Abstract
There has been much fanfare, and rightly so, heralding a revolution in the treatment of autoimmune disease using biologic agents—antibodies or other molecules that specifically target known mediators of disease. But not all patients respond to even the most successful biologic agent, which may provide clues about alternate disease mechanisms. Studies aimed at understanding the mechanism of action of biologic agents will yield significant benefits for experimental medicine.
Behind the dramatic advance of biologic agents lies the potential for another, sometimes less appreciated, revolution for experimental medicine. Biologic agents, by their nature, facilitate the dissection of the role played by the drug's target in the disease. Consequently, patient-focused investigation can begin to acquire a level of experimental sophistication that, until now, has been the exclusive domain of mouse immunologists. Not only can this form of experimental medicine add substantially to our understanding of the immunopathogenesis of disease, but it can also aide in the development of new therapies. Not all patients respond to any given biologic agent, a fact that may provide clues to alternate mechanisms of disease, and may also allow us to more accurately classify certain diseases based on response to therapy. This commentary will briefly discuss three aspects of the impact of biological therapy on experimental medicine. First, that the use of agents that target the same molecule can have very different therapeutic effects. Second, parallels can now be drawn between patients and corresponding animal models in which the same molecule is targeted. Finally, that widespread application of biological therapies to different diseases can reveal common immunologic pathways (Fig. 1).
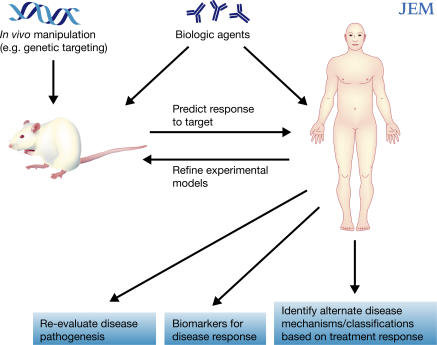
Figure 1.
Biologic therapy as a catalyst for experimental medicine and therapeutic advance. Targeted manipulation of the immune system in animal models, such as with biologic agents, has led to insights into disease pathogenesis and has contributed to the use of these agents in humans. The use of biologic agents in patients with autoimmune disease may help identify alternate disease mechanisms, establish biomarkers of disease, and reevaluate the pathogenesis of disease. Studies in humans can in turn help refine experimental animal models.
Dissecting disease mechanisms
Although a major focus of experimental medicine has been the development of new biological agents, dissecting the mechanism(s) of action of successful new agents is equally important. Removed from the regulatory and conceptual straightjacket of clinical trials, successful biologic agents are now being administered to thousands of patients, and these patients represent a tremendous resource for experimental medicine. The blockade of a specific target protein or the removal of a particular cell population in patients provides information about disease processes that cannot be gleaned from association studies. These patient populations allow the disease to be analyzed in responders and nonresponders, both before and after the intervention.
Nevertheless, the prospect of understanding the pathogenesis of autoimmune disease by studying how specific biologic treatments work has not been a familiar mindset among the clinical community. This is hardly surprising when the list of immunomodulatory drugs begins with corticosteroids, drugs that affect the transcription of 20% of proteins expressed by lymphocytes. Although efforts are being made to tease out the beneficial effects of corticosteroids from the harmful ones (1), it is unlikely that a particular molecular target will be identified that explains all of its immunomodulatory effects. A similar principle applies to other “conventional” therapies, such as the rheumatoid arthritis (RA) drugs methotrexate and sulphasalazine. We don't know exactly how they work, but we know (partly as a result of educated guesswork) that they affect multiple immunologic pathways. The fact that they do work was not predicted by an a priori hypothesis beyond a vague idea of immunosuppression. Therefore, it is difficult to make predictions about which patients (or diseases) may respond to treatment based on an understanding of the drug's mechanism of action. There are, however, a priori hypotheses behind the development of biological agents, and these hypotheses can be tested. Despite this, some believe that if a biologic therapy is effective, figuring out why is not a high priority. Indeed, the success of a particular biologic therapy often leads to further intellectual attachment to the original immunologic rationale.
The administration of biologic therapies to patients with autoimmune diseases, however, has revealed several surprises that illustrate the value in understanding how biologic agents work. For instance, it might be expected that targeted inhibition of a single cytokine, such as tumor necrosis factor (TNF), would yield a simple understanding of the role of TNF in disease pathogenesis. However, different anti-TNF drugs can appear to behave as entirely separate agents. An excellent example is etanercept (soluble TNF-α receptor) and infliximab (chimeric anti–TNF-α monoclonal antibody), of which only the latter is effective for the treatment of Crohn's disease (2, 3). In this inflammatory bowel disease, infliximab, but not etanercept, induces the apoptosis of lamina propria T cells, which could explain the differential efficacy of the two agents, though other processes have also been implicated (4, 5).
