Abstract
Although there is evidence for distinct roles of myeloid dendritic cells (DCs [mDCs]) and plasmacytoid pre-DCs (pDCs) in regulating T cell–mediated adaptive immunity, the concept of functional DC subsets has been questioned because of the lack of a molecular mechanism to explain these differences. In this study, we provide direct evidence that maturing mDCs and pDCs express different sets of molecules for T cell priming. Although both maturing mDCs and pDCs upregulate the expression of CD80 and CD86, only pDCs upregulate the expression of inducible costimulator ligand (ICOS-L) and maintain high expression levels upon differentiation into mature DCs. High ICOS-L expression endows maturing pDCs with the ability to induce the differentiation of naive CD4 T cells to produce interleukin-10 (IL-10) but not the T helper (Th)2 cytokines IL-4, -5, and -13. These IL-10–producing T cells are T regulatory cells, and their generation by ICOS-L is independent of pDC-driven Th1 and Th2 differentiation, although, in the later condition, some contribution from endogenous IL-4 cannot be completely ruled out. Thus, in contrast to mDCs, pDCs are poised to express ICOS-L upon maturation, which leads to the generation of IL-10–producing T regulatory cells. Our findings demonstrate that mDC and pDCs are intrinsically different in the expression of costimulatory molecules that drive distinct types of T cell responses.
Myeloid DCs (mDCs) and plasmacytoid pre-DCs (pDCs) represent two major subsets of DCs in humans (1, 2). mDCs selectively express Toll-like receptor (TLR) 2–6 and 8 and have the ability to produce large amounts of IL-12 during antibacterial and antiviral responses, whereas pDCs express TLR7 and TLR9 and have the ability to produce large amounts of type 1 IFNs in antiviral immune responses (3–5). The ability of pDCs to rapidly produce large amounts of type 1 IFNs upon endosomal TLR7 and TLR9 activation has been linked to their unique and constitutive expression of IRF7 (6), the ability to rapidly form Myd88–IRAK1–IRAK4–TRAF6–IRF7 signal transduction complexes (7), and the unique nature of the endosome allowing prolonged retention of microbial-derived RNA and DNA (8). These findings have provided the functional and molecular basis for the concept that mDCs and pDCs play distinct and complementary roles in innate antimicrobial immunity.
mDCs and pDCs also play distinct roles in the regulation of T cell–mediated adaptive immunity (9, 10). However, the functional plasticity of both DC subsets coupled with the lack of a direct molecular mechanism to explain these differences has made it difficult to draw a definitive conclusion regarding the concept of functional DC subsets.
Previously, it has been shown that mDCs at an immature stage have the ability to prime naive T cells to differentiate into IL-10–producing T regulatory cells (11–13). Conversely, mDCs matured by microbial ligands for TLRs or by CD40 ligand (CD40L) prime naive T cells to differentiate into T helper (Th)1 effector cells (9, 14). Paradoxically, pDCs appear to have an intrinsic capacity to prime naive T cells to differentiate into IL-10–producing T regulatory cells at a mature stage either activated by IL-3 plus CD40L or by TLR9 ligands (15, 16).
In this study, we identify the molecular mechanism underlying this phenomenon. We found that in contrast to maturing mDCs, maturing pDCs rapidly and strongly up-regulate inducible costimulator (ICOS) ligand (ICOS-L) and specifically drive the generation of IL-10–producing T regulatory cells regardless of the activation pathway. Our findings provide the molecular evidence that pDCs are different from mDCs in regulating T cell–mediated adaptive immune responses through their intrinsic ability to up-regulate ICOS-L and induce IL-10–producing T regulatory cells after differentiation into mature DCs.
RESULTS
pDCs but not mDCs preferentially up-regulate ICOS-L upon activation
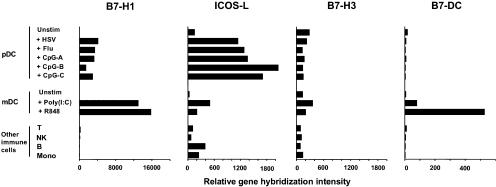
Recently identified B7-like molecules, including B7-H1, ICOS-L, B7-H3, and B7-DC, have been shown to costimulate T cells for IL-10 production (17–20). To test whether a distinct expression pattern of these B7-like molecules determines the capacity of maturing pDCs but not maturing mDCs to generate IL-10–producing T cells, we compared microarray gene expression profiles of B7 superfamily members in pDCs and mDCs activated in culture by a variety of stimuli. Maturing pDCs and maturing mDCs strongly up-regulated the expression of the classic costimulatory molecules CD80 and CD86 (not depicted). However, compared with maturing mDCs, maturing pDCs preferentially up-regulated the expression of ICOS-L but not B7-H1, B7-H3, B7-DC (Fig. 1), or B7-H4 (undetectable; not depicted).
Figure 1.
ICOS-L is preferentially expressed by activated pDCs and not mDCs. Microarray gene expression profiles of B7-like molecules B7-H1, ICOS-L, B7-H3, and B7-DC in freshly isolated unstimulated (unstim) pDCs and pDCs activated by different stimuli in comparison with freshly isolated unstimulated mDCs, mDCs activated by different stimuli, and other primary immune cells of the blood. Results of the gene expression profiles are shown as the relative gene hybridization intensity level.
Strong ICOS-L expression on activated pDCs is maintained upon differentiation into mature DCs along both TLR- and IL-3/CD40L-dependent pathways
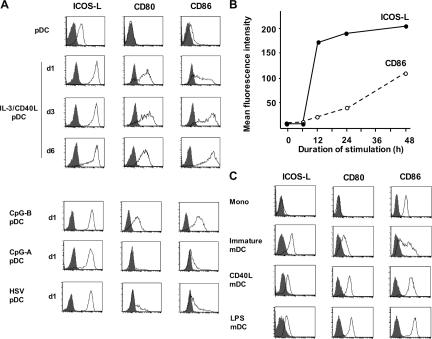
To confirm the gene expression data and gain further insights into the regulation of ICOS-L during pDC maturation, we performed flow cytometry for surface ICOS-L expression. Freshly isolated blood pDCs constitutively expressed ICOS-L (log 1 fluorescence intensity) while lacking CD80 and expressing minimal levels of CD86 (Fig. 2 A) as previously reported (21). pDCs stimulated with IL-3 plus CD40L gradually up-regulated CD80 and CD86 expression and became fully mature DCs within 6 d of culturing. In contrast, the up-regulation of ICOS-L was very strong and occurred very rapidly (reaching log 2–3 fluorescence intensity within the first 24 h of culturing; Fig. 2 A). Importantly, the high levels of ICOS-L expression persisted during the 6-d process of DC maturation (Fig. 2 A).
