Abstract
The impact of regulatory T cells (T reg cells) on the course of HIV and SIV disease is unknown. T reg cells could suppress protective antiviral responses and accelerate disease progression. Alternatively, these cells might block T cell activation and thereby limit viral replication as well as activation-associated immunopathology. Given the higher frequency of T reg cells known to be present during human fetal ontogeny, such influences may be most important in the context of perinatal infection. We found that infant macaques had higher fractions of CD4+CD25+CD127lowFoxP3+ T reg cells in the peripheral blood and in lymphoid tissues, and that these T reg cells showed greater in vitro suppressive activity on a per cell basis. Infant and adult macaques were infected with SIVmac251 to test the influence of the T reg cell compartment on SIV-specific immune responses. After infection with SIV, most (three out of four) infant macaques had persistently high viral loads, weak and transient SIV-specific CD4+ and CD8+ T cell responses, and rapid disease progression. T reg cells in the infant but not in the adult directly suppressed SIV-specific CD4+ T cell responses, which were detectable only after depletion of T reg cells. In the case of both the infant and the adult macaque, T reg cells were not able to directly suppress SIV-specific CD8+ T cell responses and had no apparent effect on T cell activation. In aggregate, these observations suggest that the T reg cell compartment of the infant macaque facilitates rapid disease progression, at least in part by incapacitating SIV-specific CD4+ T cell responses.
The impact of regulatory T cells (T reg cells) on HIV disease progression remains unclear. T reg cells comprise a heterogeneous collection of cells having in common the ability to suppress the activation and proliferation of other T cells. Because there is evidence that some effector T cell responses help to control HIV replication, such cells might reasonably be hypothesized to be harmful in the setting of HIV infection. Typical postacute reduction of plasma viral load is coincident with the appearance of adaptive T cell responses (1, 2), whereas depletion of CD8+ T cells (along with NK cells) from SIV-infected macaques prevents reduction of the viral load below peak or, in the chronic phase, leads to a rise in viral load (3). Furthermore, some MHC class I alleles are associated with either slow or rapid disease progression; these associations have recently been shown to be attributable to underlying differences in specific antiviral CD8+ T cell responses (4). Many authors have accordingly suggested that inhibition of antiviral T cell responses by T reg cells could lead to viral persistence and more rapid disease progression (5, 6).
The correlation between the total magnitude of the HIV-specific T cell response and CD4+ T cell count, however, is generally poor (7). As a possible explanation for this finding, several groups have found that some but not all subpopulations of antiviral T cells may be important for immune control. Betts et al., for instance, reported that nonprogressors maintain more highly functional HIV-specific CD8+ T cells than do nonprogressors (8). Emu et al. demonstrated that controllers of HIV replication, whether treated or untreated, exhibited high levels of HIV-specific CD4+ T cells producing both IL-2 and IFN-γ (9). Both of these reports suggest, in essence, that it is the quality and not the quantity of the anti-HIV T cell response that is important. Indeed, in lymphocytic choriomeningitis infections of mice, it has been shown that antiviral CD4+ T cell responses are required for the maintenance of effective CD8+ T cell responses, suggesting that “high-quality” CD4+ and CD8+ T cell responses could be correlated (10). Finally, Kiepiela et al. recently reported that only T cell responses directed at Gag play a role in immune control during chronic HIV infection, whereas responses directed at other viral proteins are detrimental (4). It is unknown whether T reg cells are capable of inhibiting effective, multifunctional antiviral T cell responses or whether T reg cells might differentially affect responses to various viral proteins.
Even in the face of robust adaptive immune responses, most untreated, HIV-infected patients experience CD4+ T cell depletion and disease progression. The time to disease progression is inversely correlated not only with viral load but also with the degree of T cell activation observed in peripheral blood, as assessed by expression of CD38 on CD8+ T cells (11–13). In treated patients, the level of T cell activation is inversely correlated with treatment-mediated CD4+ T cell gains (14). There are no definitive data demonstrating that T cell activation leads to T cell depletion; nevertheless, the strength of these associations has provided support for the hypothesis that chronic T cell activation is an important mechanism of HIV pathogenesis. If this hypothesis is correct, then it is possible that T reg cells might retard disease progression by inhibiting T cell activation. Indeed, Eggena et al. found that HIV-infected subjects with higher levels of T cell activation harbored a smaller number of T reg cells among CD3+ cells (15).
If T reg cells influence the course of HIV disease, then their impact should be most obvious when they are most prevalent. Three groups have shown recently that T reg cells are more common in fetal human tissue and have proposed that these cells contribute to peripheral tolerance in fetal and, perhaps, early postnatal life (16–18). It is accordingly possible that they might have a profound influence, be it positive or negative, in the context of perinatal infection. In this regard, it is notable that some 20–30% of perinatally infected children manifest a very rapid pace of disease progression (19, 20).
One explanation for faster disease progression among some infants is that a lower intensity of adaptive T cell responses allows for unchecked viral replication. Among infants, T helper responses are characterized by lower frequencies of antigen-specific T cells (21), a reduced magnitude of the response (22), and, often, a Th2 cell bias (23). The lower intensity of adaptive T cell responses among infected infants might be related to T cell–intrinsic defects or to differences in the frequency or activity of other immune cells. For example, in contrast to adult DCs, infant DCs express lower levels of MHC and adhesion molecules (24, 25), their ability to produce IL-12 is lower (26), and responses to Toll-like receptor ligation are reduced because of the limited expression of adaptor proteins and the limited activation of downstream signaling molecules (27). Therefore, the priming of the T cell response to infectious pathogens in infants is compromised.
Elucidation of the impact of T reg cells on lentiviral disease has been slowed by the difficulty of identifying T reg cells and the paucity of pharmacologic methods for altering their frequency or function. Recently, we reported that the natural T reg cells of adult macaques are CD25+FOXP3+CD127low, as are human T reg cells (28). We report in this paper that natural T reg cells are several-fold more abundant in the blood and tissues of infant macaques than those of adult macaques. We have taken advantage of this naturally occurring, age-related difference in T reg cell frequency to study the impact of T reg cells on the course of SIV disease, including their ability to suppress SIV-specific T cell responses and T cell activation.
RESULTS
Age-dependent differences in the clinical outcome of SIV infection
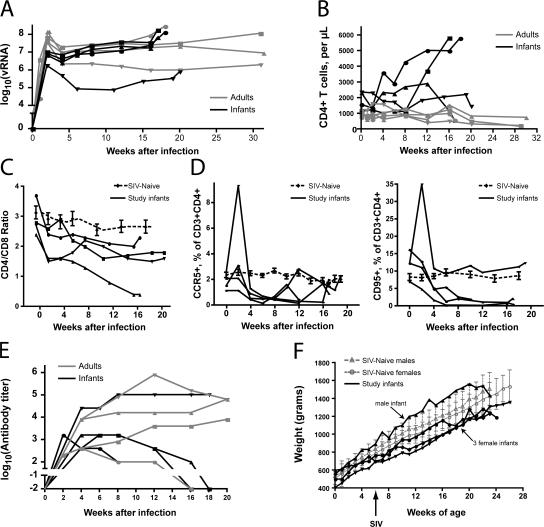
Infant and adult macaques were infected intravenously with SIVmac251 and followed longitudinally for several months. Both age groups exhibited high viral RNA (vRNA) levels during the acute phase of infection, although adults attained higher peak viral loads in plasma (P = 0.017 for effect of age group by two-way analysis of variance [ANOVA]; Fig. 1 A). A small decrease of plasma vRNA levels was observed between weeks 2 and 4 after infection in three out of four adult macaques, and these animals maintained the resulting set-point vRNA levels throughout the course of infection (Fig. 1 A). The remaining adult was a rapid progressor who maintained high viral loads, produced only low levels of anti-SIV antibodies (see below), and had to be killed 18 wk after infection because of weight loss and persistent diarrhea. Three out of four infant macaques demonstrated only minor declines (<0.5 log) in vRNA between weeks 3 and 4 after infection. After week 4, these animals experienced progressive accumulation of vRNA in plasma, with an increased level detected at nearly every measurement until death. The remaining infant (no. 37740, depicted by inverted triangles in Fig. 1 and elsewhere) effectively controlled viral replication for 3 months after infection, after which time vRNA levels increased progressively.
Figure 1.
