial t-PA release, we questioned what function neuronally released t-PA might have other than a plausible involvement in fibrinolysis, and we hypothesized that t-PA might modulate sympathetic neuronal activity.
We reasoned that if t-PA were to play such a role, it should be possible to uncover it with the use of t-PA inhibitors. For this, we used t-PAstop, a synthetic inhibitor of t-PA (28), and the recombinant form of the high affinity physiological serine protease inhibitor rPAI-1. In the isolated vas deferens of the guinea pig, a prototypical model of sympathetic neuromuscular junction (26, 27), t-PAstop, attenuated both the purinergic and adrenergic responses to EFS, demonstrating that a fully functional t-PA is required for a complete sympathetic response and suggesting that neuronal t-PA is likely to potentiate either the release or the effects of the sympathetic neurotransmitters. The first indication that sympathetic nerve terminals are the likely site of this potentiation emerged from finding that each of the two t-PA inhibitors, t-PAstop and rPAI-1, inhibited NE exocytosis as a function of its concentration in guinea pig heart synaptosomes, which is a typical model of sympathetic nerve endings (31).
As these results suggested that t-PA enhances the release of NE elicited by the depolarization of sympathetic nerve terminals, we sought to confirm this view by determining whether the administration of rt-PA to native nerve endings (cardiac synaptosomes) or neuroblastoma cells in culture directly enhanced NE release. We chose to use concentrations of t-PA approximating those clinically achieved for intraarterial thrombolysis (43). We not only found that t-PA indeed promotes NE release but particularly important was discovering that fibrin (CNBr-F), which is known to potentiate the plasmin-generating effect of t-PA (6, 7), also potentiated the NE-releasing effect of rt-PA. Inasmuch as the generation of plasmin is pivotal for the fibrinolytic activity of t-PA, we next assessed whether the NE-releasing effect of rt-PA might also be plasmin dependent. Clearly, it was not. Indeed, the administration of plasmin did not significantly affect basal NE release in guinea pig heart synaptosomes, and the administration of α2-antiplasmin (9) did not modify the NE-releasing effect of rt-PA. Moreover, the contractile response of the vas deferens to EFS and the NE exocytosis from cardiac synaptosomes isolated from plasminogen-deficient mice did not differ from those of WT mice, excluding a role for plasminogen and, thus, for plasmin in these responses. Accordingly, we concluded that t-PA acts directly at sympathetic nerve terminals to release NE by an action that is potentiated by fibrin (CNBR-F) but independent of plasmin formation.
Having determined that sympathetic responses are either attenuated when endogenous t-PA is inhibited or potentiated by the administration of rt-PA, we sought definitive proof of the relevance of t-PA actions in sympathetic neural function in animals lacking t-PA or PAI-1. Consistent with a t-PA–induced promotion of NE release, we found that in tissues isolated from t-PA–null mice, sympathetic responses and NE release were markedly reduced. Conversely, sympathetic responses and NE release were potentiated in tissues from the PAI-1–null mice. The notion that the lack of t-PA was indeed the cause of the depressed sympathetic responses was proven by our reconstitution experiments, demonstrating the restoration of sympathetic responses upon administration of a subthreshold amount of rt-PA in the vas deferens. Most importantly, postjunctional contractile responses elicited by the administration of the neurotransmitters ATP and NE in the vas deferens of mice lacking either t-PA or PAI-1 were indistinguishable from the responses recorded in their WT controls. This clearly excluded the possibility that t-PA might act at a postsynaptic site in its potentiation of sympathetic responses.
NE is known to be released from sympathetic neurons by two modalities: exocytosis and reversal of its transporter in an outward direction (i.e., carrier-mediated release; reference 44). The opening of N-type Ca2+ channels and activation of the Na+/H+ exchanger (NHE) at the axonal membrane are pivotal events initiating these two types of release, respectively (35). NHE activation leads to an increase in intracellular Na+, which is critical for the initiation of carrier-mediated NE release (45). Inasmuch as the inhibition of N-type Ca2+ channels with ω-conotoxin (34) and of NHE with the amiloride derivative EIPA each attenuated the NE-releasing effect of rt-PA in cardiac synaptosomes, both mechanisms of NE release are likely to play a role in the action of t-PA. Given that the intracellular Ca2+ chelator BAPTA-AM and cariporide (compound HOE642, a selective NHE-1 blocker; reference 35) also inhibited the NE-releasing effect of rt-PA, a transient increase in intracellular Ca2+ is probably involved in the activation of NHE-1 and the initiation of carrier-mediated NE release (35). A further indication that the NE transporter is outwardly bound under the influence of t-PA is suggested by the finding that the NE transporter inhibitor desipramine attenuated the NE-releasing effect of t-PA.