Figure 2.
In contrast to mDCs, pDCs up-regulate ICOS-L upon activation and maintain high expression levels upon differentiation into mature DCs. (A) Surface expression of ICOS-L, CD80, and CD86 in freshly isolated untreated pDCs and pDCs activated along the IL-3–dependent pathway with IL-3 plus CD40L for 6 d or along the TLR-dependent pathway with CpG-B, CpG-A, or HSV for 1 d. Open histograms represent staining of costimulatory molecules; closed histograms represent isotype controls. (B) Mean fluorescence intensity of ICOS-L and CD86 expression in pDCs activated by CpG-B monitored at different points over a 48-h period of maturation into DCs. (C) Surface expression of ICOS-L, CD80, and CD86 on blood monocytes (Mono), monocyte-derived DCs cultured for 5 d with GM-CSF and IL-4 (immature mDC), and mature mDCs activated along the CD40L pathway (CD40L-mDC) or the TLR-dependent pathway (LPS-mDC). Results in A–C are representative of at least five independent experiments.
Similarly, pDCs stimulated with three TLR9 agonists, CpG-A, CpG-B, and HSV, up-regulated ICOS-L expression very strongly (log 2–3 fluorescence intensity) within the first 24 h of culturing (Fig. 2 A). However, of the TLR9 agonists, only CpG-B considerably up-regulated CD80 and CD86 expression within 24 h of stimulation. The dissociation between the expression of ICOS-L and the expression of CD80 and CD86 was further confirmed by a time-course analysis of pDC stimulated with CpG-B. Whereas maximal ICOS-L expression levels were reached within 12 h of stimulation, the up-regulation of CD80 and CD86 was a gradual process reaching its maximum after 48 h (Fig. 2 B). As was the case in pDCs matured by IL-3 and CD40L, the high ICOS-L expression persisted during the 48-h maturation process of CpG-B–stimulated pDCs. These data indicate that irrespective of the nature of the activation stimulus, pDCs rapidly express high levels of ICOS-L and maintain the expression after differentiation into fully mature DCs.
Unlike pDCs, mDCs down-regulate the expression of ICOS-L on maturation
Next, we investigated the regulation of ICOS-L expression in monocyte-derived DCs as a model system for mDCs. ICOS-L was not expressed by freshly isolated blood monocytes and was expressed at low levels in monocyte-derived immature mDCs induced by IL-4 and GM-CSF (log 1 fluorescence intensity; Fig. 2 C). Activation of immature mDCs by CD40L or TLR4 ligand LPS strongly up-regulated the expression of CD80 and CD86 but down-regulated ICOS-L expression (Fig. 2 C; reference 11). Thus, whereas both maturing pDCs and maturing mDCs expressed high levels of CD80 and CD86, maturing pDCs but not maturing mDCs express high levels of ICOS-L. This difference is not dependent on the nature of the activation stimulus (TNF-R– vs. TLR-mediated activation) but may reflect an intrinsic difference between pDCs and mDCs.
Maturing pDCs use ICOS-L to induce the differentiation of naive CD4 T cells into IL-10–producing T cells during Th1 and Th2 responses
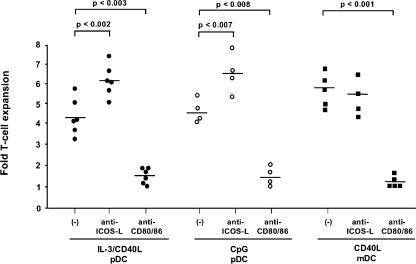
Because ICOS-L has been previously linked to the induction of IL-10 (22–24), we sought to determine whether ICOS-L on maturing pDCs is required for the differentiation of naive T cells into IL-10–producing T cells. Maturing pDCs generated in culture with CpG-B (named CpG-pDC) or IL-3 plus CD40L (named IL-3/CD40L-pDC) and maturing mDCs activated by CD40L (named CD40L-mDC) were cultured with allogeneic peripheral blood–derived naive CD4 T cells. We found that ICOS was expressed at very low levels on naive T cells but was substantially induced within 1 d of culturing with maturing pDCs and mDCs, reaching maximal expression levels within 4 d (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061660/DC1). To determine a role for ICOS-L in these primary cultures, we blocked ICOS costimulation by neutralizing mAbs against ICOS-L in comparison with a CD28 blockade by neutralizing mAbs against CD80 plus CD86. CpG-pDCs, IL-3/CD40L-pDCs, and CD40L-mDCs all induced strong expansion of naive T cells that was largely blocked by neutralizing mAbs against CD80 and CD86 but not against ICOS-L, confirming that CD28 is the most prominent costimulatory pathway for the DC-mediated expansion of naive T cells (Fig. 3). However, we observed that blocking ICOS costimulation significantly enhanced the expansion of naive T cells in cultures with CpG-pDCs and IL-3/CD40L-pDCs but not CD40L-mDCs, suggesting a role for ICOS-L in primary T cell activation by maturing pDCs (Fig. 3).
Figure 3.
Both maturing mDCs and pDCs use CD28 costimulation to expand naive T cells. Naive peripheral CD4 T cells were cocultured with IL-3/CD40L-pDCs, CpG-pDCs, or CD40L-mDCs in the presence of neutralizing antibodies against ICOS-L (anti–ICOS-L) and against CD80 plus CD86 (anti-CD80/86) or were cocultured with isotype-matched control antibodies (−). After 6 d of culturing, T cell numbers were measured using trypan blue exclusion, and the T cell expansion was calculated. Each symbol represents an independent experiment, and horizontal bars represent the mean. P-values calculated by unpaired Student's t test are indicated.