Age-dependent differences in the clinical outcome of SIV infection. (A) Plasma vRNA levels through the course of SIVmac251 infection in infants (black symbols) and adults (gray symbols). Data points from each animal are shown using a different symbol; the same symbols are used in association with the same animals throughout this paper. For example, data from infant no. 37740 are consistently shown with ▾, whereas those from the rapidly progressing adult are shown with •. Two-way ANOVA analysis of values taken at weeks 2, 4, 8, and 12 suggests a significant effect of age group (P = 0.017) and time point (P < 0.0001). Bonferroni posttests show that the peak vRNA levels at 2 wk demonstrate the largest difference between the groups, as compared with differences at other time points, but the p-value for this post-hoc test is >0.05. (B) CD4+ T cell counts during SIVmac251 infection. (C) The ratio between CD4+/CD8+ T cells in peripheral blood. The four continuous lines depict data from infants in this study, which were infected at 6 wk of age. The dashed line shows composite data from 20 age-matched uninfected controls. Error bars show the standard error of the mean. (D) Frequency of CCR5+ and CD95+ cells among CD3+CD4+ cells of infected infant macaques and uninfected controls. (E) SIV-binding antibody titers among SIVmac251-infected infants and adults. Whole, disrupted SIV was used as the target antigen. (F) Weight gain among SIV-infected infants in this study. SIV infection at 6 wk of age is indicated with an arrow. Weight gain among normal male and female infants is also shown, with error bars indicating one standard deviation from the mean.
Consistent with a previous study (29), CD4+ T cells decreased in all of the adult macaques (Fig. 1 B). In contrast, despite similar plasma vRNA levels and the development of severe clinical disease requiring death, there was no loss of CD4+ T cells among infant macaques (Fig. 1 B). In fact, in two out of four animals, absolute CD4+ T cell numbers increased markedly (Fig. 1 B). Analysis of the CD4/CD8 T cell ratio, however, revealed a gradual decline (range = 35–83%) that was more pronounced than the average decline (17%) observed in normal, age-matched infant macaques (n = 20) over the same time period because of normal T cell development (Fig. 1 C). Adult macaques, in comparison, had a decrease in the CD4/CD8 T cell ratio that ranged from 60 to 75% (unpublished data). Thus, high vRNA levels in infant macaques are not associated with the profound loss of CD4+ T cells that is observed in adult macaques.
A more detailed analysis revealed that infant macaques showed some loss of CD4+ T cells with an effector/memory phenotype, but this loss was masked by the fact that this population represented only a very small fraction in peripheral infant blood (unpublished data). Except for monkey no. 37740, who had the lowest plasma vRNA levels, infant macaques showed an early loss of activated CC chemokine receptor (CCR) 5–, CD95-, and CXC chemokine receptor (CXCR) 3–expressing CD4+ T cells (Fig. 1 D and not depicted).
SIV-binding antibody titers were followed throughout infection. All animals developed binding antibodies within the first month of infection (Fig. 1 E). Compared with other adults, the rapidly progressing animal developed only low antibody titers, as has been observed in similar cases of rapid SIV disease progression (30). Infected infants initially developed antibody responses comparable to those seen in adults. In three out of four infants, however, these titers declined slowly over the course of infection, in a pattern similar to that observed in the rapidly progressing adult. The remaining infant (no. 37740) developed robust antibody responses that were sustained until necropsy at 5 mo. This data is consistent with previous reports showing lower anti-SIV antibody titers in infant compared with adult macaques (31).
Rapid disease progression in infant macaques was further indicated by early (2 wk after infection) and frequent reports of diarrhea. In infants, diarrhea and associated weight loss (Fig. 1 F) are important clinical symptoms because weight gain is critical for development. Although occasional outbreaks of Shigella-related diarrhea (nosocomial or carried from dam) can be observed among neonates or infants, stool samples of the three infants with frequent reports of diarrhea tested negative for parasites (including Giardia and Cryptosporidium), Shigella, Salmonella, Aeromonas, Yersinia, and Campylobacter. Necropsy samples of intestine were also tested and proved negative for infectious causes of diarrhea. Therefore, diarrhea was most likely caused by SIV enterocolitis. In this context, it is noteworthy that monkey no. 37740 showed consistent weight gain throughout the first 20 wk of life and had only two reports of diarrhea. The other three infants, however, were repeatedly reported for diarrhea or plateau of weight gain. Furthermore, although these infants achieved normal weight gain during the first 10 wk of life, their weight gain per day dropped during weeks 10–20. Nutritional supplements, fluids, and antibiotics were provided in an attempt to support weight gain. Even with these interventions, three out of four infant monkeys had to be killed after ∼17 wk of infection because of weight loss, dehydration, and other clinical signs characteristic of simian AIDS (e.g., ulcerating mucosal infections). The association of progressively increasing plasma viral loads with the development of simian AIDS suggested the possibility that rapid disease progression among infants was caused by poor immune control of virus.
As compared with adult T cells, infant T cells have a reduced capacity for cytokine production
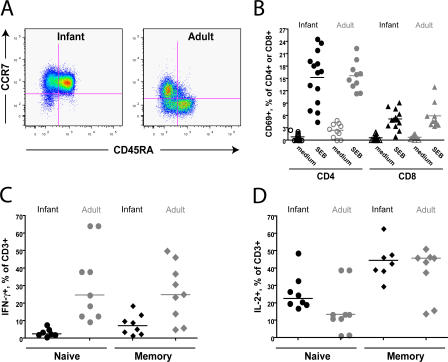
The lymphocyte population in the peripheral blood of newborn macaques undergoes enormous phenotypic and functional changes during the first 3 mo of life (32). Phenotypically, PBMCs at birth comprise ∼90% T cells and only 10% B cells. Among the T cell population, the CD4/CD8 T cell ratio is much higher in infants than in adults (32, 33); however, as previously shown, the increased availability of potential SIV target cells does not correlate with an increased viral load (33). In addition, many phenotypic markers used to define activation and disease progression in the T cells of HIV-infected adults are consistently expressed by healthy, SIV-naive infant T cells. For example, almost all peripheral blood CD4+ and CD8+ T cells express CD38 throughout the first year of life (unpublished data). We hypothesized that the functional capacity of infant macaque T cells might also be substantially different from that of adult cells.
To address this hypothesis, infant and adult PBMCs were stimulated with the superantigen Staphylococcal enterotoxin B (SEB) or with PMA/ionomycin and assessed for cytokine production by intracellular cytokine staining. Naive and memory T cell subpopulations were assessed separately because the vast majority of infant T cells are CD45RA+CCR7+ naive cells that might be expected to produce cytokine less readily (Fig. 2 A). We found that T cells from both infants and adults readily expressed the early activation marker CD69 after treatment with SEB or PMA/ionomycin (15 or 95% of CD4+ T cells, respectively, after 6 h; Fig. 2 B and not depicted), indicating successful stimulation. However, infant naive (CD45RA+CCR7+) and memory (CD45RA−CCR7+) T cells were substantially less likely than adult naive and memory T cells to produce IFN-γ (Fig. 2 C) or TNF-α (not depicted) after stimulation with SEB or PMA/ionomycin but equally likely to produce IL-2 (Fig. 2 D). This tendency toward IL-2 production was particularly pronounced in naive cells. It should be noted that SEB was used over a range of 200–1,000 ng and that the age-related differences in cytokine production were found at all doses tested (unpublished data). To rule out the possibility that infant T cells could produce cytokines at levels comparable to adult T cells but with delayed kinetics, the kinetics of cytokine induction were also tested. Although the frequencies of IFN-γ– or TNF-α–producing cells from both infants and adults were increased at 12 as compared with 6 h, production in infant T cells remained significantly less than that achieved by adult cells (P < 0.0001; unpublished data).
Figure 2.
Reduced capacity for cytokine production by infant T cells. (A) Representative flow cytograms showing the distribution of CCR7 and CD45RA on unstimulated CD3+ cells from infant (left) and adult (right) macaques. All data in A–D were produced using cells from uninfected animals. (B) The graph indicates the percentage of CD4+ or CD8+ T cells that expressed CD69 after stimulation with medium or SEB. Whole blood was stimulated for 6 h, then stained with surface antibodies before lysis and data collection. Horizontal bars represent means. (C) IFN-γ production among CD3+ cells from infants (black symbols) and adults (gray symbols) after treatment with PMA/ionomycin for 6 h. Data for cytokine-producing naive (CD45RA+CCR7+) and memory (CD45RA−CCR7+) cells are shown. The data were produced by first gating on naive and memory cell populations and then assessing cytokine production within each subset. (D) IL-2 production among CD3+ cells from infants and adults. Horizontal bars in C and D represent medians.
