Abstract
Although required for many fundamental immune processes, ranging from self-tolerance to pathogen immunity, interleukin (IL)-2 production is transient, and the mechanisms underlying this brevity remain unclear. These studies reveal that helper T cell IL-2 production is limited by a classic negative feedback loop that functions autonomously or in collaboration with other common γ chain (IL-4 and IL-7) and IL-6/IL-12 family cytokines (IL-12 and IL-27). Consistent with this model for cytokine-dependent regulation, they also demonstrate that the inhibitory effect can be mediated by several signal transducer and activator of transcription (STAT) family transcription factors, namely STAT5, STAT4, and STAT6. Collectively, these findings establish that IL-2 production is limited by a network of autocrine and paracrine signals that are readily available during acute inflammatory responses and, thus, provide a cellular and molecular basis for its transient pattern of expression.
More than 25 yr ago, IL-2 was identified as an autocrine secretory product required for long-term culture of helper (CD4+) T cells (1). Subsequently, the importance of IL-2 has been affirmed, but its effects on these cells, whether direct or indirect, are more diverse and complex than initially proposed. Early reports demonstrated that IL-2 promotes adaptive immunity by augmenting T cell proliferation, survival, and effector (Th1/Th2) differentiation (2, 3). It was later established that by programming them for apoptosis, IL-2 also participates in the contraction of inflammatory responses, and, more recently, it has been shown to promote expansion of regulatory T (T reg) cells, a lineage that is critical for maintaining self-tolerance (4, 5). Due to these latter effects, IL-2 and IL-2 receptor–deficient mice exhibit a complex autoimmune phenotype characterized by multi-organ inflammation, a lack of T reg cells, and an accumulation of autoreactive T cells (4). However, despite a growing appreciation for these antiinflammatory properties, only the stimulatory capacity of IL-2 has been considered for therapeutics, most notably for promoting T cell expansion and function in patients with advanced cancers or AIDS (6, 7).
Given the paucity of antigen-specific T cells at the onset of an immune response and the fact that IL-2 is a potent growth factor in vitro, it has long been accepted that IL-2 is necessary for clonotypic expansion (2). Consistent with this notion, IL-2 is one of the first products of activated CD4+ T cells and is required for the development, proliferation, and survival of antigen-specific effector (Th1/Th2) and T reg cell populations in vivo (2, 3, 8). Still, although it is known that IL-2–secreting T cells are pluripotent, capable of differentiating into multiple lineages (Th1/Th2/T reg cells) (9, 10), their appearance is short-lived and the molecular mechanisms underlying the transient nature of this response remain poorly defined (11).
RESULTS AND DISCUSSION
Helper T cell IL-2 production is limited by a classic negative feedback loop
To investigate the kinetics of IL-2 production, an in vitro model of T cell differentiation was used. Naive polyclonal T cells were labeled with CFSE, a vital dye that allows proliferative history to be visualized, activated with TCR and CD28-specific agonist antibodies, and, on successive days after stimulation, intracellular cytokine staining was used to quantify IL-2. The peak in IL-2 responses was noted during the first 24 h of culture, an early time point that preceded the onset of cell division and effector cytokine production (Fig. 1 A and unpublished data). At later intervals, most cells had proliferated and the percentage of IL-2+ events declined gradually: a twofold reduction after 48 h and a threefold drop at 72 h (Fig. 1, A–C). These data are consistent with many previous reports in demonstrating that helper T cells produce IL-2 quickly but transiently (9, 11).
Figure 1.
IL-2 is required for the natural decay in IL-2 production during helper T cell differentiation. (A–D) Polyclonal CD4+ T cells were CFSE labeled, stimulated with anti-CD3/anti-CD28, and cultured with or without anti–IL-2 mAb. (A–C) After 24, 48, or 72 h, cells were stained for surface CD4 and intracellular IL-2 (n = 6; five experiments pooled in B). (D) Cells were collected at 48 h (no PMA/Iono/BFA), and IL-2 mRNA was quantified by real-time PCR. (E) Monoclonal DO11.10+ CD4+ T cells were CFSE labeled and stimulated with antigen, soluble anti-CD28 mAb, and syngeneic APCs (±anti–IL-2 mAb). IL-2 production was measured after 48 or 96 h. (A–D) Only CD4+ (A and C) or CD4+ DO11.10+ (E) events are shown. RT-PCR data are pooled from five separate experiments.
