Abstract
Natural killer (NK) cell cytotoxicity involves the formation of an activating immunological synapse (IS) between the effector and target cell through which granzymes and perforin contained in lytic granules are delivered to the target cell via exocytosis. Inhibition of nonmuscle myosin II in human NK cells with blebbistatin or ML-9 impaired neither effector–target cell conjugation nor formation of a mature activating NK cell IS (NKIS; formation of an actin ring and polarization of the microtubule-organizing center and cytolytic granules to the center of the ring). However, membrane fusion of lytic granules, granzyme secretion, and NK cell cytotoxicity were all effectively blocked. Specific knockdown of the myosin IIA heavy chain by RNA interference impaired cytotoxicity, membrane fusion of lytic granules, and granzyme secretion. Thus, myosin IIA is required for a critical step between NKIS formation and granule exocytosis.
A major function of NK cells is the specific lysis of target cells, such as virally infected and tumor cells. The immunological synapse (IS) is formed at the contact site of immune cells and the cells they are recognizing. In the interaction between an NK cell and its target cell, an activating NK cell IS (NKIS) forms in distinct stages (1, 2). The NKIS, which is similar to the IS in other cells, contains a supramolecular activation cluster (SMAC). The SMAC is a distinct three-dimensional structure at the effector–target cell interface with specific clustering domains. In NK cell cytotoxicity, effector–target conjugate formation occurs first, followed by the accumulation of actin filaments and adhesion/activating receptors such as CD2 at the peripheral SMAC (pSMAC), and later by polarization of the microtubule organizing center and microtubule-dependent lytic granule polarization to the central SMAC (cSMAC) (2, 3). Polarization and exocytosis of lytic granules (a type of secretory lysosome) are key events in mature NKIS formation and function, and they are necessary for NK cell cytotoxicity.
Reorganization of filamentous actin (F-actin) is required for the formation of a mature lytic NKIS (2). Myosin motor proteins are also emerging as potentially important in IS formation. The myosin superfamily is thus far composed of at least 15 classes, with ∼40 members (4). Myosins generate ATP-dependent movement along actin, and are regulated by phosphorylation. Nonmuscle myosin II, in particular, is believed to be involved in force generation within cells via F-actin contraction. It is a hexamer consisting of two heavy chains, each with an actin-binding head region and a self-associating rodlike tail region with an α-helical coiled-coil motif, as well as two regulatory and two essential light chains. Initially, myosin was shown to play a role in molecular clustering at the T cell IS (5), but this work was performed using the relatively coarse inhibitor of myosin function 2,3-butane-dione monoxime (BDM) (6). The discovery of blebbistatin (1-phenyl-1,2,3,4-tetrahydro-4-hydroxypyrrolo[2.3-b]-7-methylquinolin-4-one), which is a specific inhibitor of myosin II ATPase activity (7), has facilitated the study of myosin II function in immune cells. Inhibition of myosin II by blebbistatin in CD4+ T cells impairs cell motility, but not IS formation (8). Moreover, inhibition of myosin II with the myosin light chain kinase inhibitor ML-9 (1-[5-chloronaphthalene-1-sulfonyl]-1H-hexahydro-1,4-diazepine hydrochloride) has been shown to inhibit NK cell cytotoxicity, but not effector–target conjugation (9). Myosin II is also especially relevant in NK cells because the myosin IIA isoform is recruited to a multiprotein complex formed during activating NKIS formation (10). This complex contains at least seven proteins, including Wiskott-Aldrich syndrome protein (WASp), which is required for F-actin reorganization at the NKIS (11).
Cytotoxic lymphocyte granule exocytosis is a unique cellular process, but has numerous features in common with the process of directed vesicle secretion at the neural synapse. The process of neurotransmitter release involves several defined steps, including movement of vesicles to the active zone, docking of vesicles at the membrane, priming, fusion, and subsequent neurotransmitter release (12). Although hundreds of proteins are believed to be involved in neural vesicle exocytosis (12), only four have thus far been identified in cytotoxic lymphocyte granule exocytosis (13). These proteins affect granule exocytosis at the stages of granule polarization (AP-3), docking (Rab27a), and priming (Munc13-4 and syntaxin11).
