Abstract
Mast cells are protective against snake venom sarafotoxins that belong to the endothelin (ET) peptide family. The molecular mechanism underlying this recently recognized innate defense pathway is unknown, but secretory granule proteases have been invoked. To specifically disrupt a single protease function without affecting expression of other proteases, we have generated a mouse mutant selectively lacking mast cell carboxypeptidase A (Mc-cpa) activity. Using this mutant, we have now identified Mc-cpa as the essential protective mast cell enzyme. Mass spectrometry of peptide substrates after cleavage by normal or mutant mast cells showed that removal of a single amino acid, the C-terminal tryptophan, from ET and sarafotoxin by Mc-cpa is the principle molecular mechanism underlying this very rapid mast cell response. Mast cell proteases can also cleave ET and sarafotoxin internally, but such “nicking” is not protective because intramolecular disulfide bridges maintain peptide function. We conclude that mast cells attack ET and sarafotoxin exactly at the structure required for toxicity, and hence sarafotoxins could not “evade” Mc-cpa's substrate specificity without loss of toxicity.
Mast cells have crucial functions in innate immunity. A protective role of mast cells is evident in mast cell–deficient mice that are unprotected from acute bacterial peritoneal infection and subsequent sepsis (1, 2). Although this protection has largely been attributed to rapid release of mast cell–derived TNF, other mechanisms also contribute. A molecule of critical importance in the pathology of sepsis is endothelin-1 (ET-1; for reviews see references 3–5). ET-1 is a highly potent blood pressure–regulating peptide. Under physiological conditions, ET-1 is produced primarily by endothelial cells, and it is mostly secreted toward the abluminal side of the vessel wall, where it regulates the blood pressure via contraction of smooth muscle cells (for review see reference 6) (7). Cell types other than endothelium, notably monocytes and tissue macrophages (8), cardiomyocytes, tracheal epithelium, and kidney and liver cells, are additional sources of ET-1 (for review see reference 4). During sepsis, ET-1 production is rapidly stimulated by inflammatory mediators, including TNF, IL1β, and TGFß (4, 9). A link between mast cells and ET has been found. Mast cells express ET receptors, and binding of ET-1 to mast cells induces potent degranulation and release of mast cell granule content (10, 11). This release protects normal mice, but not mast cell–deficient mice, from the lethal effects of intraperitoneal injection of ET-1, possibly connecting the mast cell–dependent survival of peritoneal sepsis with ET-1 (11). Direct activation of mast cells by ET-1 is important in this process because ET receptor (ETA) expression on mast cells contributes to protection (11). Because large amounts of ET-1 were applied in these studies, the physiological or pathological relevance for the “mast cell–ET link” is less clear. However, mast cells are also essential for survival of mice challenged with snake venom sarafotoxin 6b (S6b) (12), which is a peptide homologous to ET-1. The function of mast cells in antitoxin protection is thus a novel aspect of innate immunity.
Peritoneal mast cells produce a multitude of granule proteases, which include one carboxypeptidase (mast cell carboxypeptidase A [Mc-cpa; also termed Cpa3]), chymases (mast cell protease 4 [Mcp-4] and Mcp-5), and tryptases (Mcp-6 and -7; for reviews see references 13–15). Although these enzymes were identified many years ago, and studied extensively in vitro, information about their in vivo functions is scarce. A major hurdle to uncover the functions of Mcps has been the difficulty to identify relevant in vivo substrates. This problem can now be addressed in mutant mice for Mc-cpa (16), Mcp-4 (17), and Mcp-5 (18) genes. Resistance of Mcp-4−/− mice to ET-1/S6b excludes an essential protective function of this enzyme (12). Mc-cpa (19), which is a mast cell–specific enzyme related to pancreatic carboxypeptidases, preferentially cleaves C-terminal aromatic amino acids. Mc-cpa is the only mast cell enzyme with carboxypeptidase activity, but mast cells lacking Mc-cpa develop in normal numbers, and are fully competent in degranulation, histamine release, and passive cutaneous anaphylaxis (16).
Mcps are assembled in conjunction with proteoglycans during their synthesis in the secretory pathway (for review see reference 20). Mc-cpa−/− mast cells concomitantly lack Mc-cpa and Mcp-5, despite normal expression of Mcp-5 mRNA (16), and Mc-cpa is absent in Mcp-5−/− mast cells (18). This implies that Mc-cpa−/− and Mcp-5−/− mice are each functionally double-deficient for Mc-cpa and Mcp-5, and limits conclusions about the functional roles of each enzyme.
We sought to identify the mast cell enzyme that is required for degradation of ET-1 and S6b in vivo. To analyze the specific role of Mc-cpa, we generated a novel mouse mutant in which the wild-type Mc-cpa gene (21) was mutated to a Mc-cpa allele (Mc-cpaY356L,E378A) bearing two amino acid exchanges designed to render Mc-cpa catalytically inactive. We show here that homozygous mutants (termed Mc-cpaY356L,E378A mice) are, indeed, selectively deficient for Mc-cpa enzyme activity. Interestingly, loss of this enzyme function renders mice susceptible to ET-1 and S6b. Moreover, by combining the genetic defect of Mc-cpaY356L,E378A mice with mass spectrometric analyses of the substrates, we uncover the molecular basis for this mast cell–mediated defense pathway protecting against ET-related toxins.
RESULTS
Protease expression and function in Mc-cpa−/− and Mc-cpaY356L,E378A mast cells
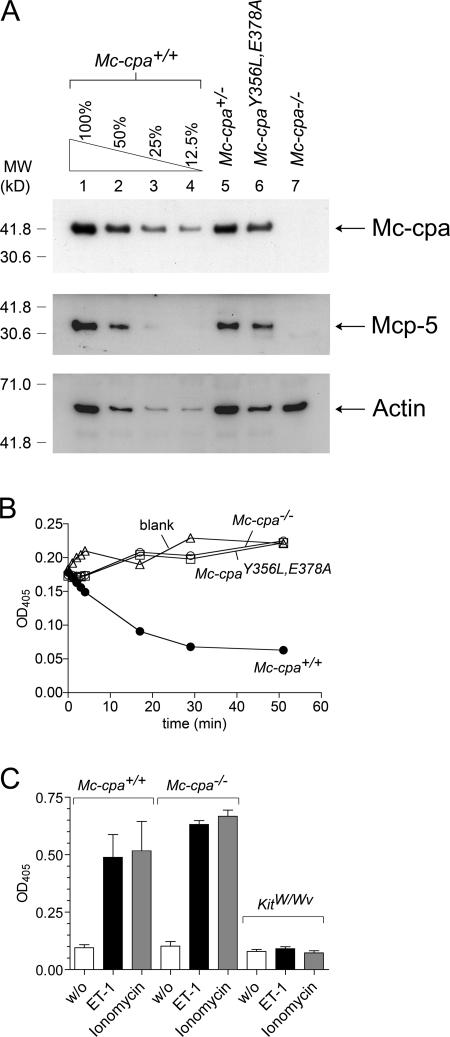
Mc-cpa–null (Mc-cpa−/−) mast cells lack Mc-cpa protein (Fig. 1 A, lane 7) and the corresponding enzyme activity on a carboxypeptidase test substrate (Fig. 1 B).Mc-cpa−/− mast cells are also devoid of Mcp-5 protein (Fig. 1 A, lane 7), although the Mcp-5 gene is functional and Mcp-5 mRNA is expressed at normal levels (16). Other connective tissue Mcps, notably chymase Mcp-4 (22) and tryptase Mcp-6 (15), are expressed in Mc-cpa−/− mast cells (16). It is likely that Mc-cpa and Mcp-5 are copackaged during synthesis and sorting into secretory granules, but the mechanism for the failure to express Mcp-5 in the absence of Mc-cpa (16), or Mc-cpa in the absence of Mcp-5 (18), remains to be determined.
Figure 1.