T reg cells are more abundant in the blood and tissues of infant than adult macaques
We and others have previously shown that T reg cells are abundant in the human fetus (16–18). We speculated that T reg cells might also be abundant in newborn macaques and that their presence could influence the immune response to SIV.
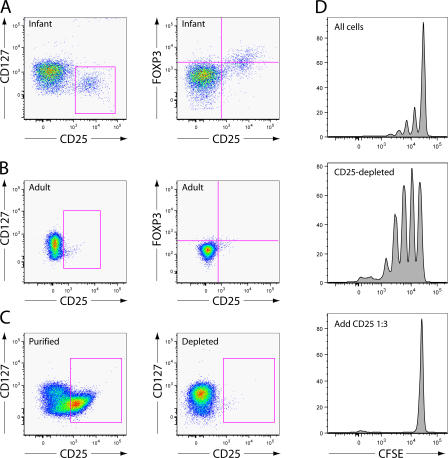
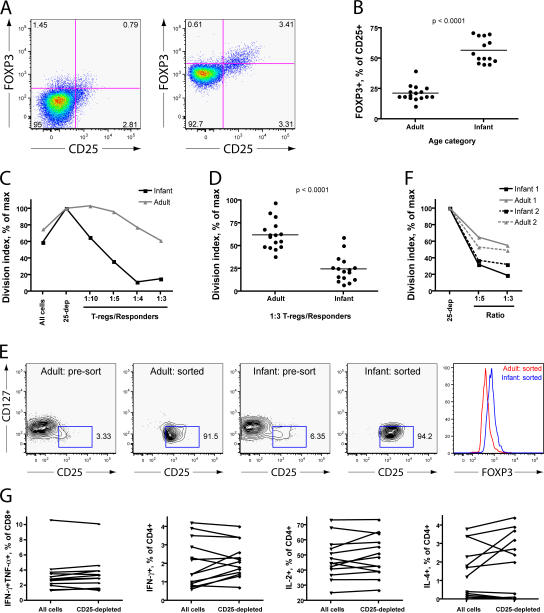
To identify T reg cells in infant macaques, we examined expression of the markers CD25, CD127, and FOXP3, which are useful for the identification of T reg cells in mice, humans, and adult macaques (28, 34). We found that virtually all CD4+CD25+ cells in infant macaque blood and tissue are CD127low and that many are FoxP3+ (Fig. 3 A). CD4+CD25+ cells of adults were similarly CD127low, although a smaller fraction of these cells were FoxP3+. Because this result suggested that CD127 expression would not be necessary or useful for the differentiation of T reg cells from activated T cells, as it is in humans, we isolated all CD25+ cells using paramagnetic beads and tested their suppressive capacity in vitro. The population of cells isolated using this method was 90% CD4+, 60% CD25+, and suppressive in vitro, whereas negatively selected cells were CD25− and not suppressive in vitro (Fig. 3, C and D; and not depicted). Therefore, for the remainder of this study, paramagnetic beads were used to isolate presumptive CD25+ T reg cells for in vitro studies.
Figure 3.
Infant macaque T reg cells are CD25+ and CD127low. (A) Representative flow cytograms show the distribution of CD25, CD127 (left), and FOXP3 (right) on infant CD3+CD4+ cells from whole blood samples. All data in A–D were produced using cells from uninfected animals. (B) Distribution of CD25, CD127, and FoxP3 on adult CD3+CD4+ cells from whole blood samples. (C) Expression of CD25 and CD127 on cells isolated by positive (left) or negative (right) selection on paramagnetic beads. The events shown are gated singlet lymphocytes expressing CD3 and CD4. 5 × 106 PMBCs were processed. (D) Histograms show CFSE dilution in cultures stimulated with plate-bound anti-CD3 antibodies. CD25+ and CD25-depleted cells were prepared according to the same methods described in Fig. 2 C. The unfractionated and CD25-depleted populations were labeled with CFSE. The unfractionated cell population (90,000 cells; top), CD25-depleted population alone (90,000 cells; middle), or CD25-depleted population with CD25+ T reg cells (90,000 and 30,000 cells, respectively; bottom) were incubated on plates coated with anti-CD3 antibodies for 5 d before analysis.
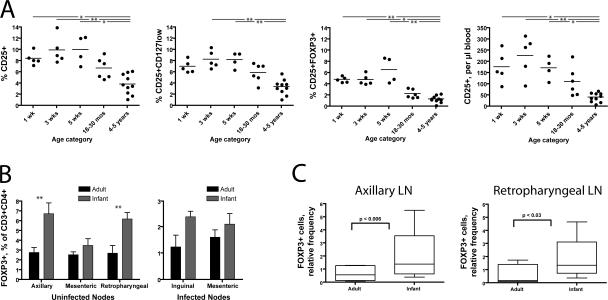
Our initial results (Fig. 3, compare A and B) suggested that T reg cells might be more abundant in the blood and tissues of infant macaques than of adults. Indeed, we found that CD25+ cells composed ∼10% of CD3+CD4+ cells from infant blood but only ∼4% of CD3+CD4+ cells from adult blood (Fig. 4 A). Using more stringent criteria to define the regulatory cell phenotype (CD25+CD127low or CD25+FoxP3+) reduced the number of regulatory cells detected by flow cytometry but confirmed that T reg cells are more frequent among infant CD4+ T cells (Fig. 4 A, second and third panels). Furthermore, because a larger fraction of infant T cells is CD4+, these differences are even more pronounced when the values are expressed as a percentage of the CD3+ population or as absolute numbers (Fig. 4 A).
Figure 4.
T reg cells are abundant in the blood and tissues of infant macaques. (A) T reg cell phenotypes in the blood of uninfected infant (1–5 wk), juvenile (18–30 mo), and adult (4–5 yr) macaques. Whole blood samples were taken from macaques at the indicated ages and stained for the markers indicated. Percentages shown (three left panels) are calculated as a fraction of the gated CD3+CD4+ population. The absolute values in the far right panel were obtained by multiplying the percentages observed in flow cytometric analysis by the absolute number of CD3+CD4+ cells obtained in a complete blood count. The groups shown in each graph have significantly different means as tested by one-way ANOVA (P < 0.001 in each case). The asterisks drawn between some groups indicate a significant difference in post-hoc testing (*, P < 0.05; and **, P < 0.01 using Dunn's multiple comparison test). Horizontal bars represent means. (B) FoxP3+ cells detected flow cytometrically in lymph node samples. Uninfected samples were frozen before analysis. Infected samples were harvested at necropsy (i.e., at different times after infection for each animal) and analyzed immediately. Among uninfected lymph node samples, two-way ANOVA shows a significant influence of age (P < 0.0001) and node (P < 0.05). **, P < 0.01 using Bonferroni posttests that suggest a significant difference between adults and infants within axillary and retropharyngeal nodes. (C) FoxP3+ cells detected by immunohistochemical staining of formalin-fixed, paraffin-embedded tissue. p-values were calculated using the Student's t test. Error bars represent means ± SEM.
Because some CD25 expression is lost after freezing, the presence of T reg cells in frozen infant and adult lymph node cells was assessed using flow cytometric staining for FoxP3. Axillary and retropharyngeal lymph nodes from uninfected infants at 4 wk of age contained a substantially larger fraction of FOXP3+ cells among CD3+ or CD4+ T cells than adult nodes (Fig. 4 B), mirroring the situation observed in whole blood samples. Adult and infant mesenteric lymph nodes contained comparable numbers of FoxP3+ cells, with a nonsignificant trend toward observation of more FoxP3+ cells in infant samples (Fig. 4 B). Among infected animals, the frequencies of CD25+ cells in whole blood and fresh lymph node tissue at necropsy were significantly correlated (P < 0.05; unpublished data). Immunohistochemical staining for FoxP3+ confirmed these flow cytometric results, demonstrating that the axillary and retropharyngeal lymph nodes of infants contain a larger number of FoxP3+ cells than those of adults (Fig. 4 C).