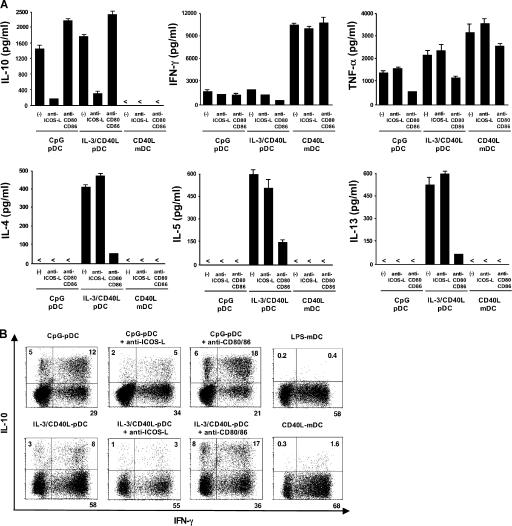
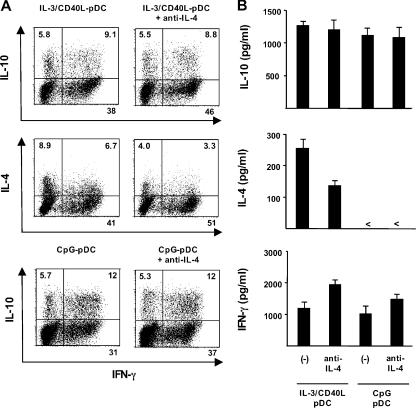
To determine whether ICOS-L on maturing pDCs is involved in the polarization of naive T cells, we tested the ability of primed T cells to secrete cytokines in response to a polyclonal restimulation. T cells primed by CpG-pDCs produced large amounts of IL-10 (800–6,400 pg/ml; n = 6), moderate levels of IFN-γ and TNF-α, but no IL-4, -5, or -13 (Fig. 4 A). This cytokine profile is consistent with a Th1-like response as previously reported (25). The blocking of ICOS costimulation by neutralizing anti–ICOS-L mAbs significantly inhibited the production of IL-10 (mean reduction of 74 ± 14%; P < 0.01) but not of IFN-γ or TNF-α (Fig. 4 A). In contrast, blocking CD28 costimulation by neutralizing anti-CD80 and -CD86 mAbs led to an increased production of IL-10 (mean increase of 40 ± 17%; P < 0.01) but not of IFN-γ or TNF-α (Fig. 4 A). T cells primed with IL-3/CD40L-pDCs produced large amounts of IL-10 (900–7,500 pg/ml; n = 6), substantial levels of IL-4, -5, and -13, and moderate levels of IFN-γ and TNF-α (Fig. 4 A). This cytokine profile is consistent with a Th2-type response as previously reported (9). Blocking ICOS costimulation significantly inhibited the production of IL-10 (mean reduction of 80 ± 10%; P < 0.01) but did not inhibit the production of IL-4, -5, or -13 nor IFN-γ or TNF-α (Fig. 4 A). In contrast, blocking CD28 costimulation led to an increased production of IL-10 (mean increase of 27 ± 12%; P < 0.01) while inhibiting the production of IL-4, -5, -13 (>70% reduction for all cytokines), and partially IFN-γ and TNF-α (Fig. 4 A). Confirming the ELISA data, we found on the single-cell level that the generation of IL-10–producing T cells from naive CD4 T cells was strongly inhibited by anti–ICOS-L mAbs in cultures with CpG-pDCs (decrease from 17 to 7% of IL-10–producing T cells) and IL-3/CD40L-pDCs (decrease from 11 to 4%) but was enhanced by anti-CD80 and -CD86 antibodies in cultures with CpG-pDCs (increase from 17 to 25%) and IL3/CD40L-pDCs (increase from 11 to 23%; Fig. 4 B). Interestingly, over two thirds of IL-10–producing T cells coexpressed IFN-γ (Fig. 4 B), but all IL-10–producing T cells were clearly negative for IL-4, -5, or -13 (not depicted). Consistent with their low expression of ICOS-L, maturing mDCs did not generate detectable numbers of IL-10–producing T cells even in the presence of anti-CD80 and -CD86 antibodies (Fig. 4, A and B). Importantly, naive T cells stimulated under neutral conditions by anti-CD3 did not produce IL-10, indicating that ICOS-L on maturing pDCs drive the de novo generation and not the expansion of preexisting IL-10–producing T cells (Fig. 5).
Figure 4.
Maturing pDCs but not mDCs use ICOS-L to specifically generate T cells producing IL-10 during the induction of both Th1 and Th2 responses. Peripheral naive CD4 T cells were cocultured with CpG-pDCs, IL-3/CD40L-pDCs, LPS-mDCs, and CD40L-mDCs in the presence of neutralizing antibodies against ICOS-L (anti–ICOS-L), CD80 plus CD86 (anti-CD80/86), or isotype-matched control antibodies (−). (A) The ability of primed T cells to secrete IL-10, -4, -5, -13, IFN-γ, and TNF-α was assayed by ELISA of supernatants collected 24 h after polyclonal stimulation with anti-CD3 and -CD28 mAbs. The results are representative of six independent experiments. < indicates that the measured value was below the detection limit of the assay experiments (<20 pg/ml). T cells primed by LPS-mDCs (not depicted) secreted an equivalent cytokine profile as T cells primed by CD40L-mDCs. Error bars represent SD. (B) Intracellular production of IL-10 and IFN-γ assayed by flow cytometry 6 h after the stimulation of primed T cells with PMA and ionomycin. The percentages of each population are indicated in the plots. One of three independent experiments is shown.
Figure 5.
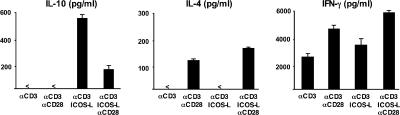
The generation of IL-10 and IL-4 are differentially regulated by ICOS-L and CD28 costimulation, respectively. Peripheral naive CD4 T cells were cultured for 6 d on parental L cells or ICOSL-L cells, which were precoated with 0.2 μg/ml anti-CD3 mAb stimulating 1 μg/ml anti-CD28 mAb or isotype-matched control antibodies were added into the culture. The ability of primed T cells to secrete IL-10, -4, and IFN-γ was assayed by ELISA of supernatants collected 24 h after polyclonal stimulation with anti-CD3 and -CD28 mAbs. The results are representative of three independent experiments. < indicates that the measured value was below the detection limit of the assay (<20 pg/ml). Error bars represent SD.
To confirm the capacity of ICOS-L on maturing pDCs to differentiate truly naive T cells into IL-10–producing T cells, we performed additional coculture experiments using naive CD4 T cells isolated from cord blood. Maturing pDCs primed naive cord blood–derived T cells to produce very large amounts of IL-10 (7,000–110,000 pg/ml primed by CpG-pDCs, n = 3; and 4,000–30,000 pg/ml primed by IL-3/CD40L-pDCs, n = 3). The blocking of ICOS costimulation significantly inhibited the ability of primed T cells to produce IL-10 (mean reduction of 60 ± 18%; P < 0.01) but not their ability to produce IFN-γ or IL-4 (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061660/DC1).
