Abstract
Axonal injury is considered the major cause of disability in patients with multiple sclerosis (MS), but the underlying effector mechanisms are poorly understood. Starting with a proteomics-based approach, we identified neurofascin-specific autoantibodies in patients with MS. These autoantibodies recognize the native form of the extracellular domains of both neurofascin 186 (NF186), a neuronal protein concentrated in myelinated fibers at nodes of Ranvier, and NF155, the oligodendrocyte-specific isoform of neurofascin. Our in vitro studies with hippocampal slice cultures indicate that neurofascin antibodies inhibit axonal conduction in a complement-dependent manner. To evaluate whether circulating antineurofascin antibodies mediate a pathogenic effect in vivo, we cotransferred these antibodies with myelin oligodendrocyte glycoprotein–specific encephalitogenic T cells to mimic the inflammatory pathology of MS and breach the blood–brain barrier. In this animal model, antibodies to neurofascin selectively targeted nodes of Ranvier, resulting in deposition of complement, axonal injury, and disease exacerbation. Collectively, these results identify a novel mechanism of immune-mediated axonal injury that can contribute to axonal pathology in MS.
Multiple sclerosis (MS) is characterized by repeated episodes of inflammation and demyelination in the central nervous system (CNS) with varying degrees of axonal loss (1). Although chronic disability in MS was traditionally attributed to demyelination, axonal loss is now regarded as the major pathological correlation to the development of permanent neurological deficits (2, 3). Axonal injury is most pronounced in regions of active inflammation and demyelination (4, 5), but it is currently unknown whether this axonal pathology is caused by loss of trophic support of the oligodendrocyte, toxic inflammatory mediators, or a specific immune response against axonal antigens (6–10).
The presence of Ig's and complement activation products in active MS lesions (11, 12) and the efficacy of therapeutic plasma exchange, or treatment with B cell–depleting antibodies, in some patients (13–15) provide circumstantial evidence for the involvement of antibodies in MS. However, the identity of antigens targeted by clinically relevant antibodies in MS remains obscure. Most studies have focused on the role of myelin-specific autoantigens such as myelin oligodendrocyte glycoprotein (MOG), galactosyl ceramide, or sulphogalactosyl ceramide that provide targets for autoantibody-mediated demyelination in experimental autoimmune encephalomyelitis (EAE), an animal model of MS (16–21). Despite several studies that have documented autoantibody responses to neuronal and axonal antigens in MS, their functional importance has gone largely unexplored (9, 22–25).
Our present study was inspired by findings obtained using a proteomic approach to explore the specificity of the myelin-reactive autoantibody repertoire in MS patients. We identified several patients who showed a conspicuous antibody response to neurofascin present in our myelin preparations.
Neurofascin exists in two isoforms: neurofascin 155 (NF155) is a myelin protein localized at the paranodal axo–glial junction, whereas NF186 is a neuronal protein exposed on the surface of myelinated axons at the axonal initial segment and node of Ranvier (26). NF186 associates with the β1 and β3 chains of voltage-gated sodium channels (27) and other nodal proteins to maintain the unique molecular architecture of the node of Ranvier necessary for saltatory conduction (26).
The neurofascin-specific autoantibody response in MS patients recognized the extracellular domain of both NF155 and NF186, prompting us to investigate the functional effects of such a panneurofascin-specific antibody response both in vitro and in an animal model. Cotransfer of a panneurofascin mAb together with MOG-reactive T cells demonstrated that antineurofascin antibodies can exacerbate disease severity in EAE by binding selectively to NF186 at the node of Ranvier. This results in acute, but reversible, axonal injury and is associated with codeposition of C9 and mouse mAb at nodes of Ranvier. In vitro, the neurofascin-specific antibody was able to induce an electrophysiological deficit in hippocampal slices only in the presence of fresh sera, indicating that its pathogenic effect in vivo is complement dependent. These observations identify NF186 as a target for autoantibody-mediated axonal injury, a novel pathomechanism that may contribute to the development of axonal pathology in MS.
RESULTS
Identification of neurofascin as a candidate autoantigen in MS
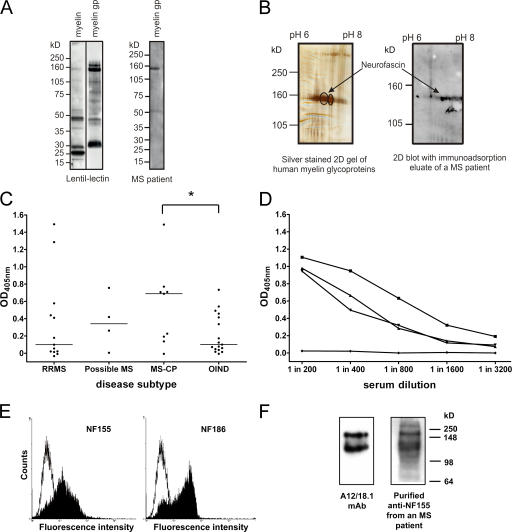
To quantitatively identify minor myelin glycoproteins recognized by autoantibodies in patients with MS, we used a glycoprotein fraction isolated from human myelin by lentil-lectin affinity chromatography that is highly enriched for known myelin antigens such as MOG, as well as other as yet poorly characterized myelin-associated glycoproteins (Fig. 1 A). Western blotting after SDS-PAGE with sera from 22 MS patients and 21 control patients (10 with other neurological diseases and 11 with other inflammatory neurological diseases [OINDs]) revealed a marked interpatient variability in the immunoreactive band pattern (not depicted); however, in ∼20% of MS samples we observed a prominent response to components migrating in the molecular mass range of 150–180 kD (Fig. 1 A). To identify this protein, myelin glycoproteins were separated by two-dimensional gel electrophoresis, and were probed after Western blotting using purified IgG (28) obtained from five MS patients and two control donors (one with an autoimmune peripheral neuropathy and the other with cardiomyopathy). This approach identified a series of spots migrating with an apparent molecular mass of 155 kD that was recognized by the IgG fractions from all five MS patients but neither of the controls (Fig. 1 B). Two spots were excised from the gels, and the immunoreactive target was identified as neurofascin by mass spectrometry (sequence coverage of 23% and a probability of 1.9 e−34).
Figure 1.
