Abstract
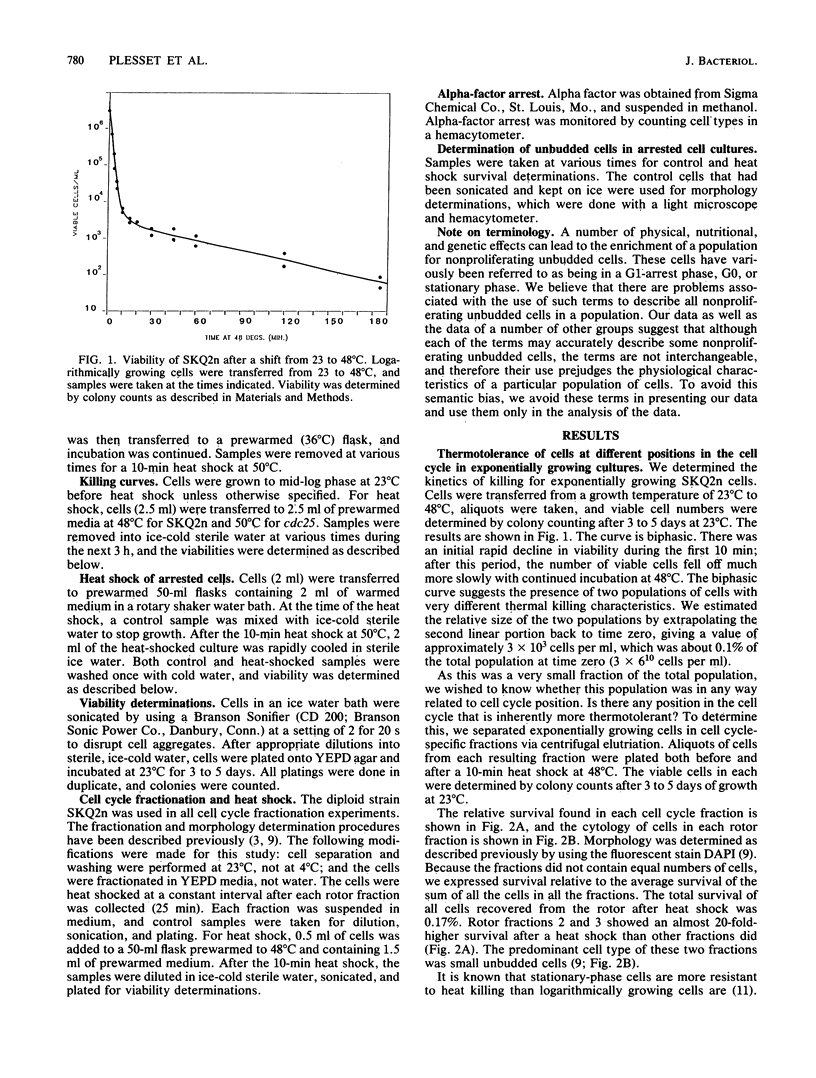
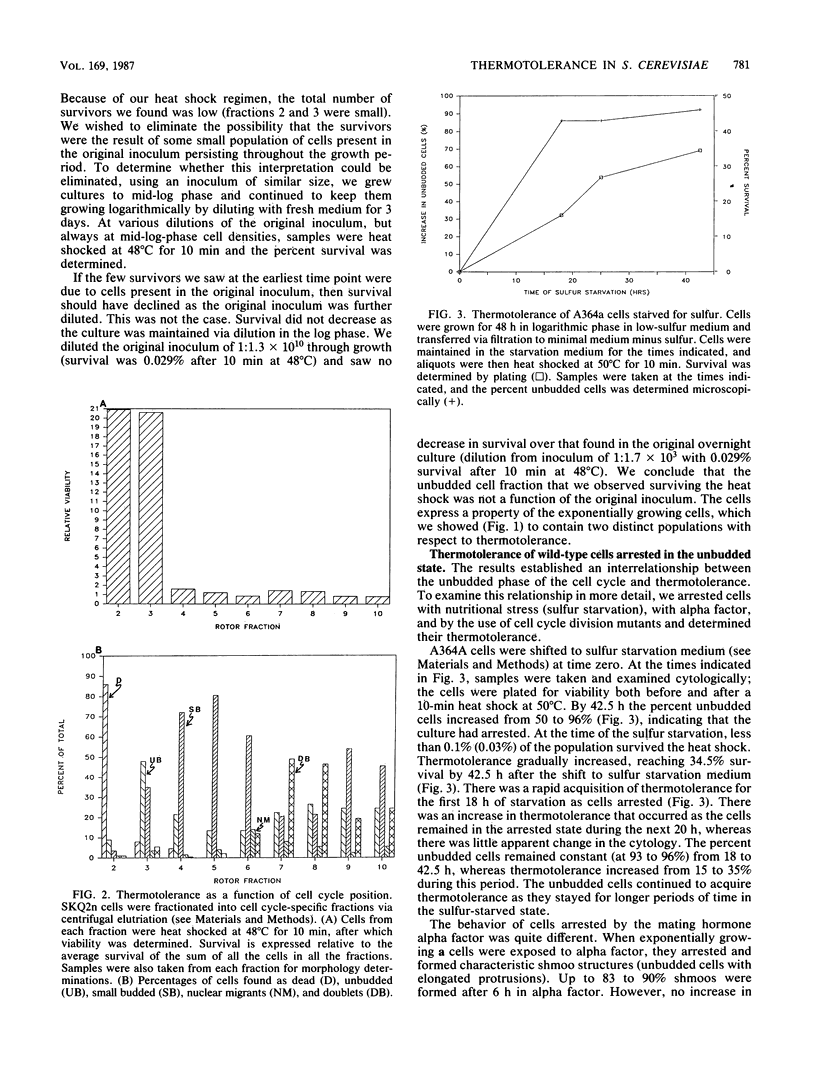
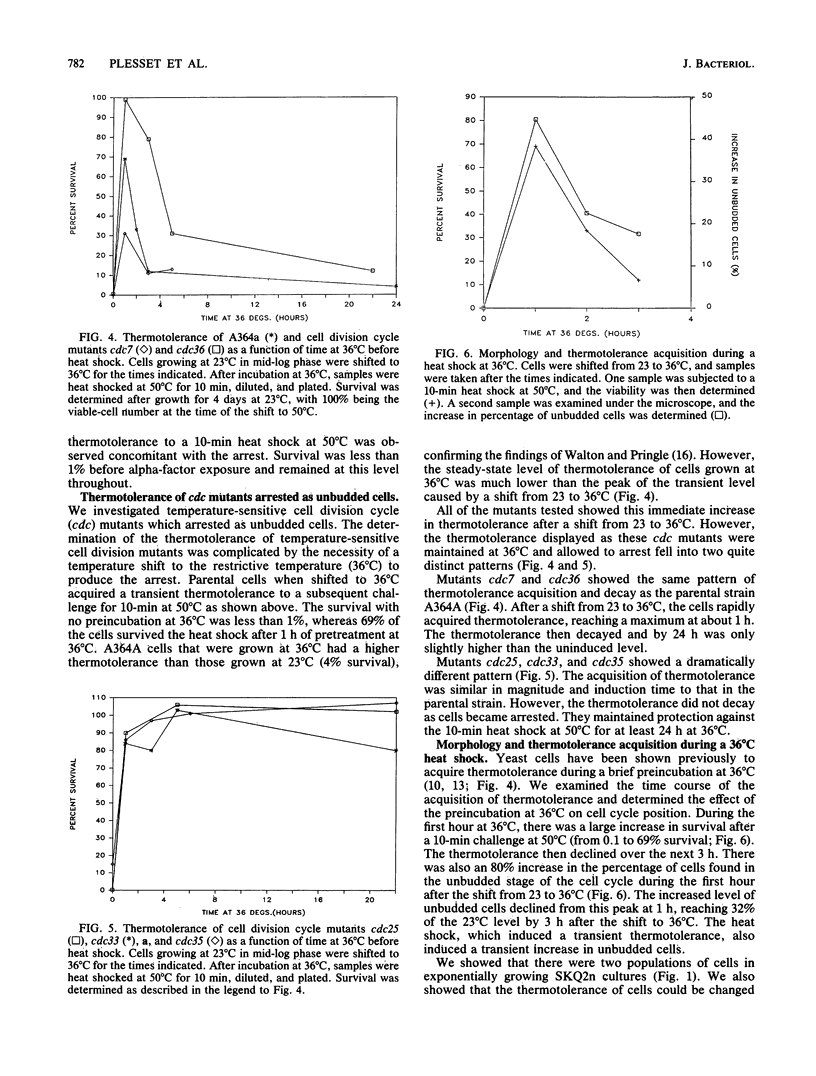
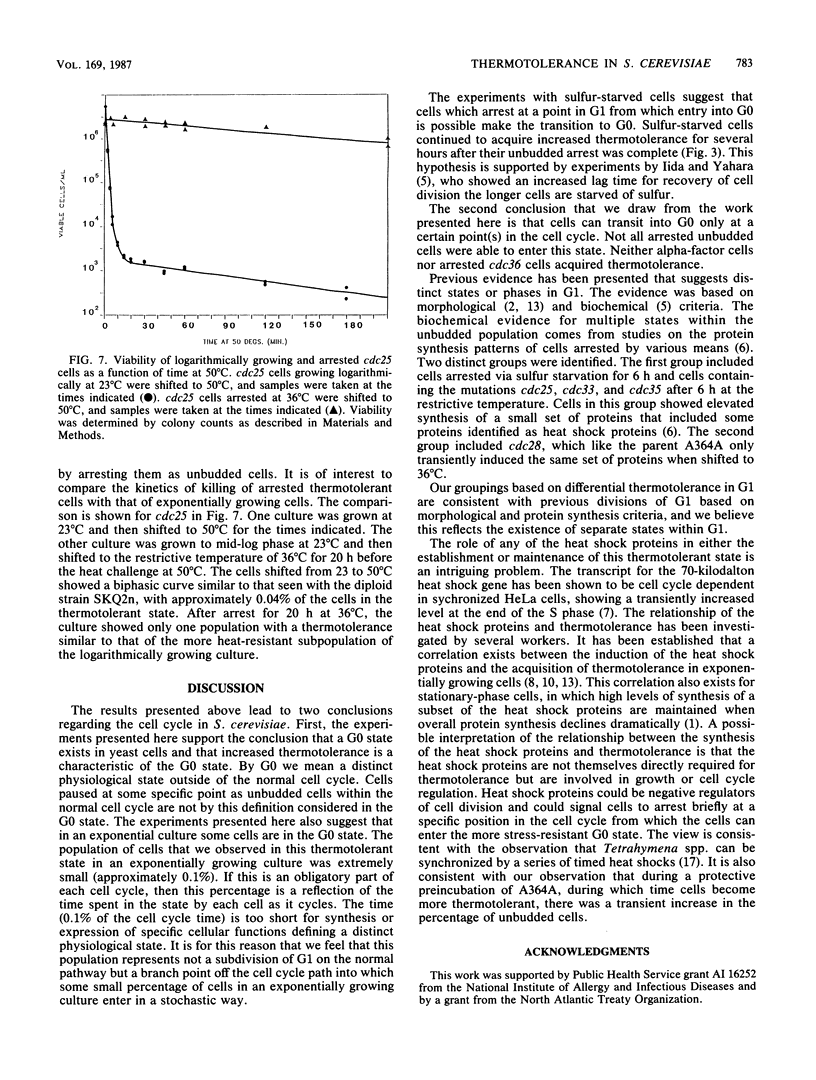
We showed that the heat killing curve for exponentially growing Saccharomyces cerevisiae was biphasic. This suggests two populations of cells with different thermal killing characteristics. When exponentially growing cells separated into cell cycle-specific fractions via centrifugal elutriation were heat shocked, the fractions enriched in small unbudded cells showed greater resistance to heat killing than did other cell cycle fractions. Cells arrested as unbudded cells fell into two groups on the basis of thermotolerance. Sulfur-starved cells and the temperature-sensitive mutants cdc25, cdc33, and cdc35 arrested as unbudded cells were in a thermotolerant state. Alpha-factor-treated cells arrested in a thermosensitive state, as did the temperature-sensitive mutant cdc36 when grown at the restrictive temperature. cdc7, which arrested at the G1-S boundary, arrested in a thermosensitive state. Our results suggest that there is a subpopulation of unbudded cells in exponentially growing cultures that is in G0 and not