Abstract
Human immunodeficiency virus (HIV) type 1 is highly efficient at evading immune responses and persisting, ultimately causing fatal immunodeficiency in some patients. Mutation in the epitopes recognized by cytolytic CD8+ T cells (CTLs) is one such escape process. A new study now shows that one HIV-1 escape mutation may also result in impaired dendritic cell (DC) activity, possibly impairing later T cell responses to the same and other epitopes. The new data complete our understanding of the mechanisms by which the CTL response to an immunodominant gag epitope presented by human histocompatibility leukocyte antigen (HLA)-B27 is evaded. The complexity of the full escape helps to explain why patients with this HLA type progress to AIDS more slowly than average.
It is now well established that HIV can escape the immune responses that could otherwise control the infection, including both antibody and T cell responses. The latter has received much attention since it was first proposed in 1991 (1), and selection of escape mutations in several HIV epitopes targeted by CD8+ T cells has been carefully documented (2–5). Epitope escape usually occurs early in infection, as the rapidly replicating virus displays almost every possible mutation enabling it to evade any suppressive force. Although such escape mutations often undermine immune control, many also have a fitness cost for the virus (2, 3) so that escape is not always bad for the patient.
The first described and best-studied HIV CTL epitope comes from the p24 gag protein and spans amino acids 263–272 (KRWIILGLNK). This epitope is presented to CD8+ T cells by HLA B27 (2, 4–8). In patients with HLA B27, the CD8+ T cell response is dominated by T cells specific for this epitope, probably because these T cells are functionally diverse, bind antigen with relatively high avidity, and proliferate well (9). This response appears early in infection as the initial peak viremia declines (10), but unusually, virus escape does not occur until late in infection (8), probably because the escape requires more than one compensating mutation. In this issue, Lichterfeld et al. (p. 2813; reference 11) show that one of the mutations involved in this escape enhances HLA B27 binding to the inhibitory receptor immunoglobulin-like transcript (ILT) molecule 4 (also known as LIR2), which is expressed on monocytes and DCs (11).
Mechanics of a mutation
Peptides that bind to HLA B27 almost always have an arginine (R) at the second position, which fits snugly into the B pocket of the peptide-binding groove of the HLA molecule. The arginine at position 264 in the HIV-1 gag263–272 epitope is encoded by the amino acid codon AGA (or more rarely, AGG). Escape can occur when this arginine is mutated. The single mutations that have been recorded at this codon include AAA (lysine), ACA (threonine), and GGA or AGG (glycine). Although peptides with these substitutions may bind sufficiently to HLA B27 in in vitro assays, they are not recognized by T cells when expressed within the cell as viral or transfected sequences (8). These mutations thus effectively remove the epitope from the immune response. Given the high replication and mutation rates of HIV-1, one might expect the virus to escape rapidly and completely from the early T cells that recognize this epitope. Escape from this epitope, however, typically occurs only after several years, happening in at least 50% of HLA B27 patients who are studied for several years (3, 11, 12, and unpublished data). According to the Los Alamos HIV sequence database, these mutations are very rare in the general population of HIV-infected individuals, 95% of whom do not have HLA B27.
A possible explanation for the late escape was provided by Kelleher et al. (7), who showed that the critical arginine-to-lysine change at position 264 (R264K) only occurred when there had been a previous change of leucine-to-methionine at position six in the epitope (L268M). Unlike the arginine at position 2, the side chain at position 6 is not involved in HLA B27 binding but rather faces and interacts with the T cell receptor (Fig. 1). More recently, Schneidewind et al. (4) showed that there is nearly always a third amino acid change that accompanies the R264K and L268M mutations: an alanine-to-serine change at position 173 (A173S). This amino acid lies outside the epitope, but the three mutated amino acids cluster in the three-dimensional structure of p24, suggesting that they might compensate for each other. The R264K mutation imparts a fitness cost on the virus, as elegantly shown by Goulder et al. (2), who found that the virus rapidly mutated this sequence back to wild-type in HLA B27− babies that had been infected by B27+ mothers carrying the mutated virus. This was directly tested in vitro by Schneidewind et al., who showed that an infectivity fitness cost for the R264K mutation was compensated for by the A173S mutation (4). This requirement for a triple mutation must be much rarer than a single mutation, and probably accounts for the delayed escape from this T cell response.