How t-PA might modulate Ca2+ entry and intracellular Ca2+ transient and NHE-1 activation, eventually culminating in enhanced NE release, is presently a matter of speculation. The action of t-PA could be initiated by protease–substrate reactions, such as those involved in plasmin formation (23, 43, 46). Indeed, the only known substrate of the remarkably specific t-PA in vivo is a single peptide bond (Arg560-Val561) within the proenzyme plasminogen, which is converted to plasmin (47). However, we found that the neuromodulatory action of t-PA is independent of plasmin formation. Therefore, it is conceivable that either an unknown substrate is activated by t-PA, leading to enhanced transmitter release, or a receptor protein is proteolytically activated by t-PA, thereby disinhibiting/augmenting NE release from nerve endings. In fact, our findings with t-PAstop and CNBr-F suggest that the activity level of t-PA is the major determinant of its neuromodulatory action. So far, a specific t-PA receptor has not been discovered, although different binding proteins for t-PA have been described previously (46, 48, 49). Nevertheless, it is likely that a specific substrate or receptor is implicated because the NE-releasing effect of rt-PA appears to involve mechanisms (i.e., intracellular Ca2+, NHE-1, and NE transporter) known to mediate NE exocytosis and carrier-mediated release, which are all modulated by receptor activation (37).
t-PA has been reported to overflow into the coronary effluent of isolated rat hearts during reperfusion after ischemia (50). Also, it was recently shown that myocardial ischemia elicits the release of t-PA in the coronary vasculature of the pig (16, 51). Despite the long-held belief that increased endogenous fibrinolysis is beneficial in cardiovascular disease (52), recent studies in the brain emphasize possible adverse effects of t-PA (25). Notably, t-PA is overexpressed in atherosclerotic coronary arteries (53), and elevated t-PA plasma concentrations correlate with the severity of coronary artery disease (54) and are an independent predictor of myocardial infarction (55, 56). Moreover, arrhythmias are often associated with the thrombolytic use of t-PA in the setting of myocardial infarction (57–61). In hyperadrenergic states such as myocardial ischemia and reperfusion, excessive NE release is a primary arrhythmogenic factor (37, 39, 40), and t-PA is released upon the stimulation of cardiac sympathetic nerves (15). Therefore, it is conceivable that t-PA may participate with NE in the generation of arrhythmias associated with ischemia and reperfusion. In fact, we found that ischemia/reperfusion hearts from t-PA knockout mice released significantly less NE (P < 0.05) and had fewer and briefer instances of VF than WT controls. Thus, endogenous t-PA is likely to contribute to arrhythmias linked to myocardial ischemia and infarction.
The administration of rt-PA for thrombolysis in acute myocardial infarction and stroke is still considered an important treatment option. Despite its beneficial effects on cerebral infarct size, rt-PA is believed to induce neurotoxic effects. In fact, rt-PA infusion has been shown to dramatically increase cerebral infarct size in t-PA–deficient mice, whereas infarct size was significantly smaller in untreated t-PA−/− mice than in WT controls (62). Supporting possible adverse cardiac effects of t-PA, we found that hearts from t-PA−/− mice were protected against reperfusion arrhythmias, a phenomenon probably associated with the reduced NE spillover. Moreover, we found that in therapeutically relevant concentrations, rt-PA increased NE release from cardiac synaptosomes and human neuroblastoma cells in culture as a function of its concentration.
In conclusion, we have obtained novel evidence that the endogenous plasminogen activator system plays an important role in promoting sympathetic transmitter release in the heart. This action is independent of plasmin formation. Most importantly, hearts from t-PA–null mice released much less NE and had fewer arrhythmias when subjected to ischemia/reperfusion than their WT controls. Thus, the plasminogen activator system, which is characterized by the activity level of t-PA, is involved in the excessive release of NE and associated arrhythmias in myocardial ischemia and reperfusion. Targeting this effect of t-PA may have valuable therapeutic potential not only in myocardial ischemia but also in other hyperadrenergic conditions such as heart failure and hypertension.