We show that inhibition of myosin II with blebbistatin and other myosin inhibitors impairs neither effector–target cell conjugation nor mature NKIS formation. However, they do inhibit membrane fusion of lytic granules, and thus also NK cell cytotoxicity. RNA interference (RNAi)–mediated knockdown of nonmuscle myosin IIA expression produces the same inhibitory effect. Therefore, myosin II inhibition blocks a step between mature synapse formation and lytic granule fusion with the cell membrane (leading to exocytosis of granule contents), pointing to a specific role for nonmuscle myosin IIA in NKIS function and showing it to be a fifth protein involved in lymphocyte lytic granule exocytosis.
RESULTS AND DISCUSSION
Myosin II inhibitors block NK cell cytotoxicity
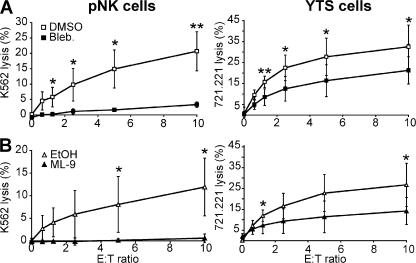
NK cell cytotoxicity requires the integrated function of multiple cytoskeletal elements (1, 2, 9, 11 ). Nonmuscle myosin IIA was focused on because it was recruited to an actin-associated multiprotein complex in NK cells after activation by target cells (10). Three different myosin II inhibitors (blebbistatin, ML-9, and BDM), which were each previously demonstrated to block myosin II function at the concentrations used (6, 7, 15), blocked NK cell cytotoxicity in a 51Cr release assay (Fig. 1 and not depicted). ML-9 was previously shown to have this effect (9). Both blebbistatin and ML-9 reduced cytotoxicity significantly compared with vehicle in several independent experiments, although YTS cells were more resistant than peripheral blood NK (pNK) cells. Although BDM also blocked NK cell cytotoxicity, only ML-9 and blebbistatin were used in further studies because of less overall specificity of BDM for myosin II (6).
Figure 1.
Inhibition of myosin II blocks NK cell cytotoxicity. pNK cells and YTS cells were pretreated with inhibitors, and their killing ability was assayed using 51Cr release from K562 or 721.221 target cells, respectively. DMSO was used as a solvent for blebbistatin (Bleb; A) and EtOH was used as a solvent for ML-9 (B), and they are the control curves for their respective inhibitors. Both solvents showed nonspecific effects on cytotoxicity. Specifically, at an E/T ratio of 10:1 DMSO at the concentration used reduced killing of pNK cells by 18% and of YTS cells by 0%, whereas EtOH at the concentration used reduced killing of pNK cells by 52% and of YTS cells by 15%. Each curve represents the mean ± the SD of three independent experiments. *, P < 0.05 and **, P < 0.01 by Student's t test.
Evaluation of NKIS formation
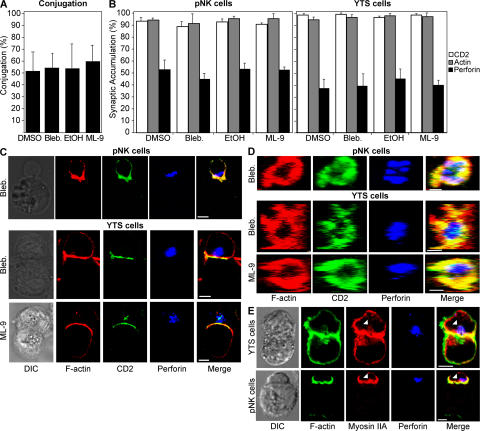
Several key steps before target cell lysis are necessary for NK cell effector function (1, 2), and inhibition of any of these steps would decrease NK cell cytotoxicity. To elucidate where myosin II plays its critical role, NK cell effector function was analyzed at each step. The first step is an adhesion molecule-mediated conjugation between the NK cell and target cell and was not affected by either of the myosin II inhibitors (Fig. 2 A). Next, the immunological synapse begins to mature. Important steps in this process are the accumulation of F-actin and adhesion molecules in the NKIS, followed by the polarization of lytic granules (2). As inhibition of actin by cytochalasin D blocks both of these steps in synapse formation (2), it is plausible that myosin II could play a role in the approximation of F-actin networks and receptor clustering (5, 16). However, inhibition of myosin II did not affect the percentage of cells that accumulated actin, CD2, or perforin at the NKIS (Fig. 2 B). The percentage of cells that accumulate perforin in the cSMAC is always less than the percentage of cells that accumulate actin or CD2 in the pSMAC (2). Cell morphology and two-dimensional synapse structure were the same as previously published images (Fig. 2 C and Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20071143/DC1) (2). Perforin, which is a component of and thus a marker for cytolytic granules, was clustered in the cSMAC, whereas actin and CD2 accumulated in the pSMAC. The appropriate localization of these key synapse proteins was also found in z sections, and the NKIS structure and size appeared unaffected by inhibition of myosin II in both YTS and pNK cells (Fig. 2 D and Fig. S1 B). A ring of F-actin and CD2 in the pSMAC was clearly formed, with perforin in the cSMAC. Thus, inhibition of myosin II affected NK cell effector function neither at the point of conjugation with a target cell nor in the formation of the pSMAC (actin ring) and cSMAC (cytolytic granule polarization), indicating that myosin II may function in a step between the formation of the NKIS and the killing of the target cell. Correspondingly, confocal microscopy of myosin II showed it to be primarily localized in the cortex with F-actin, as expected, because myosin II is a primarily F-actin–interacting protein (Fig. 2 E). However, a small amount of myosin II was found to be colocalized with granules as well, along with F-actin, indicating that some myosin II is localized in a position where it can participate in the terminal steps of granule-mediated cytotoxicity.
