Abstract
The induction of regulatory T (T reg) cells holds considerable potential as a treatment for autoimmune diseases. We have previously shown that CD4+CD25hi T reg cells isolated from patients with active rheumatoid arthritis (RA) have a defect in their ability to suppress proinflammatory cytokine production by CD4+CD25− T cells. This defect, however, was overcome after anti–tumor necrosis factor (TNF)-α antibody (infliximab) therapy. Here, we demonstrate that infliximab therapy gives rise to a CD4+CD25hiFoxP3+ T reg cell population, which mediates suppression via transforming growth factor (TGF)-β and interleukin 10, and lacks CD62L expression, thereby distinguishing this T reg cell subset from natural T reg cells present in healthy individuals and patients with active RA. In vitro, infliximab induced the differentiation of CD62L− T reg cells from CD4+CD25− T cells isolated from active RA patients, a process dependent on TGF-β. In spite of the potent suppressor capacity displayed by this CD62L− T reg cell population, the natural CD62L+ T reg cells remained defective in infliximab-treated patients. These results suggest that anti–TNF-α therapy in RA patients generates a newly differentiated population of T reg cells, which compensates for the defective natural T reg cells. Therefore, manipulation of a proinflammatory environment could represent a therapeutic strategy for the induction of T reg cells and the restoration of tolerance.
The numerous descriptions of regulatory T (T reg) cells in both animal models and, more recently, in humans have revealed a wide diversity of populations and mechanisms of action. Thymically derived CD4+CD25+ T reg cells play a crucial role in the maintenance of self-tolerance and the prevention of autoimmune disease. This “natural” subset expresses the transcription factor Foxp3, which has emerged as an important functional marker of T reg cell activity, supported by the fact that ectopic Foxp3 expression is sufficient to empower naive T cells with a regulatory function (1). CD4+ T reg cells can also be generated in the periphery (adaptive), and many studies have identified different subpopulations of CD4+ T cells based on phenotypic and functional properties (2, 3). Examples include IL-10–secreting Tr1 cells, which can inhibit colitis in mice (4, 5), and Th3 cells, which play a role in mediating tolerance via the production of TGF-β (6). TGF-β has also been shown to mediate suppression by CD4+CD25+ T reg cells in murine models of autoimmunity (7). In addition, TGF-β can also confer a suppressive phenotype on polyclonally activated naive T cells in vitro, leading to an expansion of FoxP3+ T reg cells (8–10).
The manipulation of the peripheral pool of T reg cells has been a particular focus for the treatment of autoimmune diseases and transplantation. Our previous data showing that T reg cells from patients with rheumatoid arthritis (RA) are functionally defective, and that after infliximab therapy this defect is reversed, have emphasized the potential of biological therapy (11). An intriguing observation was the increased number of peripheral blood CD4+CD25hi T reg cells seen only in RA patients responding to infliximab. Thus, TNF-α blockade might, as a consequence of the reduced inflammation, either “recall” T reg cells from previously inflamed joints or lead to the differentiation of “new” T reg cells. Here, we demonstrate that infliximab treatment induced the differentiation of a population of T reg cells expressing Foxp3 and low levels of CD62L through conversion of CD4+CD25− T cells.