Antibodies to NF155 and NF186 are present in patients with MS. (A) Human myelin and the myelin glycoprotein (gp) fraction were separated by SDS-PAGE and blotted, and glycoproteins were detected using biotinylated lentil-lectin. The myelin glycoprotein fraction is highly enriched in higher molecular mass glycoproteins (left). Using this myelin glycoprotein fraction to screen patient sera by Western blotting identified antibody responses to a variety of components, including a protein migrating with an apparent molecular mass of ∼155 kD (right). (B) Two two-dimensional gels were run in parallel. One was silver stained (left) and the other was blotted and developed with IgG obtained from an immunoadsorption eluate of an MS patient (right). The encircled spots at 155 kD were excised and identified by MALDI-MS as neurofascin. (C) Serum IgG antibody titers to recombinant NF155ED were measured by ELISA in OIND (n = 17) and MS (n = 26) patients stratified by the disease course. Patients with chronic progressive MS (MS-CP) had the highest median antibody response (horizontal lines) to NF155ED, which was significantly higher than in OIND patients. *, P < 0.05 using the Mann-Whitney U test. (D) The titrations of three sera from high titer MS patients and one negative control donor are shown. (E) Anti-NF155ECD antibodies affinity purified from plasma of an MS patient bind to both isoforms of neurofascin when expressed at the surface of transfected cells (left, NF155; right, NF186), as shown by flow cytometry. The open graph represents the staining of the control cell line, and the shaded graph represents the staining of the neurofascin transfectants. (F) Rat brain homogenate was separated by SDS-PAGE, blotted, and probed with either the neurofascin-specific mAb A12/18.1 or the purified anti-NF155ECD antibodies, indicating recognition of both the 155- and 186-kD isoforms.
Having identified NF155 as a candidate autoantigen in MS, we reexamined the disease specificity of this serum antibody response in additional MS patients (n = 26), OIND patients (n = 17), and control blood donors (n = 21) by ELISA (Fig. 1, C and D). Approximately one third of MS patients and occasional OIND patients and control donors had a high titer response to the extracellular domain of NF155 (NF155ED; Fig. 1, C and D). When MS patients were stratified according to disease course, it became evident that the highest levels of NF155ED-specific antibodies were found in the sera of patients with chronic progressive disease (median OD = 0.69) as compared with relapsing-remitting MS (median OD = 0.10), OIND (median OD = 0.10), and blood donor controls (median OD = 0.25). We next purified anti-NF155ED antibodies by immunoaffinity chromatography from the plasma of three representative high titer MS patients to determine whether these antibodies recognized the native protein when expressed at the surface of NF155-transfected cells. This was indeed the case, but purified NF155ED-specific antibodies also bound to NF186-transfected cells, as demonstrated by flow cytometry (Fig. 1 E). This cross-reactivity was confirmed by Western blotting of total brain homogenate (Fig. 1 F) and led us to speculate that recognition of NF186 at the node of Ranvier by these antibodies might exacerbate axonal injury and accelerate disease progression.
Antineurofascin antibodies exacerbate clinical disease in EAE, an animal model of MS
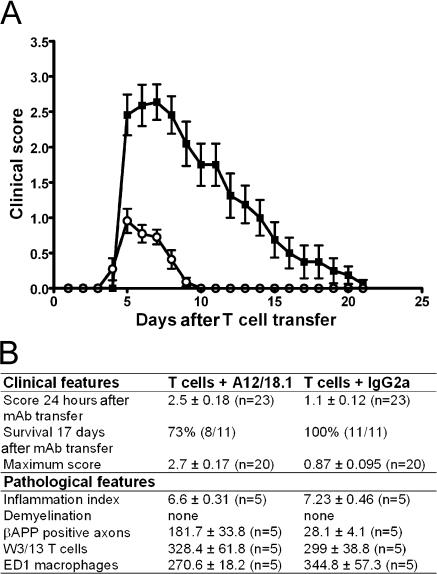
To investigate this hypothesis, we developed a cotransfer model of T cell–mediated EAE (16) in which rats were injected at disease onset with the panneurofascin IgG2a mAb A12/18.1. This antibody mimics the properties of NF155ED-specific antibodies purified from MS patients in that it recognizes the extracellular domains of both NF155 and NF186, as demonstrated by flow cytometry of live neurofascin-transfected cell lines (unpublished data). Treatment with 500 μg A12/18.1 i.p. at disease onset resulted in a rapid and substantial increase in disease severity within 24 h, by which time the clinical score of antineurofascin-treated animals was 2.5 ± 0.18 (mean ± SEM; n = 23). In comparison, control animals injected with an irrelevant mouse IgG2a antibody had a clinical score of 1 ± 0.12 (mean ± SEM; n = 23; Fig. 2 A). All animals survived this acute exacerbation of disease and began to show signs of clinical recovery 5 d after antibody transfer. The majority of animals subsequently regained complete motor function within 2 wk and exhibited no residual signs of disease. However, despite exhibiting some initial signs of clinical improvement, a proportion of the animals (27%; n = 11) treated with A12/18.1 died suddenly during this recovery period (Fig. 2 B). This was not seen in animals treated with the control mouse IgG2a mAb, nor has it been reported in other cotransfer models of EAE. In the absence of a preexisting inflammatory response in the CNS, the transfer of the neurofascin-specific mAb into naive recipients (n = 7) resulted in no clinical deficit.
Figure 2.
Passive transfer of antineurofascin antibody exacerbates adoptive transfer EAE. (A) Rats injected with 2 × 106 activated MOGIgD-specific T cells followed by 500 μg IgG2a control antibody on d 4 (open circles) reached a maximum clinical score of 1 ± 0.12 and had completely recovered by day 10 after T cell transfer. Disease activity was exacerbated in rats coinjected with antineurofascin mAb (closed squares). These animals reached a maximum clinical score of 2.7 ± 0.17, and clinical recovery was still incomplete 20 d after T cell transfer. Data are reported as the mean ± SEM. (n = 11). Pooled data from independent experiments are shown. (B) Cotransfer of the NF155/186-specific mAb in rats with adoptively transferred EAE increased disease severity without exacerbating either inflammation or demyelination in the CNS. Neither the inflammatory index (P = 0.25) nor the number of T cells identified with W3/13 (P = 0.84) or the number of macrophages identified with ED1 (P = 0.41) was increased in the animals treated with the NF155/186-specific mAb. In contrast, axonal damage was augmented, as seen by β-APP staining (P = 0.009). Clinical data are pooled from independent experiments. Histological data were obtained from animals killed 48 h after mAb injection. Data are reported as the mean ± SEM. Comparison of groups was statistically evaluated with Mann-Whitney U test.