Figure 2.
Myosin II inhibition does not affect conjugation or mature lytic synapse formation. (A) YTS-GFP cells were incubated with 721.221-RFP cells, and conjugation was quantified by FACS. Conjugates were defined as the percentage of GFP, RFP double-positive events measured by FACS compared with those seen with YTS-GFP cells alone. (B) The accumulation of F-actin, perforin, and CD2-GFP (YTS cells) or CD2-FITC (pNK cells) at the IS of an effector–target conjugate pair was evaluated for 50 conjugates in each experiment. Values are expressed as the percentage of synapses at which fluorescence was accumulated. Bar graphs represent the mean ± the SD of three independent experiments. (C) pNK cells are shown conjugated with K562 cells (top), and YTS-CD2-GFP cells are shown conjugated with 721.221 cells (bottom). NK cells are the top cells and target cells are the bottom cells in the conjugate pair. Differential interference contrast images are in the left column and fluorescent images are in the right columns. A merged overlay of all fluorescent channels is in the fifth column. (D) Representative lytic synapses were evaluated throughout their volume, and the z, x plane was reconstructed. The first row shows a lytic synapse in a pNK cell conjugated with a K562 cell. The bottom two rows show lytic synapses between YTS-CD2-GFP cells and 721.221 cells. The final column is a merged overlay of the fluorescent channels from the first three columns. The different inhibitor treatment for each row is indicated on the left of both C and D. In the case of both C and D, vehicle control images were identical in each case to those where NK cells were treated with inhibitors (solvent control for C and D are found in Fig. S1). (E) Myosin IIA colocalizes with F-actin and perforin. Representative conjugate pairs of both a YTS cell and a 721.221 target cell (top), as well as a pNK cell with a K562 cell (bottom) are shown, in the same format as C. Myosin II is found to associate with F-actin, as well as granules (white arrowhead). Bars, 5 μm.
Inhibition of granule exocytosis
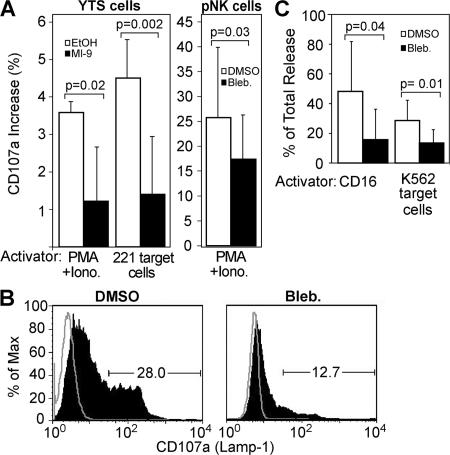
After lytic granule polarization to the synapse, granule fusion with the NK cell membrane occurs, followed by perforin and granzyme release (exocytosis) and eventual target cell lysis. Because myosin inhibitors blocked cytotoxicity, but not activating NKIS formation, the effects of myosin II inhibition on granule exocytosis were examined. First, cell surface CD107a (LAMP-1), which is found on the lumenal surface of intracellular lytic granules that are derived from lysosomes, was measured. When lytic granules fuse with the cell membrane, the lumenal surface becomes part of the cell surface (17). Thus, surface expression of CD107a was used as a marker for granule fusion with the cell membrane upon stimulation of the NK cells. Activation of YTS cells with either PMA/ionomycin or with 721.221 target cells yielded a small increase in surface CD107a that was inhibited by treatment with myosin II inhibitors (Fig. 3 A). However, pNK cells showed a much larger increase in CD107a expression upon treatment with PMA/ionomycin, as previously described (17). This expression was significantly reduced after myosin II inhibition (Fig. 3 B). Thus, myosin II plays a role in the fusion of lytic granules with the NK cell membrane.