RESULTS AND DISCUSSION
The expanded population of T reg cells from infliximab-treated patients is Foxp3+ and CD62L−
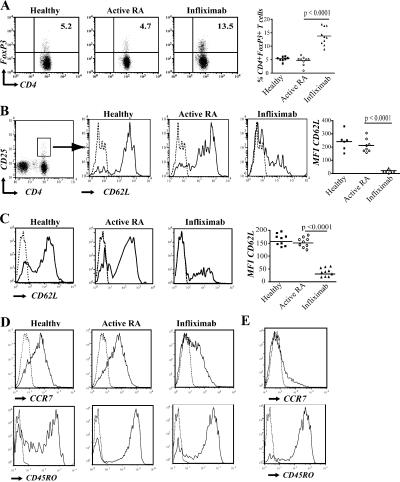
First, we addressed whether the increased number of CD4+CD25hi T cells observed in RA patients responding to infliximab therapy corresponded to an increase of T reg cells or simply reflected an increase in the percentage of activated T cells. Peripheral CD4+ T cells isolated from healthy, active RA patients and those treated with infliximab were analyzed for the expression of the transcription factor Foxp3 by intracellular staining. A two- to threefold increase in the percentage of CD4+Foxp3+ cells was observed in the PBMCs of post-infliximab patients compared with active RA patients or healthy individuals, suggesting that the increase in CD25 expression in post-anti–TNF-α–treated patients reflects increased numbers of T reg cells (Fig. 1 A). There was no significant difference in FoxP3 expression in CD4+ T cells from active RA patients compared with healthy controls. To better characterize the phenotype of the T reg cells after infliximab, we measured surface markers representative of activation, memory, and regulation. A significant increase in the percentage of CD4+CD25hiCD62L− was observed in PMBCs from infliximab-treated patients compared with RA patients with active disease before infliximab and healthy individuals (Fig. 1 B). As previous data has indicated that the most potent CD4+CD25hi T reg cell subset expressed CD62L (12), we next assayed the expression of CD62L in the CD4+Foxp3+ population. Although most CD4+Foxp3+ cells from healthy individuals and patients with active RA expressed CD62L, the profile of expression was remarkably different after infliximab treatment, where the majority of CD4+Foxp3+ cells expressed low levels of CD62L (Fig. 1 C). There was no change in the percentage of Foxp3+ cells or shift in CD62L expression in patients responding to methotrexate (not depicted). Compared with the T reg cells found in healthy individuals and patients with active disease, RA T reg cells post-infliximab expressed CD45RO but had a reduced expression of CCR7 (Fig. 1 D). Further examination of the CD4+Foxp3+CD62L− RA T reg cells post-infliximab revealed that these cells lacked CCR7 expression and remained CD45RO+ (Fig. 1 E).
Figure 1.
Increased numbers of Foxp3+CD62L−CD4+ T cells found in infliximab-treated RA patients compared with patients with active disease and healthy controls. (A) Representative FACS plots gated on the CD4+ population depicting PBMCs from healthy controls, active RA patients, and infliximab-treated RA patients stained with anti-CD4 and anti-Foxp3. The percentages of Foxp3+ cells in the CD4+ gate for individual RA patients, before and after infliximab, and healthy controls are shown in the chart. (B) Histograms were gated on CD4+CD25high cells (as indicated), and the expression of CD62L is shown. (C) PBMCs from the same groups as in A were stained with anti-CD4, anti-Foxp3, and anti-CD62L. The histograms indicate the expression of CD62L in the CD4+Foxp3+ population. Data from individual patients are shown in the chart. (D) CCR7 and CD45RO expression on the CD4+Foxp3+ T cell population in the different patient groups. (E) CCR7 and CD45RO expression on the CD4+FoxP3+CD62L− population from an RA patient treated with infliximab. Results are representative of six patients or healthy individuals for each group. The dashed line in the FACS plots represents the isotype control for the specific marker examined.
CD62L− T reg cells from infliximab-treated RA patients mediate their suppressive action through TGF-β and IL-10
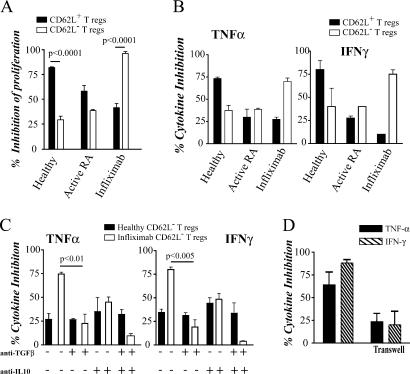
We next tested whether the CD4+CD25hiCD62L− T reg cells isolated from infliximab-treated patients were functionally suppressive. CD4+CD25hi were FACS sorted according to their expression of CD62L (identified as CD62L+ and CD62L− T reg cells; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061531/DC1), and their regulatory capacity was assessed with respect to inhibition of proliferation and TNF-α and IFN-γ production by autologous CD4+CD25− T cells. In agreement with previous studies (12), CD62L+ T reg cells isolated from healthy individuals were more potent at suppressing CD4+CD25− T cell proliferation than CD62L− T reg cells (Fig. 2 A). Conversely, CD62L− T reg cells isolated from RA patients after infliximab exhibited a more potent suppression of T cell proliferation than their CD62L+ T reg cell counterparts. Similarly, we observed a “switch” in the suppressor population from CD62L+ T reg cells in healthy individuals to CD62L− T reg cells in patients after infliximab, with respect to inhibition of IFN-γ and TNF-α production (Fig. 2 B). There was a substantial reduction in the suppressor potency of CD62L+ T reg cells isolated from patients with active RA compared with healthy individuals, confirming that CD4+CD25hi T reg cells are defective in RA patients (11). Of importance, the potency of the CD62L+ T reg cells after anti–TNF-α therapy was not restored to levels found in healthy controls (Fig. 2 B). Collectively, these results raise the intriguing possibility that the natural T reg cells (CD62L+ T reg cells) remain defective in patients even after infliximab therapy, and that the previously reported restoration of function of CD4+CD25hi T reg cells after infliximab therapy could be due to the differentiation of a new population of CD62L− T reg cells.
