Abstract
CD8 is critical for T cell recognition of peptide/class I major histocompatability complex ligands, yet is down-regulated during activation of CD8 T cells. We report that loss of CD8 expression early during in vivo responses to vaccinia virus or Listeria monocytogenes (LM) correlates with decreased T cell staining with specific class I/peptide tetramers and reduced CD8 T cell sensitivity for antigen. Loss of CD8 cell surface expression occurs despite sustained mRNA expression, and CD8 levels return to normal levels during differentiation of memory cells, indicating a transient effect. We determined that during response to LM, CD8 down-regulation is regulated by T cell reactivity to type I interferon (IFN-I) because CD8 loss was averted on IFN-I receptor–deficient T cells. IFN-I alone was not sufficient to drive CD8 down-regulation, however, as antigen was also required for CD8 loss. These results suggest that CD8 effector T cell differentiation involves a transient down-regulation of antigen sensitivity (CTL “detuning”), via reduced CD8 expression, a feature that may focus the effector response on target cells expressing high levels of antigen (e.g., infected cells), while limiting collateral damage to bystander cells.
CD8 is an important coreceptor for TCR during CD8 T cell activation by peptide/class I MHC ligands (1–4). CD8 engagement can enhance peptide sensitivity by 1 million–fold or more (2), and it is required for a stable complex between class I and the TCR (5, 6). CD8 expression has been thought to be stable during a CD8 T cell response, and it is commonly used as the marker to define cytotoxic T cells. However, CD8 expression can be inhibited by certain cytokines, including IL-2, -4, and -15, on activated T cells (7–11). Furthermore, down-regulation of CD8 has been suggested as one of the mechanisms for peripheral tolerance (12–15). CD8 low subpopulations have been reported during chronic diseases (16–18), but CD8 down-regulation also occurs during acute immune responses to pathogens (19–21) and cell lines (22). In the current study, we use both viral and bacterial infection models to investigate the underlying mechanism and consequences of transient CD8 down-regulation during acute infections.
Tetramer staining is an efficient tool to define Ag-specific CD8 T cells in infections and tumors, and tetramer binding correlates well with antigen-specific IFN-γ production and cytolytic activity (23–26). However, class I MHC tetramer binding to specific T cells can be affected by TCR expression levels, CD8 availability, and distribution of TCR on the cell surface (27–35). Some studies suggest that CD8 T cell activation enhances binding of specific peptide/MHC ligands (36) and functional avidity of the maturing CTL population (21). However, other studies report that CD8 T cell activation results in a loss of peptide/MHC ligand binding (34, 35, 37) and accompanying impaired functional sensitivity (34). However, the role of CD8 down-regulation in influencing tetramer binding and/or functional sensitivity is still unclear.
In this study, we investigate the basis and impact of CD8 down-regulation during immune responses to LM and vaccinia virus (VV). Transient down-regulation of CD8 expression was correlated with loss of specific peptide/MHC tetramer binding and impaired responsiveness of CD8 T cells to antigen. We demonstrate that down-regulation of CD8 expression (and loss of specific peptide/MHC tetramer binding) during the response to LM is dependent on CD8 T cell responsiveness to type I interferon (IFN-I), indicating that inflammatory cues may act to limit the reactivity (i.e., detune) effector CD8 T cells, potentially to limit tissue damage.
RESULTS
CD8 expression is down-regulated during immune response by nontranscriptional regulation
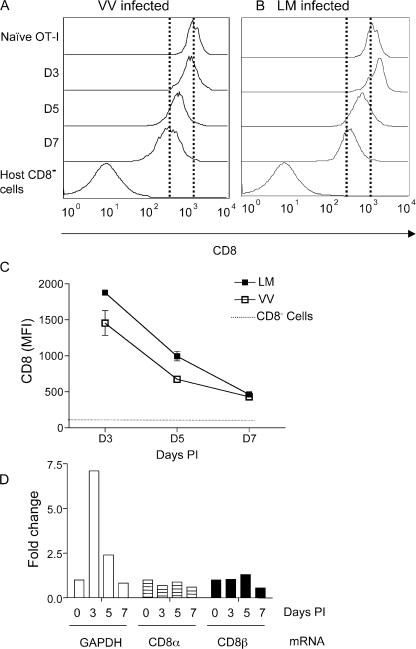
CD8 has been shown to be a critical coreceptor in TCR complex formation during T cell activation, and its expression has long been used as a marker for cytotoxic T cells. Previous studies suggested CD8 down-regulation was induced by prolonged encounter with specific antigen (12–15), but it has also been observed during acute infections (19–21). We examined the response of OT-I TCR transgenic T cells after adoptive transfer into normal B6 hosts and after infection with recombinant VV-expressing OVA peptide (VV-OVAp). As noted in previous reports (19, 21), we observed down-regulation of CD8 expression on OT-I T cells in the spleen during the in vivo response, peaking at day 7 of activation (Fig. 1 A). CD8 down-regulation was also observed on responding OT-I cells in LNs and the peritoneal cavity (unpublished data). CD8 down-regulation also occurred, with similar kinetics, after LM-OVA infection (Fig. 1 B). The extent and kinetics of CD8 down-regulation is summarized in Fig. 1 C, which shows >70% reduction in CD8 expression after VV-OVAp and LM-OVA infection (at day 7; Fig. 1 C and not depicted). Interestingly, the kinetics of CD8 down-regulation did not correlate with the kinetics of clonal expansion because clonal expansion was different in the two infections, peaking at day 5 for VV infection, and day 7 for LM infection (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062376/DC1).
Figure 1.
CD8 expression is down-regulated on responding T cells during viral and bacterial infections. Ly5.2 mice adoptively transferred with naive OT-I.PL T cells were inoculated with 5 × 106 PFU VV-OVAp per mouse, or 104 LM-OVA i.p. At the indicated time points, spleens were harvested, and OT-I and endogenous CD8 T cells were identified using CD8 and Thy 1.1 staining. Representative staining of CD8 levels on OT-I cells after VV-OVAp (A) infection or LM-OVA (B) infection are shown. Dashed lines indicate CD8 expression levels of uninfected controls and OT-I cells at day 7 of infection. (C) Kinetics of CD8 down-regulation in infections. Each of the values represents the mean of three or more mice, and error bars represent the SD. Data are representative of more than four experiments. (D) OT-I.PL cells were adoptively transferred and primed with VV-OVAp, as described. At days 3, 5, and 7 after infection, OT-I cells were sorted from the spleen and RNA isolated. Naive OT-I CD8 T cells were sorted as a control. Equivalent amounts of total RNA were used for generation of cDNA, and real-time PCR was used to quantify expression of CD8α, CD8β, and GAPDH. Each column represents the fold change compared with naive samples, and the experiment was repeated with similar results.
To address whether down-regulation of CD8 results from reduced transcription of CD8α and/or β, OT-I cells were sorted from recipient mice at various time points after infection with VV-OVAp and compared with naive OT-I cells for levels of CD8α and β transcripts, which were quantified using real-time RT-PCR. Similar to Fig. 1 A, there was uniform down-regulation of CD8 on the population of day 5–activated OT-I cells, and this effect was even more pronounced at day 7 (not depicted). However, although we observed changes in expression of GAPDH with OT-I T cell activation (as expected based on previous studies; reference [38]), there were minimal changes in mRNA levels for either CD8 chain (Fig. 1 D), suggesting that the appearance of the prominent CD8low pool by day 7 of activation was not a result of reduced CD8αβ transcript levels. Thus, although the mechanism for regulating CD8 expression levels is currently unclear, it is likely to be posttranscriptional.