Figure 3.
Myosin II inhibitors block lytic granule exocytosis. (A) pNK and YTS cells were activated with either their respective target cells or PMA and ionomycin (Iono). The percentage of increase in CD107a surface expression was evaluated by FACS and expressed as the increase over identically treated (including with appropriate inhibitors) unstimulated cells. Thus, unstimulated cells were used as a baseline to account for any nonspecific stimulation of antibody endocytosis or granule exocytosis over the incubation time, as well as any antibody endocytosis nonspecifically induced by cell treatment. Unstimulated cells also accounted for any potential shifts in fluorescence caused by drug color. Bar graphs represent the mean ± the SD of three independent experiments. (B) A representative histogram of CD107a surface expression from pNK cells stimulated with PMA/ionomycin ± blebbistatin. The percentage of pNK cells expressing a high level of CD107a was gated, and values are shown on the graph. The percentage of Max indicates the number of events at each given fluorescence intensity, where the intensity with the greatest number of cells has been normalized to be 100%. (C) pNK cells were stimulated by plate-bound anti-CD16 or K562 target cells, and their supernatant was used in a serine esterase assay. Results are expressed as a percentage of total release from cells pretreated with inhibitors or appropriate controls to account for possible background effects on the colorimetric assay caused by either intrinsic compound color or direct effects on serine esterase activity. Bar graphs represent the mean ± the SD of four to five independent experiments. Paired and unpaired Student's t tests were performed to obtain P values.
After granule fusion, the contents of the granules, most importantly perforin and granzymes, are released into the immunological synapse (18). CD16 (FcγRIIIA), which is present on a majority of pNK cells, renders them strong mediators of antibody-dependent cellular cytotoxicity on IgG–coated target cells. Moreover, this process results in a robust degranulation in the absence of target cells (19). Thus, stimulation by anti-CD16, as well as by K562 target cells, was used to activate pNK cells and induce granule release into the supernatant. Serine esterase activity (which is an assay for granzymes) was significantly decreased in both cases when stimulated pNK cells were first preincubated with blebbistatin (Fig. 3 C), indicating that, as expected, a lack of granule fusion leads to a lack of granule content release.
These results are particularly important in defining a key role for myosin II in granule exocytosis independent of other steps in NK cell cytotoxicity. Although synapse formation and conjugation were unimpaired, further experiments are required to fully evaluate the effect of myosin II inhibition on NK cell motility, especially as myosin IIA has previously been shown to regulate the motility of CD4+ T cells (8). However, both treatment with PMA/ionomycin that is uniformly distributed within media and interaction with anti-CD16 mAb-coated plates are motility independent.