Protein expression, carboxypeptidase activity, and ET-1–induced degranulation in mutant mast cells. (A) Lysates of purified peritoneal mast cells from Mc-cpa+/+ (lanes 1–4), Mc-cpa+/− (lane 5), Mc-cpaY356L,E378A (lane 6), and Mc-cpa−/− (lane 7) mice were analyzed by Western blotting for Mc-cpa (top), Mcp-5 (middle), and actin (bottom) expression. Mc-cpa+/+ cell lysates were titrated to estimate the level of Mc-cpa expression in Mc-cpaY356L,E378A mice. 100% in lane 1 corresponds to 14,000 purified peritoneal mast cells. Densitometric measurements of actin expression showed 637, 222, 69, and 46 arbitrary units in lanes 1–4, respectively, and 622, 283, and 601 in lanes 5–7, respectively. Measurements for Mc-cpa expression showed 592, 303, 130, and 70 arbitrary units in lanes 1–4, respectively, and 401, 270, and <1 in lanes 5–7, respectively. Based on these values, we estimate that Mc-cpaY356L,E378A mast cells expressed ∼80% of the Mc-cpa amount expressed in normal mast cells. (B) Lysates of 10,000 peritoneal mast cells from Mc-cpa+/+ (•), Mc-cpa−/− (○), and Mc-cpaY356L,E378A (□) mice, and the blank (without cells) control (▵) were analyzed for carboxypeptidase activity by test substrate (45). Both Mc-cpa−/− and Mc-cpaY356L,E378A mast cells lacked Mc-cpa activity. (C) Mast cell degranulation in response to ET-1 in vitro. PECs from Mc-cpa+/+, Mc-cpa−/−, and KitW/Wv mice were left without stimulus (white bars), treated with ET-1 (black bars), or treated with ionomycin (gray bars). Supernatants were analyzed for β-hexosaminidase release as a measure for degranulation by colorimetric assay (16). The release is mast cell specific, as shown by the absence of β-hexosaminidase release in PECs from KitW/Wv mice. Data summarize the mean ± one SD for three (Mc-cpa+/+ and Mc-cpa−/−) and two (KitW/Wv) independent experiments. Release was significantly different comparing unstimulated versus ET-1–stimulated Mc-cpa+/+ (P = 0.046) and unstimulated versus ET-1–stimulated Mc-cpa−/− (P = 0.001) mast cells, indicating specific degranulation of both genotypes by ET-1. In both genotypes, stimulations by ET-1 and ionomycin were not significantly different. P = 0.5 for Mc-cpa+/+ and P = 0.1 for Mc-cpa−/−.
To uncover the functions of Mc-cpa independently of Mcp-5, we generated a second mouse mutant in which Mc-cpa protein expression was permissive, but in this case the enzyme was altered with the aim to ablate its catalytic activity. To this end, we mutated by homologous recombination in embryonic stem (ES) cells the Mc-cpa gene to encode two amino acid changes. Residues Y356 and E378 (counting from the ATG of the Mc-cpa gene), correspond to Y248 and E270 in mature pancreatic carboxypeptidase, which is an enzyme homologous to Mc-cpa (23). In pancreatic carboxypeptidase, these amino acids are crucial for ligand binding and hydrolysis of substrate peptide bonds (24, 25). Therefore, we mutated Y356 to L356 (termed Y356L) and E378 to A378 (termed E378A). After germline transmission, homozygous Mc-cpaY356L,E378A/Y356L,E378A mutants (termed Mc-cpaY356L,E378A mice) were bred. Details of the generation and analyses of this mutant will be reported in future studies.
In contrast to Mc-cpa−/− mast cells (Fig. 1 A, lane 7), Mc-cpaY356L,E378A mast cells (Fig. 1 A, lane 6) expressed Mc-cpa and Mcp-5 protein. Titrated Western blots showed that Mc-cpaY356L,E378A mast cells expressed ∼80% of the amount of Mc-cpa protein compared with wild-type mast cells (Fig. 1 A). To exclude a dose effect of reduced Mc-cpa expression in Mc-cpaY356L,E378A mast cells, we compared, where appropriate, heterozygous (Mc-cpa +/−) mice to Mc-cpaY356L,E378A mice. To test whether the introduced mutations ablated catalytic activity, enzyme function of Mc-cpaY356L,E378A mast cells was analyzed in vitro (Fig. 1 B). Titration experiments showed that Mc-cpa enzyme activity was detectable in as few as 1,000 Mc-cpa+/+ mast cells, but undetectable in 20,000 Mc-cpaY356L,E378A mast cells (not depicted), the highest cell number tested, and equivalent to approximately one half of the total mast cell number present in one peritoneal cavity. Hence, Mc-cpaY356L,E378A mast cells expressed Mc-cpa protein lacking enzyme activity, and this was sufficient for Mcp-5 expression.
Mast cells express ET-1 receptors on their surface, and binding of ET-1 to ET-1 receptors is a potent stimulus for release of secretory granules (10, 11). Using RT-PCR, we compared the expression of two ET-1 receptors, the ETA and ETB, in purified peritoneal mast cells. ETA is the receptor type that is predominantly, if not exclusively, expressed on peritoneal mast cells (unpublished data), which is consistent with the requirement for ETA expression for ET-1–induced degranulation of peritoneal mast cells (11). Next, ET-1–induced degranulation, which is measured by release of β-hexosaminidase, was analyzed in mast cell–containing peritoneal exudate cells (PECs). With the exception of mast cell–deficient KitW/Wv mice (26), numbers of peritoneal mast cells were comparable in all genotypes analyzed (unpublished data). Relative to maximal signaling by ionomycin, ET-1 stimulated strong degranulation in mast cell–containing PECs from Mc-cpa +/+ and Mc-cpa −/−, but not in mast cell–deficient PECs from KitW/Wv mice (Fig. 1 C). Hence, ET-1 specifically stimulated mast cells for degranulation among PECs, and loss of Mc-cpa and Mcp-5 did not impair ET-1–stimulated granule release in mast cells.
Susceptibility of Mcp mouse mutants to ET-1
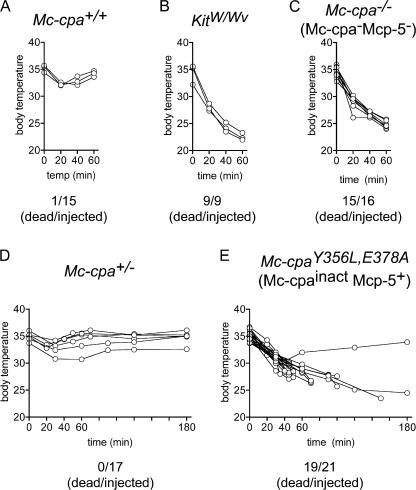
ET-1, which is the strongest endogenous blood pressure–regulating peptide, has been implicated in many pathological processes, including shock and sepsis (for reviews see references 6, 7). In an in vivo model of the pathological effects of ET-1, intraperitoneal injection of 3 μg ET-1 per mouse caused a rapid drop in body temperature and the death of two thirds of KitW/Wv, but not wild-type, mice (11). In our hands, injection of 7.5 μg ET-1 into mast cell–positive Kit +/+ mice (designated as Mc-cpa +/+ mice in Fig. 2 A) showed a transient temperature drop, and 14/15 mice fully recovered (Fig. 2 A). In contrast, the same dose caused a massive temperature drop within 20 min, and the death of 9/9 KitW/Wv mice by 60 min (Fig. 2 B). Hence, this dose was used for all subsequent experiments.
Figure 2.