The preceding studies imply that IL-2 production wanes as T cells divide and differentiate, but they do not provide a rationale for this phenomenon. Given that IL-2 was rapidly secreted and, consequently, was abundant in these cultures, it was postulated that IL-2 itself might suppress IL-2 production. To investigate that hypothesis, IL-2 was neutralized with a monoclonal anti–IL-2 antibody (mAb) and kinetic analysis was performed as before. Depletion of IL-2 had little effect on IL-2 production during the first 24 h in culture, but, remarkably, this treatment abrogated subsequent reductions in IL-2+ events and in the amount of cytokine produced per cell (mean fluorescence intensity [MFI]; Fig. 1, A–C). Consistent with changes in protein expression, IL-2 mRNA levels rose dramatically when IL-2 was neutralized (Fig. 1 D) and, fittingly, there was a dose-dependent decline in the percentage of IL-2+ cells, IL-2 MFI, and IL-2 mRNA when IL-2–deficient cultures were supplemented with recombinant human cytokine (Fig. 2, A–C). IL-2 responses were unaffected when CD25+ T reg cells were depleted from IL-2–sufficient or –deficient cultures, indicating that despite a well-established role in suppressing IL-2 production (4, 10), they have little influence during the short-term assays used here (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061198/DC1). Collectively, these studies imply that IL-2 limits itself directly via a classic negative feedback loop, not through effects on T reg cells.
Figure 2.
Common γ chain cytokines can suppress helper T cell IL-2 production. (A–C) CFSE-labeled CD4+ T cells were cultured with anti- murine IL-2 mAb and supplemented with recombinant human IL-2 (left to right: 1, 10, 50, and 100 U/ml). As in Fig. 1, flow cytometry and RT-PCR were used to quantify IL-2 production at 72 and 48 h, respectively (n = 3; three experiments pooled in B and C). (D–H) IL-2 production was measured after CD4+ T cells were cultured with recombinant IL-4, IL-7, or IL-15 (±anti–IL-2 mAb; 48 h; n = 3; three experiments pooled in D–F). (A–H) Only CD4+ events are shown.
To confirm that IL-2–dependent inhibition is applicable during physiological modes of activation, OVA-specific CD4+ T cells (DO11.10) were stimulated with OVA-pulsed APCs and IL-2 production was monitored. As with the polyclonal studies, IL-2 production was transient in this monoclonal population and, more importantly, the decline in cytokine production was dependent on IL-2 (Fig. 1 E). In concert with the above findings, these data support the idea that IL-2 itself is responsible for a “natural” decline in IL-2 production during nascent helper T cell responses.
Common γ chain cytokines limit helper T cell IL-2 production
IL-2 is the founder of a cytokine family whose members use the common γ chain as a shared component of their receptor complexes (12). Because common γ chain cytokines can have analogous functions in helper T cells, experiments were performed to determine whether IL-4, IL-7, or IL-15 suppresses IL-2 production. Similar to previous reports (13), these studies demonstrate that IL-4 is a potent inhibitor of IL-2 production, prompting significant reductions in IL-2+ events, IL-2 MFI, and IL-2 mRNA (Fig. 2, D–H). Although better known for its effects on memory cells (14), IL-7 also inhibited IL-2 production, albeit to a lesser extent than IL-4 (Fig. 2, D–H). On the other hand, despite sharing two receptor chains with IL-2 (3), IL-15 had little effect on this process, suggesting that the analogy cannot be extended to all common γ chain family members (Fig. 2, D–H).
The preceding studies demonstrate that IL-2 and IL-4 can each suppress helper T cell IL-2 production. Because both can activate STAT5 (15), the signature transcription factor of common γ chain cytokines (12), it was reasoned that their shared ability to inhibit IL-2 production could be mediated through this pathway. To test that hypothesis, cytokine-dependent IL-2 inhibition was compared between WT CD4+ T cells and those derived from mice lacking STAT5a/b in the T cell compartment (16). From these studies, it was clear that STAT5−/− cells produced more IL-2 than WT counterparts, presenting almost 10 times as many IL-2+ events and a sixfold increase in IL-2 MFI at 48 h after stimulation (Fig. 3, A and B, and Fig. S2, which is available at http://www.jem.org/cgi/content/full/jem.20061198). Moreover, although IL-2 production was comparable between WT and STAT5−/− cells when IL-2 was neutralized, the human cytokine could only suppress in the former group, thus formally establishing that the negative feedback loop is dependent on STAT5 (Fig. 3, A and B, and Fig. S2). In fact, STAT5−/− CD4+ T cells produced similar levels of IL-2 at 24 and 72 h after stimulation, demonstrating that, unlike for WT counterparts, IL-2 production was not transient in the absence of this transcription factor (Fig. S2).
Figure 3.
STAT5 is required for IL-2 and IL-4 to suppress IL-2 production. (A–D) CD4+ T cells were isolated from either WT mice (STAT5+/+) or those lacking STAT5a/b in T cells (STAT5 −/−). Cells were cultured with or without anti–IL-2 mAb and, where noted, hIL-2, IL-4, or IL-12 was added. Only CD4+ events are shown (n = 3; compiled in Fig. S2).