RNAi-mediated myosin IIA knockdown impairs NK cell cytotoxicity and granule exocytosis
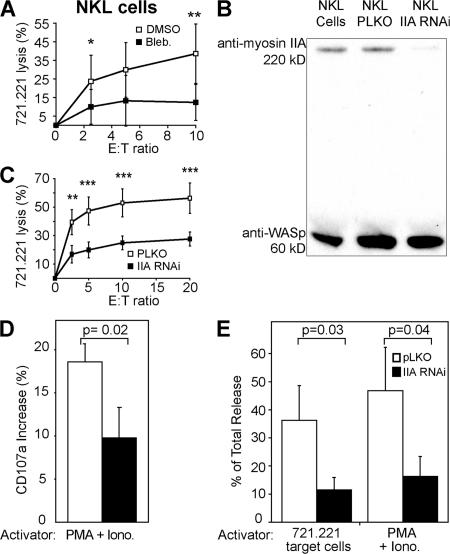
Although the specificity of blebbistatin for nonmuscle myosin II may implicate it, several isoforms of myosin II exist with differing effects. Although myosin IIA was the isoform identified as a component of the WIP–WASp–containing multiprotein complex at the synapse by vMALDI tandem mass spectroscopy (10), a smaller amount of myosin IIB was also seen by Western blot (unpublished data). In this light, nonmuscle myosin IIA and IIB have been shown to have distinct roles in vesicle exocytosis during cell membrane repair in fibroblasts (20). Myosin IIA had a proposed role in vesicle trafficking, and IIB in membrane resealing. Thus, to confirm the inhibitor specificity, and to look at the isoform-specific function of myosin II, RNAi knockdown of myosin IIA was performed in NKL cells, which also have reduced killing ability in the presence of blebbistatin (Fig. 4 A). NKL cells infected with the lentivirus for myosin IIA RNAi had a >80% reduction in myosin IIA protein expression relative to WASp expression (Fig. 4 B) compared with the pLKO vector-only control. Furthermore, myosin IIA mRNA expression was reduced by ∼75% as assessed by semiquantitative RT-PCR, whereas mRNA transcript levels of other proteins involved in granule exocytosis (syntaxin11, Munc-13-4, and Rab27a) were unaffected (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071143/DC1). Cytotoxicity was also impaired by ∼50%, which is comparable to the extent of myosin IIA knockdown (Fig. 4 C). Granule exocytosis was impaired to approximately the same extent, as assessed by lytic granule fusion with the cell membrane measured by CD107a surface expression (Fig. 4 D), as well as by serine esterase release (Fig. 4 E). Cell morphology and proliferative capacity remained similar to control cells.
Figure 4.
Knockdown of myosin IIA in NKL cells impairs cytotoxicity and granule exocytosis. (A) NKL cells were pretreated with blebbistatin, and killing ability was assayed using 51Cr release from 721.221 target cells. (B) Relative protein levels were assessed after RNAi of myosin IIA by Western blot with WASp content as the control. (C) Killing ability of NKL cells with myosin IIA knockdown was assayed using the same 51Cr release assay. NKL cells infected with the pLKO vector only were used as a control. *, P < 0.1, **, P < 0.04, and **, P < 0.02 by Student's t test for both graphs. (D) The percentage of increase in CD107a surface expression was evaluated by FACS and expressed as the increase over identically treated cells, but with no stimulation by PMA and ionomycin (Iono). (E) NKL cells were stimulated by PMA and ionomycin or 721.221 target cells, and their supernatant was used in a serine esterase assay. Results are expressed as a percentage of total release. All data represent the mean ± the SD of three independent experiments. Unpaired Student's t tests were performed to obtain P values.
These results demonstrate the key role that myosin IIA plays in NK cell effector function at some step between lytic granule polarization and granule exocytosis. However, the nature of this step remains to be defined, and there are several possibilities. In particular, the interaction between myosin IIA and actin could be force generating and facilitate vesicle fusion. Myosin II has already been hypothesized to play a role in the fusion dynamics of chromaffin cells by influencing the kinetics of exocytotic pore expansion (21), and it could have a similar role in this study. Myosin light chain kinase, which is a key regulator of myosin II, has also been implicated in vesicle release at neural synapses (22) and the ATP-dependent priming of exocytosis in chromaffin cells (23), further indicating that myosin II may play a key role in vesicle fusion.
Alternatively, myosin IIA could help build a bridge between two cytoskeletal proteins. In the NKIS, microtubules bring lytic granules when they polarize to a synapse that has already accumulated F-actin. Conceivably, myosin IIA could help lytic granules approach the synapse more closely than they are able to while attached to microtubules, and position them close enough to fuse with the NK cell membrane. Myosin II also appears to have an important role in vesicle transport (21), and thus may also have a cooperative role with microtubule motors.
Myosin IIA may therefore play a role in vesicle movement close to the synapse, and although z sections do not show a disrupted cSMAC or pSMAC, myosin IIA inhibition may have a more subtle effect on granule alignment within the actin ring, disrupting vesicle docking, priming, or fusion with the membrane. In that case, the deficiency could be similar to that seen in the mouse model of Griscelli syndrome (ashen), which is a Rab27a deficiency where CTL lytic granules become trapped behind the Golgi apparatus and fail to dock at the plasma membrane (24). Alternatively, myosin IIA may play a role in vesicle priming, as Munc13-4 does in CTL as found in patients with familial hemophagocytic lymphohistiocytosis 3 (25). NK cells in Munc13-4–deficient patients have also been found to have lower cytotoxicity caused by impaired granule fusion with the membrane, whereas granule polarization was unaffected (26). Similarly, a role for myosin IIA in lytic granule exocytosis has been definitively demonstrated here.