Susceptibility of Mc-cpa−/− and Mc-cpaY356L,E378A mutant mice to ET-1. ET-1 was injected intraperitoneally into Mc-cpa+/+ (A), KitW/Wv (B), Mc-cpa−/− (C), Mc-cpa+/− (D), and Mc-cpaY356L,E378A (E) mice, and the body temperature was tracked by rectal measurements at the indicated time points. Summarized total numbers of dead per injected mice are indicated for each genotype. For clarity, the temperature kinetic is shown for representative mice. Mc-cpa+/+ and Mc-cpa+/− showed a mild and transient temperature drop (A and D), whereas mast cell–deficient KitW/Wv (B), and mice lacking Mc-cpa and Mcp-5 (Mc-cpa−/−; C) succumbed to ET-1 injection within 60 min. Mice lacking only catalytically active Mc-cpa (Mc-cpaY356L,E378A; E) were also largely susceptible to ET-1, but the kinetic of the temperature drop was delayed compared with KitW/Wv or Mc-cpa−/− mice.
To analyze whether Mc-cpa and/or Mcp-5 are involved in protection against ET-1, Mc-cpa−/− mice were challenged by ET-1 (Fig. 2 C). Similar to mast cell–deficient mice (Fig. 2 B), 15/16 Mc-cpa−/− mice succumbed to ET-1 injection. Although experiments using KitW/Wv mice underpin the requirement for mast cells for protection (11), analyses of Mc-cpa−/− mice, which have normal numbers of mast cells (16), identify Mc-cpa and/or Mcp-5 as essential components of this defense pathway. Next, we determined by injection of ET-1 into Mc-cpaY356L,E378A mice whether Mc-cpa enzyme activity was required for protection against ET-1–induced lethality. Mc-cpa +/+ (not depicted) and heterozygous (Mc-cpa +/−) mice were injected in parallel (Fig. 2 D). Mc-cpa +/− controls were included because Mc-cpaY356L,E378A mice expressed <100% of the Mc-cpa found in normal mast cells (Fig. 1 A). Mc-cpa +/− mice were protected, which implies that a reduction in protease expression does not impair the defense against the applied dose of ET-1 (Fig. 2 D). Interestingly, Mc-cpaY356L,E378A mice were largely susceptible to ET-1 (Fig. 2 E). However, when compared with Mc-cpa −/− mice (Fig. 2 C), the kinetic of the temperature drop was delayed, i.e., 8/21 mice survived beyond 60 min, and another 2/21 survived indefinitely. Nevertheless, Mc-cpaY356L,E378A mice were clearly susceptible to ET-1, which demonstrates a nonredundant role for Mc-cpa, but not Mcp-5, in resistance to ET-1.
Mast cell–mediated degradation of ET-1
To address a mechanistic link between mast cells and survival of the mice in response to exogenously applied ET-1, peritoneal mast cells were incubated with ET-1 in vitro. Initially, we measured ET-1 degradation by an ET-1–specific ELISA. This assay has previously been reported to measure mast cell–mediated degradation of ET-1, and to reveal the mechanism behind how mast cells limit ET-1 toxicity (11). ET-1 was incubated for 60 min with PECs from Mc-cpa +/+, Mc-cpa −/−, or KitW/Wv mice (Fig. 3 A). Compared with input, the amount of ET-1 after incubation with Mc-cpa +/+ PEC was reduced by >90%. Surprisingly, a similarly strong reduction in ELISA-reactive ET-1 was found after incubation with Mc-cpa −/− PECs. Degradation of ET-1 was mostly mast cell–mediated, as shown by the inability of KitW/Wv PECs, which lack mast cells, to substantially reduce the amount of ET-1. These data suggested that not only normal mast cells but also mast cells lacking Mc-cpa and Mcp-5 proteins could degrade ET-1, a notion that is not easily reconciled with the observation that Mc-cpa +/+ mice were protected (Fig. 2 A), whereas Mc-cpa −/− mice were fully susceptible (Fig. 2 C) to ET-1. This discrepancy implied that the loss of ELISA-reactivity did not indicate whether or not ET-1 remained biologically active.
Figure 3.
Degradation of ET-1 by mast cells. (A) ELISA measurements of “residual” ET-1 after incubation of ET-1 with no cells (open bar), or with PECs from Mc-cpa+/+ (solid bar), Mc-cpa−/− (striped bar), or KitW/Wv (shaded bar) mice. Mast cell–containing PECs from Mc-cpa+/+ (P = 0.030 [significant] comparing Mc-cpa+/+ versus KitW/Wv mice), and Mc-cpa−/− (P = 0.037 [significant] comparing Mc-cpa−/− versus KitW/Wv mice) mice abolished ET-1 reactivity by ELISA, suggesting that ET-1 was degraded regardless (P = 0.78 [not significant] comparing Mc-cpa+/+ vs. Mc-cpa−/−) of the mast cell genotype. Data summarize the mean ± one standard deviation for three independent experiments. (B–F) Mass spectrometric analysis of ET-1 degradation (left), and schematic depiction of substrate products (right). ET-1 was left untreated (B) or incubated with ionomycin-stimulated purified peritoneal mast cells from Mc-cpa+/+ (C), Mc-cpa−/− (D), or Mc-cpaY356L,E378A (E and F) mice. C-terminal degradation, evident by the appearance of ET-1 fragments corresponding to the molecular masses of 1–19 and 1–20, was mediated by Mc-cpa+/+ (C), but not by Mc-cpa−/− (D) or Mc-cpaY356L,E378A (E), mast cells. However, after incubation of ET-1 with Mc-cpa−/− (D) or Mc-cpaY356L,E378A (E) mast cells, the molecular mass increased from 2,492 (ET-1; B) to 2,510 daltons (ET-1 plus water; D and E). Hydrolysis of ET-1 was demonstrated after reduction of the samples by the disappearance of the ET-1 1–21 peak, and the appearance of new fragments with molecular masses of 1,639 and 1,786 daltons (see Materials and methods) corresponding to ET-1 1–13 and 1–14, respectively (F). Data are representative of three independent experiments.
It was, therefore, of interest to obtain more direct data on mast cell–mediated ET-1 degradation to correlate specific degradation products with survival. To this end, ET-1 was incubated with mast cells of different genotypes, and the products were analyzed by mass spectrometry (Fig. 3, B-F). Purified peritoneal mast cells from Mc-cpa +/+, Mc-cpa −/−, or Mc-cpaY356L,E378A mice were incubated in the presence of ET-1 together with ionomycin for maximal stimulation. The resulting ET-1 fragments were measured by mass spectrometry. Synthetic peptides corresponding to ET-1 (1–21; Fig. 3 B), or peptides lacking one (1–20) or two (1–19) amino acids at the C terminus (not depicted) were measured separately, as standards.
In the presence of Mc-cpa +/+ mast cells, most ET-1 (1–21) was converted into shorter fragments with masses of 1–20 and 1–19 (Fig. 3 C). C-terminal degradation products were not generated by Mc-cpa −/− mast cells (Fig. 3 D). Thus, mast cells can degrade ET-1 at the C terminus, and this degradation requires expression of Mc-cpa or Mcp-5. To directly analyze the role of Mc-cpa in this process, ET-1 was incubated with Mc-cpaY356L,E378A mast cells (Fig. 3 E). Interestingly, ET-1 (1–21) was also maintained in the presence of these mutant mast cells lacking only catalytically active Mc-cpa. These experiments unequivocally identify Mc-cpa as the nonredundant enzyme required for removal of C-terminal amino acids from ET-1.