Transfer of the panneurofascin-specific antibody results in acute axonal damage in the absence of demyelination or increased inflammation in the CNS
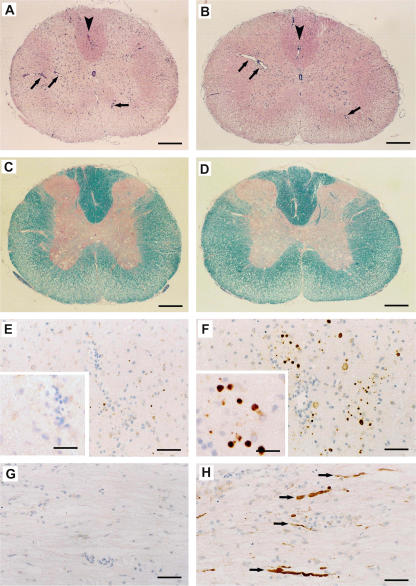
We performed extensive histological and immunopathological analysis of the CNS to determine how A12/18.1 exacerbated clinical disease in animals with EAE. We could find no evidence that the mAb had any significant effect on either inflammation or demyelination (Figs. 2 B and 3, A–D). Comparison of the inflammatory response revealed that this was similar in both A12/18.1-treated and control animals both with respect to the numbers of inflammatory foci, as well as the numbers of T cells and macrophages infiltrating the CNS (Figs. 2 B and 3, A and B). However, the disease exacerbation induced by A12/18.1 was associated with a marked increase in immunoreactivity for β-amyloid precursor protein (β-APP), a marker of acute axonal injury (Figs. 2 B and 3, E–H). The accumulation of β-APP reflects disruption of axonal transport at sites of axonal injury and was often observed in areas devoid of infiltrating inflammatory cells in animals treated with the neurofascin-specific antibody. This was not observed in control IgG2a-treated animals with EAE in which β-APP immunoreactivity was minimal and restricted to sites of perivascular and sub-pial inflammation. After complete clinical recovery, β-APP immunoreactivity had returned to background levels indicating resolution of axonal damage. Occasional degenerating myelin figures were observed in only one animal, and we observed no obvious reduction in axonal density in the spinal cord after clinical recovery.
Figure 3.
Pathology associated with autoantibody-mediated axonal injury in adoptive transfer EAE. Representative histopathology of the spinal cord 2 d after transfer of either antineurofascin antibody (B, D, F, and H) or an IgG2a control antibody (A, C, E, and G). (A and B) Hematoxylin and eosin staining reveals the presence of similar numbers of inflammatory infiltrates (arrows) in animals coinjected with (A) control mAb and (B) NF155/186-specific mAb. Arrowheads indicate areas in A and B that are shown in immunohistochemical staining for β-APP (E and F) at a higher magnification. (C and D) Luxol fast blue staining for myelin (blue/turquoise) reveals that demyelination is not enhanced by the antineurofascin mAb. In E–H, β-APP is stained brown by immunohistochemistry, and cell nuclei are counterstained blue with hematoxylin. This staining for β-APP, which indicates acute axonal injury, reveals numerous injured axons (brown dots in cross sections, F; brown fibers in longitudinal sections, H) in animals treated with the NF155/186-specific mAb. Note that in comparison with animals treated with the NF155/186-specific mAb (F and H), axonal injury is virtually absent in animals receiving the control IgG2a mAb (E and G). Arrows in H indicate brown β-APP–positive axons. Bars: (A–D) 220 mm; (E–H) 30 mm; (E and F, insets) 13 mm.
Binding of antibody to neurofascin in vivo is restricted to the node of Ranvier
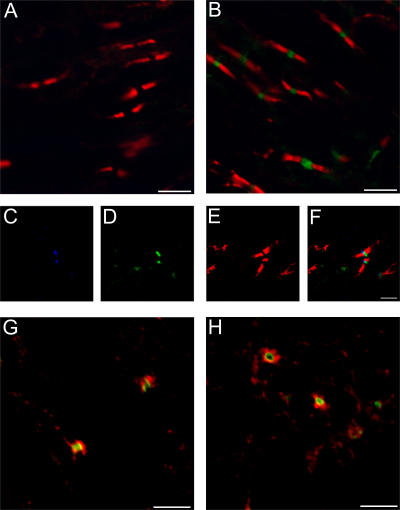
Our data indicate that the clinical exacerbation induced by A12/18.1 is mediated by a direct effect of the antibody on the axon. This suggests that the primary target is NF186 at the node of Ranvier rather than the myelin/oligodendroglial isoform of NF155. This was confirmed by identifying sites at which A12/18.1 was bound within the CNS of animals with EAE by laser scanning confocal microscopy (Fig. 4).
Figure 4.
The panneurofascin-specific mAb A12.18/1 binds selectively to the node of Ranvier in vivo in animals with EAE. Confocal microscopy of representative spinal cord tissue from animals with EAE 30 h after transfer of anti-NF155/NF186 mAb A12.18/1 (B–H) or IgG2a control antibody (A). Cotransfer of anti-NF155/NF186 mAb resulted in deposition of the antibody within discreet regions of the CNS when visualized with an Alexa Fluor 488–conjugated anti–mouse IgG2a antibody (green). Double staining with a rabbit antibody to NF155 (red) identified the paranodal domains of myelinated axons and demonstrated that the injected mAb did not colocalize with NF155 but was deposited between adjacent paranodes (B).There was no deposition of mouse antibody in the CNS of animals injected with the isotype control antibody when visualized with an Alexa Fluor 488–conjugated anti–mouse IgG2a antibody (green), as shown in relation to NF155 staining at the paranode (red, A). Triple staining (C–F) for voltage-gated sodium channels (blue, C), bound anti-NF155/NF186 mAb (green, D), and NF155 (red, E) confirm that the adoptively transferred mAb binds selectively at the node of Ranvier. (F) Merged image of (C–E). Deposition of the in vivo–injected antineurofascin mAb at the node of Ranvier is accompanied by the deposition of complement C9. Sections were stained with an Alexa Fluor 488–conjugated anti–mouse IgG2a antibody to identify bound antineurofascin mAb (green), and a rabbit anti–rat C9 antibody (red, G and H). Bars: 5 μm.