Because of limitations on tissue collection from young animals, we obtained SIV-infected infant lymph nodes only at necropsy. Comparison of these samples to adult nodes taken at necropsy revealed a nonsignificant trend toward a higher frequency of FoxP3+ cells (Fig. 4 B). However, adult and infant necropsies were performed at different times after infection, and it is unclear whether these samples are comparable to each other or indicative of conditions earlier in infection. At necropsy, both adult and infant nodes had a lower frequency of FoxP3+ cells but not a lower frequency of CD25+CD127low cells when compared with uninfected nodes (Fig. 4 B and not depicted).
T reg cells of infants are more suppressive in vitro than those of adults
Although neither infant nor adult macaque cells demonstrated a discrete population of FoxP3+ cells (Fig. 5 A), such as is often found in human samples, expression of CD25 and expression of FOXP3 were correlated (Fig. 3 A) such that more CD25+ than CD25− cells were FoxP3+. The proportion of CD25+ cells that was FoxP3+, however, differed between adult and infant cells: only 20% of adult macaque CD25+ T reg cells but >50% of infant macaque CD25+ T reg cells expressed detectable FoxP3 (Fig. 5 B). Because the level of FOXP3 expression has been linked quantitatively to suppressive function in mouse cells (35), this observation raised the possibility that infant macaque CD25+ T reg cells might be more suppressive than their adult counterparts. However, there is evidence in the literature that FoxP3 expression cannot always be equated with regulatory function (36). For this reason, we performed additional experiments aimed at testing the regulatory function of infant and adult T reg cells, defined by surface phenotype, in vitro.
Figure 5.
T reg cells of infants have greater suppressive activity than those of adults. (A) Representative flow cytograms showing intracellular FOXP3 staining in uninfected adult (left) and infant (right) peripheral blood cells. Numbers represent the percentage of gated events found in each quadrant. (B) Percentages of CD3+CD4+CD25+ cells from uninfected peripheral blood that were shown to express FOXP3 by intracellular staining and flow cytometry. Horizontal bars represent means. (C) Suppression of cell division by CD25+ T reg cells from infant and adult macaques isolated using paramagnetic beads. Data points indicate the division index achieved by CFSE-labeled cells in each sample as a percentage of the maximal value obtained in the CD25-depleted population. Cells were isolated from peripheral blood, purified using paramagnetic beads and columns, and stimulated for 5 d on anti-CD3–coated tissue culture plates. These data are representative of two experiments that tested a full range of T reg cell/responder ratios from infants and adults in parallel and are consistent with a much larger number of results testing suppression at a ratio of 1:3 and shown in D. (D) Results from a larger number of suppression assays performed on different days and testing suppression at a 1:3 ratio of T reg cells/responders. Horizontal bars represent means. (E) Adult and infant T reg cell populations purified by FACS. T reg cells represented >90% of the CD3+CD4+ cells in sorted populations. The far right panel shows the intensity of FoxP3 staining in aliquots of sorted adult and infant T reg cells (mean fluorescence intensity = 513 and 831, respectively). (F) Results of suppression assays performed using regulatory cells purified by FACS from the blood of two uninfected infants and two uninfected adults. Because of limited cell numbers, these assays were performed using only 40,000 CD25-depleted responders. Responders were depleted of CD25+ cells using paramagnetic beads. (G) Effect of T reg cell depletion on cytokine production by infant T cells in response to polyclonal stimuli. Aliquots of infant T cells were depleted of CD25+ cells using paramagnetic beads. Depleted or undepleted samples were then stimulated with PMA and ionomycin for 12 h, permeabilized, and stained with antibodies specific for cytokines or cell-surface markers. The percentage of CD8+ (far left graph) or CD4+ (right three graphs) expressing various cytokine combinations (simultaneous IFN-γ and TNF-α) or individual cytokines (IFN-γ, IL-2, or IL-4) in response to stimulation is shown. At least 12 samples are shown in each graph.
We tested the suppressive capacity of infant and adult macaque T reg cells in vitro at various responder/suppressor ratios. In replicate experiments, infant T reg cells were more suppressive than adult T reg cells at all ratios tested (Fig. 5 C). This difference was especially evident at 1:3 and 1:4 ratios of CD25+ T reg cells/responders. We accordingly examined the degree of suppression achieved by adult and infant T reg cells at a 1:3 ratio in a larger number of suppression assays using samples from uninfected adults and infants. We found that the functional difference between infant and adult T reg cells was consistent and statistically significant, with infant T reg cells suppressing an average of 75% of proliferation in vitro but adult T reg cells suppressing only 40%. (Fig. 5 D). Because a larger percentage of infant PBMCs were CD25+, we considered the possibility that bead-based purification produced a more pure population of infant than adult T reg cells. To address this concern, nearly pure populations of adult and infant T reg cells were prepared by FACS (Fig. 5 E). Infant T reg cells were more suppressive than adult T reg cells in this assay as well (Fig. 5 F).
We performed depletion assays to determine whether T reg cells were the principal cause of poor IFN-γ production among infant T cells exposed to polyclonal stimuli (Fig. 2). Depletion of T reg cells from assays using stimulation with PMA/ionomycin had no consistent effect on the production of IFN-γ or TNF-α among CD8+ T cells or on the production of IFN-γ, IL-2, or IL-4 among CD4+ T cells (Fig. 5 G and not depicted).
Higher numbers of T reg cells correlate with transient anti-SIV CD8+ T cell responses
Given that both faster disease progression and a larger number of T reg cells were observed among infant macaques, we hypothesized that T reg cells might suppress SIV-specific CD8+ T cell responses in SIV-infected infants. This prediction was tested by performing cytokine flow cytometry assays in the presence or absence of CD25+ T reg cells (Fig. 6, A and B).
Figure 6.
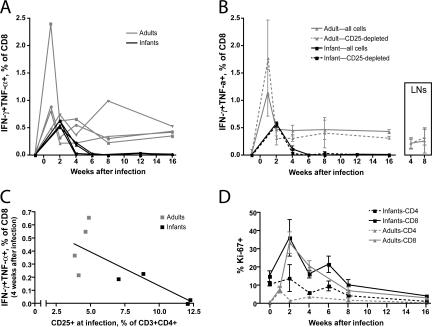
Transient CD8+ T cell responses among infected infant macaques. (A) Percentage of CD3+CD8+ T cells expressing IFN-γ and TNF-α after exposure to p27 Gag peptides. Note that infant blood was not tested during the first week after infection because of limitations on blood collection. The symbols used for individual study animals are identical to those in Fig. 1 and remain consistent in subsequent figures. (B) Influence of T reg cell depletion on CD3+CD8+ T cells expressing IFN-γ and TNF-α. The continuous lines in this graph represent averages of the lines shown in A. The dashed lines indicated the average response among study animals after depletion of CD25+ T reg cells. (inset) the influence of T reg cell depletion on CD8+ T cell responses within adult lymph nodes taken 4 and 8 wk after infection. (C) Correlation of the IFN-γ+TNF-α+ responses among CD8+ cells at 4 wk after infection with the percentage of T reg cells present before infection. The slope of this line is significantly different from zero (P < 0.05) when all animals are considered together but not when adults or infants are considered separately. (D) Intracellular Ki-67 expression among infant and adult T cells after infection with SIVmac251. Each line represents an average of four animals per study group. Error bars in B and D represent means ± SEM.
Adult macaques were found to mount detectable CD8+ T cell responses to SIV p27 peptides throughout the course of infection (Fig. 6 A). Peak SIV-specific CD8+ T cell responses were observed 1 wk after infection, coincident with peak viremia, and declined thereafter as vRNA levels also declined (Fig. 1 A). In contrast, although low SIV-specific CD8+ T cell responses were detected in the peripheral blood of infant macaques at 2 and 4 wk after infection, they were not maintained thereafter.
Surprisingly, no difference in CD8+ T cell responses was observed after the depletion of T reg cells from either infant PBMCs, adult PBMCs, or adult lymph node cells (Fig. 6 B). This finding suggested that direct suppression by T reg cells is not the most important mechanism limiting CD8+ T cell responses in either infant or adult macaques. Nevertheless, the two infants having the highest frequency of T reg cells at the time of infection produced no detectable anti-SIV responses among CD8+ cells by 4 wk afterward (Fig. 6 C), whereas the two infants with a lower frequency of T reg cells still had detectable responses at this time.