MATERIALS AND METHODS
Guinea pig and mouse vas deferens.
250–400-g male guinea pigs and 4–5-mo-old mice were anesthetized with CO2 and exsanguinated (approved by the Weill Medical College's Institutional Animal Care and Use Committee). Vasa deferentia (prostatic portion of guinea pigs and midsegment of mice) were suspended in a 20-ml bath containing Krebs-Henseleit (KH) solution at 37°C aerated with 95% O2 + 5% CO2. Mice deficient in PAI-1, plasminogen, or t-PA (63, 64) were provided by S. Strickland. The background of all mice was C57BL/6. Vasa were equilibrated for 60 min (resting tension of 1 g for guinea pigs and 250 mg for mice). EFS was applied for 15 s every 5 min (pulses of 1 ms at 4–64 Hz; supramaximal voltage). The first contractile phase (PPADS sensitive; purinergic) and second phase (prazosin sensitive; adrenergic) were expressed as percentages of the response to K+ (40 and 80 mM for guinea pigs and mice, respectively). Responses were stable to at least three to four consecutive stimulations. When used, drugs were preincubated for 15 min.
Guinea pig heart synaptosomes.
Male guinea pigs were killed as indicated above. Hearts were isolated and perfused at constant pressure (40 cm H2O) with oxygenated Ringer's solution at 37°C for 20 min. Hearts were minced in ice-cold 0.32 M sucrose containing 1 mM EGTA. Synaptosomes (pinched-off sympathetic nerve endings) were isolated as previously described (31) and incubated with various drugs at 37°C in the absence or presence of 0.1–10 μg/ml rt-PA. After centrifugation at 20,000 g for 20 min at 4°C, NE and protein contents were determined in the supernatant and pellet, respectively (31).
Expression of PAI-1 in Pichia pastoris.
rPAI-1 was prepared by yeast transfection according to the manufacturer (Invitrogen). PAI-1 was purified from culture supernatants by nickel-agarose chromatography; enzyme purity was determined by SDS/PAGE.
Measurement of PAI-1 activity.
Purified rPAI-1 was diluted in 50 mM Tris-imidazole buffer (300 mM NaCl, pH 8.4) and incubated with 5 μl rt-PA (0.1 mg/ml) for 15 min at room temperature, and the kinetics of substrate cleavage (2 mM pefachrome tPA; Pentapharm) were monitored spectrophotometrically at 405 nm in 96-well microtiter plates for 5 min.
Cross-reactivity between human reagents and guinea pig proteins.
Experiments were performed to ascertain that recombinant human proteins cross react with guinea pig substrates and, in particular, that rt-PA binds to guinea pig plasminogen and activates it. After purification (i.e., by lysine-Sepharose column separation), guinea pig plasminogen was converted to plasmin by human rt-PA in a concentration-dependent fashion, as demonstrated by SDS-PAGE containing lytic casein bands. This suggested cross-reactivity between human and cavian elements of the plasminogen activator system.
SH-SY5Y neuroblastoma cell line.
The human neuroblastoma cell line SH-SY5Y, which was provided by T.W. Lovenberg (Johnson and Johnson Pharmaceutical Research and Development, LLC, San Diego, CA), was maintained in a 1:1 ratio of Eagle's MEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C and 5% CO2. Cells were grown to confluence in six-well plates; [3H]NE release experiments were performed as described previously (65). [3H]NE loading was achieved with Hepes-buffered Na+ Ringer's solution (50 nM [3H]NE) at 37°C for 60 min. After three washings, 1 and 10 μg/ml rt-PA was added for 10 min at room temperature. When CNBr-F was used, rt-PA was incubated together with CNBr-F at room temperature for 5 min and subsequently added to the samples. Aliquots of the supernatant and cell lysates (after 30 min of 0.3% Triton X-100) were taken from each well and analyzed for [3H]NE content with a scintillation counter.
Perfusion of mouse hearts ex vivo.