Together, these results indicate that maturing pDCs use ICOS-L to drive the differentiation of naive T cells into T cells producing IL-10 in the context of Th1 and Th2 polarization. Interestingly, the functions of ICOS costimulation do not overlap with those of CD28-mediated costimulation. Whereas ICOS costimulation is critical for the generation of IL-10–producing T cells, CD28 costimulation inhibits the generation of these cells but is required for both T cell expansion and production of Th2 cytokines IL-4, -5, and -13 and, to some extent, for the production of Th1 cytokines IFN-γ and TNF-α. Importantly, the generation of IL-10–producing T cells by ICOS-L occurs regardless of the functional plasticity of pDCs to induce Th1 or Th2 polarization upon activation by a TLR-dependent or IL3/CD40L pathway.
ICOS-L is sufficient to drive the generation of IL-10–producing T cells in the absence of Th2 polarization
Historically, IL-10 has been associated with Th2 cytokines IL-4, -5, and -13 (26). However, our neutralization experiments suggested that the induction of IL-10 versus Th2 cytokines may be differentially regulated by ICOS and CD28 costimulation. To further confirm these findings, we stimulated naive CD4 T cells with anti-CD3 alone or anti-CD3 in the presence of ICOS-L and/or CD28 costimulation. Naive T cells stimulated by anti-CD3 alone did not differentiate to produce IL-10 or -4 (Fig. 5), whereas the addition of ICOS-L stimulation generated T cells producing high levels of IL-10 but not IL-4, and the addition of CD28 costimulation generated T cells producing substantial levels of IL-4 but not IL-10 (Fig. 5). These data indicate that ICOS-L is sufficient to drive the generation of IL-10–producing T cells in the absence of Th2 cytokine IL-4 and confirm that the generation of T cells producing IL-10 versus IL-4 is differentially regulated by ICOS and CD28 costimulation. Furthermore, naive T cells stimulated by anti-CD3 alone produced considerable levels of IFN-γ that was enhanced by the presence of CD28 costimulation but not by ICOS-L (Fig. 5), confirming that ICOS costimulation is not involved in Th1 polarization. Similar to what we have described in the blocking experiments, the presence of CD28 costimulation decreased the ICOS-L–driven generation of IL-10–producing T cells (Fig. 5).
IL-4 represents a master regulator of Th2 polarization (27). To ascertain that IL-4 is not involved in the generation of IL-10–producing T cells by maturing pDCs, we performed blocking experiments using neutralizing anti–IL-4 mAbs. Although pDCs are unable to produce IL-4 (9), this cytokine may originate from developing Th2 cells themselves. Blocking of IL-4 partially inhibited the ability of naive T cells primed by IL3/CD40L-pDCs to produce Th2 cytokine IL-4, but it had no effect on the generation of IL-10–producing T cells (Fig. 6). These data suggest that the development of IL-10–producing T cells is independently regulated; however, we cannot completely rule out the possibility that ICOS-L–induced IL-10 may at least in part depend on residual IL-4 given that anti–IL-4 only partially inhibited Th2 development in our culture system with IL3/CD40L-pDCs. Priming of naive T cells with CpG-pDCs in the presence of anti–IL-4– blocking mAbs did not affect IL-10 production, confirming that the ICOS-L–driven induction of IL-10 can occur in the absence of a Th2 response (Fig. 6).
Figure 6.
Blocking of IL-4 does not inhibit IL-10 induction in Th1 cells in response to ICOS-L on maturing CpG-pDCs. Peripheral naive CD4+ T cells were cocultured with IL-3/CD40L-pDCs or CpG-pDCs in the presence of neutralizing antibodies against IL-4 or isotype-matched control antibodies (−). (A) Intracellular production of IL-10 and IFN-γ (top and bottom plots) as well as IL-4 and IFN-γ (middle plots) was assayed by flow cytometry 6 h after the stimulation of primed T cells with PMA and ionomycin. The percentages of each population are indicated in the plot. (B) The ability of primed T cells to secrete IL-10, -4, or IFN-γ was assayed by ELISA of supernatants collected 24 h after polyclonal stimulation with anti-CD3 and -CD28 mAbs. One representative experiment of four independent experiments is shown. < indicates that the measured value was below the detection limit of the assay (<20 pg/ml). Error bars represent SD.
IL-10–producing T cells driven by ICOS-L on maturing pDCs are T regulatory cells
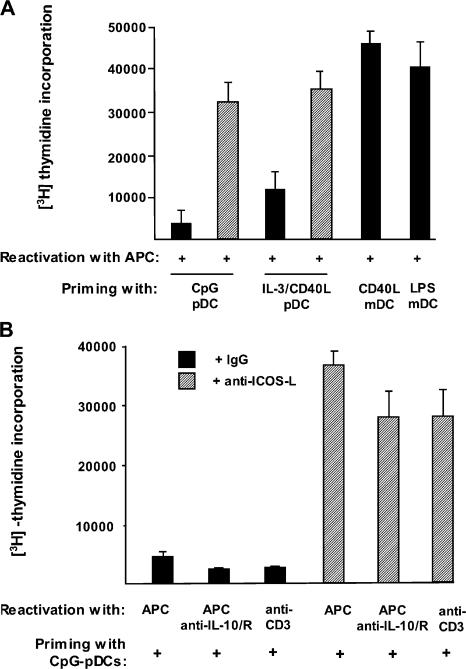
Three observations prompted us to determine whether IL-10–producing T cells primed by maturing pDCs through ICOS costimulation are T regulatory cells. First, the IL-10+IFN-γ+ T cell profile induced by ICOS-L was reminiscent of T regulatory type 1 cells (28). Second, the inhibition of ICOS costimulation enhanced the ability of maturing pDCs to expand T cells, suggesting that IL-10–producing T cells are poorly proliferative, which is a cardinal feature of T regulatory cells. Finally, ICOS costimulation has been implicated in the induction of T regulatory cell–mediated peripheral tolerance (29–31). First, we examined the ability of T cells primed with maturing pDCs to proliferate in response to an alloantigen-specific reactivation. Both T cells primed with CpG-pDCs and IL-3/CD40L-pDCs proliferated poorly in response to the reactivation (Fig. 6 A). A mean reduction of 80 ± 15% (n = 3) and 70 ± 20% (n = 3) in their proliferative response was observed compared with T cells primed with CD40L-mDCs. In contrast, T cells primed with both CpG-pDCs and IL-3/CD40L-pDCs in the presence of neutralizing anti–ICOS-L antibodies proliferated at similar levels to CD40L-mDCs in response to the reactivation (Fig. 7 A). Given that T cells primed with maturing pDCs produce IL-10, we tested whether the reduced proliferation was caused by an inhibition of APC function by IL-10 secreted during the reactivation. This was not the case because the neutralization of IL-10 during the reactivation failed to reverse the low proliferation of T cells primed with maturing pDCs (Fig. 7 B). Also, reactivation in an APC-free system by low doses of anti-CD3 antibodies failed to reverse the low proliferation of T cells primed with maturing pDCs, confirming that these T cells have the intrinsic anergic phenotype of T regulatory cells. ICOS costimulation was required to induce this state of anergy because T cells proliferated vigorously if neutralizing anti–ICOS-L antibodies were added during priming with maturing pDCs (Fig. 7 B).