ET-1 can also be cleaved at multiple positions by endopeptidases (27). Along this line, Mcps other than Mc-cpa, notably chymases (11, 28), were reported to contribute to ET-1 degradation, and cleavage by chymases has, in fact, been proposed as the protective mechanism (11) (for review see reference 29). Mast cells from Mc-cpa −/− and Mc-cpaY356L,E378A mice could not degrade the C terminus of ET-1, but we noticed a shift in the molecular mass of ET-1 from 2,492 (ET-1) to 2,510 daltons (Fig. 3, D and E). This increase in molecular weight suggested addition of a water molecule (ET-1 plus one H2O = 2,510 daltons), which pointed at hydrolysis of one peptide bond within the ET-1 molecule. ET-1 harbors two intramolecular disulfide bridges linking the cysteine in position 1 with that in position 15, and the cysteines in positions 3 and 11 (27). In the absence of C-terminal degradation, the overall maintained length of the peptide could mask internal cleavage of ET-1 between the disulfide bridges by a mast cell–derived endopeptidase. To address this possibility, ET-1 was incubated with Mc-cpaY356L,E378A mast cells, and half of the supernatant was kept under nonreducing conditions in which ET-1 remained full length (Fig. 3 E). The second half was reduced to open the disulfide bridges. Under reducing conditions, ET-1 (1–21) disappeared, and shorter peptide fragments became apparent (Fig. 3 F), which is consistent with an ET-1 cleavage at positions 13 and 14, i.e., within the two cysteines in positions 11 and 15. Thus, mast cell products attack ET-1 internally, causing a nick in the peptide that could only be revealed under reducing conditions. This internal nicking was not mediated by Mcp-5 because the same pattern of degradation products was generated by Mc-cpa−/− mast cells lacking both Mc-cpa and Mcp-5.
These data strongly suggested that the Mc-cpa–catalyzed removal of a single amino acid (tryptophan) from the C terminus of ET-1 was necessary and sufficient for the mast cell–mediated inactivation of ET-1. To prove that C-terminal degradation of ET-1 was sufficient for protection, synthetic peptides corresponding to full-length ET-1 (1-21) or C-terminally shorter peptides were injected into mast cell–deficient KitW/Wv mice (Fig. 4). Of these, only full-length ET-1 (Fig. 4 A), but neither the 1–20 (Fig. 4 B), nor the 1–19 (not depicted) amino acid long peptides induced a temperature drop or lethality in KitW/Wv mice.
Figure 4.
Removal of tryptophan from the C terminus renders ET-1 nontoxic for mast cell–deficient mice. Synthetic peptides of ET-1 (1–21; A) or of ET-1 minus tryptophan in position 21 (1–20; B) were injected intraperitoneally into mast cell–deficient KitW/Wv mice, and the body temperature was followed by rectal measurements at indicated time points. Mast cell–deficient KitW/Wv mice were resistant to ET-1 (1–20), but succumbed to ET-1 (1–21).
Collectively, Mc-cpa +/+, but not Mc-cpaY356L,E378A, mast cells degrade the C terminus of ET-1, and this Mc-cpa–mediated C-terminal shortening is highly protective because ET-1 (1–20) was not toxic in vivo (Fig. 4 B). Moreover, Mcps other than Mc-cpa and Mcp-5 can nick internal peptide bonds between cysteines 11 and 15 in ET-1. Apparently, the ELISA detects a conformation-dependent epitope that is missing in nicked ET-1. In contrast, in vivo experiments demonstrated that such heterodimers of internally cleaved, but “full-length” ET-1 remained lethal in Mc-cpa−/− and Mc-cpaY356L,E378A mice (Fig. 2).
Catalytic activity of Mc-cpa is essential to protect against snake venom sarafotoxin
Snake venom sarafotoxins share significant amino acid sequence similarity with endogenous ET-1 (27, 30). This sequence homology appears to be immunologically relevant because mast cells can enhance resistance not only to ET-1 but also to snake venom sarafotoxins (12). The molecular mechanism underlying the mast cell–mediated degradation of sarafotoxins has not been fully uncovered. Given the similarities in sequence and structure involving two disulfide bridges, it appears likely that the mechanisms of ET and sarafotoxin degradation are similar, if not identical. However, the specific role of Mc-cpa could not easily be addressed up to now because ablation of Mc-cpa expression by gene targeting, as in Mc-cpa −/− mice (16), or by RNA interference (RNAi), as in Mc-cpa +/+ BM-derived mast cells transplanted into mast cell–deficient mice (12), inevitably also lead to abrogation of Mcp-5 expression (see Introduction and Fig. 1 A) (12, 16).
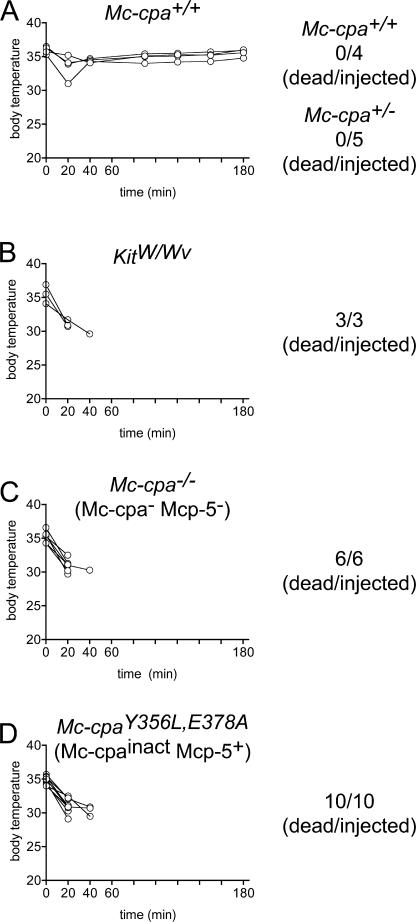
To address whether Mc-cpa or Mcp-5 are involved in the resistance against S6b, which is the most toxic representative of this group of snake venom peptides, the different Mc-cpa mouse genotypes (Mc-cpa +/+, Mc-cpa +/−, Mc-cpa −/−, and Mc-cpaY356L,E378A), as well as KitW/Wv mice, were injected with 7.7 μg S6b (Fig. 5).Mc-cpa +/+ and Mc-cpa +/− mice were protected (Fig. 5 A), but mast cell–deficient KitW/Wv mice were not (Fig. 5 B), thereby confirming a crucial role of mast cells in the protection against S6b in vivo (12). Mice lacking Mc-cpa and Mcp-5 (Mc-cpa −/−) showed a rapid temperature drop similar to KitW/Wv, and all Mc-cpa −/− mice succumbed to S6b injection (Fig. 5 C). Interestingly, Mc-cpaY356L,E378A mice were also fully susceptible to S6b (Fig. 5 D). In contrast to the delayed ET-1–induced lethality of Mc-cpaY356L,E378A mice when compared with KitW/Wv or to Mc-cpa −/− mice (Fig. 2), Mc-cpaY356L,E378A mice were equally vulnerable to S6b as KitW/Wv or Mc-cpa −/− mice (Fig. 5). These experiments establish an essential role for Mc-cpa in the mast cell defense against S6b.
Figure 5.
Susceptibility of Mc-cpa mutants to S6b. The S6b was injected intraperitoneally into Mc-cpa+/+ (A), Mc-cpa+/− (only numbers shown in A), KitW/Wv (B), Mc-cpa−/− (C), and Mc-cpaY356L,E378A (D) mice, and the body temperature was followed by rectal measurements at the indicated time points. Summarized total numbers of dead per injected mice are indicated for each genotype. The temperature kinetic of Mc-cpa+/− mice (not depicted) was similar to the one from Mc-cpa+/+ mice. All strains except for Mc-cpa+/+ and Mc-cpa+/− mice succumbed to S6b injection within 60 min. The full susceptibility of Mc-cpaY356L,E378A mice demonstrates the essential role of Mc-cpa enzyme activity for survival of snake venom S6b.
Mast cell–mediated, C-terminal degradation of S6b
S6b degradation products that were generated by incubation of S6b with ionomycin-stimulated peritoneal mast cells from Mc-cpa +/+, Mc-cpa −/−, or Mc-cpaY356L,E378A mice were analyzed by mass spectrometry (Fig. 6). We observed C-terminal shortening of S6b (Fig. 6 A) by Mc-cpa +/+ mast cells (Fig. 6 B). Consistent with the susceptibility of Mc-cpa −/− and Mc-cpaY356L,E378A mice to S6b (Fig. 5), mast cells from Mc-cpa−/− (not depicted) and Mc-cpaY356L,E378A (Fig. 6 C) mice failed to degrade S6b at the C terminus. Hence, Mc-cpa activity is not only essential for C-terminal degradation of ET-1 but also of S6b.