Figure 2.
CD62L− T reg cells from infliximab-treated RA patients are more potent suppressors than their CD62L+ counterparts and mediate their suppressive effects through IL-10 and TGF-β. CD4+CD25−, CD4+CD25hiCD62L+, and CD4+CD25hiCD62L− were FACS (MoFlo) sorted from the PBMCs of healthy controls, active RA patients, and infliximab-treated RA patients. In all experiments, cells were stimulated with 2 μg/ml of soluble anti-CD3/CD28. (A) CD4+CD25− T cells were cocultured with either CD62L+ or CD62L− T reg cells (2:1 ratio shown) for 5 d, with [3H]thymidine added in the last 18 h of culture. Mean triplicate values shown from six patients. (B) CD4+CD25− T cells were cultured alone or cocultured at a 2:1 ratio with either CD62L+ or CD62L− T reg cells for 48 h. Cells were intracellularly stained for TNF-α and IFN-γ. Data shown expressed as mean ± SE of six patients and six healthy controls, and are represented as the percentage inhibition of cytokine production compared with CD4+CD25− T cells alone. The means for percent IFN-γ+/TNF-α+ cells are as follows: healthy CD4+CD25− 3.2/3.4, CD4+CD25−/CD62L+ T reg cells 0.7/0.9, CD4+CD25−/CD62L− T reg cells 1.9/2.1; active RA CD4+CD25− 5.2/13.2, CD4+CD25−/CD62L+ T reg cells 3.6/9.0, CD4+CD25−/CD62L− T reg cells 3.1/7.8; post-infliximab CD4+CD25− 4.2/8.6, CD4+CD25−/CD62L+ T reg cells 3.8/6.3; and CD4+CD25−/CD62L− T reg cells 1.1/2.2. (C) CD4+CD25− T cells from healthy and infliximab-treated RA patients were cultured alone or with CD62L− T reg cells (2:1 ratio) for 48 h with anti-CD3/CD28 alone or in the presence of 2 μg/ml anti–TGF-β1, 0.5 μg/ml anti–IL-10, or anti–TGF-β1 and anti–IL-10 together, and stained for TNF-α and IFN-γ. Data depicted represent mean ± SE of six patients and healthy controls. (D) CD4+CD25− T cells were cocultured, either directly or separated by a transwell membrane, with CD62L− T reg cells from RA patients after infliximab, and stimulated with 2 μg/ml anti-CD3/CD28 for 48 h. Cells were intracellularly stained for TNF-α and IFN-γ, and the results are depicted as the percentage inhibition of cytokine production compared with CD4+CD25− T cells alone. Data represent mean ± SE of four patients.