Figure 7.
IL-10–producing T cells generated by ICOS-L on maturing pDCs are anergic. (A) CD4 T cells primed by CpG-pDCs, IL-3/CD40L- pDCs, LPS-mDCs, and CD40L-mDCs in the presence of neutralizing anti–ICOS-L mAbs (hatched bars) or isotype-matched control antibodies (black bars) were collected after 6 d and reactivated with allogeneic monocytes from the same donor whose cells were used for priming. The ability of primed T cells to mount a proliferative response was assessed by 3[H]thymidine incorporation after 2 d. (B) CD4 T cells primed by CpG-pDCs in the presence of neutralizing anti–ICOS-L mAbs (hatched bars) or isotype-matched control antibodies (black bars) were either reactivated by allogeneic monocytes (APC) or low doses of anti-CD3 (1 μg/ml; plate bound) plus or minus neutralizing mAbs to IL-10 and -10R. (A and B) Results are expressed as mean counts per minute + SD (error bars) of triplicate wells and are representative of three independent experiments.
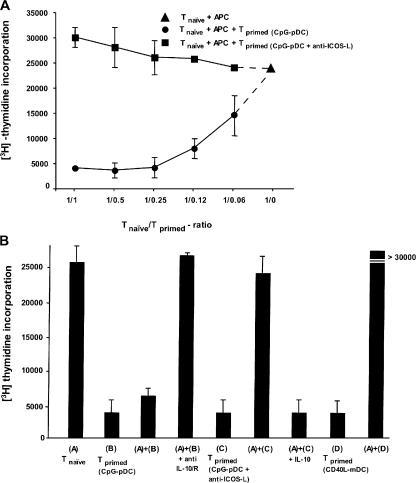
To determine whether these T cells are immunosuppressive, T cells primed with maturing pDCs were added into a coculture of autologous naive T cells with monocytes derived from the original allogeneic donor. T cells primed with maturing pDCs strongly inhibited the proliferation of naive T cells in a dose-dependent manner (Fig. 8 A). In contrast, T cells primed with maturing pDCs in the presence of neutralizing anti–ICOS-L failed to do so (Fig. 8 A). To further investigate whether IL-10 was involved in immunosuppression by T regulatory cells primed with maturing pDCs, neutralizing antibodies to IL-10 and -10R were added into the cocultures. Anti–IL-10 completely blocked the inhibitory effect of these T regulatory cells (Fig. 8 B), indicating that the suppression was mediated by IL-10. Accordingly, T cells primed with maturing pDCs in the presence of neutralizing anti–ICOS-L (which secrete low levels of IL-10) or T cells primed with maturing mDCs (which do not secrete any IL-10) failed to suppress the proliferation of naive T cells (Fig. 8 B). A role of IL-10 in suppression was further confirmed by the finding that the inhibitory effect of T cells primed with maturing pDCs could be mimicked by the addition of low doses of IL-10 into the cultures (Fig. 8 B). Thus, maturing pDCs have the unique ability to generate IL-10–producing T regulatory cells through their high expression of ICOS-L. These IL-10–producing T cells are anergic and potently suppress bystander T cell responses by secreting IL-10.
Figure 8.
IL-10–producing T cells generated by ICOS-L on maturing pDCs are T regulatory cells. (A) The capacity of primed T cells to suppress primary T cell responses was tested by stimulating naive CD4+ T cells with allogeneic monocytes (APC) in the presence of decreasing numbers of syngeneic T cells primed by CpG-pDCs alone (circles) or CpG-pDCs in the presence of anti–ICOS-L mAbs (squares). 3[H]thymidine incorporation was assessed after 6 d. Results are representative of three independent experiments. Importantly, the maximal proliferation of restimulated T cells primed with CpG-DCs or CpG-DCs plus anti–ICOS-L alone after 6 d was <5,000 cpm. (B) Naive CD4+ T cells were cultured with allogeneic monocytes alone (A), monocytes plus T cells primed by CpG-pDCs (B), monocytes plus T cells primed by CpG-pDCs in the presence of anti–ICOS-L mAbs (C), and monocytes plus T cells primed by maturing mDCs (D). The role of IL-10 in the suppressive activity of primed T cells was determined by adding anti–IL-10/IL-10R mAbs (anti-IL10/R) or recombinant human IL-10 to the suppression assay. The results are representative of five experiments. Similar to T cells primed by CpG-pDCs, T cells primed by IL-3/CD40L-pDCs suppressed primary T cell responses (not depicted). (A and B) Results are expressed as mean counts per minute + SD (error bars) of triplicate wells.
DISCUSSION
Although there is evidence for distinct roles of mDCs and pDCs in regulating T cell–mediated adaptive immunity, the concept of functional DC subsets has been questioned because of the plasticity of both subsets and the lack of a molecular correlate to explain these differences. Now, we provide the direct molecular evidence that maturing mDCs and maturing pDCs are intrinsically distinct in that they express different sets of molecules for T cell priming. Although both mDCs and pDCs up-regulate the expression of CD80 and CD86 upon activation, only pDCs up-regulate the expression of ICOS-L and maintain high expression levels upon differentiation into fully mature DCs along both TLR- and IL-3/CD40L-dependent activation pathways. The high levels of ICOS-L expression endow maturing pDCs with a dominant costimulatory pathway leading to the differentiation of naive CD4 T cells into T cells producing IL-10 but not Th2 cytokines IL-4, -5, and -13 or Th1 cytokines IFN-γ and TNF-α during the induction of Th2 and Th1 polarization. In contrast, maturing mDCs down-regulate ICOS-L expression upon activation by TLR ligands or CD40L and, accordingly, fail to induce T cells producing IL-10.