Tetramer binding is down-regulated during primary infections and correlates with decreased CD8 expression
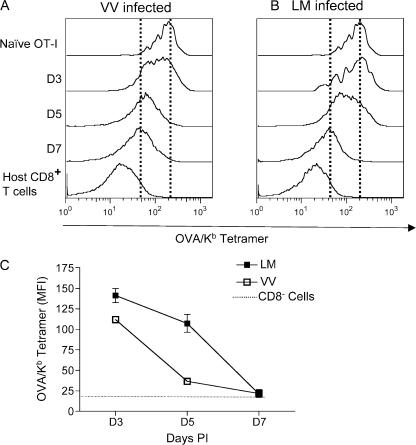
Tetramer binding to TCR has become a powerful tool for defining Ag-specific CD8 T cells (23–26). Given that specific peptide/class I MHC binding can be influenced by the availability of CD8 (29, 39), we sought to test whether the observed down-regulation of CD8 on antigen-activated T cells influences specific tetramer binding. Indeed, we observed a marked decrease in specific tetramer staining on OT-I T cells primed with VV-OVAp and LM-OVA (Fig. 2, A and B). Loss of specific tetramer staining was first noticed at day 5 and peaked at day 7 (Fig. 2, A-C). Tetramer binding was reduced by >70% in VV-OVAp and LM-OVA infection at day 7 after infection (Fig. 2 C and not depicted). Thus, in vivo–stimulated T cells show a marked loss in specific tetramer staining, and this follows the same kinetics as the loss in CD8 expression levels. Linear regression analysis demonstrated a statistically significant direct correlation between tetramer binding and CD8 expression; high tetramer binding correlated with high CD8 expression, and vice versa (Fig. S2, A and B, available at http://www.jem.org/cgi/content/full/jem.20062376/DC1). To address if this tetramer-binding down-regulation was also related to TCR expression levels, TCR expression was examined in both models (Fig. S3). Interestingly, TCR levels on stimulated OT-I cells were maintained at days 3 and 5 in the spleen, and the slight decline in TCR expression observed in the spleen at day 7 was not found in OT-I cells from LNs or the peritoneal cavity (Fig. S3, A–D). Together, these data indicate that loss of peptide/MHC tetramer binding correlates with down-regulation of CD8, rather than changes in TCR expression.
Figure 2.
Tetramer binding is down-regulated in viral and bacterial infections. OT-I adoptive transfer and infections were performed as in Fig. 1. Spleen cells were stained with Kb/OVA tetramer. Representative Kb/OVA staining on OT-I cells after VV-OVAp infection (A) or LM-OVA infection (B) are shown. Dashed lines indicate mean tetramer staining levels of uninfected controls and OT-I cells at day 7 of infection. (C) Kinetics of loss of tetramer binding during infections. Each of the values represents the mean of three or more mice, and error bars represent the SD. These data are representative of at least three experiments.
Down-regulation of CD8 expression and tetramer binding are transient
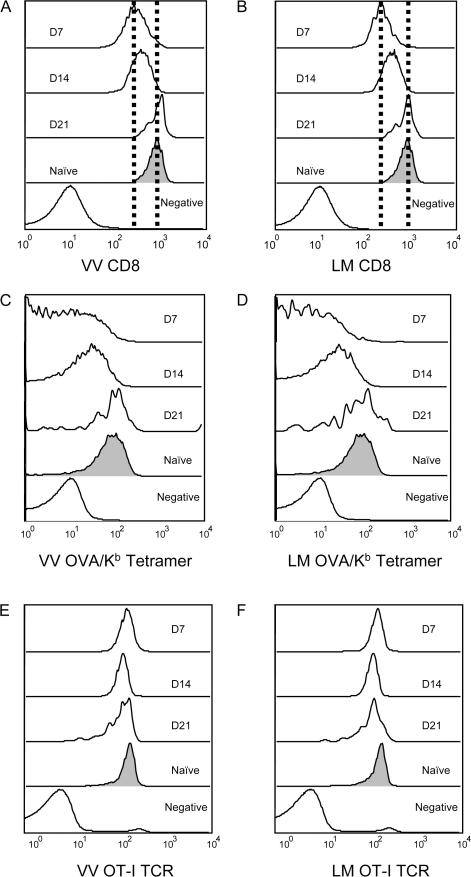
A previous study of LM-reactive T cells reported that CD8 down-regulation was sustained, lasting well into the memory phase of the response (19). However, CD8 levels on bulk memory CD8 T cells are similar to those on the naive pool (unpublished data). To address this issue in our system, we studied the kinetics of CD8 reexpression during OT-I responses to VV-OVAp and LM-OVA infection. As previously observed (Fig. 1), down-regulation of CD8 expression was most marked at day 7 after infection, but CD8 levels had partially recovered at day 14 and were back to control (naive) levels by day 21 after infection (Fig. 3, A and B).
Figure 3.
Down-regulation of CD8 expression and tetramer binding is transient. Adoptive transfer and infections were performed as in Fig. 1. Representative histograms are shown for CD8 expression (A and B) Kb/OVA tetramer binding (C and D) and OT-I TCR expression (E and F) on OT-I cells after VV-OVAp (A, C, and E) or LM-OVA (B, D, and F) for the indicated time points. These data are representative of three animals per group from two independent experiments.
Again, regulation of specific tetramer binding paralleled the changes in CD8, rather than TCR expression levels (Fig. 3, C–F), which is similar to our observations for down-regulation of tetramer binding during priming. Therefore, our data indicate recovery of CD8 expression, and also specific tetramer staining, during differentiation of CTL into the late effector/memory pool.