Figure 6.
Degradation of S6b by mast cells. (A–D) Mass spectrometric analyses (left side) of degradation products of S6b. Resulting peptides are schematically depicted on the right side. S6b was left untreated (A) or incubated with ionomycin-stimulated purified mast cells from Mc-cpa+/+ (B) or Mc-cpaY356L,E378A (C and D) mice. C-terminal degradation, which is evident from the appearance of fragments corresponding to the molecular mass of S6b (1–19; 2,266 daltons), was mediated by Mc-cpa+/+ (B), but not by Mc-cpaY356L,E378A (C) mast cells. The peak marked by the asterisk in B does not correspond to a fragment of S6b, and it was not observed in a second experiment. The molecular mass of S6b (1–21) increased after incubation with Mc-cpaY356L,E378A mast cells from 2,565 (1–21; A) to 2,583 daltons (S6b 1–21 plus water; C). This indicated hydrolysis of S6b, which was proven after reduction of the samples by the disappearance of the S6b 1–21 peak, and the appearance of residual fragments with molecular masses of 1,710 and 1,858 daltons (see Materials and methods) corresponding to S6b 1–13 and 1–14, respectively (D). Data are representative for two independent experiments.
The sequences of ET-1 and S6b are identical in 14/21 amino acids, and S6b, like ET-1, contains two disulfide bridges. The homology is even higher between positions 8 and 21 (identity in 11/14 amino acids). In ET-1, the degradation products were consistent with endopeptidase cleavage at position 13 or 14. Tyrosine (13) and phenylalanine (14) are conserved in ET-1 and S6b. To determine whether mast cell products also cleaved S6b internally, S6b was incubated with Mc-cpaY356L,E378A mast cells, and the samples were analyzed with and without reduction by mass spectrometry. Without reduction, addition of a water molecule, which is indicative of hydrolysis of as single-peptide bond, was revealed by the corresponding mass increase (Fig. 6 C). After reduction, S6b (1–21) disappeared, and reaction products consistent with the masses of 1–13 and 1–14 fragments of S6b (Fig. 6 D) pointed at an identical endopeptidase digestion pattern comparing ET-1 and S6b. The identical pattern of reduced S6b reaction products occurred when Mc-cpa−/− mast cells were incubated with S6b (not depicted). Thus, as for ET-1, the internal cleavage was not mediated by Mcp-5, and internally nicked, C-terminally intact S6b remained lethal.
DISCUSSION
In contrast to the notoriously harmful role of mast cells in allergic disease, new mast cell functions in immunity are emerging. Mast cell properties such as protection against the lethal toxicity of snake venom sarafotoxins raise questions as to which mast cell effector molecules mediate these innate immune responses, and by what mechanism. Mcps are good candidates for relevant effector molecules because they represent major protein components in mast cell granules that are secreted during signaling-dependent degranulation. However, Mcps have been commonly viewed as tissue damaging (31). In contrast, the high degree of evolutionary conservation of Mcps in various species, as well as the strong expression of proteases, suggests positive functions. Impaired worm expulsion in mice lacking a mucosal Mcp, Mcp-1 (32), is an example of a protease contributing to protection.
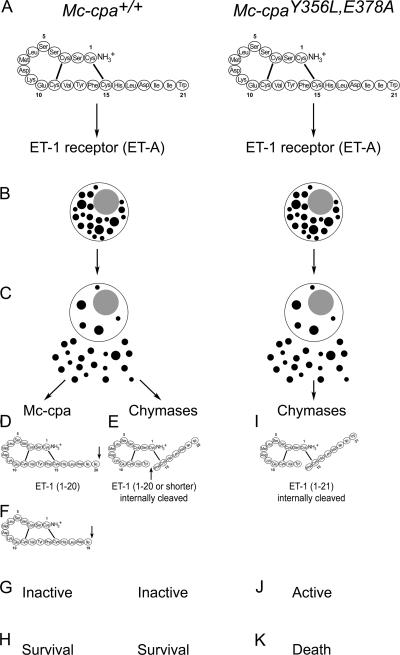
Recent reports demonstrated that mast cells must be present in the peritoneal cavity for mice to survive a challenge with the blood pressure regulating peptide ET-1 (11), and the related S6b (12). Protection against these peptides is initiated by binding of ET or sarafotoxin to ET receptors that induce rapid and potent degranulation of peritoneal mast cells (Fig. 7, A-C) (10, 11). ETA is the major ET receptor expressed on peritoneal mast cells (not depicted) (11), and expression of ETA on mast cells is important, as ETA-deficient mast cells are nonprotective (11).
Figure 7.
Molecular mechanism of ET family peptide degradation by Mc-cpa. (A–C) ET-1 and S6b, shown here for ET-1, stimulate mast cells via binding to ET receptors. The receptor type most prominently expressed on peritoneal mast cells is ETA. ETA activation leads to massive degranulation, which is comparable to stimulation via ionomycin, and release of mast cell granule content. These early events are identical in mast cells from Mc-cpa+/+ (A–C; left) and Mc-cpaY356L,E378A (A–C; right) mice. (D–H) Mast cells secrete a set of proteases that include one carboxypeptidase A (Mc-cpa) and several chymases that include Mcp-5. Mc-cpa attacks the C terminus of ETs, and removes amino acids in position 21 (Trp; D) and 20 (Ile; F). These modifications render ETs and related toxins biologically inactive by three orders of magnitude (G; 34), and the mice survive (H). Mc-cpaY356L,E378A mice, which selectively lack active Mc-cpa, are unable to attack the C terminus of ET-1 (I). Mast cell products (possibly Mcp-4) other than Mc-cpa and Mcp-5 attack ET-1 internally by hydrolysis (E and I). Although the molecular structure (46) of position 13–nicked ET-1 is likely altered, ET-1 fragmentation is prevented by the disulfide bridge (1 to 15; E and I), and the molecules retain their toxicity (J and K), unless the C terminus is truncated (E). Mc-cpaY356L,E378A mutant mice are unable to do so, and succumb to exogenous ET-1 and related toxins, thereby demonstrating the essential role of Mc-cpa for this innate immune pathway. Whether or not Mc-cpa is also involved in the degradation of endogenously produced ETs remains to be investigated.
Attempts have been made to identify the relevant effector molecules. Because mast cell stimulation by ET-1 causes degranulation, but also the production of cytokines such as TNF-α and IL-6 (10), a large number of well-known factors could be considered. Because ET-1 and S6b are peptides, the underlying mechanism could be Mcp-mediated proteolysis that abrogates the lethal action of ET-1 and S6b in vivo (33). Earlier in vitro experiments showed that mast cell enzymes can degrade ET-1 (28). Maurer et al. (11) used pharmacological protease-inhibitors to characterize the ET-1–degrading Mcp, and concluded that a mast cell chymase, but not Mc-cpa, was responsible for ET-1 inactivation in the peritoneal cavity (29). In contrast to these pharmacological blocking studies, a role for Mc-cpa in ET-1 and also S6b degradation in vivo was suggested in more recent experiments by the same group using RNA interference (RNAi) (12). It must be pointed out that the latter experiment led to a reduction of Mc-cpa expression in mast cells to ∼20% of the wild-type level, but also, as expected from our previous knockout experiments (16), to the concomitant loss of Mcp-5 expression (12). This double-deficiency of Mc-cpa and Mcp-5 in Mc-cpa −/−, but also in Mc-cpa RNAi knockdown experiments, precluded a definitive identification of the responsible Mcp up to now.