After killing, hearts of gene-inactivated mice (tPA−/–, plasminogen−/–, and PAI-1−/–) were excised and cooled in ice-cold KH solution equilibrated with 95% O2 + 5% CO2 (66). An 18-gauge steel cannula was inserted in the aorta, and the heart was perfused at constant pressure (100 cm H2O) with KH at 37°C. Coronary flow was measured by timed collections of the effluent every 5 min. Coronary NE overflow was measured by HPLC with electrochemical detection as previously described (66). The detection limit was ∼0.05 pmol. Only hearts with an initial stable sinus rhythm were considered.
EFS.
Mouse hearts were perfused with KH containing 1 μM atropine, 10 μM hydrocortisone, 0.1 μM desipramine, and 0.1 μM rauwolscine. Two stainless steel paddles were apposed to the heart with their stimulating surface parallel to the interventricular septum. ECG was recorded online. After a 20-min stabilization, three sequential stimulations (3, 6, and 9 Hz at 5 V for a duration of 60 s, with pulses lasting 2 ms) using PowerLab/8SP (ADInstruments) were applied every 15 min. For ischemia/reperfusion, after a 30-min stabilization, mouse hearts were subjected to 30-min stop-flow normothermic global ischemia followed by 30-min reperfusion. ECG was recorded online (1-kHz recording frequency) and analyzed with PowerLab/8SP. The incidence and duration of reperfusion arrhythmias were calculated according to the Lambeth Conventions (67).
Preparation of cardiac synaptosomes.
Two hearts per mouse type were perfused for 20 min as indicated above. Both hearts were minced together in ice-cold 0.32 M sucrose. Synaptosomes were isolated as described above, depolarized with K+, and NE exocytosis was measured as previously described (66).
Drugs and chemicals.
Adenosine 5′-triphosphate (disodium salt), NE-HCl, and EIPA were purchased from Sigma-Aldrich. Plasminogen, t-PAstop, and CNBr-F were purchased from American Diagnostica, Inc., and α2-antiplasmin (human plasma) was purchased from Calbiochem. Recombinant two-chain tissue t-PA was obtained from Genentech, Inc., and pefachrome t-PA (Pefa-5037) was purchased from Pentapharm. HOE642 (cariporide) was provided by B.A. Schoelkens (Hoechst Marion Roussel, Frankfurt am Main, Germany). rPAI-1 was prepared by yeast transfection.
Statistics.
Values refer to means ± SE. One-way analysis of variance followed by a Dunnett's posttest, one-sample Student's t test, and unpaired Student's t test were performed as indicated. P < 0.05 was considered significant.
Acknowledgments
We gratefully acknowledge the help of Christina J. Mackins and Eleanor M. Tyler.
This work was supported by National Institutes of Health grants HL34215, HL73400, and HL46403 to R. Levi and grants NS35704 and NS38472 to S. Strickland.
The authors have no conflicting financial interests.
Abbreviations used: CNBr-F, cyanogen bromide–digested fibrinogen; EFS, electrical field stimulation; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; KH, Krebs-Henseleit; NE, norepinephrine; NHE, Na+/H+ exchanger; PAI-1, plasminogen activator inhibitor-1; PVC, premature ventricular contraction; rPAI-1, recombinant PAI-1; rt-PA, recombinant t-PA; t-PA, tissue plasminogen activator; VF, ventricular fibrillation; VT, ventricular tachycardia.