CD8 down-regulation accompanies reduced responsiveness to antigen
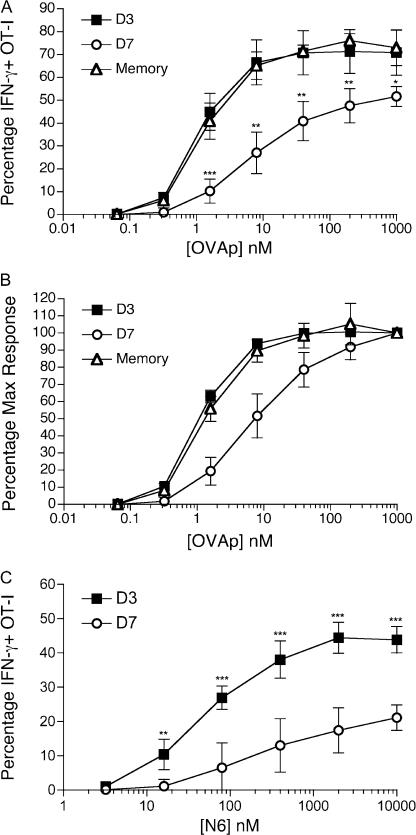
Because day 7–activated CD8 T cells showed both diminished CD8 expression and reduced tetramer-binding ability (Fig. 1 and Fig. 2), it was important to determine whether these changes lead to altered functional responses. Spleens were harvested from mice adoptively transferred with OT-I T cells and infected with VV-OVAp either 3 or 7 d earlier, and CD8 down-regulation was observed on cells from the latter time point, similar to that observed in Fig. 1 (not depicted). The splenocytes were then stimulated with serially diluted OVAp, or a variant of this peptide (called N6), which we have previously shown is a weak agonist for the OT-I TCR (40). Production of IFN-γ by the OT-I cells was determined via intracellular staining (Fig. 4). At the maximum dose of OVAp tested, the percentage of IFN-γ–positive OT-Is was ∼70% in the CD8high (day 3 after infection) and ∼50% in the CD8low (day 7 after infection) populations (Fig. 4 A), demonstrating that fewer CD8low cells were able to respond. Furthermore, a higher concentration of OVAp was required for half-maximal responsiveness by the CD8low day 7 cells compared with the CD8high day 3 pool (Fig. 4 B), leading to a slight but consistent 2–5-fold lower dose sensitivity of the day 7 effector OT-I cells, relative to the day 3 population (Fig. 4, A and B, and not depicted). Finally, the per cell production of IFN-γ was also greater in the day 3 effector pool, as revealed by the average mean fluorescence intensity (MFI) of intracellular IFN-γ staining (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20062376/DC1). Such changes in sensitivity were further magnified when the response to the OT-I N6 peptide was studied. This weak agonist variant of the OVA peptide was capable of inducing IFN-γ production by cells in the day 3 OT-I effector pool, but this response was severely reduced in the day 7 effector population (Fig. 4 C). Together, these data indicate that both the frequency of cells capable of responding to a given dose of antigen, and the efficacy of that response is decreased in effector cells from day 7 relative to the population from day 3.
Figure 4.
CD8 T cells with low CD8 expression show decreased responsiveness to antigen. OT-I effector T cells (primed as in Fig. 1) and memory OT-I cells (see Materials and methods) were challenged with indicated doses of OVAp (A and B) or N6 peptide (C) in vitro for 3.5 h, followed by intracellular staining for IFN-γ. Histograms show the percentage of IFN-γ+ OT-I cells (A and C) or the percentage of maximum response (B) as a function of peptide concentration. OVAp response of day 3 and 7 effector cells is representative of 4 experiments, and assay of memory OT-I response and N6 reactivity was repeated with similar results. Each value represents the mean plus the SD of 3–4 mice per group. Asterisks indicate statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previous studies have argued for functional avidity maturation of the CD8 response, such that the sensitivity of T cells to specific peptide/MHC ligands was proposed to increase by over 50-fold from early effector cells (day 2/3) to the late effector (day 8) stage (21). Our findings above, showing reduced functional avidity for day 7 versus day 3 OT-I effector cells, appeared to contradict this model. However, it was possible that the functional sensitivity of OT-I cells would increase as the cells completed maturation into the memory pool because previous reports indicated that high functional avidity was maintained into the memory phase (21). To address this, we also tested the sensitivity of memory OT-I cells in the same experiments (Fig. 4, A and B). These studies revealed that day 3 effector and memory OT-I cells had similar frequencies of IFN-γ–producing cells and similar dose sensitivity (both of which were high, relative to day 7 effector population). Therefore, our data suggest a transient dip in antigen sensitivity as CD8 T cells pass through the late effector phase, rather than a progressive rise in functional avidity during differentiation.
Regulation of CD8 expression is IFN-I dependent
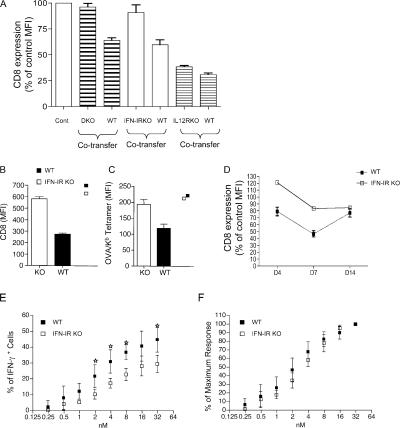
IFN-I has been shown to act directly on CD8 T cells to promote the survival of CD8 effectors (41), and both IFNα and IL-12 have been reported to be involved in the activation of CTLs as third signals (42, 43). Hence, we considered whether IFN-I and/or IL-12 were also involved in CD8 down-regulation. To test this, WT OT-I T cells and OT-I T cells deficient for IFN-IR, IL-12Rβ1, or both cytokine receptor (double KO [DKO]) T cells were cotransferred into normal B6 animals. Recipient mice were infected with LM-OVA and assayed at day 7. At this time point, the numbers of IFN-IR and DKO OT-I cells were reduced compared with their WT counterparts (by ∼20 and ∼60%, respectively; unpublished data), which is consistent with previous reports (44), but this still provided ample effector stage cells for analysis. The DKO OT-I showed almost no CD8 down-regulation, whereas cotransferred WT OT-I showed ∼40% down-regulation of CD8 expression (Fig. 5 A). CD8 down-regulation after LM-OVA infection correlated with expression of IFN-IR because IFN-IR−/− OT-I also failed to show CD8 down-regulation after activation, whereas IL-12Rβ1−/− OT-I cells exhibited CD8 down-regulation similar to the WT controls (Fig. 5 A). Relative to naive controls, day 7–activated IFN-IR−/− OT-I failed to show down-regulation of either CD8 levels (Fig. 5 B) or Kb/OVA tetramer binding (Fig. 5 C), whereas cotransferred WT OT-I showed down-regulation of staining with both reagents (Fig. 5, B and C). This autonomous resistance to CD8 loss by the IFN-IR−/− cells during LM infection was not caused by a difference in kinetics of CD8 down-regulation because the CD8 levels were maintained (or even increased) relative to controls over several time points (Fig. 5 D). However, production of IFN-γ was not enhanced in effector IFN-IR−/− OT-I compared with their WT counterparts (Fig. 5, E and F), suggesting that factors other than CD8 and peptide/MHC ligand binding may impact IFN-γ production, and/or that signals through the IFN-IR are required not only for the CD8 regulation but also for the optimal production of IFN-γ.
Figure 5.
Down-regulation of CD8 expression in response to LM-OVA infection is IFN-I dependent. Purified naive CD8 T cells from OT-I.PL and the indicated cytokine receptor KO OT-I strains were adoptively transferred into CD45.1 congenic B6 mice, and the host animals were infected with LM-OVA. 7 d later, recipient spleens were harvested, and WT and receptor KO OT-I cells (distinguished by staining for CD45.2 and Thy 1.1) were analyzed. (A) CD8 expression on cotransferred WT and receptor KO OT-I cells. The MFI of each group was expressed as the percentage of the MFI of CD8 staining of OT-I cells from uninfected mice. In a different experiment, CD8 expression (B) and Kb/OVA tetramer staining (C) were compared for cotransferred WT and IFN-IR−/− OT-I cells at day 7 after LM-OVA infection. Individual square symbols show CD8 expression and tetramer binding on WT and IFN-IR−/− OT-I cells in uninfected controls. (D) CD8 expression on WT and IFN-IR−/− OT1 cells as a function of time after LM-OVA infection. Dashed line represents WT cells, solid line represents IFN-IR−/− cells. (E and F) Cotransferred WT and IFN-IR−/− OT-I cells were analyzed for antigen sensitivity at day 7 after LM-OVA infection. Cells were stimulated as in Fig. 4, and the percentage of IFN-γ–positive OT-I cells (E) or the percentage of maximum IFN-γ responsiveness (F) was determined. The graphs show average responses (and SD) for three mice per group, and similar data were observed in three experiments. Stars indicate significant differences (P < 0.05).