MATERIALS AND METHODS
Cells and inhibitors.
The NKL (27) and YTS NK cell lines, YTS cells expressing the mouse ecotropic receptor (YTS-Eco), and YTS stably expressing GFP (YTS-GFP) or a CD2-GFP fusion protein (2) were used as model NK cell systems. Leukocyte-enriched peripheral blood was obtained from Massachusetts General Hospital or the Dana-Farber Cancer Institute with Institutional Revue Board approval, and ex vivo pNK cells were prepared by treatment with Rosette-Sep NK (StemCell Technologies) and subsequent separation via centrifugation through Ficoll-Paque (GE Healthcare). K562 erythroleukemia, 721.221 B-lymphoblastoid cells, or 721.221 cells expressing RFP (RFP-221; all MHC class I–negative) were used as target cells.
NK cells were preincubated with the following inhibitors for 30–60 min at 37°C, where indicated: 80 μM ML-9 (Sigma-Aldrich) in 0.6% EtOH, 20 mM BDM (Sigma-Aldrich) in 2% MeOH, and 75 μM blebbistatin (Toronto Research Chemicals) in 0.3–3.3% DMSO.
Myosin IIA RNAi.
Two DNA oligos designed to produce the shRNA targeting the sequence 5′-GGGACTTGTCCCAAGTCTGAC-3′ in the 3′UTR of the myosin IIA gene were inserted into the pLKO.3G (pLKO) vector containing an ampicillin resistance gene (a gift from D. Mathis and C. Benoist, Joslin Diabetes Center, Boston, MA). The constructed plasmids, together with envelope gene–encoding plasmids pHR'-CMV-δR8.20vpr and pHR'-CMV-VSV-G (a gift from R. Erikson, Harvard University, Cambridge, MA) were transfected into a 293T packaging cell line using FuGENE 6.0 Transfection Reagent (Roche) to produce recombinant lentivirus. Harvested virus was mixed with 8 μg/ml polybrene (Sigma-Aldrich) and used to infect NKL cells. Lentivirus-infected cells were selected by GFP expression with flow cytometry, and myosin IIA expression was assessed by Western blot (10) and semiquantitative RT-PCR.
Semiquantitative RT-PCR.
Total mRNA was extracted from lentivirus-infected NKL cells, quantified, and diluted to a concentration of 100 ng/ml. After reverse transcription, PCR was performed in limiting dilutions of 1/25 and 1/125 using the Thermoscript RT-PCR kit (Invitrogen). Primers used for the evaluation of myosin IIA heavy chain (MYH9) expression were 5′-CAAAGGAGCCCTGGCGTTAGAG-3′ and 5′-CCCCATCCGCTTTGCCATCTAC-3′. For hMunc13-4, the primers used were 5′-TACTTCTGCAGCCGAATCCA-3′ and 5′-CCCAGCGTGTCGTAGTCCA-3′. For hRab27a, the primers used were 5′-ATGTCTGATGGAGATTATGATTACCTC-3′ and 5′-TTGCTTGGCTTATGTTTGTCCCATTGGCA-3′. For hSyntaxin11, the primers used were 5′-ACTACAACCAGGCCGAGATGAA-3′ and 5′-TTGTACGTTGAGCTCGATGACG-3′. For hHPRT, the primers used were 5′-CCTGCTGGATTACATTAAAGCACTG-3′ and 5′-GTCAAGGGCATATCCAACAACAAAC-3′.
NK cell functional assays.
Cytolytic activity was assayed by 51Cr release assay, as previously described (11).
To measure conjugation, YTS-GFP cells (5 × 106 cells/ml) were mixed with an equal amount of RFP-221 cells, combined by centrifugation at 200 g for 1 min, and incubated at 37°C for 20 min. Cells were then fixed with 0.5% paraformaldehyde and evaluated by FACS.