References
- 1.Levin, E.G., and G.J. del Zoppo. 1994. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. Am. J. Pathol. 144:855–861. [PMC free article] [PubMed] [Google Scholar]
- 2.Eijnden-Schrauwen, Y., T. Kooistra, R. E. de Vries, and J.J. Emeis. 1995. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 85:3510–3517. [PubMed] [Google Scholar]
- 3.Wang, Y., X. Jiang, A.R. Hand, C. Gilles, J. Kirk, R.E. Cone, and J. O'Rourke. 2002. Additional evidence that the sympathetic nervous system regulates the vessel wall release of tissue plasminogen activator. Blood Coagul. Fibrinolysis. 13:471–481. [DOI] [PubMed] [Google Scholar]
- 4.O'Rourke, J., X. Jiang, Z. Hao, R.E. Cone, and A.R. Hand. 2005. Distribution of sympathetic tissue plasminogen activator (tPA) to a distant microvasculature. J. Neurosci. Res. 79:727–733. [DOI] [PubMed] [Google Scholar]
- 5.Collen, D. 1999. The plasminogen (fibrinolytic) system. Thromb. Haemost. 82:259–270. [PubMed] [Google Scholar]
- 6.Yakovlev, S., E. Makogonenko, N. Kurochkina, W. Nieuwenhuizen, K. Ingham, and L. Medved. 2000. Conversion of fibrinogen to fibrin: mechanism of exposure of tPA- and plasminogen-binding sites. Biochemistry. 39:15730–15741. [DOI] [PubMed] [Google Scholar]
- 7.Medved, L., and W. Nieuwenhuizen. 2003. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb. Haemost. 89:409–419. [PubMed] [Google Scholar]
- 8.Potempa, J., E. Korzus, and J. Travis. 1994. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J. Biol. Chem. 269:15957–15960. [PubMed] [Google Scholar]
- 9.Robbie, L.A., N.A. Booth, A.M. Croll, and B. Bennett. 1993. The roles of alpha 2-antiplasmin and plasminogen activator inhibitor 1 (PAI-1) in the inhibition of clot lysis. Thromb. Haemost. 70:301–306. [PubMed] [Google Scholar]
- 10.Peng, T., X. Jiang, Y.F. Wang, A. Hand, C. Gillies, R.E. Cone, and J. O'Rourke. 1999. Sympathectomy decreases and adrenergic stimulation increases the release of tissue plasminogen activator (t-PA) from blood vessels: functional evidence for a neurologic regulation of plasmin production within vessel walls and other tissue matrices. J. Neurosci. Res. 57:680–692. [PubMed] [Google Scholar]
- 11.Jiang, X., Y. Wang, A.R. Hand, C. Gillies, R.E. Cone, J. Kirk, and J. O'Rourke. 2002. Storage and release of tissue plasminogen activator by sympathetic axons in resistance vessel walls. Microvasc. Res. 64:438–447. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, X., A.R. Hand, S. Shen, R.E. Cone, and J. O'Rourke. 2003. Enhanced tissue plasminogen activator synthesis by the sympathetic neurons that innervate aging vessels. J. Neurosci. Res. 71:567–574. [DOI] [PubMed] [Google Scholar]
- 13.Bashkov, G.V., I.Y. Sergeev, and N.A. Medvedeva. 1993. Role of sympathetic cholinergic pathway in the neurogenous control of tissue-type plasminogen activator release into the blood. Blood Coagul. Fibrinolysis. 4:993–998. [PubMed] [Google Scholar]
- 14.Jern, C., L. Selin, and S. Jern. 1994. In vivo release of tissue-type plasminogen activator across the human forearm during mental stress. Thromb. Haemost. 72:285–291. [PubMed] [Google Scholar]
- 15.Björkman, J.A., S. Jern, and C. Jern. 2003. Cardiac sympathetic nerve stimulation triggers coronary t-PA release. Arterioscler. Thromb. Vasc. Biol. 23:1091–1097. [DOI] [PubMed] [Google Scholar]
- 16.Aspelin, T., M. Eriksen, A.K. Lindgaard, T. Lyberg, and A. Ilebekk. 2005. Cardiac fibrinolytic capacity is markedly increased after brief periods of local myocardial ischemia, but declines following successive periods in anesthetized pigs. J. Thromb. Haemost. 3:1947–1954. [DOI] [PubMed] [Google Scholar]
- 17.Leprince, P., B. Rogister, P. Delree, J.M. Rigo, B. Andre, and G. Moonen. 1991. Modulation of proteolytic activity during neuritogenesis in the PC12 nerve cell: differential control of plasminogen activator and plasminogen activator inhibitor activities by nerve growth factor and dibutyryl-cyclic AMP. J. Neurochem. 57:665–674. [DOI] [PubMed] [Google Scholar]
- 18.Pittman, R.N., and A.J. DiBenedetto. 1995. PC12 cells overexpressing tissue plasminogen activator regenerate neurites to a greater extent and migrate faster than control cells in complex extracellular matrix. J. Neurochem. 64:566–575. [DOI] [PubMed] [Google Scholar]