IFN-I–regulated CD8 loss is antigen dependent
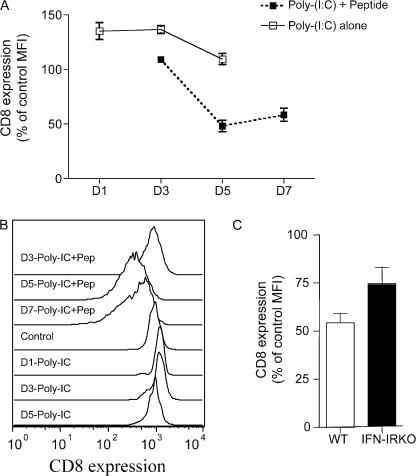
The aforementioned data suggested that response to IFN-I was involved in CD8 down-regulation. We considered that this may be a direct response to IFN-I that is independent of TCR stimulation. To test this, we used poly I:C as a potent inducer of IFN-I (45), injected with or without antigen (OVAp) into mice adoptively transferred with OT-I T cells. IFN-I is known to induce CD69 up-regulation on T cells, regardless of TCR specificity (46, 47), and we observed that all CD8 T cells became CD69+ at 1 d after immunization (unpublished data), indicating induction of IFN-I by the injected poly I:C. However, CD8 down-regulation only occurred in OT-I T cells from mice receiving poly I:C with peptide, not in mice receiving poly I:C alone (Fig. 6, A and B), suggesting that CD8 down-regulation requires antigen stimulation. The kinetics of CD8 down-regulation was similar to that observed in VV- and LM-infected animals (compare Fig. 6 to Fig. 1). We could also show, using IFN-IR−/− OT-I T cells, that the CD8 down-regulation after poly I:C plus peptide immunization required IFN-I sensitivity by the T cells themselves (Fig. 6 C), which is consistent with the results in LM-OVA infection (Fig. 5).
Figure 6.
CD8 down-regulation is not induced by IFN-I alone. (A and B) Mice were adoptively transferred with WT OT-I cells and immunized with poly I:C with or without OVAp. At the indicated time point, spleens were harvested and CD8 expression and levels were analyzed. (A) The average CD8 expression levels (the mean ± the SD) on splenocytes from 3–4 mice per group are shown. Open symbols represent immunization with poly I:C alone, whereas closed symbols show responses induced by poly I:C plus OVAp. (B) Representative histograms from these same groups. (C) WT and IFN-IR−/− OT-I cells were cotransferred and studied for CD8 expression levels 5 d after poly I:C plus OVA peptide immunization.
Endogenous CD8 T cells exhibit down-regulation of CD8 expression, tetramer binding, and responsiveness during activation
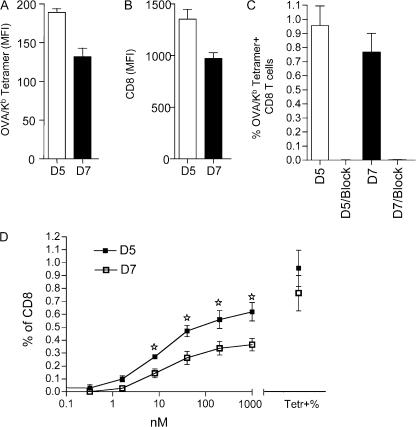
Although TCR transgenic cell adoptive transfer models are valuable for tracking immune response in vivo, it was possible that the loss of CD8 expression, engagement with tetramer, and reduced responsiveness of day 7–activated OT-I cells related to their abnormal frequency in the immune response. Thus, we also studied endogenous CD8 T cell responses to Kb/OVA. Normal B6 mice were infected with VV-OVAp and studied for CD8 expression levels and Kb/OVA tetramer binding at day 5 (when sufficient antigen-specific cells could first be detected) and day 7 after infection. As for the OT-I experiments, we observed down-regulation of both CD8 expression and Kb/OVA tetramer binding at day 7 relative to day 5 (Fig. 7, A and B). Similar to what has been reported previously for OT-I cells (31), tetramer binding to the polyclonal responder CD8 T cells was strictly CD8 dependent, being completely blocked by antibody to CD8 (Fig. 7 C). In addition, we tested responsiveness of endogenous Kb/OVA-specific CD8 T cells at days 5 and 7 after VV-OVAp infection (Fig. 7 D). As was the case for OT-I cells, the frequency of IFN-γ–producing CD8 cells was significantly higher in cells assayed at day 5 compared with day 7, whereas the frequency of antigen-specific cells (i.e., OVA/Kb tetramer staining cells) was not significantly different between the groups (Fig. 7 D). Together with the observation that tetramer binding to day 7–responsive cells was also reduced (Fig. 7 A), these data suggest a marked decrease in the frequency of antigen-specific cells capable of producing IFN-γ in response to antigen at this time point. Thus, antigen-specific endogenous CD8 T cells behave similarly to OT-I cells in regard to CD8 expression, tetramer binding, and changes in responsiveness during in vivo responses to VV-OVAp.
Figure 7.
Endogenous CD8 T cells exhibit regulation of CD8 expression, tetramer binding, and antigen sensitivity. B6 mice were infected with VV-OVAp and spleens were analyzed 5 and 7 d after infection. Peptide-specific CD8 T cells (defined by Kb/OVA tetramer staining) were analyzed for the level of tetramer binding (A) and CD8 expression level (B). (C) The CD8 dependence of tetramer binding at both time points was assessed by addition of blocking anti-CD8α antibody. Each column or dot represents an average of three or four mice, and error bars indicate the SD. (D) Antigen sensitivity of Kb/OVA-specific endogenous CD8 T cells was assessed by stimulation for IFN-γ production, as in Fig. 4. The percentage of IFN-γ producing CD8 T cells as a function of OVAp concentration is indicated on the left side of the graph. On the right side, the percentage of Kb/OVA tetramer-positive cells is indicated, to reflect the frequency of antigen-specific cells in the populations. Data show the average (plus the SD) of three mice per group, and similar findings were observed in an independent experiment.
DISCUSSION
In this study, we showed that CD8 expression, peptide/MHC class I tetramer binding, and functional reactivity are all transiently down-regulated during effector differentiation of CD8 T cells responding to pathogens in vivo. This “detuning” of effector cells was observed for both TCR transgenic OT-I cells and endogenous polyclonal Kb/OVA-specific CD8 T cells, and in response to both VV and LM infections. Additional studies suggest OT-I T cells responding to OVA-expressing tumor cells show a similar decline in CD8 staining and tetramer binding (unpublished data), arguing that these changes occur with diverse immune challenges.