To determine CD107a (LAMP-1) surface expression, YTS cells were mixed at a 1:1 ratio with target cells, 2.5 μg/ml PMA (Sigma-Aldrich), 0.5 μg/ml ionomycin (Sigma-Aldrich), or only media for 6 h at 37°C in the presence of PE-conjugated anti-CD107a mAb (BD PharMingen), after which cells were washed and resuspended in PBS + 1% BSA. 3 mM monensin (Golgi-Stop; BD PharMingen) was added after the first hour. pNK cells were treated similarly, except for a 4-h incubation with 2 ng/ml PMA, 0.5 μg/ml ionomycin, and 0.6 mM. Monensin was used and cells were resuspended in PBS + 2% BSA and 0.5 mM EDTA. Both cell types were incubated with FITC or APC-conjugated CD56 mAb (BD PharMingen) on ice for 30 min, washed twice, fixed with 1% paraformaldehyde, and evaluated by FACS. GFP-expressing NKL cells were stimulated with 20 ng/ml PMA, 0.5 μg/ml ionomycin for 4 h at 37°C in the presence of PE-conjugated anti-CD107a mAb (BD PharMingen), washed, resuspended in PBS + 2% BSA and then evaluated by flow cytometry. Only GFP-positive cells were included in the analysis. Unstimulated NK cells were used as a negative control for all cell lines. Equal numbers of events were collected for both unstimulated and stimulated samples, as well as for control and experimental NK cell lines.
Nα-benzyloxycarbonyl-L-lysine thiobenzyl ester assay for serine esterase was performed as previously described (14), except that pNK cells were preincubated at least overnight with 100 U/ml IL-2. PNK cells were stimulated with plate-bound anti-CD16 and K562 target cells (E/T ratio 1:1). NKL cells were stimulated with 20 ng/ml PMA and 0.5 μg/ml ionomycin, and 721.221 target cells. Stimulation was for 5 h at 37°C. Total release was obtained by repeated freeze–thaw of NK cells.
Confocal microscopy.
Confocal microscopy was performed as previously described (2), except that rabbit anti–human myosin IIA (Sigma-Aldrich) and goat anti–rabbit (Invitrogen) were additionally used. For evaluation of synapses throughout their volume, 20–30 images were acquired through the z axis at 0.5–1-μm intervals (total volume of z, x reconstructions was 10–20 μm).
Online supplemental material.
Fig. S1 shows that NK cells preincubated with solvent alone form normal two- and three-dimensional mature lytic synapses identical to inhibitor-treated cells. Fig. S2 shows that myosin IIA RNAi reduces the expression of myosin IIA mRNA in NK cells, but not that of the granule exocytosis–related proteins hSyntaxin11, hRab27a, and hMunc13-4. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071143/DC1.
Supplemental Material
Acknowledgments
We thank Dennis Keefe and Derin Keskin for advice and members of the Strominger laboratory for help at all stages.
This work was supported by National Institutes of Health grants AI-50207 (to J.L. Storminger), AI-55602 (to J.S. Orange), and grants from the Harvard College Research Program (to M.M. Andzelm).
The authors have no conflicting financial interests.
Abbreviations used: BDM, 2,3-butane-dione monoxime; cSMAC, central SMAC; IS, immunological synapse; NKIS, NK cell IS; pNK, peripheral blood NK; pSMAC, peripheral SMAC; RNAi, RNA interference; SMAC, supramolecular activation cluster; WASp, Wiskott-Aldrich syndrome protein.
References
- 1.Wülfing, C., B. Purtic, J. Klem, and J.D. Schatzle. 2003. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc. Natl. Acad. Sci. USA. 100:7767–7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orange, J.S., K.E. Harris, M.M. Andzelm, M.M. Valter, R.S. Geha, and J.L. Strominger. 2003. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc. Natl. Acad. Sci. USA. 100:14151–14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopcow, H.D., D.S. Allan, X. Chen, B. Rybalov, M.M. Andzelm, B. Ge, and J.L. Strominger. 2005. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc. Natl. Acad. Sci. USA. 102:15563–15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellers, J.R. 2000. Myosins: a diverse superfamily. Biochim. Biophys. Acta. 1496:3–22. [DOI] [PubMed] [Google Scholar]
- 5.Wülfing, C., and M.M. Davis. 1998. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 282:2266–2269. [DOI] [PubMed] [Google Scholar]
- 6.Ostap, E.M. 2002. 2,3-Butanedione monoxime (BDM) as a myosin inhibitor. J. Muscle Res. Cell Motil. 23:305–308. [DOI] [PubMed] [Google Scholar]