- 19.Gualandris, A., T.E. Jones, S. Strickland, and S.E. Tsirka. 1996. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 16:2220–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmer, R.J., M. Mahata, S. Mahata, M.T. Sebald, D.T. O'Connor, and L.A. Miles. 1997. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J. Biol. Chem. 272:1976–1982. [DOI] [PubMed] [Google Scholar]
- 21.Parmer, R.J., and L.A. Miles. 1998. Targeting of tissue plasminogen activator to the regulated pathway of secretion. Trends Cardiovasc. Med. 8:306–312. [DOI] [PubMed] [Google Scholar]
- 22.Lupu, F., D.A. Heim, F. Bachmann, M. Hurni, V.V. Kakkar, and E.K. Kruithof. 1995. Plasminogen activator expression in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 15:1444–1455. [DOI] [PubMed] [Google Scholar]
- 23.Chen, Z.L., and S. Strickland. 1997. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 91:917–925. [DOI] [PubMed] [Google Scholar]
- 24.Castellino, F.J., and V.A. Ploplis. 2005. Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 93:647–654. [DOI] [PubMed] [Google Scholar]
- 25.Melchor, J.P., and S. Strickland. 2005. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost. 93:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sneddon, P. 2000. Electrophysiology of autonomic neuromuscular transmission involving ATP. J. Auton. Nerv. Syst. 81:218–224. [DOI] [PubMed] [Google Scholar]
- 27.Westfall, T.D., and D.P. Westfall. 2001. Pharmacological techniques for the in vitro study of the vas deferens. J. Pharmacol. Toxicol. Methods. 45:109–122. [DOI] [PubMed] [Google Scholar]
- 28.Renatus, M., W. Bode, R. Huber, J. Sturzebecher, and M.T. Stubbs. 1998. Structural and functional analyses of benzamidine-based inhibitors in complex with trypsin: implications for the inhibition of factor Xa, tPA, and urokinase. J. Med. Chem. 41:5445–5456. [DOI] [PubMed] [Google Scholar]
- 29.Lambrecht, G., T. Friebe, U. Grimm, U. Windscheif, E. Bungardt, C. Hildebrandt, H.G. Baumert, G. Spatz-Kumbel, and E. Mutschler. 1992. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur. J. Pharmacol. 217:217–219. [DOI] [PubMed] [Google Scholar]
- 30.Hess, H.J. 1975. Prazosin: biochemistry and structure-activity studies. Postgrad. Med. Spec No:9–17. [PubMed]
- 31.Seyedi, N., T. Win, H.M. Lander, and R. Levi. 1997. Bradykinin B2-receptor activation augments norepinephrine exocytosis from cardiac sympathetic nerve endings. Mediation by autocrine/paracrine mechanisms. Circ. Res. 81:774–784. [DOI] [PubMed] [Google Scholar]
- 32.Verheijen, J.H., E. Mullaart, G.T. Chang, C. Kluft, and G. Wijngaards. 1982. A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb. Haemost. 48:266–269. [PubMed] [Google Scholar]
- 33.Vaughan, P.F., C. Peers, and J.H. Walker. 1995. The use of the human neuroblastoma SH-SY5Y to study the effect of second messengers on noradrenaline release. Gen. Pharmacol. 26:1191–1201. [DOI] [PubMed] [Google Scholar]
- 34.Sher, E., E. Biancardi, M. Passafaro, and F. Clementi. 1991. Physiopathology of neuronal voltage-operated calcium channels. FASEB J. 5:2677–2683. [DOI] [PubMed] [Google Scholar]
- 35.Reid, A.C., C.J. Mackins, N. Seyedi, R. Levi, and R.B. Silver. 2004. Coupling of angiotensin II AT1 receptors to neuronal NHE activity and carrier-mediated norepinephrine release in myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 286:H1448–H1454. [DOI] [PubMed] [Google Scholar]
- 36.Hatta, E., K. Yasuda, and R. Levi. 1997. Activation of histamine H3-receptors inhibits carrier-mediated norepinephrine release in a human model of protracted myocardial ischemia. J. Pharmacol. Exp. Ther. 283:494–500. [PubMed] [Google Scholar]
- 37.Levi, R., and N.C.E. Smith. 2000. Histamine H3-receptors: a new frontier in myocardial ischemia. J. Pharmacol. Exp. Ther. 292:825–830. [PubMed] [Google Scholar]
- 38.Sesti, C., M. Koyama, M.J. Broekman, A.J. Marcus, and R. Levi. 2003. Ectonucleotidase in sympathetic nerve endings modulates ATP and norepinephrine exocytosis in myocardial ischemia. J. Pharmacol. Exp. Ther. 306:238–244. [DOI] [PubMed] [Google Scholar]
- 39.Podrid, P.J., T. Fuchs, and R. Candinas. 1990. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation. 82:I103–I113. [PubMed] [Google Scholar]
- 40.Selwyn, A.P., and E. Braunwald. 2001. Ischemic heart disease. In Harrison's Principles of Internal Medicine. E. Braunwald, A.S. Fauci, D.L. Kasper, S.L. Hauser, D.L. Longo, and J.L. Jameson, editors. McGraw-Hill, New York. 1399–1410.