In contrast, both infliximab and etanercept are effective in patients with RA. But patients who fail to respond to one type of anti-TNF drug are only marginally less likely to respond to a different anti-TNF drug (6, 7) compared to “anti-TNF naive” patients, again suggesting that the drugs' mechanism(s) of action differ. Infliximab induces the differentiation of regulatory T cells in responsive patients with RA, highlighting another potential mechanism of action (8). Efforts to discover which disease mechanisms are associated with a clinical response may help identify new therapeutic targets. This could also reveal therapeutic biomarkers that predict clinical responsiveness, allowing physicians to target therapies only to those patients who are likely to respond (Fig. 1). Apart from being cost-effective, such clinical biomarkers would spare many patients the substantial risks associated with agents such as anti-TNF drugs.
One therapeutic target, two species
The efficacy of biologic therapies has also focused attention on the experimental models we use to identify potential targets for the treatment of human disease. Although the relevance of animal models for human disease has been much debated, these models have yielded important insights. Manipulation of mice using, for example, blocking antibodies and genetic targeting has provided definitive answers about the role of specific molecular targets in a given disease setting. The introduction of blocking antibodies for the treatment of human disease has allowed direct comparisons to be drawn between patients and the corresponding disease models. To illustrate the interrelationship between experimental models and their human disease counterparts, we will discuss two different biologic therapies (B cell depletion and anti-TNF therapy) in the context of two disease models, collagen-induced arthritis (CIA), a model of RA, and experimental allergic encephalomyelitis (EAE), a model of multiple sclerosis (MS).
In the CIA model, the absence of B cells prevents disease and the blockade of TNF ameliorates disease (9–11). Of interest, although arthritis cannot be induced in B cell–deficient mice, it can be induced (albeit mildly) in TNF-deficient mice (12). Surprisingly, whereas the demonstration that TNF promotes disease in the CIA model has been used to support clinical trials (13), the role of B cells in CIA was long considered of little relevance to human therapy. The importance of B cells was also demonstrated in a T cell transgenic model of arthritis, in which no joint inflammation occurred in the absence of B cells (14).
The success of the B cell–depleting drug rituximab (anti-CD20 antibody) in treating patients with RA has provided proof that B cells are important in RA, and has lead to a reemphasis on the role of B cells in various experimental models of arthritis. So far, little is known about whether B cells contribute to RA pathogenesis by producing antibodies and/or presenting antigen to T cells. But this question could be addressed by studying patients receiving rituximab and other B cell–targeting agents. The hope is that data generated from such studies will facilitate a more interactive dialogue between those studying patients and those studying animal models. One recent example of this was the administration of anti-CD20 to mice with CIA, which demonstrated that B cell depletion before, but not after, collagen administration diminished disease severity (15). Whether this result provides further insight into the pathogenesis of RA, or is simply specific for that experimental model, remains to be determined.
Studies conducted in the past two decades examining the role of TNF in EAE have generated sufficient data to justify the use of TNF-blocking agents for the treatment of MS. In mice, disease severity is reduced when TNF is blocked using antibodies (16, 17), and disease is delayed in mice genetically deficient for TNF (18). In general, overexpression of TNF exacerbates disease in the EAE model (19). Humans, however, have proven to be a different story. In 1999, a randomized, placebo-controlled study demonstrated that blocking TNF worsened disease in patients with MS (20). TNF has a complex role in the pathology of EAE in mice and acts at several stages of disease. Nevertheless, the message from the human data is clear: blocking TNF is detrimental to patients with MS. This observation has resulted in a reexamination of the animal data.
Contrasting the failure of anti-TNF agents in patients with MS, a recent clinical trial indicated that B cell depletion is beneficial in these patients—an outcome that will clearly influence the ongoing controversy regarding the role of B cells in EAE. Several studies have demonstrated that EAE is unaffected or gets worse when B cells are absent (21, 22), although both the nature of the immunogen and the strain of mouse can dramatically influence the result (23). The ongoing use of rituximab in patients with MS provides an important opportunity to enhance our understanding of the different roles of B cells in this complex disease (24). It will be critical to design experiments in patients being treated with biological therapies to dissect their mechanism of action, and these results can inform experiments in animal models.
Additional opportunities for experimental medicine will also arise from studies aimed at understanding the basis of side effects of biologic agents, such as the increased risk of tuberculosis with anti-TNF (25). Information gleaned from patients with primary immunodeficiency can also aide in the prediction of possible adverse effects of the corresponding biological agents. For example, data gathered from a registry of patients with X-linked agammaglobulinemia (XLA), who genetically lack B cells, revealed an increased risk of recurrent bacterial and viral infection (26). This information can be used to inform us on the safety of rituximab, although long-term registries are also required for rituximab-treated patients. One of the key immunological differences between patients with XLA and those treated with rituximab is the maintenance of immunoglobulin (Ig) concentrations in the latter. However, repeated B cell depletion can lead to a significant reduction in Ig levels, particularly IgM (27). This may necessitate the use of Ig infusions for rituximab-treated patients, currently standard therapy for XLA. Of importance, B cells are not an absolute requirement for the generation of T cell memory in response to vaccination either in patients with XLA (28) or in patients treated with rituximab (29).