30 h after antibody transfer, deposition of A12/18.1 was restricted to distinct bands in longitudinal sections (Fig. 4 B) or appeared as occasional circular profiles in transverse sections. No deposition of mouse IgG2a was detected in the CNS of animals injected with the control antibody (Fig. 4 A). Triple staining using antibodies to specific nodal and paranodal antigens demonstrated that deposition of the mouse neurofascin-specific mAb was restricted to nodes of Ranvier, where it colocalized with voltage-gated sodium channels (Fig. 4, C–F). We observed no colocalization of adoptively transferred A12/18.1 with NF155 within the paranodal domain of myelinated fibers (Fig. 4 B), whereas the mAb will bind both isoforms of neurofascin in vitro and can be used to identify NF155 within the paranodal domains of myelinated fibers in fixed tissue sections (not depicted).
In animals treated with A12/18.1, we observed a distinct halo of C9 deposition restricted to the immediate vicinity of antibody bound to the node (Fig. 4, G and H). This was not observed in animals with EAE treated with the control mouse IgG2a antibody and suggests that complement may play an important role in antibody-mediated axonal injury in this model system.
Antineurofascin antibodies inhibit neurotransmission in vitro
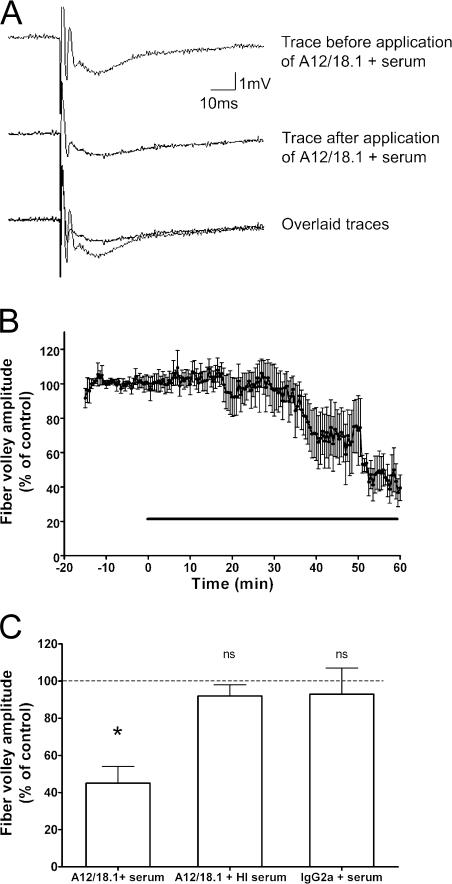
The ability of the neurofascin-specific mAb to disrupt nerve conduction was examined in rat hippocampal slices in vitro. Perfusion of hippocampal slices with 20 μg/ml A12/18.1 in the presence of 2.5% fresh rat serum had a considerable effect on axonal conduction as measured by the presynaptic fiber volley (essentially a compound action potential) recorded from the Schaffer collateral-commissural fibers. After a variable delay of between 15 and 50 min (Fig. 5, A and B), we observed a >50% reduction in the amplitude of the fiber volley (Fig. 5 C). This effect was abolished if the serum was heat inactivated before the experiment at 56°C for 30 min to destroy complement (Fig. 5 C). In addition, there was no effect when the hippocampal slices were perfused with fresh serum and a control mouse IgG2a mAb (Fig. 5 C).
Figure 5.
Antineurofascin antibody disrupts nerve conduction in vitro. (A) An example of the field potential recorded under control conditions from the CA1 region of a rat hippocampal slice in a single slice using a single stimulus of the Schaffer collateral-commissural fibers every 30 s (top trace) is shown. Perfusion of the antineurofascin mAb A12/18.1 plus normal rat serum causes a decrease in the fiber volley amplitude (middle trace), which can be clearly seen when the traces are overlaid (bottom trace). (B) Time-course data showing the effect of A12/18.1 antibody and serum on the amplitude of the fiber volley (mean ± SEM; n = 5). The horizontal line indicates application of antibody. (C) A decrease in fiber volley amplitude after a 1-h perfusion of the treatment is only seen when A12/18.1 is applied in the presence of fresh rat serum. There is no effect on the fiber volley amplitude if A12/18.1 and heat inactivated (HI) serum are applied or if a control IgG2a antibody and fresh serum are applied (n = 5 for all treatments). The dashed line indicates the control value against which the other values are measured. Significance is determined by a paired t test on the raw (not normalized) data. P = 0.012 for A12/18.1 antibody and fresh serum.
DISCUSSION
Axonal loss is responsible for the development of chronic neurological deficits in MS, but the pathomechanisms that initiate or exacerbate axonal injury in this inflammatory demyelinating disease remain obscure. Previous studies demonstrated that axonal injury is most pronounced in areas of inflammatory demyelination. This led to the concept that axonal loss was a nonspecific “bystander” response to inflammatory demyelination mediated by soluble factors generated by activated macrophages such as nitric oxide or T cells (10, 29). However, although inflammatory demyelination is a ubiquitous feature of MS, axonal loss does not necessarily correlate with lesion distribution and load in defined spinal cord tracts (30) and continues despite resolution of CNS inflammation in patients with progressive disease (31, 32). These observations led to suggestions that, in addition to the effects of inflammation, other mechanisms are involved in the development of axonal pathology in MS.
Using an unbiased proteomic approach, we identify neurofascin as a novel target of autoantibodies in MS. Neurofascin is a member of the L1 family of cell adhesion molecules, and its two isoforms play essential roles in maintaining the structural and functional integrity of myelinated fibers (26). These isoforms are derived from a single gene by alternative splicing, and their extracellular domains contain six identical Ig domains and a variable number of identical fibronectin-like repeats, differing only in that NF155 uses an alternative fibronectin type III repeat and lacks a mucin-like domain (33). As a consequence, mAbs raised against these proteins are commonly cross-reactive. This is also the case for the autoantibody response to neurofascin in patients with MS, which recognizes the intact extracellular domains of both NF155 and NF186, as we demonstrate by flow cytometry.