To address the exclusive function of Mc-cpa, we generated a mouse homozygous for a mutated Mc-cpa allele. Based on data from previous structure–function experiments that addressed the catalytic mechanism in pancreatic Cpa (24, 25), we mutated Y356 to L356 and E378 to A378. The observation that Mc-cpaY356L,E378A mast cells expressed Mc-cpa protein that lacked detectable carboxypeptidase activity (Fig. 1) showed that pancreatic and mast cell carboxypeptidases are not only homologous in their amino acid sequences but that homologous amino acids are also functionally conserved. One important aspect of Mc-cpaY356L,E378A mast cells was the stoichiometrically normal expression of Mcp-5 (Fig. 1), which demonstrates a structural, rather than an enzymatic, Mc-cpa requirement for Mcp-5 expression. This, in turn, was a prerequisite to specifically evaluate the function of Mc-cpa.
Previous experiments addressing the mast cell–mediated degradation of ET-1 led to the conclusions that chymases and Mc-cpa (28), or chymases but not Mc-cpa (11), or Mc-cpa but not chymases (12) degrade ET-1. Challenging Mc-cpaY356L,E378A mice by ET-1 and S6b now revealed directly that the enzyme activity of Mc-cpa is essential for protection against ET-1 (Fig. 2) and S6b (Fig. 5). Identification of Mc-cpa as the functionally relevant enzyme also helped to clarify which proteolytic mechanism is protective. Combining the genetic defects of Mc-cpa −/− or Mc-cpaY356L,E378A mast cells with mass spectrometric analyses of substrates showed two sites of attack. The first is C-terminal removal of one (Fig. 7 D) or two (Fig. 7 F), or more (28) amino acids. This step is exclusively catalyzed by Mc-cpa as shown by intact C termini after contact with Mc-cpaY356L,E378A mast cells (Fig. 7 I). That this is the protective reaction was further supported by the lack of toxicity of synthetic ET-1 (1–20) in mast cell–deficient mice (Fig. 4). The second site of attack is internal cleavage at positions 13 or 14 (Fig. 7 E). This could be conclusively detected in the absence of C-terminal degradation, i.e., in the presence of mutant mast cells lacking Mc-cpa protein (Mc-cpa −/−; not depicted) or activity (Mc-cpaY356L,E378A; Figs. 3 F and 6 D) under reducing conditions. Because hydrolysis was seen using Mc-cpa −/− mast cells (Fig. 3 D), the reaction is not catalyzed by Mcp-5. The consequence of internal attack on ET-1 by mast cells remains elusive because only C-terminal degradation correlated with abrogation of in vivo toxicity. It remains to be determined whether internal cleavage has absolutely no effect on the biological activity of ET-1 in vivo.
The in vivo toxicity of mast cell–mediated degradation products of ET-1 is in agreement with previous structure–function analyses of ETs. ETs nicked by endopeptidase maintains toxicity (27), whereas the two C-terminal residues (Ile and Trp) are important for receptor binding and biological activity of ETs (34, 35). A note of caution should be made regarding ELISA measurements of ET-1 degradation. This assay, used before to correlate in vitro degradation with in vivo toxicity (11), was, in our hands, misleading because it indicated degradation even by mast cells that could not protect mice in vivo (Fig. 2 versus Fig. 3). It is possible that immunoreactivity in this assay was abrogated by the internal nicks, or by conformational changes. In any case, it may be prudent to consider the biological activity of ET-1 when further studying the mast cell–mediated degradation of ET-1 in the future.
Collectively, we have shown that Mc-cpa is an essential effector molecule within the “mast cell–ET link” providing a very rapid (minutes) and life-saving response of toxin neutralization in vivo. Several sites on ET-1 are attacked by mast cells but only the C-terminal degradation is a safeguard. It is remarkable that the protective Mcp, Mc-cpa, “targets” exactly that amino acid in snake venom sarafotoxin that is essential for toxicity. This substrate specificity of Mc-cpa, as well as the fast circuit composed of binding of sarafotoxin to ET receptors, expressed on the cell surface of mast cells, followed by the rapid degranulation, release of the protective enzyme, and cleavage of the toxin, may represent an evolutionarily well conserved, and hence old, function of mast cells. ET-1 levels in plasma, liver, and peritoneal cavity rapidly rise during experimental sepsis, such as in cecal ligation and puncture models (36–38). Moreover, in human sepsis patients, high levels of ET-1 are correlated with morbidity and mortality (4, 39). ET-1 is thought to be involved in the pathological manifestations of sepsis in many organs, including heart, lung, liver, kidney, and intestines (4, 9). Massive local or systemic ET-1–mediated vasoconstriction (40) is a major pathological mechanism in sepsis as shown by beneficial effects of ET receptor antagonist treatment in models of sepsis (41). The observation that mast cells are closely located, among other sites, to blood vessels, and present in the peritoneal cavity, suggests that mast cells are exposed systemically or locally to changes in ET-1 levels (42). Mast cells are not only activated by ET-1 via ETA (10) but also have a potent mechanism to inactivate ET-1 via secretion of Mc-cpa. Hence, mast cells may play an important role in either promoting an inflammatory cascade in response to ETA stimulation by releasing mediators such as TNF or in dampening the deleterious pathophysiological effects of ET-1 on the vascular system by Mc-cpa–mediated rapid cleavage of ET-1. It remains to be determined under which conditions of local or systemic inflammation the effect of ET-1 on mast cells and the reciprocal inactivation of ET-1 by mast cells prevail.
MATERIALS AND METHODS
Mice and gene targeting.
WBKitW/+, C57BL/6KitWv/+ mice (Japan-SLC) and WBB6F1KitW/Wv mice were bred as previously reported (43). Mc-cpa−/− mice (16) were backcrossed for 12 generations to the C57BL/6 strain, and maintained on this background. Mc-cpaY356L,E378A mice were generated by gene targeting (“knock-in”) in E14.1 ES cells (129/OlaHsd). The targeting construct was assembled in a pBSK-based vector bearing a loxP-flanked neomycin (Neo) resistance gene and the HSV thymidine kinase gene (TK; provided by H. Luche and H.J. Fehling, University of Ulm, Ulm, Germany). The NotI-linearized vector consisted, from 5′ to 3′, of a 1.6-kb homologous “short arm” (nucleotides 24,438 to 26,059 of the Mc-cpa gene, according to Ensembl gene number ENSMUSG00000001865), the Neo gene under the TK promotor in the opposite orientation to the Mc-cpa locus, a 5.6-kb homologous “long arm” (nucleotide 26,077 to +5,141 of Mc-cpa gene) and the TK gene in the orientation of the Mc-cpa locus. The long arm harbored exon 11, which was mutated from tac→ttg (nucleotide 1,072–1,074, according to National Center for Biotechnology Information Entrez mRNA number NM_007753), resulting in a substitution of tyrosine 356 to leucine, and from gag→gcg (nucleotide 1138–1140 [NM_007753]), resulting in a substitution of glutamic acid 378 to alanine. PCR-based mutagenesis was done as previously described (44). Gene targeting led to insertion of the Neo gene into intron 10. Gene targeting, ES cell selection, Neo gene excision in ES cells, and the generation of chimeric mice were done as previously described (16). All animal experiments were performed in accordance with institutional and government regulations and were approved by Regierungspräsidium Tübingen, Germany.
Western blots.
Protease expression was analyzed as previously described (16). Briefly, proteins were separated by 11.5% SDS-PAGE, followed by blotting onto PVDF membranes (Millipore). Rat anti–mouse antisera specific for Mc-cpa and Mcp-5 (provided by Lars Hellman, Uppsala University, Sweden) were used at 1:200 dilutions. Blots were developed by anti–rat–horseradish peroxidase (1:100,000 dilution; GE Healthcare) as the second step, followed by ECL substrate (Pierce Chemical Co.). Actin expression was analyzed using monoclonal mouse anti–β-actin (clone AC-15; 1:3,000 dilution; Sigma-Aldrich), followed by polyclonal rabbit anti–mouse– horseradish peroxidase (1:1,000 dilution; Dako Cytomation).
Mc-cpa enzyme assay.