T cell activation levels were also evaluated in both age groups of animals. Despite harboring a larger population of T reg cells, infant animals displayed higher levels of T cell activation among CD4+ and CD8+ cells whether measured by Ki-67 or HLA-DR expression (Fig. 6 D and not depicted). In fact, we observed a trend, which did not reach statistical significance, toward more frequent expression of Ki-67 by infant than adult CD4+ T cells.
Infant but not adult T reg cells suppress SIV-specific CD4+ T cell responses
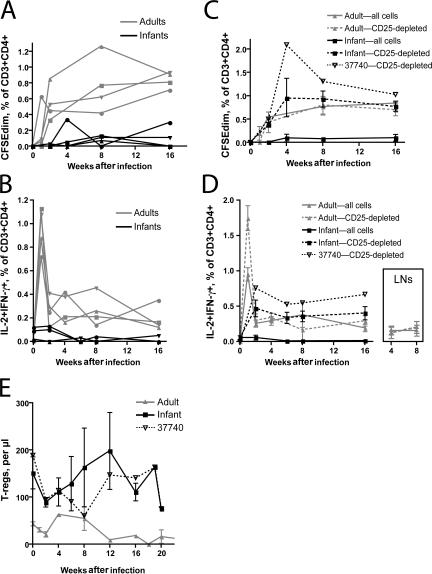
Because transient CD8+ T cell responses can be caused by the absence of CD4+ T cell help (10), we investigated the relationship between SIV-specific CD4+ T cells and CD25+ T reg cells in infected animals. Infected adult macaques mounted modest responses to SIV antigens among CD4+ T cells, which were detected in both proliferation and cytokine flow cytometry assays (Fig. 7, A and B, respectively). Proliferative responses increased gradually throughout the first 4 mo, whereas responses detected by cytokine flow cytometry peaked 1 wk after infection. Among adults, these responses were sustained throughout infection and were augmented only minimally by the depletion of T reg cells (Fig. 7, C and D). Whole, undepleted PBMC samples from infected infants, on the other hand, demonstrated no or very low CD4+ T cell responses to SIV throughout infection (Fig. 7, A and B). Depletion of T reg cells from these samples, however, revealed proliferative and IL-2 and IFN-γ responses among CD4+ T cells that were comparable to those observed in adults (Fig. 7, C and D, respectively). Depletion of T reg cells from infant lymph node samples taken at necropsy also revealed responses among CD4+ T cells that were not observed in undepleted samples (unpublished data). Despite the apparent presence of T reg cells actively suppressing CD4 responses to SIV, few or no CD4+ T cells producing IL-10 or TGF-β in response to SIV were detected (unpublished data).
Figure 7.
Suppression of SIV-specific CD4+ T cells by infant but not adult T reg cells. (A) Cell division assessed by CFSE dilution among CD3+CD4+ T cells. Purified PBMCs were labeled with CFSE and stimulated for 5 d by incubation of cultures with AT2-inactivated SIV. The percentages shown on the graph indicate the fraction of live CD3+CD4+ T cells found below the primary, undivided peak of CFSE intensity. Background cell division observed after treatment of cultures with microvesicles has been subtracted. (B) Percentage of CD3+CD4+ cells expressing IL-2 and IFN-γ after stimulation with antigen, assessed by cytokine flow cytometry. Note that infant blood was not tested during the first week after infection because of limitations on blood collection. (C) Influence of T reg cell depletion on CD3+CD4+ T cells that divide after exposure to AT2-SIV. The continuous lines in this graph represent averages of the four lines shown in panel A. The dashed lines indicate the average response among all four study animals after depletion of CD25+ T reg cells. Finally, the dotted line indicates the response of an individual infant (no. 37740), which is discussed in Results. (D) Influence of T reg cell depletion on CD3+CD4+ cells that express IL-2 and IFN-γ. The continuous and dashed lines shown represent averages of all four study animals, as described in C. The dotted line indicates the response of infant no. 37740. (inset) The influence of T reg cell depletion on CD4+ T cell responses within adult lymph nodes taken 4 and 8 wk after infection. (E) T reg cells (CD3+CD4+CD25+CD127low) in adult an infant peripheral blood after infection with SIV mac251. As in C and D, the continuous lines represent averages of four study animals, and the dotted line represents the number of cells found in infant no. 37740. Error bars in C, D, and E represent means ± SEM.
The finding of suppressed CD4+ T cell responses among infants is consistent with the lower and transient antibody responses mounted by these animals (Fig. 1 D). Although only whole SIV antibody responses were measured in this study, a previous study has shown that Gag antibody titers are reduced with the progression to simian AIDS and concurrent loss of CD4+ T cells, whereas anti-Env antibodies develop relatively normally or increase (31). We noted that one infant macaque (no. 37740) developed higher antibody titers, lower plasma vRNA levels, and apparently slower disease progression. This animal, like other infants, had poor SIV-specific responses among undepleted CD4+ T cells (Fig. 7, A and B). The frequencies and absolute numbers of T reg cells were not significantly different in this animal than in other infants, although we noted a trend toward fewer T reg cells during postinfection weeks 6–12 (Fig. 7 E). However, this infant had the most robust response to SIV antigens among CD25-depleted cells of any animal, infant or adult (Fig. 7, C and D).
DISCUSSION
The influence of T reg cells on lentiviral disease progression remains unclear. In this study, we document not only a higher frequency but also a greater suppressive capacity of T reg cells among infant macaques as compared with adults. We took advantage of this natural difference to evaluate the impact of T reg cell function on SIV disease progression. In three out of four infant macaques, rapid disease progression was associated with low and transient CD4+ and CD8+ T cell responses. Although SIV-specific CD8+ T cell responses were not revealed by T reg cell depletion in vitro, such depletion did reveal sustained SIV-specific CD4+ T cell responses. Strikingly, T reg cell suppression of SIV-specific CD4+ responses was only observed in infant, not in adult, macaques. Among infant macaques, the presence of a larger number of T reg cells did not lead to reduced levels of T cell activation. These observations thus highlight a close association between T reg cell–mediated suppression of SIV-specific CD4+ T cell responses and rapid disease progression in infant macaques.
It is possible that a higher degree of T reg cell activity is responsible for key differences between infant and adult immune systems, particularly the apparently lower reactivity of infant T cells to vaccines and infectious agents (21, 22). Indeed, the current study documents an example of such low reactivity, in that SIV-infected infants mounted a CD8+ T cell response that was of low intensity and short duration. A similar response to vaccination would be considered suboptimal, and our study suggests the possibility that interference with T reg cell function might augment the efficacy of vaccines in infants. Nonetheless, we also observed deficiencies in cytokine production among infant T cells that are independent of T reg cell activity. Interventional studies that specifically alter T reg cell function in infant macaques will be required before the impact of T reg cells on infant immune responses can be completely understood.
We noted that low-intensity and transient CD8+ T cell responses among infants are associated with active suppression of CD4+ T cells expressing IL-2 and IFN-γ. We hypothesize that CD8+ T cell responses in infants failed because of a lack of effective support from CD4+ T cells. Other groups have reported that chronic viral infections lead to exhaustion of the CD8+ T cell response and have discovered that the exhausted CD8+ cells are not deleted but remain in circulation, where the cells express programmed death 1 and possibly other inhibitory receptors that prevent effector function (37). This model could not be tested directly in this study because of the lack of tetramer reagents reactive to the MHC haplotypes of the animals. However, Matloubian et al. found that mice depleted of CD4+ T cells demonstrate intense, permanent CD8+ T cell exhaustion (10), whereas Dittmer et al. showed that T reg cells lead to functional impairment of CD8+ T cells in Friend retrovirus infection (38). Possibly, T reg cells render CD8+ T cells vulnerable to exhaustion and programmed death 1 expression by depriving them of adequate CD4+ T cell help.
Our data demonstrate consistent, active suppression of CD4+ T cell responses in infants by CD25+ T reg cells. Emu et al. found that multifunctional (IL-2+IFN-γ+) CD4+ T cell responses are more common in HIV-infected patients having apparently effective anti-HIV immune responses: untreated elite controllers with viral loads <50 and treated patients with low viral loads despite the presence of drug-resistant virus (9). Our data raise the possibility that these important effector cells are inhibited by T reg cells, which become increasingly prevalent among CD3+CD4+ cells throughout the course of HIV disease. Other authors have reported that highly functional CD8+ T cells are prevalent in nonprogressors and are associated with low viral load (8). Neither group examined the possibility that highly functional CD8+ T cells and CD4+ T cells are associated with each other in the same patients.