Historically, IL-10 production has been linked to the production of Th2 cytokines IL-4, -5, and -13 (26). However, recent studies have reported that IL-10 is not involved in Th2-mediated allergic responses (32, 33) and may even inhibit such responses (34). Our study now provides evidence for a distinct regulation of IL-10 and Th2 cytokine induced by maturing pDCs. First, we show that IL-10 and Th2 cytokines are differentially induced by ICOS-L and CD28 costimulation. Blocking ICOS-L on maturing pDCs inhibits the specific production of IL-10 without affecting the induction of Th2 cytokines IL-4, -5, and -13. Conversely, blocking CD28 costimulation inhibits the production of Th2 cytokines without affecting IL-10. Second, we show that TLR-activated pDCs, which inhibit Th2 development and drive Th1 polarization through the secretion of type 1 IFNs (35), retain the ability to generate IL-10–producing T cells. Finally, we show that blocking IL-4, a cytokine that has an instructive role in directing Th2 development, decreases the generation of Th2 cells but does not affect the generation of IL-10–producing cells. Although we cannot exclude that under Th2 conditions, ICOS-L–induced IL-10 may depend at least in part on IL-4, these data strongly suggest that the ICOS-L–driven induction of IL-10 and the generation of Th2 cells are independently regulated on maturing pDCs. In line with our findings, ICOS-deficient patients were found to have decreased numbers of IL-10–producing T cells but normal numbers of Th2 cells and IFN-γ–producing T cells (36). Furthermore, on the transcriptional level, it was demonstrated that the induction of IL-10 can occur in the absence of IL-4–driven Th2 development (37). However, our study is in contrast to a recent publication showing that blocking ICOS-L inhibits the production of IL-10 as well as Th2 cytokines by CD45RO+ memory T cells cultured with pDCs, suggesting that there may be differences in the role of ICOS costimulation in primary versus memory T cell responses (38).
IL-10–producing T cells induced by ICOS-L on maturing pDCs have characteristics of T regulatory cells with the ability to suppress bystander T cell activation through the secretion of IL-10. In recent years, there has been accumulating evidence that there may be a specialized role for pDCs in peripheral tolerance, although the molecular basis for this has not been identified. pDCs were shown to suppress inflammatory responses to inhaled allergens (39), microbial infections (40), and tumors (41), to promote allogeneic stem cell engraftment (42), and to mediate tolerance to solid grafts by inducing T regulatory cells (43). Our study now identifies a specialized molecular pathway that intimately links maturing pDCs to the generation of T regulatory cells through ICOS-L. ICOS-L has been previously implicated in the generation of IL-10–producing T regulatory cells in both human studies (31) and mouse disease models (29, 30, 44). Furthermore, a specific defect in T regulatory cell–mediated mucosal tolerance has been shown in ICOS-deficient (ICOS−/−) mice (45), and increased susceptibility to autoimmune disease has been described in ICOS-deficient patients (36). The fact that pDCs are intimately linked to the generation of IL-10–producing T regulatory cells upon activation and differentiation into mature DCs is in striking contrast to the fact that mDCs drive the generation of IL-10–producing T regulatory cells at immature stages and lose this ability upon maturation (11–13). These findings may suggest a specialized role for pDCs in immunoregulation during infection and inflammation.
There is indisputable evidence that pDCs can drive protective antiviral inflammation through their ability to produce type 1 IFNs, which promotes the function of bystander mDCs (46–48), B cells (49), T cells (50), and NK cells (51, 52). However based on our findings, maturing pDCs have an intrinsic property to inhibit immune responses by inducing IL-10–producing T regulatory cells when directly presenting Ag to T cells. These IL-10–producing T regulatory cells may prevent excessive inflammation, which could damage the host. In support of our hypothesis, the depletion of pDCs during viral infection exacerbates immunopathology of the infected host (53), and the absence of IL-10 leads to uncontrolled lethal immune responses to infections (54).
In conclusion, we define the molecular pathway underlying the specialized property of pDCs but not mDCs to generate IL-10–producing T regulatory cells at a mature differentiation stage. We show that in contrast to mDCs, pDCs are poised to express ICOS-L upon maturation, which directly leads to the generation of IL-10–producing regulatory T cells independently of Th2 or Th1 polarization. Our data provide the formal proof that pDCs and mDCs represent distinct DC subsets that evolved to perform different functions in adaptive immunity.
MATERIALS AND METHODS
Media and reagents.
For cell stimulation, we used 10 ng/ml IL-3 (R&D Systems), 2 μM CpG type A (2216), type B (2006), and type C (C274), 1 μg/ml LPS (from Escherichia coli; 0111:B4; InvivoGen), 25 μg/ml poly(I:C) (InvivoGen), 1 μg/ml R848 (InvivoGen), γ-irradiated HSV-1 (KOS strain; 10 PFU/cell), and the influenza virus (PR8 strain; 10 PFU/cell). For CD40 stimulation, we used CD40L-transfected L cells as previously described (9).
Generation of maturing pDCs and maturing mDCs.
This study was approved by the Institutional Review Board for Human Research at the M.D. Anderson Cancer Center. pDCs were isolated from the buffy coat of healthy adult volunteers as previously described (5). In brief, pDCs were first enriched by positive immunoselection using anti–BDCA-4 microbeads (Miltenyi Biotec) and were sorted by FACS (Aria; BD Biosciences) according to their CD4+, CD123+, CD11c−, CD3−, CD14−, CD15−, CD16−, CD19−, and CD56− phenotype to reach >99% purity according to their BDCA-2 expression. The sorted cells were seeded at a density of 105 cells/200 μl of medium in flat-bottomed 96-well plates with each activation stimulus. For T cell coculture experiments, maturing pDCs were generated from pDCs by culturing with IL-3 in the presence of irradiated CD40L-transfected L cells for 6 d or with 2 μM of type B CpG (2006) for 2 d. Immature mDCs were generated from blood monocytes obtained from total PBMCs using anti-CD14 microbeads cultured for 5 d with GM-CSF and IL-4. Maturing mDCs were then generated by stimulating immature mDCs with LPS or CD40L-transfected L cells for 1 d.
Microarray analysis and bioinformatics.
Total RNA was immediately isolated with the RNeasy kit (QIAGEN) and used to generate cDNA according to the Expression Analysis Technical Manual from Affymetrix, Inc. Complementary RNA samples were generated with the BioArray HighYield RNA Transcript Labeling kit (Enzo Life Sciences) and the human genome U133 plus 2.0 array (Affymetrix, Inc.) according to the manufacturers' protocols. The scanned images were aligned and analyzed using GeneChip software (Microarray Suite 5.0; Affymetrix, Inc.) according to the manufacturer's instructions. The signal intensities were normalized to the mean intensity of all of the genes represented on the array, and global scaling (scaling to all probe sets) was applied before performance of the comparison analysis.
Analyses of surface ICOS-L expression.
To evaluate surface ICOS-L expression, maturing pDCs and mDCs were stained with PE-labeled anti–ICOS-L mAbs (eBioscience) or isotype-matched control mAbs after being harvested at different times during the culture and subjected to FACS (FACSCalibur; BD Biosciences). For comparison, the cells were also stained with mAbs to CD80 and CD86 (both from BD Biosciences).