Mast cells were lysed in PBS, 2 M NaCl, and 1% Triton X-100 for 15 min on ice. The lysate volume corresponding to 10,000 cells/well was adjusted to 60 μl with lysis buffer and mixed with 40 μl water and 20 μl chromogenic substrate (1.8 mM; N-[4-methoxyphenylazoformyl]-Phe-OH; Bachem). Substrate degradation was measured as the change over time in absorbance at OD405 with a SpectraMax 250 (MWG Biotech) spectrophotometer.
Mast cell degranulation assay.
PECs were resuspended in 120 μl Tyrode's buffer (10 mM Hepes, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA, pH 7.4) and stimulated with either 2 μM ionomycin or 1.25 μg ET-1 for 15 min at 37°C. The cells were pelleted at 500 g for 3 min before collection of the supernatants. To measure β-hexosaminidase release, 50 μl of the supernatant was mixed with an equal volume of substrate (p-nitrophenyl-N-acetyl-β-d-glucosaminide). A 25-mM stock of p-nitrophenyl-N-acetyl-β-d-glucosaminide in DMSO was diluted 1:25 in 0.1 M citrate buffer, pH 4.5, before use. After 90 min of incubation at 37°C, the reaction was stopped by addition of 125 μl of 1 M Tris, and the substrate conversion was measured photometrically at OD405. Data were analyzed by Student's t test.
In vivo toxicity.
Mice were injected intraperitoneally with ET-1 (7.5 μg in 300 μl/mouse; 1–21), ET-1 (6.9 μg in 300 μl/mouse; 1–20), or S6b (7.7 μg in 300 μl/mouse). To verify proper intraperitoneal injection, the injected fluid contained 0.1 μg/ml Evans blue. At the end of the experiment, the peritoneal cavity was opened in each mouse to identify free blue peritoneal fluid as an indicator of successful injection. Mice lacking this fluid were excluded from the analysis. Changes in body temperature were followed by periodic measurements of rectal temperature using a Qtemp 200 (VWR International) thermometer.
Mass spectrometry.
15,000 PMCs (Kit+ PEC) in 120 μl acetate buffer (150 mM NH4Ac, pH 7.4, 130 mM NaCl, and 1.4 mM CaCl2) were prewarmed for 10 min at 37°C, and stimulated by the addition of 2 μM ionomycin and 1.25 μg ET-1, or by the addition of 2 μM ionomycin and 1.25 μg S6b. After incubation at 37°C for 60 min, the cells were pelleted at 500 g for 3 min and 100 μl of supernatant was split into 2 50-μl aliquots. For reducing conditions, the sample was dried again under vacuum, resuspended in 10 μl DTT solution (2.5 mM DTT in 10 mM NH4HCO3), and incubated overnight at 4°C. After addition of 2.5 μl IAA solution (10 mM IAA in 10 mM NH4HCO3), the sample was incubated for 30 min in the dark. Under reducing conditions, the molecular mass of the fragments was increased by the binding of one IAA molecule (molecular weight, 58 daltons) to each sulfate group. Nonreduced and reduced samples were desalted with PerfectPure C18 tips (Eppendorf), eluted with 10 μl acetonitril/0.1% TFA, and mixed 1:1 with α-cyano-4-hydroxycinnaminic acid matrix before analysis. Mass spectrometry was done on a REFLEX IV MALDI-TOF mass spectrometer (Bruker-Daltonics). Full-length ET-1 was purchased by Sigma-Aldrich or custom made by Bachem (ET-1 in the length of 1–19, 1–20, and 1–21), and S6b was purchased from Alexis.
ET ELISA.
To measure the potential degradation of ET-1 by ELISA, mast cell–containing PECs were resuspended in 120 μl Tyrode's buffer (see Mast cell degranulation assay), and incubated with 1.25 μg ET-1 for 30 min at 37°C to stimulate degranulation, as shown in Fig. 1 C. At the end of the incubation period that allowed mast cell products to “attack” ET-1, cells were pelleted at 500 g for 3 min at 4°C, and supernatants were collected. Samples of these supernatants were tested in the β-hexosaminidase assay to control for degranulation, and analyzed by ELISA for residual ET-1. The ELISA kit (Biomedica) was used according to the manufacturer's instructions. Serial dilutions of the samples were analyzed in parallel. The amount of input versus output ET-1 was taken as a measure for mast cell–mediated ET-1 degradation. Data were analyzed by Student's t test.
Acknowledgments
We thank Dr. S. Müller for mass spectrometry, Dr. L. Hellman for generously providing protease-specific antibodies, C. Blum for expert help in the generation of mutant mice, and Dr. H.J. Fehling for discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG-RO754/2-2 to H.R. Rodewald), by the Medical Faculty, University of Ulm (to L.A. Schneider), and by the Landesgraduierten-Förderung Baden-Württemberg (to S.M. Schlenner).
The authors have no conflicting financial interests.
Abbreviations used: ET, endothelin; Mc-cpa, mast cell carboxypeptidase A; Mcp, mast cell protease; PEC, peritoneal exudate cell; S6b, sarafotoxin 6b.
L.A. Schneider, S.M. Schlenner, and T.B. Feyerabend contributed equally to this paper.
References
- 1.Echtenacher, B., D.N. Mannel, and L. Hultner. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 381:75–77. [DOI] [PubMed] [Google Scholar]
- 2.Malaviya, R., T. Ikeda, E. Ross, and S.N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 381:77–80. [DOI] [PubMed] [Google Scholar]
- 3.Wort, S.J., and T.W. Evans. 1999. The role of the endothelium in modulating vascular control in sepsis and related conditions. Br. Med. Bull. 55:30–48. [DOI] [PubMed] [Google Scholar]
- 4.Wanecek, M., E. Weitzberg, A. Rudehill, and A. Oldner. 2000. The endothelin system in septic and endotoxin shock. Eur. J. Pharmacol. 407:1–15. [DOI] [PubMed] [Google Scholar]
- 5.Magder, S., and P. Cernacek. 2003. Role of endothelins in septic, cardiogenic, and hemorrhagic shock. Can. J. Physiol. Pharmacol. 81:635–643. [DOI] [PubMed] [Google Scholar]
- 6.Levin, E.R. 1995. Endothelins. N. Engl. J. Med. 333:356–363. [DOI] [PubMed] [Google Scholar]
- 7.Highsmith, R.F. 1998. Endothelin: Molecular Biology, Physiology, and Pathology. Humana Press, Totowa, NJ.