To explore the effector mechanisms of the CD62L− T reg cells derived from post-infliximab RA patients, we examined the cytokine dependency of their suppressive effect. T reg cells were cocultured with autologous CD4+CD25− T cells in the presence of neutralizing mAbs to TGF-β and IL-10, previously recognized as regulatory cytokines involved in the suppressive mechanism of adaptive T reg cells (2). The data shown in Fig. 2 C demonstrate that in healthy individuals, the suppressive effect of CD62L− T reg cells was unaltered by the neutralization of TGF-β or IL-10. In contrast, the neutralization of TGF-β, and to a lesser extent IL-10, significantly impaired the suppressive capacity of the CD62L− T reg cells from infliximab-treated patients. When the action of both TGF-β and IL-10 was blocked, the suppressive activity of these CD62L− T reg cells was almost abolished. The potency of CD62L+ T reg cells from healthy individuals was unaltered by blockade of IL-10 or TGF-β (not depicted). The results reported here appear at odds with our previous findings showing that suppression required contact between T reg cells isolated from infliximab patients and their autologous CD4+CD25− T cells (11). However, the two results may not be directly comparable because in the previous work, suppression was assayed without separating the CD62L+ and CD62L− T reg cell subsets. Therefore, we assayed the functional properties of purified CD62L− T reg cells isolated from RA patients after infliximab therapy to dissect out their mode of action. The data shown in Fig. 2 D demonstrate that cell contact is required to effect maximal suppression of TNF-α and IFN-γ production by CD4+CD25− T cells, although some cytokine suppression is still observed when cell contact is prevented. The fact that the T reg cells isolated from patients after infliximab required both cell contact and cytokines for their suppressive effect could be explained by data indicating that TGF-β can mediate suppression through cell contact, when the predominant form of TGF-β is membrane bound (13). In addition, in some experimental systems, IL-10 production also depends on cell contact between T reg cells and CD4+CD25− T cells (14). Collectively, these results suggest that infliximab therapy gives rise to, or recruits from the periphery, a population of T reg cells that phenotypically and functionally differs from natural T reg cells present in healthy individuals or in patients with active RA.
In vitro infliximab stimulation of CD4+CD25− T cells from RA patients induced a CD62L− T reg cell population
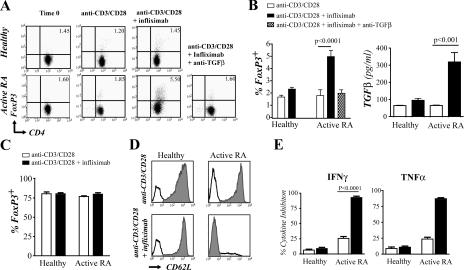
To investigate the possible provenance of CD62L− T reg cells, infliximab was added in vitro to CD4+CD25− T cells isolated from active RA or healthy individuals, and the expression of Foxp3 was measured by intracellular staining. The addition of infliximab to purified active RA CD4+CD25− T cells resulted in a substantial increase in the percentage of CD4+Foxp3+ cells (Fig. 3 A), whereas no such effect was seen when CD4+CD25− T cells were isolated from healthy individuals. Neutralization of TGF-β in this culture system completely prevented the differentiation of the CD4+Foxp3+ T cell population from the CD4+CD25− T cells (Fig. 3 A). Moreover, infliximab induced a significant increase in TGF-β production from CD4+CD25− T cells from patients with active RA, but not healthy individuals (Fig. 3 B). To determine whether infliximab modulates natural T reg cell Foxp3 expression, CD4+CD25hi T cells were isolated from healthy individuals or patients with active RA and cultured with infliximab. The results in Fig. 3 C show that infliximab did not affect the expression of FoxP3 on CD4+CD25hi T reg cells, supporting the evidence that this agent targets CD4+CD25− T cells rather than modulating preexisting T reg cells. We next measured CD62L expression after culture of CD4+CD25− T cells with infliximab. The addition of infliximab to CD4+CD25− T cells from active RA patients, but not from healthy individuals, led to a reduction in CD62L expression (Fig. 3 D). Thus, these in vitro infliximab-generated T cells share reduced CD62L expression as a common feature with the T reg cells present in the PBMCs of RA patients that had received infliximab therapy.
Figure 3.
The addition of infliximab in vitro to activated RA CD4+CD25− T cells induced a population of FoxP3+ T cells that were functionally suppressive. (A) Purified CD4+CD25− T cells from both healthy controls and active RA patients were stimulated with 2 μg/ml anti-CD3/CD28, ±10 μg/ml infliximab. In some wells, 2 μg/ml anti–TGF-β was added. Cells were cultured for 24 h and then intracellularly stained for FoxP3. Representative FACS plots are shown as well as the pooled data (n = 8 patients and 8 healthy individuals). Percentages shown are of the purified CD4+CD25− fraction. (B) Supernatants were collected from cells cultured in A before staining and were tested for TGF-β production by ELISA. (C) Purified CD4+CD25high T cells from healthy individuals and patients with active RA were incubated with 2 μg/ml anti-CD3/CD28, ±10 μg/ml infliximab, and the percentages of Foxp3+ cells are shown. (D) Histograms depicting CD62L expression in the T cells after culture as in A. Black line, isotype control; shaded area, CD62L. (E) CD4+CD25+ from the cultures in A were isolated, recultured at a 1:2 ratio with freshly isolated autologous CD4+CD25− T cells for an additional 2 d, and stained for TNF-α and IFN-γ. Data are depicted as the percentage of cytokine inhibition (n = 8).