In vivo in animals with EAE, we demonstrate that antibodies with this specificity bind selectively to NF186 at the node of Ranvier to initiate axonal injury in the CNS and exacerbate clinical disease. The inability of A12/18.1 to recognize NF155 in these animals is presumably related to its localization within the paranodal axo–glial junctional complex. The antibody may be unable to penetrate into the junctional complexes or, alternatively, access to the target epitope is blocked owing to local protein–protein interactions. How antibody binding to the node leads to loss of function in EAE remains to be clarified. Unlike autoantibody-mediated demyelination, the clinical deficit in this model is associated with neither enhanced inflammation nor demyelination. However, the codeposition of C9 with antibody suggests involvement of complement, as is the case in other autoantibody-mediated neurological diseases (34, 35). This supposition is supported by our demonstration that A12/18.1 only mediates a functional deficit in vitro in the presence of fresh serum, and that this effect is abolished if the serum is heated at 56°C before the experiment. However, irrespective of the effector mechanisms involved, antibody-mediated axonal injury and the resulting functional deficit are reversible. Not only did the majority of animals recover clinically, but β-APP-immunoreactivity also returns to background levels with recovery, and there was no evidence of axonal loss. This suggests that the node of Ranvier is relatively resistant to antibody-mediated damage. This may involve clearance of antibody–complement complexes by endocytosis or ectocytosis (36), or up-regulation of complement inhibitors such as CD55 by neurons in EAE lesions (37).
Our experiments demonstrate that neurofascin-specific antibodies mediate axonal injury after recognition of NF186, but we cannot exclude they do not have other pathological effects. The molecular architecture of the paranode is disrupted after both experimental demyelination (38) and in MS plaques (39–41), indicating that NF155 may become accessible to bind antibody in demyelinating lesions. Neurofascin-specific antibodies may also inhibit remyelination by binding to NF155 reexpressed on the surface of remyelinating oligodendrocytes/oligodendrocyte processes (42). Moreover, NF186 is not lost from the axonal surface after demyelination but is simply more diffusely distributed (40), suggesting that it will continue to provide a target for autoantibody-mediated attack in demyelinated lesions. Neurofascin is also expressed on myelinated fibers in the peripheral nervous system, suggesting that it could also be involved in the pathogenesis of autoimmune-mediated peripheral neuropathies. In the current study, we did not observe any enhanced β-APP immunoreactivity or C9 deposition in the peripheral nervous system, although some antibody was detected bound to nodes in root entry zones. The blood–nerve barrier is known to have a limited permeability to serum Ig's at these sites (43), and in the absence of a local inflammatory response, diffusion of antibody into the nerve is unable to initiate a functional deficit (44). In the absence of peripheral nerve damage, the sudden death of some animals during the recovery phase of the disease cannot be explained satisfactorily, although it might reflect central autonomic dysfunction.
We demonstrate that neurofascin-specific antibodies mediate axonal damage, impair neuronal conduction, and exacerbate clinical disease in an animal model of MS; however, are they relevant in human disease? For a circulating autoantibody response to mediate tissue damage within the CNS, two criteria must be met. First, the blood–brain barrier must be breached so that autoantibodies circulating in the blood can gain access to the CNS parenchyma (12, 16, 45), and second, once within the CNS compartment, the antibody must be able to access and bind to its target antigens. In the case of the neurofascin-specific autoantibody response in MS, these criteria are both met. First, MS is associated with an increased permeability of the blood–brain barrier to serum Ig's (46). In the initial inflammatory phase of MS, this is most prominent in areas of perivascular inflammation. However, there is also evidence that as the disease progresses patients develop chronic blood–brain barrier abnormalities that result in increased leakage of serum protein into the CNS (47, 48). This indicates that antineurofascin antibodies in the periphery will gain access to the CNS parenchyma. Second, the polyclonal neurofascin-specific autoantibody response recognizes the native extracellular domain of NF186. As we demonstrate using mAb A12/18.1, these antibodies will bind to NF186 in vivo to exacerbate axonal injury and associated functional deficits in patients with MS. These effects will be most pronounced in patients with high titer antibody responses to NF155/186 and may have a considerable impact on disease progression. This may be the case in the 20–30% of MS with high titer antibody responses, but it should be noted that neurofascin-specific antibodies were also detected in occasional control donors. This lack of absolute disease specificity is not unexpected and is similar to that reported for autoimmune responses to other CNS autoantigens in MS (46). In summary, we identify neurofascin as a novel autoantigen in a subset of MS patients and demonstrate that neurofascin-specific antibodies can induce reversible axonal injury and conduction block in inflammatory demyelinating diseases of the CNS. These findings should open new perspectives for therapeutic inhibition of antibody-mediated axonal injury.
MATERIALS AND METHODS
Patients and control donors.
Sera from 74 patients were analyzed. These included 26 patients with MS, 17 patients with OINDs, 10 patients with noninflammatory other neurological diseases, and 21 healthy blood donors. The MS patient group included 13 patients with definite relapsing-remitting MS, 4 patients with a clinical isolated syndrome suggestive of MS, and 9 patients with chronic progressive MS. The IgG fractions used to identify NF155 as a candidate autoantigen were isolated from patients undergoing immunoadsorption therapy (28). Plasmapheresis samples were provided by L.-A. Hoffmann, M. Krumbholz, T. Kümpfel, and H. Pellkofer (Ludwig-Maximilians-University, Munich, Germany). This study was approved by an Ethical Committee of the University of Munich.
Purification of myelin glycoproteins.
Myelin was prepared according to the protocol described by Norton and Poduslo (49). The myelin pellet was lyophilized and stored at −80°C. A myelin glycoprotein fraction was obtained by lentil-lectin affinity chromatography according to the manufacturer's guidelines (GE Healthcare).
Gel electrophoresis, immunodetection, and mass spectrometry.
Myelin glycoproteins were separated in 4–12% polyacrylamide gels (Novex; Invitrogen) and blotted to polyvinyldene difluoride membrane. Patients' sera were diluted 1:250 and detected with peroxidase-conjugated secondary reagents and enhanced chemiluminescence.
For two-dimensional gel electrophoresis, myelin glycoproteins were solubilized in a buffer containing 7 M urea, 2 M thiourea, 4% CHAPS, 100 mM dithiothreitol, and 2% ampholytes. Glycoproteins were resolved on 17-cm IEF strips (pH range 3–10; Bio-Rad Laboratories) and separated in the second dimension with a 7% PAGE. After electrophoresis of duplicate gels that were run in parallel, one was silver stained (50) and the other was blotted to polyvinyldene difluoride membranes and immunoreactive proteins detected as described in the previous paragraph. Spots corresponding to immunoreactive regions of the blot were excised from the silver-stained gel, digested with trypsin, and analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-MS). Proteins corresponding to the cleaved fragments were identified by screening the National Center for Biotechnology Information database using the program ProFound (Genomic Solutions).
ELISA.