Primed CD8 T cell populations exhibiting low CD8 expression levels have been previously noted, in both humans and mice (7–11, 15–22). However, the consequences of this down-regulation for antigen recognition have not been extensively studied. We demonstrate that the magnitude and kinetics of CD8 down-regulation correlated well with decreased binding of specific peptide/MHC tetramers (whereas the small changes in TCR expression levels did not correlate with loss of tetramer staining; Fig. 2 and Fig. S3). We and others have previously reported that CD8 T cells cultured in vitro showed a transient decrease of specific tetramer staining (34, 35, 37). Drake et al. and Spencer and Braciale also demonstrated loss of tetramer binding to CD8 T cells primed by influenza virus infection in vivo (34, 37), and found that this resulted in diminished functional sensitivity (34, 37). However, these studies correlated changes in tetramer binding to altered surface TCR organization (34) or CD8 glycosylation (35), rather than changes in CD8 (or TCR) expression levels. This might suggest that there are multiple pathways that can regulate tetramer binding and functional sensitivity, of which CD8 down-regulation is but one mechanism. However, the observation that CD8 down-regulation is routinely observed on stimulated T cells responding to pathogens in vivo (19–21), speaks to its potential significance.
Our data suggest that activated CD8 T cells pass through a phase (peaking at approximately day 7 of activation) during which their ability to engage and respond to peptide/MHC antigens is impaired. This appears to contrast with a previous study suggesting increased sensitivity of CD8 T cells as they differentiate. Slifka and Whitton, studying the response to LCMV, reported a progressive increase in functional avidity (i.e., peptide/MHC sensitivity) throughout the effector phase, reaching peak sensitivity at day 8 of infection, which was then sustained into the memory pool (21). For endogenous, polyclonal CD8 T cells, this lead to a substantial increase (>50-fold) in antigen sensitivity between days 4 and 8 after infection (21). This finding may involve a strong component of TCR repertoire selection because the increase in functional avidity of a monoclonal T cell population (P14 TCR transgenic T cells) was much less substantial, increasing only approximately threefold between the same time points (21). Nevertheless, our data with Kb/OVA CD8 T cells (both monoclonal [Fig. 4] and polyclonal [Fig. 7] populations), demonstrated reduced, rather than enhanced, sensitivity of the day 7 effector pool. Furthermore, we were unable to demonstrate an increase in functional avidity, as OT-I T cells progress from the early effector phase (day 3 of activation) into the memory phase (Fig. 4), suggesting that functional avidity maturation is not a feature of our system. The basis for these distinct outcomes is currently unclear. Slifka and Whitton correlated functional avidity maturation with progressively increased CD8 independence (21). However, we find that both OT-I T cells (31, 35) and polyclonal Kb/OVA-reactive cells (Fig. 7) exhibit substantial CD8 dependence for peptide/MHC tetramer binding. The different MHC class I alleles used in our model (Kb) versus Slifka and Whitton's study (Db) may impact the CD8 dependence of the T cell response. However, CD8 dependence in peptide/MHC ligand engagement and/or responsiveness has been reported for cells restricted through many mouse class I alleles (10, 48–50), including the Db/LCMV gp33-responsive P14 clone (33), and has also been observed for human CTL (51, 52). This raises the important point that CD8-independent clones might be expected to show a selective advantage in overcoming the transient decrease in peptide/MHC ligand recognition reported here. On the other hand, the proposed decreased CD8 dependence during maturation of the effector response (21) does not appear to apply in all systems, as some authors report the opposite trend (50, 53). In summary, our data do not support a model in which functional avidity progressively increases during the effector response, but rather argues for a transient “dip” in functional sensitivity in the late effector pool.
Fahmy et al. reported that the avidity of TCR engagement was enhanced by 20- to 50-fold on in vitro–activated 2C CD8 T cells compared with naive T cells, which was not related to changed expression of CD8 and TCR level, but rather to increased cross-linking of TCR on activated T cells (36). This also contrasts with the decreased tetramer binding and sensitivity of effector Kb/OVA-reactive cells reported in this study. However, as previously discussed (35), the 2C TCR ligands used in that study were relatively CD8 independent, which may contribute to these divergent outcomes. Further work will be needed to determine how the CD8 dependency of individual T cell clones impacts their sensitivity at the late effector phase.
Our findings indicating reduced antigen sensitivity of late effector cells raises the question of why this would be useful for the host. As proposed in the previous two paragraphs, this phase may present a restraint on cells that are the most CD8 dependent. In addition, however, this regulatory mechanism may detune effector responsiveness to avoid overstimulation and/or potential damage to normal cells through excessive production of cytokine (54). Interestingly, others have reported that repeated antigen exposure generates a population of CD8low T cells (10), which show impaired antigen sensitivity and which have the capacity to restrain responsiveness of their CD8high counterparts, potentially operating through TGF-β production (55). This response to sustained antigen encounter might represent a mechanism of locking cells into the CD8 down-regulated phase we observe transiently during acute antigen exposure. In addition, our data indicated that early effector (day 3) OT-I cells respond well to a weak agonist ligand, although this reactivity is impaired by day 7 of the response (Fig. 4 C). Similarly, previous studies have indicated a brief kinetic “window” after CD8 T cell activation, during which CTL transiently acquire sensitivity to low-affinity ligands, followed by decreased sensitivity of the cells (56). The decline in this sensitivity as the response matures may ensure that only high affinity/avidity CTL responses are maintained during the late effector phase.
The mechanism of CD8 down-regulation was also studied. IFN-I and IL-12 have been shown to be important for the activation of CTLs (43), and we show that autonomous reactivity to IFN-I is required for CD8 down-regulation during LM-OVA infection (Fig. 5 and Fig. 6). Stimulation of OT-I T cells with antigen plus the potent IFN-I inducer poly I:C–induced CD8 down-regulation (Fig. 6), yet poly I:C alone was unable to provoke the response (Fig. 6), suggesting that cells only become susceptible to IFN-I–induced CD8 down-regulation after TCR activation. IFN-I is important in antiviral responses, but has also been implicated as a negative factor in LM infection, causing lymphocytes to be more sensitive to apoptosis and impairing resistance of mice to LM infection (57–59). At the same time, IFN-IR−/− CD8 T cells exhibit slightly reduced proliferation after in vivo challenge with LM (unpublished data) (44). Despite the observation that IFN-IR−/− OT-Is avoid down-regulation of CD8 and tetramer binding, these cells still exhibit impaired IFN-γ production at day 7 of activation. This illustrates the complexity of deciphering the impact of CD8 down-regulation, compared with other effects of depriving CTL of IFN-I signals, especially because expression of IFN-γ may be enhanced by IFN-I under these assay conditions.
A related issue is whether IFN-IR plays a direct role on CD8 down-regulation, or whether it is indirect; for example, the cytokine receptor might enhance overall T cell activation (leading to CD8 loss via other signaling pathways), or may promote survival of CD8low T cells at the effector stage. These hypotheses are not excluded by our current data, although it is worth reiterating that IFN-IR deficiency only moderately impairs expansion of CD8 T cells after LM infection (unpublished data) (44), arguing against dramatic differences in initial T cell stimulation or survival of effector cells. Nonetheless, although our data show that reactivity to IFN-I is involved in loss of CD8 expression during LM infection, its mode of action is unclear. The kinetics of IFN-I production relative to induced CD8 down-regulation (and loss in tetramer binding) are puzzling; previous studies have indicated that serum levels of IFN-I peak at ∼24–48 h after stimulation with various pathogens or poly I:C (44), whereas we observe the peak of CD8 loss to occur approximately on day 7. These differences might indicate that early IFN-IR signals (on the responsive CD8 T cells themselves) program loss of CD8 expression some days later. In this context, it is important to note that our data suggest loss of surface CD8 was not a consequence of decreased mRNA expression (Fig. 1 D), suggesting the regulation may relate to protein production or turnover. Further studies will be required to determine the mechanism of cell surface CD8 loss, and whether IFN-I signals are directly involved in this process.