IL-2 production is inhibited by Th1- and Th2-polarizing factors
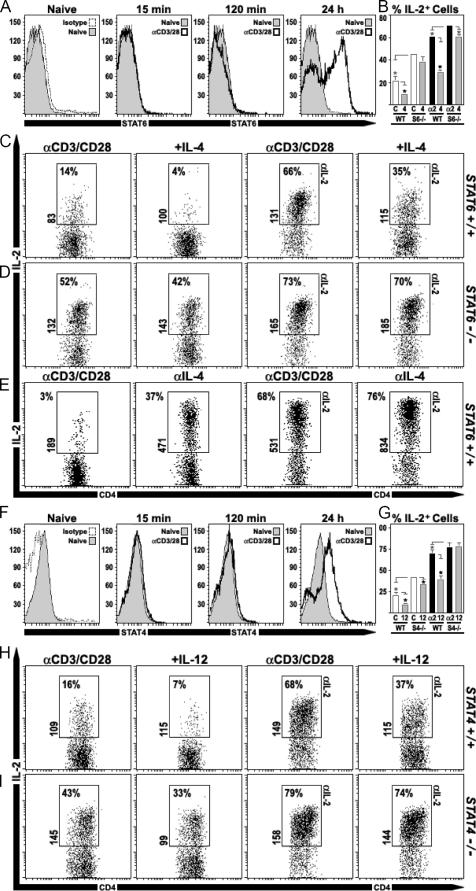
The preceding studies demonstrate that, similar to IL-2, the ability of IL-4 to suppress IL-2 production was diminished in STAT5−/− T cells. However, it was also noted that IL-4 could still prompt small, yet consistent reductions in IL-2+ events, and it remained possible that IL-4 could use additional signaling pathways to suppress. One obvious candidate for these activities was STAT6, the transcription factor that drives its ability to promote Th2 responses (17). To assess whether the culture conditions used here triggered STAT6 activation, WT CD4+ T cells were stimulated with anti-CD3/CD28 and flow cytometry was used to detect the active, phosphorylated form of this protein. As expected, naive cells were devoid of phospho-STAT6 and remained so during the first 2 h in culture. However, phospho-STAT6 was clearly evident at 24 h after stimulation, suggesting that activating factors did become available (Fig. 4 A). To determine whether STAT6 participates in suppressing IL-2, cytokine production was compared between WT and STAT6−/− CD4+ T cells. After 48 h in culture, STAT6−/− cells produced twice as much IL-2 as WT counterparts (Fig. 4, B–D). Moreover, although IL-2 production was similar when IL-2 was depleted, indicating that IL-2/STAT5-dependent inhibition was functional in both groups, the ability of exogenous IL-4 to suppress was indeed lost in STAT6−/− cells (Fig. 4, B–D). Together with the above experiments, these findings establish that STAT5 and STAT6 are required for IL-4 to inhibit IL-2 production and, given that a considerable loss of activity is observed when either transcription factor is deleted, they are likely to cooperate in this process.
Figure 4.
Helper T cell IL-2 production is limited by Th1- and Th2-polarizing factors. (A) CD4+ T cells were stimulated with anti-CD3 and anti-CD28. At 15 min, 2 h, and 24 h after activation, cells were fixed and permeabilized, and phospho-STAT6 was detected by flow cytometry (not shown: 5, 30, and 60 min; n = 2). (B–D) WT or STAT6−/− CD4+ T cells were cultured as described above (±anti–IL-2 mAb) and, where noted, IL-4 was added (n = 2: two experiments pooled in B). (E) CD4+ T cells were cultured with or without anti–IL-4 mAb (±anti–IL-2 mAb), and IL-2 production was monitored. (F) Phospho-STAT4 was measured after cells were stimulated and stained as in A (n = 2). (G–I) WT or STAT4−/− CD4+ T cells were cultured with or without IL-12 (n = 3: three experiments pooled in G). (A–I) Only CD4+ events are shown.
Given the role of IL-2/STAT5 in promoting Th2 responses (8), it is likely that anti–IL-2 mAb greatly diminished IL-4 production in the culture system used here. Consequently, it may be reasoned that the rise in IL-2 production associated with blocking IL-2 could be a secondary consequence of its effects on IL-4, itself a potent inhibitor. To address that issue, IL-2 production was measured after WT CD4+ T cells were cultured with or without neutralizing anti–IL-4 mAb. Consistent with the idea that helper T cells are a primary source of IL-4, depletion of this cytokine had at least two effects: an increase in the percentage of IFN-γ+ cells and, relevant to this work, a rise in IL-2+ cells (unpublished data and Fig. 4 E). These data affirm that autocrine IL-4 production can have a significant impact on IL-2 responses and imply that IL-4 is likely responsible for the robust STAT6 activation noted in the cultures (Fig. 4 A). However, because the effect of anti–IL-2 mAb was more profound than that of anti–IL-4 mAb, a reduction in IL-4 cannot wholly account for the efficacy of the former. Furthermore, there was a cumulative effect when both cytokines were removed, suggesting that they may cooperate in regulating IL-2 production (Fig. 4 E).