96-well polyvinyl ELISA plates (Costar) were coated with 100 μl of 5 μg/ml rat NF155ED (R&D Systems) or BSA (Sigma-Aldrich) in PBS overnight at 4°C. Patient sera were diluted 1:200, and binding was detected with anti–human IgG labeled with alkaline phosphatase (Chemicon). The antigen-specific response is plotted as (mean NF155ED OD − NF155ED SD) − (mean BSA OD + 3 BSA SD).
Affinity purification of anti-NF155ED antibodies.
500 μg of recombinant rat NF155ED was bound to an activated NHS column (GE Healthcare), according to the manufacturer's instructions. Plasma was adjusted to pH 7.4 and run over the NF155ED column, followed by extensive washing with PBS. Bound Ig's were eluted with 0.1 M glycine, pH 2.7, and neutralized immediately with 1 M Tris, pH 9.
Flow cytometry analysis.
HeLa cells were transfected with NF186 in pEGFP–N1 (Clontech) or NF155 in pCDNA3.1 (Invitrogen; provided by P. Brophy, University of Edinburgh, Edinburgh, UK) using Lipofectamine (Invitrogen), according to the manufacturer's instructions. Stable high expressing cell lines were selected using G418. Surface expression of NF155 and NF186 was confirmed by immunocytochemistry and flow cytometry using the panneurofascin mAb A12/18.1. For flow cytometry, NF155-transfected, NF186-transfected, or untransfected HeLa cells were diluted in FACS buffer (PBS/3% FCS) and plated out in 96-well plates at a density of 105 cells per well in the presence of mAb A12/18.1 or human antibody diluted to a concentration of 10 μg/ml. After incubation for 30 min on ice, the cells were washed with FACS buffer and incubated for a further 30 min on ice with anti–mouse IgG Alexa Fluor 488 (dilution 1:250; Invitrogen) or anti–human IgG Alexa Fluor 488 (dilution 1:250; Invitrogen). After washing with FACS buffer, the staining was analyzed immediately on a flow cytometer (FACSCalibur; BD Biosciences) using CellQuest Pro software (BD Biosciences).
Animal experiments.
All animal experiments were performed in Aberdeen with institutional (University of Aberdeen) and British Home Office approval. EAE was induced in female DA rats (6–8 wk old; Harlan) by adoptive transfer of 2 × 106 activated MOGIgD-specific T cells, as previously described (51). At the onset of clinical disease, rats were injected i.p. with either the NF155/186-specific mAb A18/12 or a control mouse IgG2a antibody (Sigma-Aldrich). Animals were weighed and examined daily for clinical signs of EAE, which was scored using the following scale: 0.5, partial loss of tail tone; 1, complete tail atony; 2, hind limb weakness; 3, hind limb paralysis; 4, fore limb paralysis; and 5, moribund. In addition, seven naive DA rats were injected i.p. with 500 μg antineurofascin mAb to investigate any possible effects mediated in the absence of a preexisting CNS inflammation.
Immunohistology.
Animals were fixed in 4% paraformaldehyde via cardiac perfusion. Brains and spinal cords were left in 4% paraformaldehyde for 30 min before immersion in 30% sucrose. Tissue was then embedded in optimal cutting temperature compound and snap frozen in isopentane/liquid nitrogen. To evaluate inflammation, sections were incubated with the antibodies W3/13 (Serotec) and ED1 (Serotec) to stain infiltrating T cells and macrophages, respectively. The inflammatory index was calculated as the mean number of perivascular inflammatory infiltrates derived from an average of 15 complete cross sections of spinal cord of an individual animal. Axonal injury was visualized by β-APP staining (Chemicon).
For paranodal staining, sections were postfixed for 1 min in Bouin's fixative. Before staining, 10-μm longitudinal and transverse spinal cord cryosections were incubated in 10% normal goat serum/0.3% Triton X-100 in PBS for 1 h. Primary antibodies were applied for 1 h at room temperature or at 4°C overnight. After washing, the secondary antibodies were applied for 1 h at room temperature, followed by washing and mounting in VECTASHIELD Hardset (Vector Laboratories). The antibodies used in this study were as follows: rabbit NF155-F3 (1:1,000, specific for the third fibronectin type III domain of NF155; a gift from P. Brophy) (52), mouse pan-antisodium channel (1:100, IgG1; Sigma-Aldrich), and rabbit anti–rat C9 (1:1,000; gift from P, Morgan, Cardiff University, Cardiff, UK). Anti–mouse IgG2a Alexa Fluor 488 (1:500), anti–mouse IgG1 Alexa Fluor 633 (1:500), and anti–rabbit Alexa Fluor 568 (1:500) were obtained from Invitrogen. Paraffin sections were prepared and evaluated as previously described (53).
Confocal microscopy.
Images were captured using a laser scanning confocal system (LSM510 META; Carl Zeiss MicroImaging, Inc.) coupled to an upright microscope (Axioplan2; Carl Zeiss MicroImaging, Inc.). Images were analyzed using LSM Image Viewer software (Carl Zeiss MicroImaging, Inc.).
Electrophysiology.
Young adult female Lewis rats (4–6 wk old) were killed, and the brains were removed and placed in chilled (4–5°C) oxygenated artificial cerebrospinal fluid (aCSF). The aCSF contained 124 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 10 mM d-glucose, 1 mM MgSO4, and 2 mM CaCl2 and was continuously bubbled with 95% O2/5% CO2. After dissecting free the hippocampus, 350-μm transverse slices were cut using a tissue chopper (McIlwain). Slices were stored in a holding chamber at room temperature before being transferred to an interface-type recording chamber. Within the interface chamber, aCSF was continually perfused below the slice at a rate of 1–2 ml/min and at a constant temperature of 27–29°C.
Supplemental Material
Acknowledgments
We are grateful to Prof. H. Wekerle for continuous excellent advice and support. We thank Dr. I. Sinicina for valuable aid; Drs. T. Kümpfel, L.-A. Hoffmann, H. Pellkofer, and M. Krumbholz for providing plasmapheresis samples; and Drs. A. Flügel and M. Kerschensteiner for critical appraisal of the manuscript.
This work was supported by grants from the Multiple Sclerosis Society (to C. Linington), the Deutsche Forschungsgemeinschaft (SFB 571), the Gemeinnützige Hertie-Foundation, and the Verein zur Therapieforschung für MS-Kranke. The Institute for Clinical Neuroimmunology is supported by the Hermann and Lilly Schilling Foundation. A. Al-Hayani was supported by an International Brain Research Organization travel grant. M.N. Rasband was supported by a grant from the National Multiple Sclerosis Society.