- 7.Straight, A.F., A. Cheung, J. Limouze, I. Chen, N.J. Westwood, J.R. Sellers, and T.J. Mitchison. 2003. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 299:1743–1747. [DOI] [PubMed] [Google Scholar]
- 8.Jacobelli, J., S.A. Chmura, D.B. Buxton, M.M. Davis, and M.F. Krummel. 2004. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat. Immunol. 5:531–538. [DOI] [PubMed] [Google Scholar]
- 9.Ito, M., F. Tanabe, A. Sato, E. Ishida, Y. Takami, and S. Shigeta. 1989. Inhibition of natural killer cell-mediated cytotoxicity by ML-9, a selective inhibitor of myosin light chain kinase. Int. J. Immunopharmacol. 11:185–190. [DOI] [PubMed] [Google Scholar]
- 10.Krzewski, K., X. Chen, J.S. Orange, and J.L. Strominger. 2006. Formation of a WIP-, WASp-, actin-, and myosin IIA–containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J. Cell Biol. 173:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orange, J.S., N. Ramesh, E. Remold-O'Donnell, Y. Sasahara, L. Koopman, M. Byrne, F.A. Bonilla, F.S. Rosen, R.S. Geha, and J.L. Strominger. 2002. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc. Natl. Acad. Sci. USA. 99:11351–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Südhof, T.C. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27:509–547. [DOI] [PubMed] [Google Scholar]
- 13.Hong, W. 2005. Cytotoxic T lymphocyte exocytosis: bring on the SNAREs! Trends Cell Biol. 15:644–650. [DOI] [PubMed] [Google Scholar]
- 14.Galandrini, R., F. Micucci, I. Tassi, M.G. Cifone, B. Cinque, M. Piccoli, L. Frati, and A. Santoni. 2005. Arf6: a new player in FcγRIIIA lymphocyte-mediated cytotoxicity. Blood. 106:577–583. [DOI] [PubMed] [Google Scholar]
- 15.Thomas, M.G., T.A. Santa Coloma, J. Correale, and G.L. Boccaccio. 2002. Myosin light chain kinase inhibitors induce retraction of mature oligodendrocyte processes. Neurochem. Res. 27:1305–1312. [DOI] [PubMed] [Google Scholar]
- 16.Dustin, M.L., and J.A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23–29. [DOI] [PubMed] [Google Scholar]
- 17.Alter, G., J.M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 294:15–22. [DOI] [PubMed] [Google Scholar]
- 18.Ortaldo, J.R., R.T. Winkler-Pickett, K. Nagashima, H. Yagita, and K. Okumura. 1992. Direct evidence for release of pore-forming protein during NK cellular lysis. J. Leukoc. Biol. 52:483–488. [DOI] [PubMed] [Google Scholar]
- 19.Bryceson, Y.T., M.E. March, D.F. Barber, H.-G. Ljunggren, and E.O. Long. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 202:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Togo, T., and R.A. Steinhardt. 2004. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol. Biol. Cell. 15:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ñeco, P., D. Giner, S. Viniegra, R. Borges, A. Villarroel, and L.M. Gutiérrez. 2004. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J. Biol. Chem. 279:27450–27457. [DOI] [PubMed] [Google Scholar]
- 22.Mochida, S. 1995. Role of myosin in neurotransmitter release: functional studies at synapses formed in culture. J. Physiol. (Paris). 89:83–94. [DOI] [PubMed] [Google Scholar]
- 23.Kumakura, K., K. Sasaki, T. Sakurai, M. Ohara-Imaizumi, H. Misonou, S. Nakamura, Y. Matsuda, and Y. Nonomura. 1994. Essential role of myosin light chain kinase in the mechanism for MgATP-dependent priming of exocytosis in adrenal chromaffin cells. J. Neurosci. 14:7695–7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stinchcombe, J.C., D.C. Barral, E.H. Mules, S. Booth, A.N. Hume, L.M. Machesky, M.C. Seabra, and G.M. Griffiths. 2001. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldmann, J., I. Callebaut, G. Raposo, S. Certain, D. Bacq, C. Dumont, N. Lambert, M. Ouachée-Chardin, G. Chedeville, H. Tamary, et al. 2003. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 115:461–473. [DOI] [PubMed] [Google Scholar]
- 26.Marcenaro, S., F. Gallo, S. Martini, A. Santoro, G.M. Griffiths, M. Aricò, L. Moretta, and D. Pende. 2006. Analysis of natural killer cell function in familial hemophagocytic lymphohistiocytosis (FHL). Defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 108:2316–2323. [DOI] [PubMed] [Google Scholar]
- 27.Robertson, M.J., K.J. Cochran, C. Cameron, J.M. Le, R. Tantravahi, and J. Ritz. 1996. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 24:406–415. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.