Although IL-2 is known to promote Th1 and Th2 responses, it is traditionally considered a Th1 cytokine (17). In contrast to this model, a recent report has shown that IL-2 production is inhibited by IL-12, a member of the IL-6/IL-12 cytokine family that is key in promoting Th1 differentiation (18). This present study supports these findings by demonstrating that IL-12 can prompt substantial reductions in IL-2+ events and IL-2 MFI (Fig. 4 H). This inhibitory property was not dependent on STAT5 or STAT6 (Fig. 3 and unpublished data) but, as shown previously (18), was mediated through STAT4, the same transcription factor that it uses to promote Th1 responses (19). IL-12 could not suppress IL-2 production in STAT4−/− cells, and, even when exogenous IL-12 was not added, it was clear that lacking STAT4 predisposed them to enhanced IL-2 production (Fig. 4, H and I). Consistent with the latter point, STAT4 activation was confirmed by flow cytometry (Fig. 4 F), but because neutralizing IL-12 has little effect on IL-2 production (unpublished data), the STAT4 stimulus in the nonpolarizing cultures remains unknown. Nevertheless, taken with the above results, these studies imply that, despite prevailing dogma, IL-2 production can be limited by factors traditionally associated with polarizing both Th1 and Th2 responses.
IL-2 can suppress helper T cell IL-2 production after immunization
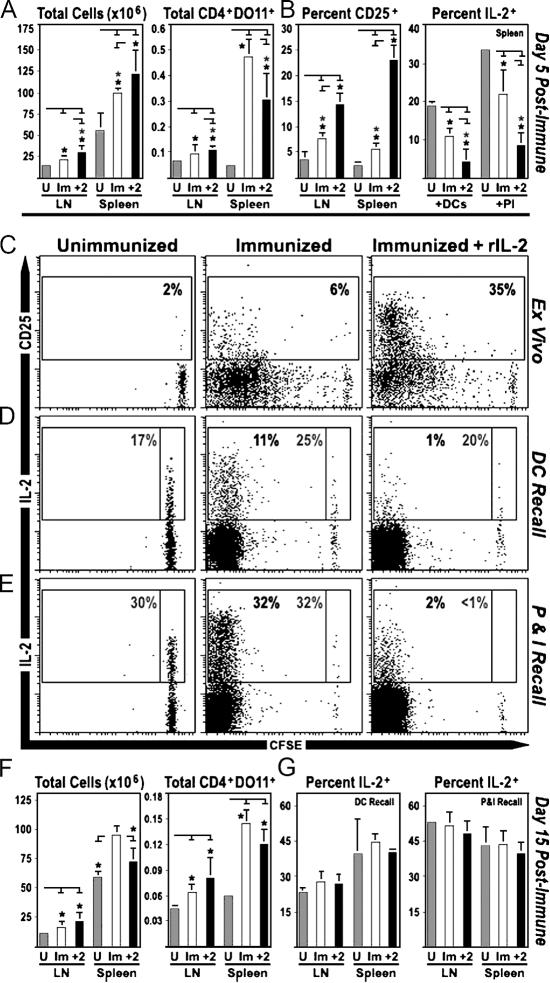
To determine whether IL-2 can suppress in vivo IL-2 responses, DO11.10 CD4+ T cells were transferred into WT mice that were then immunized with OVA-pulsed DCs and treated with either IL-2 or PBS. At 5 d after immunization, both groups displayed a significant increase in the overall cellularity of lymphoid organs (LN/spleen), thereby confirming the efficacy of the DCs as immunogens. This effect was slightly greater in IL-2–treated mice than in control animals, but the absolute numbers of antigen-specific T cells remained comparable, suggesting that this dose of IL-2 did not lead to unwarranted T cell expansion or depletion (Fig. 5 A). However, as expected, the IL-2 regimen did have two significant effects on DO11+CD4+ cells: it enhanced expression of CD25, a known IL-2/STAT5 target (2, 12), and greatly reduced their ability to produce IL-2 during ex vivo recall assays (Fig. 5, B–E). Given that the percentage of FoxP3+ cells was similar in both groups, these effects were not likely mediated through IL-2–dependent T reg cell expansion (unpublished data and Fig. S3, which is available at http://www.jem.org/cgi/content/full/jem.20061198/DC1). Instead, as with the in vitro experiments, these studies demonstrate that IL-2 production is suppressed when T cells are primed in an IL-2–rich environment.