We did not observe in vitro suppression of CD4+ or CD8+ T cell responses by T reg cells in adult macaques. We do not know whether this suppression does not occur in vivo or whether it is simply more difficult to detect in vitro because the T reg cells depleted from assays of adult cells are less suppressive. We find that T reg cell numbers vary widely between different adult humans and macaques (28); possibly, suppression of CD4+ T cell responses occurs only in adult macaques having the most T reg cells.
Our data suggest that infant T reg cells suppressed SIV-specific CD4+ T cells but not “bystander” CD4+ T cells. The explanation for this difference might involve the responding CD4+ T cells, the T reg cells, or both. With respect to responding CD4+ T cells, SIV-specific T cells and bystander T cells are being activated in vivo by different mechanisms. It is possible that these two mechanisms of T cell activation render the responding cells differently sensitive to suppression. With respect to the T reg cells themselves, the simplest explanation for differential regulation is the outgrowth of SIV-specific T reg cells. Despite clear suppression of anti-SIV responses, we did not detect SIV-specific regulatory cells secreting IL-10 or TGF-β in response to SIV p27 peptides or AT2-inactivated SIV. It is possible that SIV-specific T reg cells were present but that the techniques used were not sufficiently sensitive to detect the cells. Alternatively, polyclonal T reg cells may have been activated and become suppressive in response to the inflammatory stimuli associated with infection.
In addition to active suppression by T reg cells, we found that the intrinsic functional ability of infant CD4+ T cells to produce certain cytokines was reduced. Altered CD4+ T cell function was still detected at 9 mo of age (unpublished data). Consistent with our finding that IFN-γ production by infant CD4+ T cells is reduced, it has been documented that the IFN-γ locus of human infant CD4+ T cells is hypermethylated (39). Thus, the kinetics of IFN-γ induction could differ between infants and adults, and the time necessary to produce IFN-γ in quantities sufficient to promote Th1 cell commitment in infants might be extended. Interestingly, the SIV-specific CD4 as well as the CD8 T cell response was indeed much lower and of a transient nature when compared with the SIV-specific T cell response observed in adult macaques. However, the in vitro depletion of T reg cells resulted in in vitro SIV-specific CD4+ T cell responses that were comparable to those observed in adults. With the important caveat that the direct translation of in vitro findings to the in vivo situation and a direct comparison of results after polyclonal stimulation versus antigen-specific assays is problematic, it appears that the in vivo suppression of infant CD4+ T cell function by T reg cells is more important than the reduced capacity of infant CD4+ T cells to produce IFN-γ in the prevention of effective SIV T cell immunity in infants.
Infant macaques infected with SIVmac251 in this study experienced rapid disease progression, and three out of four were killed by 4 mo after infection. This finding, together with our other published results (40), suggests that the SIVmac251 infection of infants can be an appropriate model for rapidly progressive HIV disease in perinatally infected humans. It is important to note, however, than most (70–80%) HIV-infected human infants do not progress rapidly in the absence of therapy (19, 20). The influence of T reg cells might well be different in the setting of slowly or rapidly progressive disease. For example, in rapidly progressive disease, effector T cell responses that limit viral replication might be of utmost importance; under these conditions, a large population of T reg cells would accelerate progression. In slowly progressive disease, in contrast, suppression of bystander T cell activation by T reg cells could be beneficial.
This difference may underlie the results found in the one infant macaque (no. 37740) that progressed more slowly after SIV infection. Furthermore, we noted that this infant had a very potent CD4+ T cell response to SIV among T reg cell–depleted populations, as well as robust SIV-binding antibody responses. We speculate that SIV-responsive CD4+ T cells in this animal may have been able to provide help to B cells in vivo, despite their apparent lack of reactivity in the presence of T reg cells in vitro.
In conclusion, we find that infant macaques have a large number of highly suppressive T reg cells and that these cells directly inhibit the CD4+ T cell response to SIV. Suppression of CD4+ T cell responses was associated with a transient CD8+ T cell response and limited antibody production. If these mechanisms are at work in human patients, then T reg cell activity might partially explain the vulnerability of human infants to chronic viral infections. In the setting of HIV infection, effective and persistent CD8+ T cell responses are likely important for control. In hepatitis B virus infection, which proceeds to chronicity in 90% of neonates but only 5% of adults, sustained high-titer antibody production may be required for the elimination of the virus (41). Further study will be required to determine whether interference with the regulatory control of CD4+ T cells can assist in control of these diseases.
MATERIALS AND METHODS
Animals.
All rhesus macaques were housed in accordance with the regulations of the American Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) at the California National Primate Research Center (CNPRC). Newborn rhesus macaques were born to female rhesus macaques of the CNPRC colony that are negative for HIV-2 (serology), SIV (serology), type D retrovirus (serology and PCR), and simian T cell lymphotropic virus type 1 (serology). All adult animals were also negative for HIV-2, SIV, type D retrovirus, and simian T cell lymphotropic virus type 1 at study entry. SIV-infected animals were housed in the infectious housing unit in pairs of two animals per cage. SIV-naive infants were housed under comparable conditions, whereas SIV-naive adult monkeys were housed in outdoor corrals. Infant animals were 6 wk old at infection; adult animals were 3.4, 3.6, 3.6, and 3.7 yr old (approximately the age of reproductive maturity) (42). Animal experiments were approved by the Institutional Animal Use and Care Committee of the University of California, Davis.
SIV inoculation.
SIV infection was performed by intravenous inoculation of 1 ml of 100 TCID50 of SIVmac251. The virus stock was obtained form the ImmunoCore at the CNPRC (internal reference no. 06/04). Infant macaques were 6 wk of age and adults were 3–5 yr of age at the time of SIV inoculation. The infant animals were the same animals tested for T reg cell levels and function at 5 wk of age (Fig. 2 A). Two of the adult animals received treatment with G-CSF 1 and 5 wk before infection as part of a separate study. G-CSF treatment did not affect the number of T reg cells present in peripheral blood before or after infection, the number of T reg cells in lymph nodes before or after infection, or the apparent progress of SIV disease relative to historical controls (unpublished data).
Virus load measurement.
Plasma RNA samples were analyzed for vRNA by a quantitative branched DNA assay (43). Virus load in plasma samples is reported as log10 vRNA copy numbers per milliliter of plasma. The detection limit of this assay is 125 vRNA copies.
Measurement of anti-SIV antibody titers.
Anti-SIV binding antibody titers in serum were measured as previously described (44). Whole, disrupted SIV was used as the target antigen. The results of the anti-SIV antibody ELISAs are reported as the dilution of a sample that produced OD values above the cut-off value.
Phenotypic characterization of lymphocyte populations.
50 μl of whole blood from SIV-naive animals were stained with 3 μl of each of the relevant antibodies (all obtained from BD Biosciences) to determine the frequencies of CD3+ T cells (clone SP34-2; BD Biosciences), CD4 and CD8+ T cells (clones L200 and RTP-8, respectively), and CD20+ B cells (clone 2H7). T cell subpopulations were further characterized by using the following antibodies: CD62L (clone SK11), CD95 (DX2), CD27 (M-T271), CD28 (L293), CCR5 (3A9), and CXCR3 (1C6/CXCR3). The antibody for CD38 was obtained through the National Institutes of Health (NIH) Nonhuman Primate Reagent Resource (NPRR) from K. Reimann (Harvard University, Cambridge, MA).
Regulatory cell phenotyping was performed by staining 50-μl whole blood samples with antibodies reactive to FOXP3 (clone 206D, Pacific blue labeled; BioLegend), CD4 (L200, AmCyan; NPRR), CD127 (hIL-7R–M21, PE; BD Biosciences), CD25 (MA-251, PE-Cy7; BD Biosciences), CD3 (SP34-2, Alexa Fluor 700; BD Biosciences), and CD8 (allophycocyanin [APC]–Alexa Fluor 750, 3B5, Invitrogen). Staining for cell-surface markers was performed for 20 min at room temperature, the samples were lysed in FACS Lysing Buffer (BD Biosciences), and cells were collected by centrifugation. The collected cells were stained for intracellular FOXP3 according to the protocol provided by BioLegend. A second flow cytometry panel was used to evaluate T cell activation. This panel included antibodies reactive to CD3 (clone SP34-2, Pacific blue labeled), CD4 (L200, AmCyan), CD38 (PE; NPRR), HLA-DR (Immu-357, ECD; Beckman Coulter), CD45RA (L48, PE-Cy7; BD Biosciences), CCR5 (3A9, APC; BD Biosciences), CD8 (RPA-T8, Alexa Fluor 700; BD Biosciences), and CD27 (O323, APC-Cy7; eBioscience).