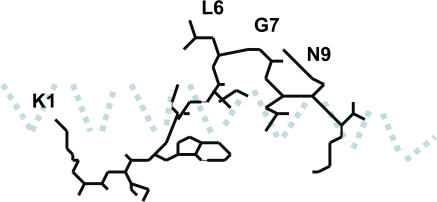
Figure 1.
Diagrammatic representation of the side chains of the HIV-1 gag peptide KRWIILGLNK bound to HLA-B27, according to the structure determined by Stewart-Jones et al. (reference 13). The α-1 helix is shown as a light blue dotted line. The floor of the peptide-binding groove below the peptide and α 2 helix are not depicted. Only the side chains of leucine 268 (L6) and asparagine 271 (N9) are exposed to the T cell receptor.
Mutation disarms DCs
In this issue, Lichterfeld et al. (11) report a longitudinal study, which shows that the L268M change occurs early in infection, suggesting that it is the first in the sequence of changes that leads to escape, and that the mutation becomes fixed (Table I). Fixation implies that the mutation confers some advantage for the virus in the presence of the T cell response. The leucine at this position is likely to interact with the T cell receptor (Fig. 1), and the initial T cell response tends to be specific for the L-containing epitope. This response then drives selection of the M-containing mutant, which in turn stimulates a new cross-reactive T cell response (11). Any advantage of the M-containing epitope would therefore seem to be transient, but the fact that this mutation persists suggests that it confers some other, as yet unknown, advantage to the virus.
Table I.
Triple bypass
Lichterfeld et al. now give an intriguing insight into what this advantage might be. They show that complexes of the L268M mutant peptide bound to HLA B27 bind to ILT4 with three times higher affinity than do complexes of the wild-type peptide with HLA B27. ILT4 is present on monocytes and DCs, and contains a cytoplasmic ITIM motif that delivers an inhibitory signal to the cell. Thus, the mutant peptide–B27 complex could impair the function of monocytes and DCs. One might expect this to have a negative effect on T cell function. The authors show, however, that after the L268M mutation occurs, a vigorous new primary T cell response develops against the new epitope. This fact is difficult to square with impaired DC function, but the authors provide a potential explanation. They show that ILT4 expression on monocytes and DCs is low in early HIV infection and increases as the infection progresses. They argue that if the L268M mutation arises early in the infection, the DCs can still prime new T cell responses, but as the infection progresses and ILT4 expression increases, other new T cell responses may be impaired by the ILT4-mediated inhibition of DCs. This could give the mutant virus a longer-term advantage. It will thus be interesting to compare the breadth of T cell responses late in infection in B27+ patients who do or do not have this epitope mutation.
Complexity of HIV escape
These observations imply a novel escape mechanism involving the inhibitory ILT4 receptor. This is the first demonstration that mutation in an HIV-1 CTL epitope can contribute to virus escape by influencing the interaction between HLA class I and a molecule other than the T cell receptor, although it is known that different epitope peptides (none yet known to escape) can influence the interaction between HLA molecules and other inhibitory receptors, such as the KIR family (12).
This is an important story for understanding HIV infection. The virus must be under considerable immune pressure to select this complex mosaic of mutations (Table I). Yet ultimately the virus evades immune control with the triple mutation and the virus load rises (8). Because this requires such a long period of time, other factors such as the loss of CD4 T cells must also contribute to the progression to AIDS. However, patients with B27 do progress to disease more slowly than the average HIV-infected individual, possibly because the B27-restricted T cells concentrate on a conserved part of the virus that requires this complicated and slow evasion process.