Figure 5.
IL-2 can suppress IL-2 responses after immunization. (A–E) CFSE-labeled, DO11.10+ CD4+ T cells were transferred into WT mice, which were immunized 2 d later with antigen-pulsed DCs. At days 2 and 3 after immunization, mice were treated with PBS or IL-2, and 2 d later (day 5 after immunization), LNs and spleens were isolated. (A) Lymphocytes and splenocytes were enumerated by microscopy. Percentages of DO11.10+ CD4+ T cells were determined by flow cytometry and used to calculate the absolute number of antigen-specific cells. (B and C) Surface CD25 was measured directly ex vivo. (D and E) Intracellular IL-2 was measured after restimulation with either (D) OVA-pulsed DCs (16 h) or (E) PMA/ionomycin (4 h). Gray numbers are the percentage of undivided IL-2+ cells, whereas the back numbers denote the percentage of proliferating IL-2+ cells (two mice per group; three experiments pooled in A and B). (F and G) WT mice were populated with DO11.10+ CD4+ T cells, immunized, and treated with IL-2 as above. LNs and spleens were processed at 15 d after immunization (three mice per group; two experiments pooled in F and G). (A–H) For cytokine analysis, all groups were treated with BFA for 2 h before intracellular staining. Only CD4+ DO11.10+ events are displayed.
To investigate the long-term effects of IL-2 treatment on IL-2 production, WT mice were populated with DO11+CD4+ cells, immunized, and treated with IL-2 as described above. However, instead of analyzing the response at day 5 after immunization, when cells had recently been exposed to antigen and high doses of IL-2, LNs and spleens were isolated at day 15 after immunization, when T cell responses had contracted and levels of antigen, costimulation, and IL-2 were greatly diminished. As above, immunization of control and IL-2–treated animals led to increases in primary lymphoid organ cellularity and absolute numbers of DO11+ cells. However, unlike the earlier time point, both groups were fully capable of producing IL-2 when stimulated ex vivo (Fig. 5, F and G, and Fig. S4, which is available at http://www.jem.org/cgi/content/full/jem.20061198/DC1). In the context of the preceding observations, these studies imply that the effects of IL-2 on helper T cell IL-2 production are transient, likely requiring continued availability of the cytokine.
Because of its central role in the life and death of helper T cells, the need to regulate IL-2 has long been appreciated. However, although the stimuli that induce IL-2 production are well understood (20), there is no consensus on why this process is so short-lived. The present studies elaborate on the latter issue by characterizing a negative feedback loop that is necessary and sufficient to limit IL-2 production during helper T cell differentiation. Not only do these findings establish IL-2 as a potent IL-2 inhibitor, they also demonstrate, together with previous work (18), that this property is shared by other common γ chain cytokines (IL-4 and IL-7) and select members of the IL-6/IL-12 family (IL-12 and IL-27). Each can suppress individually, albeit with varying potencies (IL-2>IL-4>IL-27>IL-12>IL-7), but the most efficient IL-2 inhibition is seen when two or more of these cytokines are combined. Given this synergy, it can now be proposed that, depending on the inflammatory conditions present at the time of priming, T cell IL-2 production is limited by a combination of negative feedback and cytokine-dependent inhibition (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20061198/DC1). However, it should be emphasized that this regulatory network curtails the duration of IL-2 production but does not affect the initial, antigen-driven signals that induce the response. As a result, there will always be a short burst of IL-2 that precedes the suppression, thus guaranteeing a brief exposure to this growth factor. Consistent with that idea, cytokine-dependent IL-2 inhibition is not seen when T cells are cultured in vitro for <24 h or when their proliferation is arrested (Fig. S6). Therefore, while providing a cellular and molecular rationale for its transient pattern of expression, the data presented here are consistent with established models that hold that IL-2 production is required for the expansion, survival, and or differentiation of clonotypic T cells (2, 3, 12). Moreover, they are in accordance with studies showing that rapid epigenetic changes promote IL-2 gene accessibility and transcription before cell cycle entry (21).
The studies presented here establish that STAT5 is required for IL-2 and IL-4 to inhibit helper T cell IL-2 production. In addition, they implicate other STAT family members in this process, namely STAT6 and STAT4. Whether all of these STAT signals converge on a single inhibitory mechanism remains to be determined, but because there is no evidence that STATs bind the IL-2 promoter and directly obstruct gene expression, it is likely that they induce additional factors that, in turn, act as transcriptional repressors. Consistent with this hypothesis, it has long been known that blocking protein synthesis during T cell differentiation results in super-induction of IL-2 mRNA (22). Several nuclear proteins, like Tob and p27kip1, have been shown to prohibit IL-2 production in anergic T cells, and it is possible that analogous mechanisms are used during effector T cell responses (23, 24). On the other hand, the culture conditions used here do not induce anergy, and it is just as likely that cytokine-dependent IL-2 inhibition does not invoke anergy-related pathways. Furthermore, given that IL-2 production can be regulated by changes in mRNA stability (25), it is also possible that cytokine-dependent inhibition is a posttranscriptional phenomenon.