We sought to confirm that the Foxp3+ T cells differentiated upon infliximab stimulation possess suppressor activity. Because the majority of purified CD4+CD25− T cells had acquired CD25 expression when stimulated with anti-CD3/CD28, with or without infliximab, the CD4+CD25+ T cells were purified and cultured with freshly isolated autologous CD4+CD25− T cells. Only the CD4+CD25+ T cells derived from active RA patients, but not healthy individuals, which had been cocultured with infliximab, were able to suppress production of IFN-γ and TNF-α from freshly isolated CD4+CD25− T cells (Fig. 3 E).
The potential of T reg cells to modulate immune responses has led to considerable interest in their use for the treatment of autoimmune disease. Two broad therapeutic approaches have been considered; first, to expand T reg cells in vitro with the intention of infusing these cells into patients, and second, to manipulate the immune system in vivo resulting in an increase in T reg cells. Infliximab, a monoclonal anti–TNF-α antibody, appears to be an example of the latter and demonstrates that inhibition of a proinflammatory cytokine can result in an expanded T reg cell population. Infliximab led to the differentiation of a population of T reg cells identified as FoxP3+CD25hiCD62L−CCR7− in patients with RA. These T reg cells are phenotypically distinct from natural T reg cells, which express CD62L and CCR7, but also differ functionally as their suppressor capacity depends on the production of TGF-β and IL-10. Murine studies have demonstrated the importance of IL-10 and/or TGF-β in providing increased regulatory potency to CD4+CD25+ T reg cells in the face of an inflammatory response (7, 15, 16) or in the prevention of graft rejection (17). In addition, IL-10–mediated suppression is used by CD4+CD25hiHLA-DR− T reg cells found in healthy individuals (18). Of relevance to this work, CD4+CD25+CD62L− T reg cells, producing both IL-10 and TGF-β, have been identified in diabetic mice treated with a combination of anti-CD3 and insulin (19). In patients with diabetes, anti-CD3 therapy expanded a population of CD4+CD25+CD62L− T cells (20). Thus, anti-CD3 treatment in the context of diabetes and anti–TNF-α therapy for RA both induce a population of T reg cells with a memory phenotype (CCR7−CD62L−) (21), perhaps suggesting a similar provenance.
The T reg cells found in post-infliximab RA patients may have arisen either from CD4+CD25− T cells that had already begun to differentiate along a Foxp3+ pathway in vivo, or from CD4+CD25− responder T cells. The purified CD4+CD25− T cells still contain a small fraction of Foxp3+ cells, which could be targeted by infliximab. CD127, which negatively correlates with Foxp3 expression, could be used to further exclude Foxp3+ cells in the CD4+CD25− T cells (22). However, TGF-β appeared to mediate the infliximab-driven increase in Foxp3+ T reg cells, which is known to target CD4+CD25− T cells rather than natural T reg cells (8–10). Moreover, the data shown in Fig. 3 C demonstrate that infliximab has no effect on CD4+CD25hiFoxp3+ T cells. Recent findings have revealed that TGF-β can also have a proinflammatory role dependent on the immunological environment (23). Thus, in the presence of proinflammatory cytokines, TGF-β contributes to the generation of immunopathogenic IL-17–secreting T cells (24–26). Consequently, the prerequisite for the generation of T reg cells, rather than IL-17–secreting T cells, by TGF-β is the removal of proinflammatory cytokines, specifically IL-6, but also TNF-α and IL-1. Infliximab can serve this function well as it is known to rapidly reduce levels of IL-6 (27), the critical partner for TGF-β in the production IL-17–producing T cells.
Although Foxp3 expression has been clearly linked to T reg cell activity in murine studies, this association has been less well established in humans. This is particularly true in the context of in vitro activation studies where transient expression of Foxp3 can occur (28). The ability of infliximab to confer a suppressive capacity on CD4+CD25− T cells could be explained by the presence of activated T cells producing the immunoregulatory cytokines IL-10 and TGF-β. These T reg cells could be similar to activated Tr1 or Th3 cells identified by others (4–6).