CD4 T cell stimulation.
Fresh peripheral blood naive CD4 T cells (>99% pure) were isolated using a CD4 T cell isolation kit (T cell Isolation Kit II; Miltenyi Biotec) followed by cell sorting as CD4+ CD45RA+ CD45RO− CD25− Lineage− (CD14−, CD16−, CD20−, CD56−, γδTCR−, and BDCA2−) cells as previously described (33). Alternatively, fresh cord blood–derived naive CD4 T cells were isolated from fresh cord blood by using T cell Isolation Kit II as previously described (11). This procedure was followed by a depletion of CD25+ cells to deplete T regulatory cells. The resulting cells were routinely >96% of CD4+ CD45RA+ CD45RO− CD25− T cells. 2 × 104 freshly purified allogeneic naive CD4 T cells were then cultured with maturing pDCs or maturing mDCs (DC/T cell ratio of 1:5) in round-bottomed 96-well culture plates for 6 d. We used 50 μg/ml anti–ICOS-L mAb (eBioscience) and a combination of 5 μg/ml anti-CD80 and 10 μg/ml anti-CD86 mAbs (R&D Systems) in our cultures. Mouse IgG1 was used as an isotype control. For IL-4–blocking experiments, 10 μg/ml anti–IL-4 mAb (MP4-25D2; BD Biosciences) was added to the cultures. For restimulation experiments, primed CD4+ T cells collected after 6 d were recultured with monocytes from the same allogeneic donor used for priming (monocyte/T cell ratio of 1:1) for 3 d.
Analyses of T cell cytokine production.
Primed CD4 T cells were collected and washed after 6 d of primary stimulation with DCs. For the detection of cytokine production in the culture supernatants, the T cells were restimulated with 5 μg/ml of plate-bound anti-CD3 (OKT3) and 1 μg/ml of soluble anti-CD28 mAb at a concentration of 106 cells/ml for 24 h. The levels of IL-10, -4, -5, -13, TNF-α, and IFN-γ were measured by ELISA (all kits were obtained from R&D Systems). For intracellular cytokine production, the primed CD4 T cells were restimulated with 50 ng/ml PMA plus 2 μg/ml ionomycin for 6 h. 10 μg/ml brefeldin A was added during the last 2 h. The cells were stained with the combination of fluorochrome-labeled mAbs to IL-10, -4, and IFN-γ.
Generation of human ICOS-L–expressing L cells.
Human ICOS-L–expressing L cells were generated by transducing parental CD32 L cells with a mouse stem cell virus–based retroviral vector (pMIGW2) carrying the full-length human ICOS-L coding sequence (GenBank EMBL/DDBJ accession no. NM_015259). A high level and stable expression of human ICOS-L in the transduced cells were confirmed by FACS analysis with PE-labeled anti–human ICOS-L mAbs (eBioscience).
Suppressor assay.
In direct coculture experiments, 4 × 104 naive CD4 T cells were cultured in round-bottomed 96-well plates with 4 × 104 allogeneic monocytes in the presence of equal numbers of syngeneic CD4 T cells primed by pDC-derived DCs. After 6 d of culture, 1 μCi [3H]thymidine was added to each well, and cellular incorporation was determined after 12 h. In some experiments, 10 ng/ml of neutralizing anti–IL-10 mAb plus 10 ng/ml anti–IL-10R mAb (R&D Systems) or recombinant human IL-10 was added.
Online supplemental material.
Fig. S1 shows the rapid upregulation of ICOS on naive T cells cultured with maturing pDCs and maturing mDCs. Fig. S2 shows that ICOS-L on maturing pDCs induces IL-10 production in cultured naive cord blood–derived T cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061660/DC1.
Supplemental Material
Acknowledgments
We thank K. Ramirez, Z. He, and E. Wieder for cell sorting. We also thank C. Dong for critical reading of the manuscript.
The authors have no conflicting financial interests.
Abbreviations used: CD40L, CD40 ligand; ICOS, inducible costimulator; ICOS-L, ICOS ligand; mDC, myeloid DC; pDC, plasmacytoid pre-DC; Th, T helper; TLR, Toll-like receptor.
References
- 1.Liu, Y.J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 106:259–262. [DOI] [PubMed] [Google Scholar]
- 2.Colonna, M., G. Trinchieri, and Y.J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 5.Ito, T., H. Kanzler, O. Duramad, W. Cao, and Y.J. Liu. 2006. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 107:2423–2431. [DOI] [PubMed] [Google Scholar]
- 6.Kawai, T., S. Sato, K.J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068. [DOI] [PubMed] [Google Scholar]
- 7.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K.J. Ishii, T. Kawai, et al. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda, K., Y. Ohba, H. Yanai, H. Negishi, T. Mizutani, A. Takaoka, C. Taya, and T. Taniguchi. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 434:1035–1040. [DOI] [PubMed] [Google Scholar]
- 9.Rissoan, M.C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y.J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 10.Liu, Y.J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585–589. [DOI] [PubMed] [Google Scholar]
- 11.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar, M.V., R.M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levings, M.K., S. Gregori, E. Tresoldi, S. Cazzaniga, C. Bonini, and M.G. Roncarolo. 2005. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 105:1162–1169. [DOI] [PubMed] [Google Scholar]
- 14.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilliet, M., and Y.J. Liu. 2002. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moseman, E.A., X. Liang, A.J. Dawson, A. Panoskaltsis-Mortari, A.M. Krieg, Y.J. Liu, B.R. Blazar, and W. Chen. 2004. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173:4433–4442. [DOI] [PubMed] [Google Scholar]
- 17.Dong, H., G. Zhu, K. Tamada, and L. Chen. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 18.Witsch, E.J., M. Peiser, A. Hutloff, K. Buchner, B.G. Dorner, H. Jonuleit, H.W. Mages, and R.A. Kroczek. 2002. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur. J. Immunol. 32:2680–2686. [DOI] [PubMed] [Google Scholar]
- 19.Zhang, G.B., Y.J. Chen, Q. Shi, H.B. Ma, Y. Ge, Q. Wang, Z. Jiang, Y. Xu, and X.G. Zhang. 2004. Human recombinant B7-H3 expressed in E. coli enhances T lymphocyte proliferation and IL-10 secretion in vitro. Acta Biochim. Biophys. Sin. (Shanghai). 36:430–436. [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan, S., K. Iijima, T. Kobayashi, M. Rodriguez, H. Kita, and L.R. Pease. 2004. Blockade of allergic airway inflammation following systemic treatment with a B7- dendritic cell (PD-L2) cross-linking human antibody. J. Immunol. 173:1360–1365. [DOI] [PubMed] [Google Scholar]