in G1 and that some but not all methods which cause arrest as unbudded cells lead to arrest in G0 as opposed to G1. It has been shown previously that yeast cells acquire thermotolerance to a subsequent challenge at an otherwise lethal temperature during a preincubation at 36 degrees C. We showed that this acquisition of thermotolerance was corrected temporally with a transient increase in the percentage of unbudded cells during the preincubation at 36 degrees C. The results suggest a relationship between the heat shock phenomenon and the cell cycle in S. cerevisiae and relate thermotolerance to transient as well as to more prolonged residence in the G0 state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985 Jan;161(1):385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. N., Elliott S. C. Fractionation of Saccharomyces cerevisiae cell populations by centrifugal elutriation. J Bacteriol. 1977 Jan;129(1):97–100. doi: 10.1128/jb.129.1.97-100.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Yahara I. Durable synthesis of high molecular weight heat shock proteins in G0 cells of the yeast and other eucaryotes. J Cell Biol. 1984 Jul;99(1 Pt 1):199–207. doi: 10.1083/jcb.99.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Yahara I. Specific early-G1 blocks accompanied with stringent response in Saccharomyces cerevisiae lead to growth arrest in resting state similar to the G0 of higher eucaryotes. J Cell Biol. 1984 Apr;98(4):1185–1193. doi: 10.1083/jcb.98.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. T., Capasso O., Heintz N., Nevins J. R. Cell cycle control of the human HSP70 gene: implications for the role of a cellular E1A-like function. Mol Cell Biol. 1985 Apr;5(4):628–633. doi: 10.1128/mcb.5.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Petersen N. S., Mitchell H. K. Induced thermal tolerance and heat shock protein synthesis in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):63–67. doi: 10.1016/0360-3016(82)90386-8. [DOI] [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Foy J. J., Elliott S. G., McLaughlin C. S. Synthesis of specific identified, phosphorylated, heat shock, and heat stroke proteins through the cell cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):117–126. doi: 10.1128/mcb.2.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Paris S., Pringle J. R. Saccharomyces cerevisiae: heat and gluculase sensitivities of starved cells. Ann Microbiol (Paris) 1983 Nov-Dec;134B(3):379–385. [PubMed] [Google Scholar]