- 41.Wall, U., C. Jern, and S. Jern. 1997. High capacity for tissue-type plasminogen activator release from vascular endothelium in vivo. J. Hypertens. 15:1641–1647. [DOI] [PubMed] [Google Scholar]
- 42.Huber, D., E.M. Cramer, J.E. Kaufmann, P. Meda, J.M. Masse, E.K. Kruithof, and U.M. Vischer. 2002. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 99:3637–3645. [DOI] [PubMed] [Google Scholar]
- 43.Cipolla, M.J., N. Lessov, W.M. Clark, and E.C. Haley Jr. 2000. Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 31:940–945. [DOI] [PubMed] [Google Scholar]
- 44.Schömig, A., T. Kurz, G. Richardt, and E. Schomig. 1988. Neuronal sodium homoeostasis and axoplasmic amine concentration determine calcium-independent noradrenaline release in normoxic and ischemic rat heart. Circ. Res. 63:214–226. [DOI] [PubMed] [Google Scholar]
- 45.Dart, A.M., and R.A. Riemersma. 1989. Effects of acidosis on anoxic and exocytotic noradrenaline release from the heart. J. Mol. Cell. Cardiol. 21:75–83. [DOI] [PubMed] [Google Scholar]
- 46.Benchenane, K., V. Berezowski, C. Ali, M. Fernandez-Monreal, J.P. Lopez-Atalaya, J. Brillault, J. Chuquet, A. Nouvelot, E.T. MacKenzie, G. Bu, et al. 2005. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 111:2241–2249. [DOI] [PubMed] [Google Scholar]
- 47.Madison, E.L., G.S. Coombs, and D.R. Corey. 1995. Substrate specificity of tissue type plasminogen activator. Characterization of the fibrin independent specificity of t-PA for plasminogen. J. Biol. Chem. 270:7558–7562. [DOI] [PubMed] [Google Scholar]
- 48.Hajjar, K.A. 1991. The endothelial cell tissue plasminogen activator receptor. Specific interaction with plasminogen. J. Biol. Chem. 266:21962–21970. [PubMed] [Google Scholar]
- 49.Fernandez-Monreal, M., J.P. Lopez-Atalaya, K. Benchenane, M. Cacquevel, F. Dulin, J.P. Le Caer, J. Rossier, A.C. Jarrige, E.T. MacKenzie, N. Colloc'h, C. Ali, and D. Vivien. 2004. Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J. Biol.Chem. 279:50850–50856. [DOI] [PubMed] [Google Scholar]
- 50.Winnerkvist, A., B. Wiman, G. Valen, and J. Vaage. 1996. Release of tissue plasminogen activator during reperfusion after different times of ischaemia in isolated, perfused rat hearts. Thromb. Res. 82:533–542. [DOI] [PubMed] [Google Scholar]
- 51.Osterlund, B., B. Andersson, S. Haggmark, C. Jern, G. Johansson, H. Seeman-Lodding, and B. Biber. 2002. Myocardial ischemia induces coronary t-PA release in the pig. Acta. Anaesthesiol. Scand. 46:271–278. [DOI] [PubMed] [Google Scholar]
- 52.Pretorius, M., D. Rosenbaum, D.E. Vaughan, and N.J. Brown. 2003. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation. 107:579–585. [DOI] [PubMed] [Google Scholar]
- 53.Steins, M.B., T. Padro, C.X. Li, R.M. Mesters, H. Ostermann, D. Hammel, H.H. Scheld, W.E. Berdel, and J. Kienast. 1999. Overexpression of tissue-type plasminogen activator in atherosclerotic human coronary arteries. Atherosclerosis. 145:173–180. [DOI] [PubMed] [Google Scholar]