- 21.Grouard, G., M.C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y.J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutloff, A., A.M. Dittrich, K.C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R.A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 397:263–266. [DOI] [PubMed] [Google Scholar]
- 23.Beier, K.C., A. Hutloff, A.M. Dittrich, C. Heuck, A. Rauch, K. Buchner, B. Ludewig, H.D. Ochs, H.W. Mages, and R.A. Kroczek. 2000. Induction, binding specificity and function of human ICOS. Eur. J. Immunol. 30:3707–3717. [DOI] [PubMed] [Google Scholar]
- 24.Lohning, M., A. Hutloff, T. Kallinich, H.W. Mages, K. Bonhagen, A. Radbruch, E. Hamelmann, and R.A. Kroczek. 2003. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J. Exp. Med. 197:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadowaki, N., S. Antonenko, J.Y. Lau, and Y.J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann, T.R., and R.L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. [DOI] [PubMed] [Google Scholar]
- 27.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 8:275–283. [DOI] [PubMed] [Google Scholar]
- 28.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 29.Akbari, O., G.J. Freeman, E.H. Meyer, E.A. Greenfield, T.T. Chang, A.H. Sharpe, G. Berry, R.H. DeKruyff, and D.T. Umetsu. 2002. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 30.Herman, A.E., G.J. Freeman, D. Mathis, and C. Benoist. 2004. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeiren, J., J.L. Ceuppens, M. Van Ghelue, P. Witters, D. Bullens, H.W. Mages, R.A. Kroczek, and S.W. Van Gool. 2004. Human T cell activation by costimulatory signal-deficient allogeneic cells induces inducible costimulator-expressing anergic T cells with regulatory cell activity. J. Immunol. 172:5371–5378. [DOI] [PubMed] [Google Scholar]
- 32.Borish, L., A. Aarons, J. Rumbyrt, P. Cvietusa, J. Negri, and S. Wenzel. 1996. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97:1288–1296. [DOI] [PubMed] [Google Scholar]
- 33.Ito, T., Y.H. Wang, O. Duramad, T. Hori, G.J. Delespesse, N. Watanabe, F.X. Qin, Z. Yao, W. Cao, and Y.J. Liu. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh, J.W., C.M. Seroogy, E.H. Meyer, O. Akbari, G. Berry, C.G. Fathman, R.H. Dekruyff, and D.T. Umetsu. 2002. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J. Allergy Clin. Immunol. 110:460–468. [DOI] [PubMed] [Google Scholar]
- 35.Ito, T., R. Amakawa, M. Inaba, T. Hori, M. Ota, K. Nakamura, M. Takebayashi, M. Miyaji, T. Yoshimura, K. Inaba, and S. Fukuhara. 2004. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J. Immunol. 172:4253–4259. [DOI] [PubMed] [Google Scholar]
- 36.Warnatz, K., L. Bossaller, U. Salzer, A. Skrabl-Baumgartner, W. Schwinger, M. van der Burg, J.J. van Dongen, M. Orlowska-Volk, R. Knoth, A. Durandy, et al. 2006. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 107:3045–3052. [DOI] [PubMed] [Google Scholar]
- 37.Shoemaker, J., M. Saraiva, and A. O'Garra. 2006. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J. Immunol. 176:3470–3479. [DOI] [PubMed] [Google Scholar]
- 38.Janke, M., E.J. Witsch, H.W. Mages, A. Hutloff, and R.A. Kroczek. 2006. Eminent role of ICOS costimulation for T cells interacting with plasmacytoid dendritic cells. Immunology. 118:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Heer, H.J., H. Hammad, T. Soullie, D. Hijdra, N. Vos, M.A. Willart, H.C. Hoogsteden, and B.N. Lambrecht. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldwin, T., S. Henri, J. Curtis, M. O'Keeffe, D. Vremec, K. Shortman, and E. Handman. 2004. Dendritic cell populations in Leishmania major-infected skin and draining lymph nodes. Infect. Immun. 72:1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei, S., I. Kryczek, L. Zou, B. Daniel, P. Cheng, P. Mottram, T. Curiel, A. Lange, and W. Zou. 2005. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 65:5020–5026. [DOI] [PubMed] [Google Scholar]
- 42.Fugier-Vivier, I.J., F. Rezzoug, Y. Huang, A.J. Graul-Layman, C.L. Schanie, H. Xu, P.M. Chilton, and S.T. Ildstad. 2005. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J. Exp. Med. 201:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochando, J.C., C. Homma, Y. Yang, A. Hidalgo, A. Garin, F. Tacke, V. Angeli, Y. Li, P. Boros, Y. Ding, et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662. [DOI] [PubMed] [Google Scholar]
- 44.Greve, B., L. Vijayakrishnan, A. Kubal, R.A. Sobel, L.B. Peterson, L.S. Wicker, and V.K. Kuchroo. 2004. The diabetes susceptibility locus Idd5.1 on mouse chromosome 1 regulates ICOS expression and modulates murine experimental autoimmune encephalomyelitis. J. Immunol. 173:157–163. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto, K., C.I. Kingsley, X. Zhang, C. Jabs, L. Izikson, R.A. Sobel, H.L. Weiner, V.K. Kuchroo, and A.H. Sharpe. 2005. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J. Immunol. 175:7341–7347. [DOI] [PubMed] [Google Scholar]
- 46.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco, P., A.K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 294:1540–1543. [DOI] [PubMed] [Google Scholar]
- 48.Fonteneau, J.F., M. Larsson, A.S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y.J. Liu, J.D. Lifson, D.R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jego, G., A.K. Palucka, J.P. Blanck, C. Chalouni, V. Pascual, J. Banchereau. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 19:225–234. [DOI] [PubMed] [Google Scholar]
- 50.Kolumam, G.A., S. Thomas, L.J. Thompson, J. Sprent, K. Murali-Krishna. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. 2005. J. Exp. Med. 202:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krug, A., A.R. French, W. Barchet, J.A. Fischer, A. Dzionek, J.T. Pingel, M.M. Orihuela, S. Akira, W.M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 21:107–119. [DOI] [PubMed] [Google Scholar]
- 52.Hanabuchi, S., N. Watanabe, Y.H. Wang, Y.H. Wang, T. Ito, J. Shaw, W. Cao, F.X. Qin, and Y.J. Liu. 2006. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood. 107:3617–3623. [DOI] [PubMed] [Google Scholar]
- 53.Smit, J.J., B.D. Rudd, and N.W. Lukacs. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 203:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gazzinelli, R.T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798–805. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.