- Parry J. M., Davies P. J., Evans W. E. The effects of "cell age" upon the lethal effects of physical and chemical mutagens in the yeast, Saccharomyces cerevisiae. Mol Gen Genet. 1976 Jul 5;146(1):27–35. doi: 10.1007/BF00267979. [DOI] [PubMed] [Google Scholar]
- Plesset J., Palm C., McLaughlin C. S. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1340–1345. doi: 10.1016/0006-291x(82)92147-7. [DOI] [PubMed] [Google Scholar]
- Schenberg-Frascino A., Moustacchi E. Lethal and mutagenic effects of elevated temperature on haploid yeast. I. Variations in sensitivity during the cell cycle. Mol Gen Genet. 1972;115(3):243–257. doi: 10.1007/BF00268888. [DOI] [PubMed] [Google Scholar]
- Walton E. F., Pringle J. R. Effect of growth temperature upon heat sensitivity in Saccharomyces cerevisiae. Arch Microbiol. 1980 Feb;124(2-3):285–287. doi: 10.1007/BF00427739. [DOI] [PubMed] [Google Scholar]
- Zeuthen E. Synchrony in Tetrahymena by heat shocks spaced a normal cell generation apart. Exp Cell Res. 1971 Sep;68(1):49–60. doi: 10.1016/0014-4827(71)90585-4. [DOI] [PubMed] [Google Scholar]