The authors have no conflicting financial interests.
Abbreviations used: aCSF artificial cerebrospinal fluid; β-APP, β-amyloid precursor protein; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NF155 and NF186, neurofascin 155 and 186, respectively; OIND, other inflammatory neurological disease.
E.K. Mathey and T. Derfuss contributed equally to this work.
E. Meinl and C. Linington contributed equally to this work.
S. Velhin's present address is Clinical and Experimental Laboratory for Chronic Neuroinfections, Research Institute for Epidemiology and Microbiology, 220114 Minsk, Belarus.
References
- 1.Steinman, L. 2001. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2:762–764. [DOI] [PubMed] [Google Scholar]
- 2.Hauser, S.L., and J.R. Oksenberg. 2006. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 52:61–76. [DOI] [PubMed] [Google Scholar]
- 3.Trapp, B.D., R. Ransohoff, and R. Rudick. 1999. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 12:295–302. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson, B., M.K. Matyszak, M.M. Esiri, and V.H. Perry. 1997. Axonal damage in acute multiple sclerosis lesions. Brain. 120:393–399. [DOI] [PubMed] [Google Scholar]
- 5.Trapp, B.D., J. Peterson, R.M. Ransohoff, R. Rudick, S. Mork, and L. Bo. 1998. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338:278–285. [DOI] [PubMed] [Google Scholar]
- 6.Redford, E.J., R. Kapoor, and K.J. Smith. 1997. Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain. 120:2149–2157. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins, A., H. Majed, R. Layfield, A. Compston, and S. Chandran. 2003. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 23:4967–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medana, I., Z. Li, A. Flügel, J. Tschopp, H. Wekerle, and H. Neumann. 2001. Fas ligand (CD95L) protects neurons against perforin-mediated T lymphocyte cytotoxicity. J. Immunol. 167:674–681. [DOI] [PubMed] [Google Scholar]
- 9.Lily, O., J. Palace, and A. Vincent. 2004. Serum autoantibodies to cell surface determinants in multiple sclerosis: a flow cytometric study. Brain. 127:269–279. [DOI] [PubMed] [Google Scholar]
- 10.Bjartmar, C., J.R. Wujek, and B.D. Trapp. 2003. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J. Neurol. Sci. 206:165–171. [DOI] [PubMed] [Google Scholar]
- 11.Lucchinetti, C., W. Brück, J. Parisi, B. Scheithauer, M. Rodriguez, and H. Lassmann. 2000. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47:707–717. [DOI] [PubMed] [Google Scholar]
- 12.Genain, C.P., B. Cannella, S.L. Hauser, and C.S. Raine. 1999. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 5:170–175. [DOI] [PubMed] [Google Scholar]
- 13.Weinshenker, B.G., P.C. O'Brien, T.M. Petterson, J.H. Noseworthy, C.F. Lucchinetti, D.W. Dodick, A.A. Pineda, L.N. Stevens, and M. Rodriguez. 1999. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann. Neurol. 46:878–886. [DOI] [PubMed] [Google Scholar]
- 14.Keegan, M., F. Konig, R. McClelland, W. Bruck, Y. Morales, A. Bitsch, H. Panitch, H. Lassmann, B. Weinshenker, M. Rodriguez, et al. 2005. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 366:579–582. [DOI] [PubMed] [Google Scholar]
- 15.Meinl, E., M. Krumbholz, and R. Hohlfeld. 2006. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 59:880–892. [DOI] [PubMed] [Google Scholar]
- 16.Linington, C., M. Bradl, H. Lassmann, C. Brunner, and K. Vass. 1988. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 17.Ozawa, K., T. Saida, K. Saida, H. Nishitani, and M. Kameyama. 1989. In vivo CNS demyelination mediated by anti-galactocerebroside antibody. Acta Neuropathol. (Berl.). 77:621–628. [DOI] [PubMed] [Google Scholar]
- 18.Kanter, J.L., S. Narayana, P.P. Ho, I. Catz, K.G. Warren, R.A. Sobel, L. Steinman, and W.H. Robinson. 2006. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 12:138–143. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor, K.C., K.A. McLaughlin, P.L. De Jager, T. Chitnis, E. Bettelli, C. Xu, W.H. Robinson, S.V. Cherry, A. Bar-Or, B. Banwell, et al. 2007. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 13:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou, D., R. Srivastava, S. Nessler, V. Grummel, N. Sommer, W. Bruck, H.P. Hartung, C. Stadelmann, and B. Hemmer. 2006. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl. Acad. Sci. USA. 103:19057–19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ercolini, A.M., and S.D. Miller. 2006. Mechanisms of immunopathology in murine models of central nervous system demyelinating disease. J. Immunol. 176:3293–3298. [DOI] [PubMed] [Google Scholar]
- 22.Sadatipour, B.T., J.M. Greer, and M.P. Pender. 1998. Increased circulating antiganglioside antibodies in primary and secondary progressive multiple sclerosis. Ann. Neurol. 44:980–983. [DOI] [PubMed] [Google Scholar]
- 23.Eikelenboom, M.J., A. Petzold, R.H. Lazeron, E. Silber, M. Sharief, E.J. Thompson, F. Barkhof, G. Giovannoni, C.H. Polman, and B.M. Uitdehaag. 2003. Multiple sclerosis: Neurofilament light chain antibodies are correlated to cerebral atrophy. Neurology. 60:219–223. [DOI] [PubMed] [Google Scholar]
- 24.DeVries, G.H. 2004. Cryptic axonal antigens and axonal loss in multiple sclerosis. Neurochem. Res. 29:1999–2006. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Y., R.R. Da, L.G. Hilgenberg, W.W. Tourtellotte, R.A. Sobel, M.A. Smith, M. Olek, R. Nagra, G. Sudhir, S. van den Noort, and Y. Qin. 2005. Clonal expansion of IgA-positive plasma cells and axon-reactive antibodies in MS lesions. J. Neuroimmunol. 167:120–130. [DOI] [PubMed] [Google Scholar]
- 26.Sherman, D.L., S. Tait, S. Melrose, R. Johnson, B. Zonta, F.A. Court, W.B. Macklin, S. Meek, A.J. Smith, D.F. Cottrell, and P.J. Brophy. 2005. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 48:737–742. [DOI] [PubMed] [Google Scholar]