- 8.Ehrenreich, H., R.W. Anderson, C.H. Fox, P. Rieckmann, G.S. Hoffman, W.D. Travis, J.E. Coligan, J.H. Kehrl, and A.S. Fauci. 1990. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J. Exp. Med. 172:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMillen, M.A., and M.E. Cunningham. 1996. Origin of endothelin in sepsis. Crit. Care Med. 24:721–722. [DOI] [PubMed] [Google Scholar]
- 10.Matsushima, H., N. Yamada, H. Matsue, and S. Shimada. 2004. The effects of endothelin-1 on degranulation, cytokine, and growth factor production by skin-derived mast cells. Eur. J. Immunol. 34:1910–1919. [DOI] [PubMed] [Google Scholar]
- 11.Maurer, M., J. Wedemeyer, M. Metz, A.M. Piliponsky, K. Weller, D. Chatterjea, D.E. Clouthier, M.M. Yanagisawa, M. Tsai, and S.J. Galli. 2004. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 432:512–516. [DOI] [PubMed] [Google Scholar]
- 12.Metz, M., A.M. Piliponsky, C.C. Chen, V. Lammel, M. Abrink, G. Pejler, M. Tsai, and S.J. Galli. 2006. Mast cells can enhance resistance to snake and honeybee venoms. Science. 313:526–530. [DOI] [PubMed] [Google Scholar]
- 13.Huang, C., A. Sali, and R.L. Stevens. 1998. Regulation and function of mast cell proteases in inflammation. J. Clin. Immunol. 18:169–183. [DOI] [PubMed] [Google Scholar]
- 14.Caughey, G.H. 2002. New developments in the genetics and activation of mast cell proteases. Mol. Immunol. 38:1353–1357. [DOI] [PubMed] [Google Scholar]
- 15.Hallgren, J., and G. Pejler. 2006. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 273:1871–1895. [DOI] [PubMed] [Google Scholar]
- 16.Feyerabend, T.B., H. Hausser, A. Tietz, C. Blum, L. Hellman, A.H. Straus, H.K. Takahashi, E.S. Morgan, A.M. Dvorak, H.J. Fehling, and H.R. Rodewald. 2005. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol. Cell. Biol. 25:6199–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrink, M., M. Grujic, and G. Pejler. 2004. Serglycin is essential for maturation of mast cell secretory granule. J. Biol. Chem. 279:40897–40905. [DOI] [PubMed] [Google Scholar]
- 18.Stevens, R.L., D. Qui, H.P. McNeil, D.S. Friend, J.E. Hunt, K.F. Austen, and J. Zhang. 1996. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) cannot store carboxypeptidase A in their granules. FASEB J. 10:1307. [Google Scholar]
- 19.Serafin, W.E., E.T. Dayton, P.M. Gravallese, K.F. Austen, and R.L. Stevens. 1987. Carboxypeptidase A in mouse mast cells. Identification, characterization, and use as a differentiation marker. J. Immunol. 139:3771–3776. [PubMed] [Google Scholar]
- 20.Kolset, S.O., K. Prydz, and G. Pejler. 2004. Intracellular proteoglycans. Biochem. J. 379:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds, D.S., R.L. Stevens, D.S. Gurley, W.S. Lane, K.F. Austen, and W.E. Serafin. 1989. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J. Biol. Chem. 264:20094–20099. [PubMed] [Google Scholar]
- 22.Karlson, U., G. Pejler, G. Froman, and L. Hellman. 2002. Rat mast cell protease 4 is a beta-chymase with unusually stringent substrate recognition profile. J. Biol. Chem. 277:18579–18585. [DOI] [PubMed] [Google Scholar]
- 23.Reznik, S.E., and L.D. Fricker. 2001. Carboxypeptidases from A to Z: implications in embryonic development and Wnt binding. Cell. Mol. Life Sci. 58:1790–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bukrinsky, J.T., M.J. Bjerrum, and A. Kadziola. 1998. Native carboxypeptidase A in a new crystal environment reveals a different conformation of the important tyrosine 248. Biochemistry. 37:16555–16564. [DOI] [PubMed] [Google Scholar]
- 25.Cho, J.H., D.H. Kim, K.J. Lee, and K.Y. Choi. 2001. The role of Tyr248 probed by mutant bovine carboxypeptidase A: insight into the catalytic mechanism of carboxypeptidase A. Biochemistry. 40:10197–10203. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura, Y., S. Go, and K. Hatanaka. 1978. Decrease in mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 52:447–452. [PubMed] [Google Scholar]
- 27.Skolovsky, M., R. Galron, Y. Kloog, A. Bdolah, F.E. Indig, S. Blumberg, and G. Fleminger. 1990. Endothelins are more sensitive than sarafotoxins to neutral endopeptidase: possible physiological significance. Proc. Natl. Acad. Sci. USA. 87:4702–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metsarinne, K.P., P. Vehmaan-Kreula, P.T. Kovanen, O. Saijonmaa, M. Baumann, Y. Wang, T. Nyman, F.Y. Fyhrquist, and K.K. Eklund. 2002. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted Peptide. Arterioscler. Thromb. Vasc. Biol. 22:268–273. [DOI] [PubMed] [Google Scholar]
- 29.Hultner, L., and H. Ehrenreich. 2005. Mast cells and endothelin-1: a life-saving biological liaison? Trends Immunol. 26:235–238. [DOI] [PubMed] [Google Scholar]
- 30.Kloog, Y., I. Ambar, M. Sokolovsky, E. Kochva, Z. Wollberg, and A. Bdolah. 1988. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 242:268–270. [DOI] [PubMed] [Google Scholar]
- 31.Klein, J. 1997. Allergies and other hypersensitivities. In Immunology. J. Klein and V. Hoerjsi, editors. Blackwell Science, Oxford. pp. 608-634.
- 32.Knight, P.A., S.H. Wright, C.E. Lawrence, Y.Y. Paterson, and H.R. Miller. 2000. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192:1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera, J. 2006. Snake bites and bee stings: the mast cell strikes back. Nat. Med. 12:999–1000. [DOI] [PubMed] [Google Scholar]
- 34.Kimura, S., Y. Kasuya, T. Sawamura, O. Shinmi, Y. Sugita, M. Yanagisawa, K. Goto, and T. Masaki. 1988. Structure-activity relationships of endothelin: importance of the C-terminal moiety. Biochem. Biophys. Res. Commun. 156:1182–1186. [DOI] [PubMed] [Google Scholar]
- 35.Orry, A.J., and B.A. Wallace. 2000. Modeling and docking the endothelin G-protein-coupled receptor. Biophys. J. 79:3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundblad, R., and K.E. Giercksky. 1995. Endothelin concentrations in experimental sepsis: profiles of big endothelin and endothelin 1-21 in lethal peritonitis in rats. Eur. J. Surg. 161:9–16. [PubMed] [Google Scholar]
- 37.Ornan, D.A., I.H. Chaudry, and P. Wang. 2000. The dissociation between upregulated endothelins and hemodynamic responses during polymicrobial sepsis. Biochim. Biophys. Acta. 1501:211–218. [DOI] [PubMed] [Google Scholar]
- 38.Baveja, R., N. Kresge, J.H. Ashburn, S. Keller, Y. Yokoyama, N. Sonin, J.X. Zhang, T. Huynh, and M.G. Clemens. 2002. Potentiated hepatic microcirculatory response to endothelin-1 during polymicrobial sepsis. Shock. 18:415–422. [DOI] [PubMed] [Google Scholar]
- 39.Figueras-Aloy, J., L. Gomez-Lopez, J.M. Rodriguez-Miguelez, Y. Jordan-Garcia, M.D. Salvia-Roiges, W. Jimenez, and X. Carbonell-Estrany. 2004. Plasma endothelin-1 and clinical manifestations of neonatal sepsis. J. Perinat. Med. 32:522–526. [DOI] [PubMed] [Google Scholar]
- 40.Siren, A.L., and G. Feuerstein. 1989. Hemodynamic effects of endothelin after systemic and central nervous system administration in the conscious rat. Neuropeptides. 14:231–236. [DOI] [PubMed] [Google Scholar]
- 41.Oldner, A., M. Wanecek, M. Goiny, E. Weitzberg, A. Rudehill, K. Alving, and A. Sollevi. 1998. The endothelin receptor antagonist bosentan restores gut oxygen delivery and reverses intestinal mucosal acidosis in porcine endotoxin shock. Gut. 42:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szalay, L., J. Kaszaki, S. Nagy, and M. Boros. 2000. Endothelin-1 induces mucosal mast cell degranulation in the rat small intestine. Life Sci. 67:1947–1958. [DOI] [PubMed] [Google Scholar]
- 43.Waskow, C., G. Terszowski, C. Costa, M. Gassmann, and H.R. Rodewald. 2004. Rescue of lethal c-KitW/W mice by erythropoietin. Blood. 104:1688–1695. [DOI] [PubMed] [Google Scholar]
- 44.Sawano, A., and A. Miyawaki. 2000. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 28:E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henningsson, F., J. Ledin, C. Lunderius, M. Wilen, L. Hellman, and G. Pejler. 2002. Altered storage of proteases in mast cells from mice lacking heparin: a possible role for heparin in carboxypeptidase A processing. Biol. Chem. 383:793–801. [DOI] [PubMed] [Google Scholar]
- 46.Janes, R.W., D.H. Peapus, and B.A. Wallace. 1994. The crystal structure of human endothelin. Nat. Struct. Biol. 1:311–319. [DOI] [PubMed] [Google Scholar]