Aside from the STAT-dependent mechanism described here, many factors are known to suppress IL-2 production. These observations support the idea that IL-2 responses are tightly regulated, but the relationship between STAT-dependent inhibition and other “IL-2 inhibitors” remains unclear. Various regulatory pathways may work together to limit IL-2 or, alternatively, each may operate independently. Consistent with the latter idea, NF-κBp50 and Tbet have been shown to suppress IL-2 production (26, 27), but, in their absence, IL-2, IL-4, and IL-12 are still potent inhibitors (unpublished data and 18). Even so, this result does not exclude the possibility that STAT-dependent signals cooperate with other factors, such as TGF-β/Smad3, CTLA-4, and BTLA, all of which are known to suppress IL-2 production (28, 29). Whatever the case, it is likely that this process is always limited by STAT-dependent means and, as such, it can now be proposed that helper T cells are “hard-wired” to produce all of the IL-2 they need as a short burst after activation. This idea is supported by a recent study demonstrating that brief exposure to IL-2 during priming is both necessary and sufficient to potentiate memory CD8+ T cell responses (30). Coupled with this present study, those data argue that, although essential, IL-2 responses are meant to be brief. Given this new insight, suppressive effects on helper T cell IL-2 production must be considered as clinical applications for cytokines are explored.
MATERIALS AND METHODS
Mice.
WT C57B/6 mice were purchased from The Jackson Laboratory. Mice lacking STAT5a/b were generated as described previously (16) and, along with littermate controls (C57B/6 background), were provided by J. O'Shea and L. Hennighausen (NIH). In brief, mice were engineered with LoxP sites flanking both STAT5 genes, and then bred with transgenic animals expressing Cre recombinase under control of the CD4 promoter. The resulting offspring lacked STAT5a and STAT5b in all CD4+ T cells with >90% deletion efficiency (Fig. S2). The official nomenclature for these conditional knockout mice is Stat5flx, cd4 cre (16), but, for simplicity, they are referred to here as STAT5 −/−. Mice deficient in STAT4 (STAT4 −/−) and STAT6 (STAT6 −/−) were purchased along with the corresponding BALB/c WT controls from The Jackson Laboratory. Transgenic mice (BALB/c) expressing the DO11.10 αβTCR-specific for chicken OVA peptide (OVA323–339) in the context of MHC class II molecule I-Ad were generated by K. Murphy (Washington University, St. Louis, MO) and provided by A.A.K. Abbas (University of California, San Francisco, San Francisco, CA). All experiments were performed according to the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
In vitro T cell differentiation.
For polyclonal studies, splenocytes were isolated from WT or STAT-deficient mice and depleted of CD8+ and NK1.1+ cells by magnetic bead separation (Polysciences Inc.). 2 × 106/ml cells were then labeled with 5 μg/ml CFSE (Sigma-Aldrich) and stimulated with 1 μg/ml of soluble anti-CD3 (clone 17A2) and 1 μg/ml anti-CD28 antibodies (clone 37.51; both were either generated in house or purchased from eBioscience). Where stated, cultures were supplemented with 10 μg/ml of monoclonal, anti-murine IL-2 antibodies (clones S4B6 or JES6-1; generated in house or from eBioscience) and/or the following cytokines: 1, 10, 50, or 100 U/ml of human IL-2 (NIH/NCI BRB Preclinical Repository), 50 ng/ml of murine IL-4 (eBioscience), 10 ng/ml murine IL-7 (eBioscience), 5 ng/ml of murine IL-12 (Genetics Institute), or 10 ng/ml of murine IL-15 (eBioscience). 10 μg/ml anti-murine IL-4 was used in one set of experiments (clone 11B11; NIH/NCI BRB Preclinical Repository).
For antigen-specific studies, spleens and LNs were isolated from DO11.10 transgenic mice. CD4+ T cells were isolated using positive selection beads (Dynal), CFSE labeled, and stimulated with WT syngeneic, mitomycin C–treated splenocytes in the presence of 100 ng/ml OVA peptide (2.5 × 106 mito-APCs vs. 0.25 × 106 DO11.10 CD4+ T cells).
Flow cytometry.