We have previously shown that CD4+CD25hi T reg cell function in RA patients was defective and that this defect was reversed after infliximab treatment (11). However, the data presented here lead to an important reinterpretation of our previous finding, specifically that the naturally derived T reg cells, defined as CD4+CD25hiCD62L+, appear to remain defective after treatment with anti–TNF-α therapy. The restoration of function of the CD4+CD25hi population after infliximab therapy is most likely to be due to the suppressive effects of the newly differentiated CD62L− T reg cells. In patients with RA receiving infliximab, the differentiation of adaptive T reg cells could be further amplified by their ability to confer a further suppressive activity on CD4+CD25− T cells (29). One could envisage a feedback mechanism in which infliximab drives the production of TGF-β by T cells, which would then induce their differentiation into FoxP3+CD62L− T reg cells. Why natural T reg cells remain defective after the neutralization of the proinflammatory environment by infliximab is unclear. It is possible that the reversal of the proinflammatory milieu by TNF-α blockade may only be partial, sufficient to allow the generation of T reg cells from CD4+CD25− T cells but insufficient to restore function to the natural CD62L+ T reg cells. It is tempting to speculate that the induction of T reg cells after infliximab therapy could lead to restoration of tolerance and might partly explain the exciting observation that infliximab can induce remission in some patients with early RA after cessation of therapy (30). In conclusion, our results indicate that T reg cells can be induced, and tolerance restored, by targeting specific proinflammatory cytokines such as TNF-α.
MATERIALS AND METHODS
Patient population.
31 patients with active RA, fulfilling the revised classification criteria of the American College of Rheumatology for RA, were evaluated before and 4–6 mo after anti–TNF-α therapy (see reference 11 for infliximab regime and further details for patient selection and assessment of disease activity, all of which remained the same for this study). 20 healthy individuals were used as controls. This study was approved by the University College London Hospital (UCLH) Ethics Committee.
Antibodies.
The following antibodies were used: FITC–anti-CD4 (RPA-T4), PE-Cy5–anti-CD25 (M-A251), PE–anti-CD62L (Dreg-56), PE–CY7-anti–TNF-α (Mab11), PE–Cy7-anti–IFN-γ (45.B3), PE-FoxP3 (PCH-101), APC–anti-CCR7 (3D12), and PE–anti-CD45RO (UCLH1). T cells were activated with soluble anti-CD3 (HIT-3a) and anti-CD28 (CD28.2) as indicated. All antibodies were from BD Biosciences, except FoxP3, which was from eBioscience. For neutralization experiments, anti–TGF-β1 (9016.2) and anti–human IL-10 (25209) were used (R&D Systems). Infliximab, a chimeric IgG1 anti–TNF-α mAb, was donated by Schering-Plough.
Cell isolation.
Human blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (GE Healthcare) and cultured in RPMI 1640 with 100 U/μg/ml penicillin/streptomycin (Life Technologies) and 10% FCS (Sera Laboratories International, Ltd.). Magnetic bead separation was performed as described previously (11), with all magnetic columns and magnetically labeled beads purchased from Miltenyi Biotec. To purify cells using FACS sorting (MoFlo), cells were stained with the above conjugated antibodies (CD4, CD25, and CD62L). Cells were sorted according to gates as indicated in Fig. S1.
Flow cytometric analysis and cytokine detection.
Cells were surface stained as described previously (11) using the above mentioned conjugated antibodies (CD4, CD25, and CD62L). For intracellular analysis of TNF-α and IFN-γ production, cells were cultured at 2 × 105 for 48 h with PMA, ionomycin, and Golgi Plug added in the final 5 h of culture. TGF-β1 was quantified using an ELISA kit (R&D Systems). In some experiments, 24-well transwell plates (Costar) with 0.4-μm membrane supports were used.
Proliferation assays.
In all experiments, T reg cells were cultured at 2 × 105 cells/ml, with CD4+CD25− T cell numbers adjusted accordingly for ratio experiments. Cells were cultured in 96-well U-bottomed plates (Nunc) for 5 d with [3H]thymidine added in last 18 h of culture. Proliferation was measured using a liquid scintillation counter.
Statistical analysis.
Statistical significance was determined using Student's t test, with p-values <0.05 regarded as statistically significant.
Supplemental Material
Acknowledgments
We thank Ayad Eddaoudi for providing technical assistance with MoFlo FACS sorting.
S. Nadkarni and C. Mauri have been supported by a Medical Research Council PhD studentship and the Wellcome Trust (grant no. 068629), respectively. This work was supported by UCLH Charity Trust and Schering-Plough Corporation.