Although our results argue that IFN-I plays an important role in driving CD8 down-regulation in response to LM, they do not exclude a role for alternative cytokines in other responses. We have found that coinjection of peptide antigen, along with recombinant IL-12, stimulates some down-regulation of CD8 and tetramer binding (unpublished data), although further work will be required to determine whether this involves a direct response of the T cells to IL-12. Because CD8 down-regulation has been observed in a variety of responses (7–11, 15–22), it might be anticipated that more than one inflammatory cytokine could contribute to the process.
In summary, our data indicate that the CD8 T cells undergoing in vivo response to pathogens undergo a transient decline in CD8 expression, specific tetramer binding, and functional sensitivity. These data argue that the reactivity of effector CTL is subject to detuning during normal immune responses, which may enhance maintenance of the most “fit” responder cells and limit collateral damage to the host.
MATERIALS AND METHODS
Mice, cell lines, and reagents.
OT-I mice with a transgenic TCR specific for H-2Kb/OVA257–264 were a gift from F. Carbone (University of Melbourne, Melbourne, Australia). OT-I mice were also crossed with Thy-1 congenic B6.PL-Thy1a/Cy (Thy-1.1) mice (Jackson ImmunoResearch Laboratories) and bred to homozygosity, generating OT-I.PL mice. IL-12Rβ1KO mice were obtained by crossing OT-I mice with IL-12Rβ1KO B6 mice, and IFN-IR KO (IFN-IR−/−) OT-I mice were obtained in a similar way. IL-12Rβ1−/− and IFN-IR−/− DKO OT-I mice were generated via crossing the single-receptor KO OT-I mice. The OT-I and OT-I.PL breeding colonies were maintained under specific pathogen-free conditions at the University of Minnesota. C57BL/6NCr and CD45.1 congenic “B6-Ly5.2” mice were purchased from the National Cancer Institute. All experiments involving animals were approved by the Institutional Animal Care and Usage Committee at the University of Minnesota. Generation of Kb/OVA tetramers was carried out as previously described (31). OVA peptide (OVAp: sequence SIINFEKL) and its variant N6 (sequence SIINFNKL) were synthesized by Invitrogen. All directly conjugated fluorescent antibodies were purchased from BD Biosciences or eBioscience.
Naive T cell purification and adoptive transfer.
Inguinal, axillary, brachial, cervical, and mesenteric LNs were harvested from OT-I.PL or receptor KO mice. LNs were pooled and disrupted to obtain a single-cell suspension, and the cells were enriched for CD8+ CD44low cells by negative selection using MACS magnetic cell sorting (Miltenyi Biotec). In brief, cells were coated with FITC-labeled antibodies specific for CD4, B220, I-Ab, and CD44. Anti-FITC magnetic MicroBeads (Miltenyi Biotech) were added to the cells, which were passed over separation columns attached to the MACS magnet. The cells that did not bind to the column were collected and were >95% CD8+ and <0.5% CD44high. Enriched cells (106, unless otherwise stated) were adoptively transferred i.v. (via the tail vein) into C57BL/6 recipients.
Infection and immunization.
The recombinant VV VV-GFP-JAW-OVA (VV-OVAp) was provided by J. Yewdell (National Institutes of Health, Bethesda, MD). This virus encodes the OVA257–264–restricted epitope, fused C-terminally to GFP and the transmembrane region of JAW-1. Mice were infected i.p. with 5 × 106 PFU of the indicated virus (viral titer was determined by plaque assays performed on 143B cells). Recombinant LM, which encodes full-length OVA (LM-OVA), was a gift from H. Shen (University of Pennsylvania, Philadelphia, PA). Infection was performed i.p. at a dose of 104 CFU/mouse. For other immunizations, mice were injected with poly I:C (150 μg/mouse) with or without SIINFEKL peptide (50 μg/mouse) i.v. via the tail vein. In some experiments, OT-I.PL cells were adoptively transferred, and the host mice were infected with LM-OVA, but the mice were then rested for >30 d to allow for generation of an OT-I memory pool.
Intracellular cytokine staining after in vitro rechallenge.
Spleen cells harvested from adoptively transferred mice were incubated at a concentration of 2 × 106 cells/ml in RP-10 with OVAp or N6 peptide (at the indicated concentrations) and 1 μl GolgiPlug (BD Biosciences) for 3.5 h at 37°C. Cells were washed and stained with the antibodies to CD8 and CD45.2 and/or Thy 1.1 to mark the OT-I and OT-I.PL cells. Cells were fixed in Cytofix buffer (BD Biosciences) for 15 min at 4°C, and permeabilized in saponin-containing Perm/Wash buffer (BD Biosciences) for 15 min at 4°C before staining with (FITC- or PE-conjugated) antibody to IFN-γ for 30 min at 4°C. Cells were washed once with Perm/Wash buffer and once with PBS containing 2% FBS.
Flow cytometric analysis of transferred cells.
Mice were killed at the indicated times after adoptive transfer and viral or bacterial infection. Spleen cells, peritoneal cells, and LN cells (pooled from axillary, brachial, cervical, inguinal, and mesenteric nodes) were counted by trypan blue dye exclusion to determine total viable cell counts, and were stained with the antibodies to CD8, CD45.2, and/or Thy 1.1 to detect the transferred OT-I cells. Stained cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences) to determine the percentage and total number of OT-I cells in the transferred mice. Kb/OVA tetramer staining was performed in the presence of the enhancing anti-CD8a antibody 53.6.72 or (in Fig. 7) the blocking anti-CD8b antibody CT-CD8a. Antibody specific for TCR Va2 (B20.1), which binds the OT-I TCR, was included during tetramer staining to simultaneously monitor TCR levels. These staining conditions reduce overall tetramer binding, but allow for clearer resolution of changes in tetramer staining.
Statistical analysis.
Data was graphed and analyzed using a two-tailed Student's t test (GraphPad Prism 3.0 software). Comparisons with a P value of <0.05 were considered significantly different.
Real-time RT-PCR.
Naive OT-I.PL CD8 T cells were adoptively transferred into B6.SJL hosts, which were then infected with VV-OVAp, as described in Infection and immunization. At the indicated time points after infection, donor OT-I cells were enriched by magnetic bead isolation based on anti-CD45.2 staining (Miltenyi MACS system), and then sorted based on Thy-1.1 and CD8 staining (FACSAria; Becton Dickinson). Purity of sorted cells was >95% by postsort analysis. RNA was isolated (Qiagen RNeasy kit), and 8 ng of RNA (quantitated using Ribogreen; Invitrogen) was used to synthesize cDNA (using the SuperScript III Platinum TwoStep qRT-PCR kit; Invitrogen). Quantitation was performed on 1 μl cDNA (equivalent to ∼400 pg of starting RNA) using a Smart Cycler real-time PCR machine (Cepheid). Primers used were as follows: CD8α 5′ left primer, 5′-GTTCTGTCGTGCCAGTCCTT-3′; CD8α 3′ right primer, 5′-GCCGACAATCTTCTGGTCTC-3′; CD8β 5′ left primer, 5′-CGCTGATCATTTGTGAAACTGTTT-3′; CD8β 3′ right primer, 5′-GAGTGGCCGTCTACTTTTACTGTGT-3′; GAPDH 5′ left primer, 5′-TGTCTCCTGCGACTTCAACAGC-3′; GAPDH 3′ right primer, 5′-TGTAGGCCATGAGGTCCACCAC-3′. Details of the real-time PCR conditions used are available upon request.