References
- 1.Phillips, R.E., S. Rowland-Jones, D.F. Nixon, F.M. Gotch, J.P. Edwards, A.O. Ogunlesi, J.G. Elvin, J.A. Rothbard, C.R. Bangham, C.R. Rizza, et al. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 354:453–459. [DOI] [PubMed] [Google Scholar]
- 2.Goulder, P.J., C. Brander, Y. Tang, C. Tremblay, R.A. Colbert, M.M. Addo, E.S. Rosenberg, T. Nguyen, R. Allen, A. Trocha, et al. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 412:334–338. [DOI] [PubMed] [Google Scholar]
- 3.Peyerl, F.W., H.S. Bazick, M.H. Newberg, D.H. Barouch, J. Sodroski, and N.L. Letvin. 2004. Fitness costs limit viral escape from cytotoxic T lymphocytes at a structurally constrained epitope. J. Virol. 78:13901–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneidewind, A., M.A. Brockman, R. Yang, R.I. Adam, B. Li, S. Le Gall, C.R. Rinaldo, S.L. Craggs, R.L. Allgaier, K.A. Power, et al. 2007. Escape from the dominant HLA-B27 restricted CTL response in Gag is associated with a dramatic reduction in HIV-1 replication. J. Virol. 81:12382–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frater, A.J., H. Brown, A. Oxenius, H.F. Gunthard, B. Hirschel, N. Robinson, A.J. Leslie, R. Payne, H. Crawford, A. Prendergast, et al. 2007. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J. Virol. 81:6742–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feeney, M.E., Y. Tang, K.A. Roosevelt, A.J. Leslie, K. McIntosh, N. Karthas, B.D. Walker, and P.J. Goulder. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 78:8927–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelleher, A.D., C. Long, E.C. Holmes, R.L. Allen, J. Wilson, C. Conlon, C. Workman, S. Shaunak, K. Olson, P. Goulder, et al. 2001. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27–restricted cytotoxic T lymphocyte responses. J. Exp. Med. 193:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulder, P.J., R.E. Phillips, R.A. Colbert, S. McAdam, G. Ogg, M.A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, et al. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212–217. [DOI] [PubMed] [Google Scholar]
- 9.Almeida, J.R., D.A. Price, L. Papagno, Z.A. Arkoub, D. Sauce, E. Bornstein, T.E. Asher, A. Samri, A. Schnuriger, I. Theodorou, et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson, J.D., G.S. Ogg, R.L. Allen, C. Davis, S. Shaunak, J. Downie, W. Dyer, C. Workman, S. Sullivan, A.J. McMichael, and S.L. Rowland-Jones. 2000. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS. 14:225–233. [DOI] [PubMed] [Google Scholar]
- 11.Lichterfeld, M., D. Kavanagh, K.L. Williams, B. Moza, S.K. Mui, T. Miura, R. Sivamurthy, R.L. Allgaier, F. Pereyra, A. Trocha, et al. 2007. A viral CTL escape mutation leading to immunoglobulin-like transcript 4–mediated functional inhibition of myelomonocytic cells. J. Exp. Med. 204:2813–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thananchai, H., G. Gillespie, M.P. Martin, A. Bashirova, N. Yawata, M. Yawata, P. Easterbrook, D.W. McVicar, K. Maenaka, P. Parham, et al. 2007. Allele-specific and peptide-dependent interactions between KIR3DL1 and HLA-A and HLA-B. J. Immunol. 178:33–37. [DOI] [PubMed] [Google Scholar]
- 13.Stewart-Jones, G.B., K. di Gleria, S. Kollnberger, A.J. McMichael, E.Y. Jones, and P. Bowness. 2005. Crystal structures and KIR3DL1 recognition of three immunodominant viral peptides complexed to HLA-B*2705. Eur. J. Immunol. 35:341–351. [DOI] [PubMed] [Google Scholar]