The authors have no conflicting financial interests.
C. Mauri and M.R. Ehrenstein contributed equally to this work.
References
- 1.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 2.Levings, M.K., and M.G. Roncarolo. 2005. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr. Top. Microbiol. Immunol. 293:303–326. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. [DOI] [PubMed] [Google Scholar]
- 4.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 5.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukaura, H., S.C. Kent, M.J. Pietrusewicz, S.J. Khoury, H.L. Weiner, and D.A. Hafler. 1996. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J. Clin. Invest. 98:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green, E.A., L. Gorelik, C.M. McGregor, E.H. Tran, and R.A. Flavell. 2003. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. USA. 100:10878–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantini, M.C., C. Becker, G. Monteleone, F. Pallone, P.R. Galle, and M.F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng, S.G., J.D. Gray, K. Ohtsuka, S. Yamagiwa, and D.A. Horwitz. 2002. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 169:4183–4189. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenstein, M.R., J.G. Evans, A. Singh, S. Moore, G. Warnes, D.A. Isenberg, and C. Mauri. 2004. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu, S., A.C. Yopp, X. Mao, D. Chen, N. Zhang, M. Mao, Y. Ding, and J.S. Bromberg. 2004. CD4+ CD25+ CD62+ T-regulatory cell subset has optimal suppressive and proliferative potential. Am. J. Transplant. 4:65–78. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact–dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface–bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieckmann, D., C.H. Bruett, H. Ploettner, M.B. Lutz, and G. Schuler. 2002. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10–producing, contact-independent type 1–like regulatory T cells. J. Exp. Med. 196:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, H., B. Hu, D. Xu, and F.Y. Liew. 2003. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J. Immunol. 171:5012–5017. [DOI] [PubMed] [Google Scholar]
- 16.Uhlig, H.H., J. Coombes, C. Mottet, A. Izcue, C. Thompson, A. Fanger, A. Tannapfel, J.D. Fontenot, F. Ramsdell, and F. Powrie. 2006. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177:5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingsley, C.I., M. Karim, A.R. Bushell, and K.J. Wood. 2002. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168:1080–1086. [DOI] [PubMed] [Google Scholar]
- 18.Baecher-Allan, C., E. Wolf, and D.A. Hafler. 2006. MHC class II expression identifies functionally distinct human regulatory T cells. J. Immunol. 176:4622–4631. [DOI] [PubMed] [Google Scholar]
- 19.Bresson, D., L. Togher, E. Rodrigo, Y. Chen, J.A. Bluestone, K.C. Herold, and M. von Herrath. 2006. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 116:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold, K.C., J.B. Burton, F. Francois, E. Poumian-Ruiz, M. Glandt, and J.A. Bluestone. 2003. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala). J. Clin. Invest. 111:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huehn, J., K. Siegmund, J.C. Lehmann, C. Siewert, U. Haubold, M. Feuerer, G.F. Debes, J. Lauber, O. Frey, G.K. Przybylski, et al. 2004. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 199:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, W., A.L. Putnam, Z. Xu-Yu, G.L. Szot, M.R. Lee, S. Zhu, P.A. Gottlieb, P. Kapranov, T.R. Gingeras, B.F. de St Groth, et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver, C.T., L.E. Harrington, P.R. Mangan, M. Gavrieli, and K.M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 25.Mangan, P.R., L.E. Harrington, D.B. O'Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, and C.T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 27.Feldmann, M., F.M. Brennan, and R.N. Maini. 1996. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14:397–440. [DOI] [PubMed] [Google Scholar]
- 28.Gavin, M.A., T.R. Torgerson, E. Houston, P. DeRoos, W.Y. Ho, A. Stray-Pedersen, E.L. Ocheltree, P.D. Greenberg, H.D. Ochs, and A.Y. Rudensky. 2006. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. USA. 103:6659–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng, S.G., J.H. Wang, J.D. Gray, H. Soucier, and D.A. Horwitz. 2004. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 172:5213–5221. [DOI] [PubMed] [Google Scholar]
- 30.Quinn, M.A., P.G. Conaghan, P.J. O'Connor, Z. Karim, A. Greenstein, A. Brown, C. Brown, A. Fraser, S. Jarret, and P. Emery. 2005. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 52:27–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.