Online supplemental material.
In Fig. S1, the kinetics of OT-I CD8 T cell expansion after infection with LM-OVA or VV-OVAp is shown. Fig. S2 shows the correlation between CD8 expression levels and OVA/Kb tetramer staining on OT-I CD8 T cells stimulated in vivo with LM-OVA or VV-OVAp. In Fig. S3, the expression level of the OT-I TCR was determined at various times after in vivo stimulation. Fig. S4 illustrates the reduced IFN-γ production capacity of day 7 versus day 3 effector OT-I T cells. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20062376/DC1.
Supplemental Material
Acknowledgments
The authors thank members of the Mescher and “Jamequist” laboratories for helpful input on experimental design and interpretation.
This work was supported by awards from the National Institutes of Health (R01AI52163 and U01AI070380 to S.C. Jameson; and R01 AI34824 to M.F. Mescher).
The authors have no conflicting financial interests.
Abbreviations used: DKO, double KO; LM, Listeria monocytogenes; MFI, mean fluorescence intensity; VV, vaccinia virus.
References
- 1.Gao, G.F., and B.K. Jakobsen. 2000. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol. Today. 21:630–636. [DOI] [PubMed] [Google Scholar]
- 2.Holler, P.D., and D.M. Kranz. 2003. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 18:255–264. [DOI] [PubMed] [Google Scholar]
- 3.Couedel, C., M. Bodinier, M.A. Peyrat, M. Bonneville, F. Davodeau, and F. Lang. 1999. Selection and long-term persistence of reactive CTL clones during an EBV chronic response are determined by avidity, CD8 variable contribution compensating for differences in TCR affinities. J. Immunol. 162:6351–6358. [PubMed] [Google Scholar]
- 4.Alexander, M.A., C.A. Damico, K.M. Wieties, T.H. Hansen, and J.M. Connolly. 1991. Correlation between CD8 dependency and determinant density using peptide-induced, Ld-restricted cytotoxic T lymphocytes. J. Exp. Med. 173:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schott, E., and H.L. Ploegh. 2002. Mouse MHC class I tetramers that are unable to bind to CD8 reveal the need for CD8 engagement in order to activate naive CD8 T cells. Eur. J. Immunol. 32:3425–3434. [DOI] [PubMed] [Google Scholar]
- 6.Naeher, D., I.F. Luescher, and E. Palmer. 2002. A role for the alpha-chain connecting peptide motif in mediating TCR-CD8 cooperation. J. Immunol. 169:2964–2970. [DOI] [PubMed] [Google Scholar]
- 7.Erard, F., M.T. Wild, J.A. Garcia-Sanz, and G. Le Gros. 1993. Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science. 260:1802–1805. [DOI] [PubMed] [Google Scholar]
- 8.Kienzle, N., K. Buttigieg, P. Groves, T. Kawula, and A. Kelso. 2002. A clonal culture system demonstrates that IL-4 induces a subpopulation of noncytolytic T cells with low CD8, perforin, and granzyme expression. J. Immunol. 168:1672–1681. [DOI] [PubMed] [Google Scholar]
- 9.Kienzle, N., S. Olver, K. Buttigieg, P. Groves, M.L. Janas, A. Baz, and A. Kelso. 2005. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J. Immunol. 174:2021–2029. [DOI] [PubMed] [Google Scholar]
- 10.Maile, R., C.A. Siler, S.E. Kerry, K.E. Midkiff, E.J. Collins, and J.A. Frelinger. 2005. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J. Immunol. 174:619–627. [DOI] [PubMed] [Google Scholar]
- 11.Kambayashi, T., E. Assarsson, B.J. Chambers, and H.G. Ljunggren. 2001. IL-2 down-regulates the expression of TCR and TCR-associated surface molecules on CD8(+) T cells. Eur. J. Immunol. 31:3248–3254. [DOI] [PubMed] [Google Scholar]
- 12.Zhang, L., W. Fung-Leung, and R.G. Miller. 1995. Down-regulation of CD8 on mature antigen-reactive T cells as a mechanism of peripheral tolerance. J. Immunol. 155:3464–3471. [PubMed] [Google Scholar]
- 13.Rocha, B., and H. von Boehmer. 1991. Peripheral selection of the T cell repertoire. Science. 251:1225–1228. [DOI] [PubMed] [Google Scholar]
- 14.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A.M. Schmitt-Verhulst, B. Malissen, G.J. Hammerling, and B. Arnold. 1991. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 65:293–304. [DOI] [PubMed] [Google Scholar]
- 15.Maile, R., B. Wang, W. Schooler, A. Meyer, E.J. Collins, and J.A. Frelinger. 2001. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J. Immunol. 167:3708–3714. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz, J.E., M.A. Forman, M.A. Lifton, O. Concepcion, K.A. Reimann Jr., C.S. Crumpacker, J.F. Daley, R.S. Gelman, and N.L. Letvin. 1998. Expression of the CD8alpha beta-heterodimer on CD8(+) T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus− and human immunodeficiency virus+ individuals. Blood. 92:198–206. [PubMed] [Google Scholar]
- 17.Grisotto, M.G., M.R. D'Imperio Lima, C.R. Marinho, C.E. Tadokoro, I.A. Abrahamsohn, and J.M. Alvarez. 2001. Most parasite-specific CD8+ cells in Trypanosoma cruzi-infected chronic mice are down-regulated for T-cell receptor-alphabeta and CD8 molecules. Immunology. 102:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kizaki, T., S. Kobayashi, K. Ogasawara, N.K. Day, R.A. Good, and K. Onoe. 1991. Immune suppression induced by protoscoleces of Echinococcus multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J. Immunol. 147:1659–1666. [PubMed] [Google Scholar]
- 19.Busch, D.H., I.M. Pilip, S. Vijh, and E.G.P. Am. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 8:353–362. [DOI] [PubMed] [Google Scholar]
- 20.Harrington, L.E., R. Most Rv, J.L. Whitton, and R. Ahmed. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 76:3329–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slifka, M.K., and J.L. Whitton. 2001. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat. Immunol. 2:711–717. [DOI] [PubMed] [Google Scholar]
- 22.Walker, P.R., T. Ohteki, J.A. Lopez, H.R. MacDonald, and J.L. Maryanski. 1995. Distinct phenotypes of antigen-selected CD8 T cells emerge at different stages of an in vivo immune response. J. Immunol. 155:3443–3452. [PubMed] [Google Scholar]
- 23.Altman, J.D., P.A. Moss, P.J. Goulder, D.H. Barouch, M.G. McHeyzer-Williams, J.I. Bell, A.J. McMichael, and M.M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274:94–96. [DOI] [PubMed] [Google Scholar]
- 24.Blattman, J.N., R. Antia, D.J. Sourdive, X. Wang, S.M. Kaech, K. Murali-Krishna, J.D. Altman, and R. Ahmed. 2002. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 195:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murali-Krishna, K., J.D. Altman, M. Suresh, D.J. Sourdive, A.J. Zajac, J.D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. [DOI] [PubMed] [Google Scholar]