In vitro stimulation of T cells with polyclonal stimuli.
100 μl of whole blood was stimulated with varying doses of SEB (Toxin Technologies) or 50 ng/ml PMA and 1 μg/ml ionomycin (Sigma-Aldrich). Cultures were stimulated for 6 h at 37°C, 5% CO2 in the presence of brefeldin A at 10 μg/ml. At the end of the culture period, cells were stained according to the Alternative Protocol for Intracellular Cytokine Staining (according to BD Biosciences) using the following antibodies: CD3-PE-Cy7, CD4-PE, CD8–Pacific blue, CD45RA-FITC (clone ALB11; Beckman-Coulter), CCR7-PE-Cy7 (3D12), IFN-γ–Alexa Fluor 700 (B27), and IL-2–APC (MQ1-17H12) or TNF-α–APC (mAb11). A minimum of 150,000 events were acquired on a FACSAria (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Inc.). Samples from SIV-naive infant macaques were provided to K. Abel for analysis by N. Lerche (CNPRC, University of California, Davis, Davis, CA).
In vitro suppression assays.
250 ng anti-CD3 antibody in 50 μl PBS (clone SP34-2) was added to wells of 96-well, U-bottom plates. The plates were incubated for at least 4 h and washed three times with PBS before use. Responder cells were prepared by depletion of purified PBMCs using anti-CD25–labeled paramagnetic microbeads and MS columns, according to the manufacturer's instructions (Miltenyi Biotec). The cells were resuspended in PBS containing 5% FBS at concentrations from 0.5–10 × 106 cells per ml. One-ninth volume of diluted CFSE stock (50 μM) was added, and the cell suspension was inverted several times to mix. The cells were incubated for 5 min at room temperature, 10 volumes of PBS containing 5% FBS were added, and the cells were washed at least three times in the same solution. Finally, labeled responder cells were resuspended in medium (RPMI 1640 supplemented with 2 nM l-glutamine, 5 mM Hepes, and 100 U/ml penicillin/mg/ml streptomycin). Candidate T reg cells were prepared either by flow sorting, as described in the next section, or by positive selection on MS columns. These cells were not labeled with CFSE. 90,000 responder cells were added to antibody-coated wells of a 96-well plate alone or together with a variable number of candidate regulatory cells. Plates were placed in the incubator for 5 d before harvesting, staining, and analysis using the LSRII (BD Biosciences) and FlowJo software.
Fluorescence-activated flow sorting.
Four-color sorts were performed using a sorter (FACSVantage) with the FACSDiva option (both from BD Biosciences). Cells were labeled using the anti-CD3, -CD4, -CD25, and -CD127 antibodies described.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded tissues were cut into 5-mm-thick sections and deparaffinized with Histosol, followed by rehydration in graded ethanol. The sections were incubated with hydrogen peroxide (3% in PBS), followed by a protein block (DakoCytomation) for 30 min at room temperature. The sections were incubated overnight at 4°C with a rabbit antiscurfin pAb (1:2000 dilution). After primary incubation, the sections were washed in TBS/0.5% Tween 20 and incubated for 45 min with a mouse anti–rabbit secondary antibody (1:200 dilution; DakoCytomation), followed by washing with TBS/Tween 20 and a 45-min incubation with streptavidin-conjugated horseradish peroxidase (1:200 dilution; DakoCytomation). The sections were developed with diaminobenzidine (DakoCytomation), counterstained with Gil's hematoxylin (Sigma-Aldrich), followed by a final dehydration step, and mounted for analysis.
Cytokine flow cytometry assays.
Lymph node mononuclear cells were prepared by mincing and disassociating tissue, followed by filtration of the cell suspension through a 45-μm mesh. PBMCs were prepared using Ficoll-Hypaque step gradients. Some samples were depleted of CD25+ cells, as described in In vitro suppression assays. 1.2 × 106 cells were placed in control or stimulation wells and resuspended in 120 μl of complete medium (RPMI 1640 containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 μM 3TC). Anti-CD28 (clone 28.2) and anti-CD49d (clone 9F10) were added to all wells at a concentration of 2 μg/ml for each antibody. Various stimuli were added to the experimental wells, and plates were transferred to the incubator. A peptide pool comprising peptides from p27 of SIVmac239 was used at a final concentration of 5 μg/ml for each peptide (NIH AIDS Research & Reference Reagent Program). AT2-inactivated SIVmac239 and control microvesicles were used at a final concentration of 70 ng of protein per milliliter of medium (provided by J. Lifson, National Cancer Institute, Bethesda, MD). 2 h after the addition of stimuli, GolgiPlug (BD Biosciences) was added to each well, according to the manufacturer's instructions. 12 h after the addition of GolgiPlug, samples were harvested by centrifugation and stained using fixable live/dead stain (Aqua; Invitrogen), as well as antibodies reactive to CD3 (SP34-2, Pacific blue), CD4 (19Thy-5D7, Qdot605; NPRR), CD8 (3B5, APC-Alexa Fluor 750), IL-2 (MQ1-17H12, APC; BD Biosciences), IL-4 (MP4-25D2, Alexa Fluor 488; Invitrogen), IL-10 (B-T10, PE; Miltenyi Biotec), IFN-γ (B27, PE-Cy7; BD Biosciences), IL-17 (eBio64DEC17, Alexa Fluor 488; eBioscience), TGF-β1 (TB21, PE; BioTest Diagnostics Corp.), and TNF-α (mAb11, Alexa Fluor 700; BD Biosciences). Live/dead and surface staining for CD4 and CD8 were performed before permeabilization. The samples were then split into two aliquots for intracellular staining in two panels (CD3/IL-2/IL-4/IL-10/IFN-γ and CD3/IFN-γ/TNF-α/IL-17/TGF-β). The cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences), washed in Perm Buffer (BD Biosciences), incubated with the appropriate antibodies in Perm Buffer, washed, and resuspended in PBS containing 2% FBS and 1% paraformaldehyde.
SIV-specific proliferation assays.
Unfractioned and CD25-depleted cell populations were prepared and labeled with CFSE as described in In vitro suppression assays. Stimuli including AT2-inactivated SIVmac239 and 70 ng/ml of control microvesicles were then added. The plates were incubated for 5 d before harvesting and analysis as described in In vitro suppression assays.
Statistical analysis.
Results were analyzed using ANOVA or the Student's t test using the GraphPad Prism and InStat software programs (version 4; GraphPad Software, Inc.).
Acknowledgments
The authors thank Juliet Easlick and Joseph Moore for technical support. We are grateful to Marta L. Marthas, Koen K. A. van Rompay, and Paul Baum for helpful discussions. Samples of SIV-naive infant macaques were provided to K. Abel for analysis through grant U42 RR 16043.
This work was supported in part by NIH awards R37 AI40312 and to J.M. McCune, NIH award F32 AI067088 to D.J. Hartigan-O'Connor, a Pilot Award from the CNPRC to D.J. Hartigan-O'Connor as part of a program supported by NIH award RR00169, and NIH award R21 DE016541 to K. Abel. J.M. McCune is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research and the NIH Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant DPI OD00329.
The authors have no conflicting financial interests.
Abbreviations used: ANOVA, analysis of variance; APC, allophycocyanin; CCR, CC chemokine receptor; CXCR, CXC chemokine receptor; SEB, Staphylococcal enterotoxin B; vRNA, viral RNA.
D.J. Hartigan-O'Connor and K. Abel contributed equally to this work.