One biologic therapy for many diseases
From a therapeutic perspective, the “conflicts” between the predictions arising from experimental models and the results of biological therapies could be used to support a trial-and-error approach, in which a given biological therapy is applied to a wide range of autoimmune diseases regardless of the available animal data. Indeed, the same biologic drugs are now being considered for the treatment of autoimmune diseases that have very different conventional treatments. This “disease-hopping” is motivated both by clinical need and the potential for financial profit (biologic therapy is also a revolution in money making). The potential benefits for experimental medicine are substantial. Investigators have tended to limit their research (experimental model or patient-based) to one clinical field of interest. Biologic therapies are not so particular and provide an additional impetus to bring together investigators interested in a variety of autoimmune diseases (30).
In addition to the increasing number of diseases now treated with B cell depletion and anti-TNF agents, anti-CD3 therapy is also being considered for several autoimmune diseases. Treatment with anti-CD3 has been championed, and debated, as a potent method of restoring tolerance in patients with diabetes (31), and this has increased its attractiveness for the treatment of other autoimmune diseases. This has led to debates, both about how tolerance (and its restoration) should be defined and which diseases should be considered autoimmune and thus likely to benefit from anti-CD3 therapy. For example, it is possible that not all inflammatory diseases are autoimmune, and in these cases, tolerance induction may not be an optimal therapeutic approach. One possible example is Crohn's disease. It has been proposed that immunodeficiency is the primary defect in this chronic inflammatory bowel disease exacerbated by an overzealous immune response (32).
Although we are only at the beginning of this process, studies involving biologic therapies emphasize the “two-way street” nature of experimental medicine in patients and studies in experimental animal models. There is a plethora of new agents arriving in the clinic that has been spurred on by the success of the early biologic agents. Experimental medicine must continue to thrive in the clinical setting where successful biologic therapies offer the prospect of great insight into the mechanisms of disease. Inevitably, this will promote the introduction of more specific, long-lasting, and perhaps less expensive therapies. Thus, the answer to the question of whether we need to know why the treatment works is a resounding “Yes!”
Acknowledgments
We thank Dr. Lucy Wedderburn for critical reading of this commentary.
M.R.E. and C.M. are at Department of Medicine, University College London, London W1T 4JF, UK.
References
- 1.Rhen, T., and J.A. Cidlowski. 2005. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 353:1711–1723. [DOI] [PubMed] [Google Scholar]
- 2.Brown, S.J., and M.T. Abreu. 2005. Antibodies to tumor necrosis factor-alpha in the treatment of Crohn's disease. Curr. Opin. Drug Discov. Devel. 8:160–168. [PubMed] [Google Scholar]
- 3.Sandborn, W.J., S.B. Hanauer, S. Katz, M. Safdi, D.G. Wolf, R.D. Baerg, W.J. Tremaine, T. Johnson, N.N. Diehl, and A.R. Zinsmeister. 2001. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 121:1088–1094. [DOI] [PubMed] [Google Scholar]
- 4.Van den Brande, J.M., H. Braat, G.R. van den Brink, H.H. Versteeg, C.A. Bauer, I. Hoedemaeker, C. van Montfrans, D.W. Hommes, M.P. Peppelenbosch, and S.J. van Deventer. 2003. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 124:1774–1785. [DOI] [PubMed] [Google Scholar]
- 5.Mitoma, H., T. Horiuchi, N. Hatta, H. Tsukamoto, S. Harashima, Y. Kikuchi, J. Otsuka, S. Okamura, S. Fujita, and M. Harada. 2005. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology. 128:376–392. [DOI] [PubMed] [Google Scholar]
- 6.van Vollenhoven, R., A. Harju, S. Brannemark, and L. Klareskog. 2003. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann. Rheum. Dis. 62:1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyrich, K.L., M. Lunt, K.D. Watson, D.P. Symmons, and A.J. Silman. 2006. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 56:13–20. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni, S., C. Mauri, and M.R. Ehrenstein. 2007. Anti–TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J. Exp. Med. 204:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson, L., J. Jirholt, R. Holmdahl, and L. Jansson. 1998. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin. Exp. Immunol. 111:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams, R.O., M. Feldmann, and R.N. Maini. 1992. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA. 89:9784–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori, L., S. Iselin, G. De Libero, and W. Lesslauer. 1996. Attenuation of collagen-induced arthritis in 55-kDa TNF receptor type 1 (TNFR1)-IgG1-treated and TNFR1-deficient mice. J. Immunol. 157:3178–3182. [PubMed] [Google Scholar]