To assay in vitro cytokine production, CD4+ T cells were cultured for 24, 48, 72, or 96 h and pulsed with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich). 2 h later, cells were treated with brefeldin A (BFA; 2 h at 10 μg/ml; Sigma-Aldrich) and stained for intracellular IL-2 in combination with surface CD4 (eBioscience). Rectangular gates indicate specific IL-2 staining compared with control mAb. Percentages of IL-2+ CD4+ cells are displayed within or beside their corresponding gates, and MFIs of IL-2+ events are presented vertically. For detection of phsopho-STAT4, phospho-STAT6, and FoxP3, intracellular staining was performed according to the manufacturer's specifications (kits by eBioscience).
Immunizations.
For immunizations, bone marrow–derived DCs (BMDCs) were generated from WT BALB/c mice. In brief, marrow was isolated, depleted of red blood cells, and cultured with GM-CSF to enrich for DCs (7 d), which were then matured (IL-4 plus LPS) and loaded with 1 μg/ml OVA peptide (>70% MHCIIhighB7.2high by flow cytometry). In corresponding preparations, 2 × 106 CFSE-labeled, DO11.10 CD4+ T cells were adoptively transferred (intravenous) into WT BALB/c recipients that were immunized 72 h later with the mature BMDCs. At days 2 and 3 after immunization, mice were treated (intraperitoneal) with either PBS (control) or recombinant human IL-2 (0.8 × 106 U/dose in 800 μl PBS). LNs and spleens were isolated 2 (day 4 after immunization) or 12 (day 15 after immunization) d later, and surface staining for CD4, DO11 TCR, and CD25 was performed directly ex vivo. For each mouse, absolute numbers of antigen-specific cells were calculated with the following formula: (percentage of CD4+DO11+ cells × total cellularity of LNs or spleens)/100 = total CD4+DO11+ cells. IL-2 production was assayed after lymphocytes/splenocytes were stimulated with either PMA/ionomycin (4 h) or mature BMDCs (16 h; 10:1 lymphocyte/splenocyte/DC ratio). All groups were treated with BFA for 2 h before intracellular cytokine staining.
Real-time PCR.
mRNA was isolated from cultured cells (no PMA/Iono/BFA) using Trizol reagent and converted to cDNA using SuperScript II reverse transcriptase (300–400 ng RNA per reaction; Invitrogen). For real-time PCR, IL-2–specific primers and probes were purchased from Applied Biosystems, and amplification was performed according to the manufacturer's specifications. IL-2 mRNA levels were normalized with respect to ubiquitous 18S ribosomal mRNA, and expression of IL-2 is represented as the fold induction over naive (unstimulated) controls.
Statistics.
Statistical variations between experimental groups were determined by two-tailed, paired Student's t test. Significant differences, P < 0.05, are denoted by an asterisk. Error bars denote standard deviations.
Online supplemental material.
Fig. S1 shows that the auto-inhibitory properties of IL-2 are not dependent on T reg cells. Fig. S2 shows that IL-2 production is not transient in the absence of STAT5. Fig. S3 illustrates that treatment with IL-2 does not enhance the expansion of T reg cells after immunization. Fig. S4 shows that treatment with IL-2 does not impose a permanent block on helper T cell IL-2 production after immunization. Fig. 5 is a model for the regulation of IL-2 production during primary helper T cell responses, and Fig. S6 displays temporal and cell cycle requirements for inhibiting IL-2 production. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061198/DC1.
Supplemental Material
Acknowledgments
The authors acknowledge members of the Hunter laboratory for intellectual input during the course of these studies. We also thank Drs. Abul Abbas, Matthew Krummel, Jens Lohr, Phil Scott, Lawrence Turka, and Andrew Wells for reagents and discussions.
This work funded by the State of Pennsylvania and grants from the NIH (NIH42334, AI41158 with a minority supplement to A.V. Villarino, and A10662).
The authors have no conflicting financial interests.
A.V. Villarino's present address is Dept. of Pathology, University of California San Francisco School of Medicine, San Francisco, CA 94143.