- 26.Lukacher, A.E., J.M. Moser, A. Hadley, and J.D. Altman. 1999. Visualization of polyoma virus-specific CD8+ T cells in vivo during infection and tumor rejection. J. Immunol. 163:3369–3378. [PubMed] [Google Scholar]
- 27.Zanders, E.D., J.R. Lamb, M. Feldmann, N. Green, and P.C. Beverley. 1983. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 303:625–627. [DOI] [PubMed] [Google Scholar]
- 28.Valitutti, S., S. Muller, M. Cella, E. Padovan, and A. Lanzavecchia. 1995. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 375:148–151. [DOI] [PubMed] [Google Scholar]
- 29.Luescher, I.F., E. Vivier, A. Layer, J. Mahiou, F. Godeau, B. Malissen, and P. Romero. 1995. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 373:353–356. [DOI] [PubMed] [Google Scholar]
- 30.Valitutti, S., S. Muller, M. Dessing, and A. Lanzavecchia. 1996. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183:1917–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels, M.A., and S.C. Jameson. 2000. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 191:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake, D.R., III, and T.J. Braciale. 2001. Cutting edge: lipid raft integrity affects the efficiency of MHC class I tetramer binding and cell surface TCR arrangement on CD8+ T cells. J. Immunol. 166:7009–7013. [DOI] [PubMed] [Google Scholar]
- 33.Kerry, S.E., J. Buslepp, L.A. Cramer, R. Maile, L.L. Hensley, A.I. Nielsen, P. Kavathas, B.J. Vilen, E.J. Collins, and J.A. Frelinger. 2003. Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement. J. Immunol. 171:4493–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake, D.R., III, R.M. Ream, C.W. Lawrence, and T.J. Braciale. 2005. Transient loss of MHC class I tetramer binding after CD8+ T cell activation reflects altered T cell effector function. J. Immunol. 175:1507–1515. [DOI] [PubMed] [Google Scholar]
- 35.Kao, C., M.A. Daniels, and S.C. Jameson. 2005. Loss of CD8 and TCR binding to Class I MHC ligands following T cell activation. Int. Immunol. 17:1607–1617. [DOI] [PubMed] [Google Scholar]
- 36.Fahmy, T.M., J.G. Bieler, M. Edidin, and J.P. Schneck. 2001. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 14:135–143. [PubMed] [Google Scholar]
- 37.Spencer, J.V., and T.J. Braciale. 2000. Incomplete CD8+ T lymphocyte differentiation as a mechanism for subdominant cytotoxic T lymphocyte responses to a viral antigen. J. Exp. Med. 191:1687–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu, C., A.G. Heaps, V. Cerundolo, A.J. McMichael, C.R. Bangham, and M.F. Callan. 2007. Early acquisition of cytolytic function and transcriptional changes in a primary CD8+ T-cell response in vivo. Blood. 109:1086–1094. [DOI] [PubMed] [Google Scholar]
- 39.Daniels, M.A., L. Devine, J.D. Miller, J.M. Moser, A.E. Lukacher, J.D. Altman, P. Kavathas, K.A. Hogquist, and S.C. Jameson. 2001. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 15:1051–1061. [DOI] [PubMed] [Google Scholar]
- 40.Davey, G.M., S.L. Schober, B.T. Endrizzi, A.K. Dutcher, S.C. Jameson, and K.A. Hogquist. 1998. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 188:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolumam, G.A., S. Thomas, L.J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtsinger, J.M., C.M. Johnson, and M.F. Mescher. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165–5171. [DOI] [PubMed] [Google Scholar]
- 43.Curtsinger, J.M., D.C. Lins, and M.F. Mescher. 2003. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, L.J., G.A. Kolumam, S. Thomas, and K. Murali-Krishna. 2006. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of cd8 t cells for clonal expansion and memory formation. J. Immunol. 177:1746–1754. [DOI] [PubMed] [Google Scholar]
- 45.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. [DOI] [PubMed] [Google Scholar]
- 46.Sun, S., X. Zhang, D.F. Tough, and J. Sprent. 1998. Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 188:2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang, J., D. Gross, S. Nogusa, P. Elbaum, and D.M. Murasko. 2005. Depletion of T cells by type I interferon: differences between young and aged mice. J. Immunol. 175:1820–1826. [DOI] [PubMed] [Google Scholar]
- 48.La Gruta, N.L., S.J. Turner, and P.C. Doherty. 2004. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 172:5553–5560. [DOI] [PubMed] [Google Scholar]
- 49.Doucey, M.A., D.F. Legler, N. Boucheron, J.C. Cerottini, C. Bron, and I.F. Luescher. 2001. CTL activation is induced by cross-linking of TCR/MHC-peptide-CD8/p56lck adducts in rafts. Eur. J. Immunol. 31:1561–1570. [DOI] [PubMed] [Google Scholar]
- 50.Gray, P.M., S. Arimilli, E.M. Palmer, G.D. Parks, and M.A. Alexander-Miller. 2005. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. J. Virol. 79:3339–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denkberg, G., C.J. Cohen, and Y. Reiter. 2001. Critical role for CD8 in binding of MHC tetramers to TCR: CD8 antibodies block specific binding of human tumor-specific MHC-peptide tetramers to TCR. J. Immunol. 167:270–276. [DOI] [PubMed] [Google Scholar]
- 52.Campanelli, R., B. Palermo, S. Garbelli, S. Mantovani, P. Lucchi, A. Necker, E. Lantelme, and C. Giachino. 2002. Human CD8 co-receptor is strictly involved in MHC-peptide tetramer-TCR binding and T cell activation. Int. Immunol. 14:39–44. [DOI] [PubMed] [Google Scholar]
- 53.Gray, P.M., G.D. Parks, and M.A. Alexander-Miller. 2003. High avidity CD8+ T cells are the initial population elicited following viral infection of the respiratory tract. J. Immunol. 170:174–181. [DOI] [PubMed] [Google Scholar]
- 54.Slifka, M.K., and J.L. Whitton. 2000. Clinical implications of dysregulated cytokine production. J. Mol. Med. 78:74–80. [DOI] [PubMed] [Google Scholar]
- 55.Maile, R., S.M. Pop, R. Tisch, E.J. Collins, B.A. Cairns, and J.A. Frelinger. 2006. Low-avidity CD8lo T cells induced by incomplete antigen stimulation in vivo regulate naive higher avidity CD8hi T cell responses to the same antigen. Eur. J. Immunol. 36:397–410. [DOI] [PubMed] [Google Scholar]
- 56.Altan-Bonnet, G., and R.N. Germain. 2005. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 3:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrero, J.A., B. Calderon, and E.R. Unanue. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auerbuch, V., D.G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D.A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Connell, R.M., S.K. Saha, S.A. Vaidya, K.W. Bruhn, G.A. Miranda, B. Zarnegar, A.K. Perry, B.O. Nguyen, T.F. Lane, T. Taniguchi, et al. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.