- 54.Olofsson, B.O., G. Dahlen, and T.K. Nilsson. 1989. Evidence for increased levels of plasminogen activator inhibitor and tissue plasminogen activator in plasma of patients with angiographically verified coronary artery disease. Eur. Heart J. 10:77–82. [DOI] [PubMed] [Google Scholar]
- 55.Ridker, P.M., D.E. Vaughan, M.J. Stampfer, J.E. Manson, and C.H. Hennekens. 1993. Endogenous tissue-type plasminogen activator and risk of myocardial infarction. Lancet. 341:1165–1168. [DOI] [PubMed] [Google Scholar]
- 56.Thompson, S.G., J. Kienast, S.D. Pyke, F. Haverkate, and J.C. van de Loo. 1995. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N. Engl. J. Med. 332:635–641. [DOI] [PubMed] [Google Scholar]
- 57.Linnik, W., J.E. Tintinalli, and R. Ramos. 1989. Associated reactions during and immediately after rtPA infusion. Ann. Emerg. Med. 18:234–239. [DOI] [PubMed] [Google Scholar]
- 58.Mehta, J.L., W.W. Nichols, T.G. Saldeen, V.K. Chandna, F.A. Nicolini, D.L. Lawson, and M.F. ter Riet. 1990. Superoxide dismutase decreases reperfusion arrhythmias and preserves myocardial function during thrombolysis with tissue plasminogen activator. J. Cardiovasc. Pharmacol. 16:112–120. [DOI] [PubMed] [Google Scholar]
- 59.Lepley-Frey, D. 1991. Dysrhythmias and blood pressure changes associated with thrombolysis. Heart Lung. 20:335–341. [PubMed] [Google Scholar]
- 60.Wilcox, R.G., J. Eastgate, E. Harrison, and A.M. Skene. 1991. Ventricular arrhythmias during treatment with alteplase (recombinant tissue plasminogen activator) in suspected acute myocardial infarction. Br. Heart J. 65:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newby, K.H., T. Thompson, A. Stebbins, E.J. Topol, R.M. Califf, and A. Natale. 1998. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 98:2567–2573. [DOI] [PubMed] [Google Scholar]
- 62.Wang, Y.F., S.E. Tsirka, S. Strickland, P.E. Stieg, S.G. Soriano, and S.A. Lipton. 1998. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 4:228–231. [DOI] [PubMed] [Google Scholar]
- 63.Carmeliet, P., J.M. Stassen, L. Schoonjans, B. Ream, J.J. van den Oord, M. De Mol, R.C. Mulligan, and D. Collen. 1993. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J. Clin. Invest. 92:2756–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carmeliet, P., R.C. Mulligan, and D. Collen. 1994. Transgenic animals as tools for the study of fibrinolysis in vivo. J. Intern. Med. 236:455–459. [DOI] [PubMed] [Google Scholar]
- 65.Murphy, N.P., S.G. Ball, and P.F. Vaughan. 1991. Potassium- and carbachol-evoked release of [3H]noradrenaline from human neuroblastoma cells, SH-SY5Y. J. Neurochem. 56:1810–1815. [DOI] [PubMed] [Google Scholar]
- 66.Koyama, M., N. Seyedi, W.P. Fung-Leung, T.W. Lovenberg, and R. Levi. 2003. Norepinephrine release from the ischemic heart is greatly enhanced in mice lacking histamine H3 receptors. Mol. Pharmacol. 63:378–382. [DOI] [PubMed] [Google Scholar]
- 67.Walker, M.J., M.J. Curtis, D.J. Hearse, R.W. Campbell, M.J. Janse, D.M. Yellon, S.M. Cobbe, S.J. Coker, J.B. Harness, D.W. Harron, et al. 1988. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc. Res. 22:447–455. [DOI] [PubMed] [Google Scholar]