References
- 1.Mier, J.W., and R.C. Gallo. 1980. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc. Natl. Acad. Sci. USA. 77:6134–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith, K.A. 1988. Interleukin-2: inception, impact, and implications. Science. 240:1169–1176. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann, T.A., S. Dubois, and Y. Tagaya. 2001. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 14:105–110. [PubMed] [Google Scholar]
- 4.Malek, T.R., and A.L. Bayer. 2004. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 4:665–674. [DOI] [PubMed] [Google Scholar]
- 5.Lenardo, M.J. 1991. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 353:858–861. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs, J.A., S. Vogel, J.A. Metcalf, M. Baseler, R. Stevens, J. Adelsberger, R. Lempicki, R.L. Hengel, I. Sereti, L. Lambert, et al. 2001. Interleukin-2 induced immune effects in human immunodeficiency virus-infected patients receiving intermittent interleukin-2 immunotherapy. Eur. J. Immunol. 31:1351–1360. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg, S.A., M.T. Lotze, L.M. Muul, S. Leitman, A.E. Chang, S.E. Ettinghausen, Y.L. Matory, J.M. Skibber, E. Shiloni, J.T. Vetto, et al. 1985. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313:1485–1492. [DOI] [PubMed] [Google Scholar]
- 8.Zhu, J., J. Cote-Sierra, L. Guo, and W.E. Paul. 2003. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 19:739–748. [DOI] [PubMed] [Google Scholar]
- 9.Wang, X., and T. Mosmann. 2001. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-γ, and can subsequently differentiate into IL-4– or IFN-γ–secreting cells. J. Exp. Med. 194:1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoechel, B., J. Lohr, E. Kahn, J.A. Bluestone, and A.K. Abbas. 2005. Sequential development of interleukin 2–dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 202:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sojka, D.K., D. Bruniquel, R.H. Schwartz, and N.J. Singh. 2004. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J. Immunol. 172:6136–6143. [DOI] [PubMed] [Google Scholar]
- 12.Leonard, W.J. 2001. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 1:200–208. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka, T., J. Hu-Li, R.A. Seder, B. Fazekas de St Groth, and W.E. Paul. 1993. Interleukin 4 suppresses interleukin 2 and interferon gamma production by naive T cells stimulated by accessory cell-dependent receptor engagement. Proc. Natl. Acad. Sci. USA. 90:5914–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley, L.M., L. Haynes, and S.L. Swain. 2005. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 26:172–176. [DOI] [PubMed] [Google Scholar]
- 15.Lischke, A., R. Moriggl, S. Brandlein, S. Berchtold, W. Kammer, W. Sebald, B. Groner, X. Liu, L. Hennighausen, and K. Friedrich. 1998. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J. Biol. Chem. 273:31222–31229. [DOI] [PubMed] [Google Scholar]
- 16.Yao, Z., Y. Cui, W.T. Watford, J.H. Bream, K. Yamaoka, B.D. Hissong, D. Li, S.K. Durum, Q. Jiang, A. Bhandoola, et al. 2006. Stat5a/b are essential for normal lymphoid development and differentiation. Proc. Natl. Acad. Sci. USA. 103:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liew, F.Y. 2002. T(H)1 and T(H)2 cells: a historical perspective. Nat. Rev. Immunol. 2:55–60. [DOI] [PubMed] [Google Scholar]
- 18.Villarino, A.V., J.S. Stumhofer, C.J. Saris, R.A. Kastelein, F.J. de Sauvage, and C.A. Hunter. 2006. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 176:237–247. [DOI] [PubMed] [Google Scholar]
- 19.Hunter, C.A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5:521–531. [DOI] [PubMed] [Google Scholar]
- 20.Jain, J., C. Loh, and A. Rao. 1995. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7:333–342. [DOI] [PubMed] [Google Scholar]
- 21.Bruniquel, D., and R.H. Schwartz. 2003. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat. Immunol. 4:235–240. [DOI] [PubMed] [Google Scholar]
- 22.Efrat, S., and R. Kaempfer. 1984. Control of biologically active interleukin 2 messenger RNA formation in induced human lymphocytes. Proc. Natl. Acad. Sci. USA. 81:2601–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussiotis, V.A., G.J. Freeman, P.A. Taylor, A. Berezovskaya, I. Grass, B.R. Blazar, and L.M. Nadler. 2000. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat. Med. 6:290–297. [DOI] [PubMed] [Google Scholar]
- 24.Tzachanis, D., G.J. Freeman, N. Hirano, A.A. van Puijenbroek, M.W. Delfs, A. Berezovskaya, L.M. Nadler, and V.A. Boussiotis. 2001. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2:1174–1182. [DOI] [PubMed] [Google Scholar]
- 25.Lindstein, T., C.H. June, J.A. Ledbetter, G. Stella, and C.B. Thompson. 1989. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 244:339–343. [DOI] [PubMed] [Google Scholar]
- 26.Kang, S.M., A.C. Tran, M. Grilli, and M.J. Lenardo. 1992. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 256:1452–1456. [DOI] [PubMed] [Google Scholar]
- 27.Hwang, E.S., J.H. Hong, and L.H. Glimcher. 2005. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J. Exp. Med. 202:1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKarns, S.C., R.H. Schwartz, and N.E. Kaminski. 2004. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J. Immunol. 172:4275–4284. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald, R.J., G.J. Freeman, and A.H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548. [DOI] [PubMed] [Google Scholar]
- 30.Williams, M.A., A.J. Tyznik, and M.J. Bevan. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 441:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.