- 12.Campbell, I.K., K. O'Donnell, K.E. Lawlor, and I.P. Wicks. 2001. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF. J. Clin. Invest. 107:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann, M., and R.N. Maini. 2003. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 9:1245–1250. [DOI] [PubMed] [Google Scholar]
- 14.Kouskoff, V., A.S. Korganow, V. Duchatelle, C. Degott, C. Benoist, and D. Mathis. 1996. Organ-specific disease provoked by systemic autoimmunity. Cell. 87:811–822. [DOI] [PubMed] [Google Scholar]
- 15.Yanaba, K., Y. Hamaguchi, G.M. Venturi, D.A. Steeber, E.W. St Clair, and T.F. Tedder. 2007. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J. Immunol. 179:1369–1380. [DOI] [PubMed] [Google Scholar]
- 16.Ruddle, N.H., C.M. Bergman, K.M. McGrath, E.G. Lingenheld, M.L. Grunnet, S.J. Padula, and R.B. Clark. 1990. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 172:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selmaj, K., C.S. Raine, and A.H. Cross. 1991. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann. Neurol. 30:694–700. [DOI] [PubMed] [Google Scholar]
- 18.Korner, H., D.S. Riminton, D.H. Strickland, F.A. Lemckert, J.D. Pollard, and J.D. Sedgwick. 1997. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J. Exp. Med. 186:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens, T., H. Wekerle, and J. Antel. 2001. Genetic models for CNS inflammation. Nat. Med. 7:161–166. [DOI] [PubMed] [Google Scholar]
- 20. 1999. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 53:457–465. [PubMed] [Google Scholar]
- 21.Wolf, S.D., B.N. Dittel, F. Hardardottir, and C.A. Janeway Jr. 1996. Experimental autoimmune encephalomyelitis induction in genetically B cell–deficient mice. J. Exp. Med. 184:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fillatreau, S., C.H. Sweenie, M.J. McGeachy, D. Gray, and S.M. Anderton. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944–950. [DOI] [PubMed] [Google Scholar]
- 23.Lyons, J.A., M. San, M.P. Happ, and A.H. Cross. 1999. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur. J. Immunol. 29:3432–3439. [DOI] [PubMed] [Google Scholar]
- 24.Antel, J., and A. Bar-Or. 2006. Roles of immunoglobulins and B cells in multiple sclerosis: from pathogenesis to treatment. J. Neuroimmunol. 180:3–8. [DOI] [PubMed] [Google Scholar]
- 25.Winthrop, K.L. 2006. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat. Clin. Pract. Rheumatol. 2:602–610. [DOI] [PubMed] [Google Scholar]
- 26.Winkelstein, J.A., M.C. Marino, H.M. Lederman, S.M. Jones, K. Sullivan, A.W. Burks, M.E. Conley, C. Cunningham-Rundles, and H.D. Ochs. 2006. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore). 85:193–202. [DOI] [PubMed] [Google Scholar]
- 27.Popa, C., M.J. Leandro, G. Cambridge, and J.C. Edwards. 2007. Repeated B lymphocyte depletion with rituximab in rheumatoid arthritis over 7 yrs. Rheumatology (Oxford). 46:626–630. [DOI] [PubMed] [Google Scholar]
- 28.Paroli, M., D. Accapezzato, V. Francavilla, A. Insalaco, A. Plebani, F. Balsano, and V. Barnaba. 2002. Long-lasting memory-resting and memory-effector CD4+ T cells in human X-linked agammaglobulinemia. Blood. 99:2131–2137. [DOI] [PubMed] [Google Scholar]
- 29.Neelapu, S.S., L.W. Kwak, C.B. Kobrin, C.W. Reynolds, J.E. Janik, K. Dunleavy, T. White, L. Harvey, R. Pennington, M. Stetler-Stevenson, et al. 2005. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat. Med. 11:986–991. [DOI] [PubMed] [Google Scholar]
- 30.Hafler, D. 2007. Cytokines and interventional immunology. Nat. Rev. Immunol. 7:423. [Google Scholar]
- 31.Chatenoud, L. 2005. CD3-specific antibodies restore self-tolerance: mechanisms and clinical applications. Curr. Opin. Immunol. 17:632–637. [DOI] [PubMed] [Google Scholar]
- 32.Marks, D.J., M.W. Harbord, R. MacAllister, F.Z. Rahman, J. Young, B. Al-Lazikani, W. Lees, M. Novelli, S. Bloom, and A.W. Segal. 2006. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 367:668–678. [DOI] [PubMed] [Google Scholar]