References
- 1.Borrow, P., H. Lewicki, B.H. Hahn, G.M. Shaw, and M.B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup, R.A., J.T. Safrit, Y. Cao, C.A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D.D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz, J.E., R.P. Johnson, H.M. McClure, K.H. Manson, M.S. Wyand, M.J. Kuroda, M.A. Lifton, R.S. Khunkhun, K.J. McEvers, J. Gillis, et al. 2005. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J. Virol. 79:8131–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53. [DOI] [PubMed] [Google Scholar]
- 5.Hasenkrug, K.J., and U. Dittmer. 2007. Immune control and prevention of chronic Friend retrovirus infection. Front. Biosci. 12:1544–1551. [DOI] [PubMed] [Google Scholar]
- 6.Rouse, B.T., and S. Suvas. 2004. Regulatory cells and infectious agents: detentes cordiale and contraire. J. Immunol. 173:2211–2215. [DOI] [PubMed] [Google Scholar]
- 7.Betts, M.R., D.R. Ambrozak, D.C. Douek, S. Bonhoeffer, J.M. Brenchley, J.P. Casazza, R.A. Koup, and L.J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts, M.R., M.C. Nason, S.M. West, S.C. De Rosa, S.A. Migueles, J. Abraham, M.M. Lederman, J.M. Benito, P.A. Goepfert, M. Connors, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood. 107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emu, B., E. Sinclair, D. Favre, W.J. Moretto, P. Hsue, R. Hoh, J.N. Martin, D.F. Nixon, J.M. McCune, and S.G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79:14169–14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matloubian, M., R.J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bofill, M., A. Mocroft, M. Lipman, E. Medina, N.J. Borthwick, C.A. Sabin, A. Timms, M. Winter, L. Baptista, M.A. Johnson, et al. 1996. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 10:827–834. [DOI] [PubMed] [Google Scholar]
- 12.Giorgi, J.V., L.E. Hultin, J.A. McKeating, T.D. Johnson, B. Owens, L.P. Jacobson, R. Shih, J. Lewis, D.J. Wiley, J.P. Phair, et al. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870. [DOI] [PubMed] [Google Scholar]
- 13.Liu, Z., L.E. Hultin, W.G. Cumberland, P. Hultin, I. Schmid, J.L. Matud, R. Detels, and J.V. Giorgi. 1996. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry. 26:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Hunt, P.W., J.N. Martin, E. Sinclair, B. Bredt, E. Hagos, H. Lampiris, and S.G. Deeks. 2003. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 15.Eggena, M.P., B. Barugahare, N. Jones, M. Okello, S. Mutalya, C. Kityo, P. Mugyenyi, and H. Cao. 2005. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 174:4407–4414. [DOI] [PubMed] [Google Scholar]
- 16.Cupedo, T., M. Nagasawa, K. Weijer, B. Blom, and H. Spits. 2005. Development and activation of regulatory T cells in the human fetus. Eur. J. Immunol. 35:383–390. [DOI] [PubMed] [Google Scholar]
- 17.Darrasse-Jeze, G., G. Marodon, B.L. Salomon, M. Catala, and D. Klatzmann. 2005. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 105:4715–4721. [DOI] [PubMed] [Google Scholar]
- 18.Michaelsson, J., J.E. Mold, J.M. McCune, and D.F. Nixon. 2006. Regulation of T cell responses in the developing human fetus. J. Immunol. 176:5741–5748. [DOI] [PubMed] [Google Scholar]
- 19.Auger, I., P. Thomas, V. De Gruttola, D. Morse, D. Moore, R. Williams, B. Truman, and C.E. Lawrence. 1988. Incubation periods for paediatric AIDS patients. Nature. 336:575–577. [DOI] [PubMed] [Google Scholar]
- 20.Commenges, D., A. Alioum, P. Lepage, P. Van de Perre, P. Msellati, and F. Dabis. 1992. Estimating the incubation period of paediatric AIDS in Rwanda. AIDS. 6:1515–1520. [DOI] [PubMed] [Google Scholar]
- 21.Rowe, J., C. Macaubas, T. Monger, B.J. Holt, J. Harvey, J.T. Poolman, R. Loh, P.D. Sly, and P.G. Holt. 2001. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic th1 function. J. Infect. Dis. 184:80–88. [DOI] [PubMed] [Google Scholar]
- 22.Vekemans, J., M.O. Ota, E.C. Wang, M. Kidd, L.K. Borysiewicz, H. Whittle, K.P. McAdam, G. Morgan, and A. Marchant. 2002. T cell responses to vaccines in infants: defective IFNgamma production after oral polio vaccination. Clin. Exp. Immunol. 127:495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott, S.L., C. Macaubas, B.J. Holt, T.B. Smallacombe, R. Loh, P.D. Sly, and P.G. Holt. 1998. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J. Immunol. 160:4730–4737. [PubMed] [Google Scholar]
- 24.Marchant, A., and M. Goldman. 2005. T cell-mediated immune responses in human newborns: ready to learn? Clin. Exp. Immunol. 141:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petty, R.E., and D.W. Hunt. 1998. Neonatal dendritic cells. Vaccine. 16:1378–1382. [DOI] [PubMed] [Google Scholar]
- 26.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141–2146. [DOI] [PubMed] [Google Scholar]
- 27.Marodi, L. 2006. Innate cellular immune responses in newborns. Clin. Immunol. 118:137–144. [DOI] [PubMed] [Google Scholar]
- 28.Hartigan-O'Connor, D.J., C. Poon, E. Sinclair, and J.M. McCune. 2007. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J. Immunol. Methods. 319:41–52. [DOI] [PubMed] [Google Scholar]
- 29.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C.J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, C.J., M. Genesca, K. Abel, D. Montefiori, D. Forthal, K. Bost, J. Li, D. Favre, and J.M. McCune. 2007. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J. Virol. 81:5024–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Rompay, K.K., J.L. Greenier, K.S. Cole, P. Earl, B. Moss, J.D. Steckbeck, B. Pahar, T. Rourke, R.C. Montelaro, D.R. Canfield, et al. 2003. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J. Virol. 77:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMaria, M.A., M. Casto, M. O'Connell, R.P. Johnson, and M. Rosenzweig. 2000. Characterization of lymphocyte subsets in rhesus macaques during the first year of life. Eur. J. Haematol. 65:245–257. [DOI] [PubMed] [Google Scholar]
- 33.Abel, K., B. Pahar, K.K. Van Rompay, L. Fritts, C. Sin, K. Schmidt, R. Colon, M. McChesney, and M.L. Marthas. 2006. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J. Virol. 80:6357–6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira, L.E., F. Villinger, N. Onlamoon, P. Bryan, A. Cardona, K. Pattanapanysat, K. Mori, S. Hagen, L. Picker, and A.A. Ansari. 2007. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J. Virol. 81:4445–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 36.Allan, S.E., L. Passerini, R. Bacchetta, N. Crellin, M. Dai, P.C. Orban, S.F. Ziegler, M.G. Roncarolo, and M.K. Levings. 2005. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 115:3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 38.Dittmer, U., H. He, R.J. Messer, S. Schimmer, A.R. Olbrich, C. Ohlen, P.D. Greenberg, I.M. Stromnes, M. Iwashiro, S. Sakaguchi, et al. 2004. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 20:293–303. [DOI] [PubMed] [Google Scholar]
- 39.White, G.P., P.M. Watt, B.J. Holt, and P.G. Holt. 2002. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO− T cells. J. Immunol. 168:2820–2827. [DOI] [PubMed] [Google Scholar]
- 40.Marthas, M.L., K.K. van Rompay, M. Otsyula, C.J. Miller, D.R. Canfield, N.C. Pedersen, and M.B. McChesney. 1995. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J. Virol. 69:4198–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beasley, R.P., L.Y. Hwang, G.C. Lee, C.C. Lan, C.H. Roan, F.Y. Huang, and C.L. Chen. 1983. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 2:1099–1102. [DOI] [PubMed] [Google Scholar]
- 42.Maple, T., J. Erwin, and G. Mitchell. 1973. Age of sexual maturity in laboratory-born pairs of rhesus monkeys (Macaca mulatta). Primates. 14:427–428. [Google Scholar]
- 43.Urdea, M.S., J.C. Wilber, T. Yeghiazarian, J.A. Todd, D.G. Kern, S.J. Fong, D. Besemer, B. Hoo, P.J. Sheridan, R. Kokka, et al. 1993. Direct and quantitative detection of HIV-1 RNA in human plasma with a branched DNA signal amplification assay. AIDS. 7(Suppl. 2):S11–S14. [DOI] [PubMed] [Google Scholar]
- 44.Lu, X., H. Kiyono, D. Lu, S. Kawabata, J. Torten, S. Srinivasan, P.J. Dailey, J.R. McGhee, T. Lehner, and C.J. Miller. 1998. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]