- 27.Ratcliffe, C.F., R.E. Westenbroek, R. Curtis, and W.A. Catterall. 2001. Sodium channel β1 and β3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moldenhauer, A., J. Haas, C. Wascher, T. Derfuss, K.T. Hoffmann, H. Kiesewetter, and A. Salama. 2005. Immunoadsorption patients with multiple sclerosis: an open-label pilot study. Eur. J. Clin. Invest. 35:523–530. [DOI] [PubMed] [Google Scholar]
- 29.Smith, K.J., and H. Lassmann. 2002. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 1:232–241. [DOI] [PubMed] [Google Scholar]
- 30.DeLuca, G.C., K. Williams, N. Evangelou, G.C. Ebers, and M.M. Esiri. 2006. The contribution of demyelination to axonal loss in multiple sclerosis. Brain. 129:1507–1516. [DOI] [PubMed] [Google Scholar]
- 31.Parry, A., R. Corkill, A.M. Blamire, J. Palace, S. Narayanan, D. Arnold, P. Styles, and P.M. Matthews. 2003. Beta-Interferon treatment does not always slow the progression of axonal injury in multiple sclerosis. J. Neurol. 250:171–178. [DOI] [PubMed] [Google Scholar]
- 32.Coles, A.J., A. Cox, E. Le Page, J. Jones, S.A. Trip, J. Deans, S. Seaman, D.H. Miller, G. Hale, H. Waldmann, and D.A. Compston. 2006. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J. Neurol. 253:98–108. [DOI] [PubMed] [Google Scholar]
- 33.Davis, J.Q., S. Lambert, and V. Bennett. 1996. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain−) and NrCAM at nodal axon segments. J. Cell Biol. 135:1355–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Hanlon, G.M., J.J. Plomp, M. Chakrabarti, I. Morrison, E.R. Wagner, C.S. Goodyear, X. Yin, B.D. Trapp, J. Conner, P.C. Molenaar, et al. 2001. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain. 124:893–906. [DOI] [PubMed] [Google Scholar]
- 35.Nakano, S., and A.G. Engel. 1993. Myasthenia gravis: quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 43:1167–1172. [DOI] [PubMed] [Google Scholar]
- 36.Morgan, B.P., J.R. Dankert, and A.F. Esser. 1987. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J. Immunol. 138:246–253. [PubMed] [Google Scholar]
- 37.van Beek, J., M. van Meurs, B.A. ‘t Hart, H.P. Brok, J.W. Neal, A. Chatagner, C.L. Harris, N. Omidvar, B.P. Morgan, J.D. Laman, and P. Gasque. 2005. Decay-accelerating factor (CD55) is expressed by neurons in response to chronic but not acute autoimmune central nervous system inflammation associated with complement activation. J. Immunol. 174:2353–2365. [DOI] [PubMed] [Google Scholar]
- 38.Arroyo, E.J., E.E. Sirkowski, R. Chitale, and S.S. Scherer. 2004. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J. Comp. Neurol. 479:424–434. [DOI] [PubMed] [Google Scholar]
- 39.Wolswijk, G., and R. Balesar. 2003. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 126:1638–1649. [DOI] [PubMed] [Google Scholar]
- 40.Howell, O.W., A. Palser, A. Polito, S. Melrose, B. Zonta, C. Scheiermann, A.J. Vora, P.J. Brophy, and R. Reynolds. 2006. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 129:3173–3185. [DOI] [PubMed] [Google Scholar]
- 41.Coman, I., M.S. Aigrot, D. Seilhean, R. Reynolds, J.A. Girault, B. Zalc, and C. Lubetzki. 2006. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 129:3186–3195. [DOI] [PubMed] [Google Scholar]
- 42.Charles, P., S. Tait, C. Faivre-Sarrailh, G. Barbin, F. Gunn-Moore, N. Denisenko-Nehrbass, A.M. Guennoc, J.A. Girault, P.J. Brophy, and C. Lubetzki. 2002. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr. Biol. 12:217–220. [DOI] [PubMed] [Google Scholar]
- 43.Azzi, G., J.F. Bernaudin, C. Bouchaud, B. Bellon, and J. Fleury-Feith. 1990. Permeability of the normal rat brain, spinal cord and dorsal root ganglia microcirculations to immunoglobulins G. Biol. Cell. 68:31–36. [DOI] [PubMed] [Google Scholar]
- 44.Spies, J.M., K.W. Westland, J.G. Bonner, and J.D. Pollard. 1995. Intraneural activated T cells cause focal breakdown of the blood-nerve barrier. Brain. 118:857–868. [DOI] [PubMed] [Google Scholar]
- 45.Kowal, C., L.A. DeGiorgio, T. Nakaoka, H. Hetherington, P.T. Huerta, B. Diamond, and B.T. Volpe. 2004. Cognition and immunity; antibody impairs memory. Immunity. 21:179–188. [DOI] [PubMed] [Google Scholar]
- 46.Sospedra, M., and R. Martin. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23:683–747. [DOI] [PubMed] [Google Scholar]
- 47.Hochmeister, S., R. Grundtner, J. Bauer, B. Engelhardt, R. Lyck, G. Gordon, T. Korosec, A. Kutzelnigg, J.J. Berger, M. Bradl, et al. 2006. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J. Neuropathol. Exp. Neurol. 65:855–865. [DOI] [PubMed] [Google Scholar]
- 48.Leech, S., J. Kirk, J. Plumb, and S. McQuaid. 2007. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol. Appl. Neurobiol. 33:86–98. [DOI] [PubMed] [Google Scholar]
- 49.Norton, W.T., and S.E. Poduslo. 1973. Myelination in rat brain: method of myelin isolation. J. Neurochem. 21:749–757. [DOI] [PubMed] [Google Scholar]
- 50.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850–858. [DOI] [PubMed] [Google Scholar]
- 51.Stefferl, A., A. Schubart, M. Storch, A. Amini, I. Mather, H. Lassmann, and C. Linington. 2000. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J. Immunol. 165:2859–2865. [DOI] [PubMed] [Google Scholar]
- 52.Tait, S., F. Gunn-Moore, J.M. Collinson, J. Huang, C. Lubetzki, L. Pedraza, D.L. Sherman, D.R. Colman, and P.J. Brophy. 2000. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo–glial junction. J. Cell Biol. 150:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Storch, M.K., A. Stefferl, U. Brehm, R. Weissert, E. Wallstrom, M. Kerschensteiner, T. Olsson, C. Linington, and H. Lassmann. 1998. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 8:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.