Abstract
Recent data have indicated that an important instructive class of signals regulating the immune response is Notch ligand–mediated activation. Using quantitative polymerase chain reaction, we observed that only Delta-like 4 (dll4) was up-regulated on bone marrow–derived dendritic cells after respiratory syncytial virus (RSV) infection, and that it was dependent on MyD88-mediated pathways. Using a polyclonal antibody specific for dll4, the development of RSV-induced disease was examined. Animals treated with anti-dll4 had substantially increased airway hyperresponsiveness compared with control antibody-treated animals. When the lymphocytic lung infiltrate was examined, a significant increase in total CD4+ T cells and activated (perforin+) CD8+ T cells was observed. Isolated lung CD4+ T cells demonstrated significant increases in Th2-type cytokines and a decrease in interferon γ, demonstrating an association with increased disease pathogenesis. Parellel in vitro studies examining the integrated role of dll4 with interleukin-12 demonstrated that, together, both of these instructive signals direct the immune response toward a more competent, less pathogenic antiviral response. These data demonstrate that dll4-mediated Notch activation is one regulator of antiviral immunity.
Notch signaling regulates a wide range of cellular developmental and activation events in multicelled organisms (1–6). There are four mammalian Notch receptors (Notch1–4) that appear to activate a common pathway. Upon binding to Jagged or Delta-like (dll) ligands on a neighboring cell, proteolysis by γ secretase releases the intracellular domain of Notch, and it is translocated to the nucleus, where it converts the transcription factor CSL/RBP-J from a transcriptional repressor to an activator. In the nucleus, Mastermind-like (MAML) proteins bind the Notch–CSL/RBP-J complex and act as a platform to recruit coactivators (2, 7, 8). MAML is required for CSL/RBP-J–dependent Notch signaling, and inhibiting the ability of MAML proteins to recruit coactivators blocks transcriptional activation by Notch. In recent years, Notch-mediated responses have been shown to be involved in T cell lineage maturation from double-negative (DN) pro–T cells to double-positive CD4/CD8+ T cells in the thymus (3, 9, 10). Studies have mapped out this latter mechanism in the thymus and help to explain how T cells develop through the early stages, whereas more recent studies have identified a complex relationship in mature peripheral T cells during antigen-specific responses (2, 7, 8).
In the mature immune system, the Notch pathway has been described as a signaling mechanism involved in regulating cell lineage choices for T cells. Amsen et. al. demonstrated that MyD88-dependent Th1 stimuli up-regulate the Notch ligand dll4 in DCs and polarizes Th1 cells, whereas MyD88-independent Th2 stimuli up-regulate the Notch ligand Jagged and polarize Th2 cells (11). The importance of Notch activation has been supported using a γ secretase inhibitor, which is a pharmacologic inhibitor of Notch signaling pathways, to block the induction of Th1-type responses (7, 12). However, with multiple Notch receptors on naive Th cells (Notch 1, 2, 3, 4) and ligands on DCs (dll1, dll4, Jagged1, and Jagged2), the interactions are complex and still poorly understood. Others have suggested the Notch ligands may also play a role in the activation or inhibition of proliferation of T cells, which further underlies the complexity of these interactions. Several interesting and instructive studies have separately demonstrated that Notch activation is responsible for the generation of Th1, Th2, or T reg cell generation, suggesting that the role of Notch activation pathways may be a contextual mechanism and depend on the immune environment (11, 13–16). A recent study indicated that one aspect of Notch signaling may be to prolong survival of T cells by altering apoptotic processes (17). The role of Notch in differentiation of an immune response is relatively uncharacterized, and it may include roles in survival and differentiation of a developing immune response. In this study, we have examined the role of dll4 Notch ligand for the activation of the appropriate antiviral responses and examined it in relationship to IL-12, which is a classic instructive immune signal. The link between IL-12 and Notch ligand instructive signal expression patterns on DCs has recently been established in respiratory syncytial virus (RSV) by our laboratory, using MyD88−/− mice, and the expression of the two are independent of one another (18). Overall, the role of dll4 appears to be a regulating signal for Th2 cytokine production, as well as IFNγ expression, and therefore dll4 helps to maintain a Th1 environment.
RESULTS
RSV induces dll4 in DCs
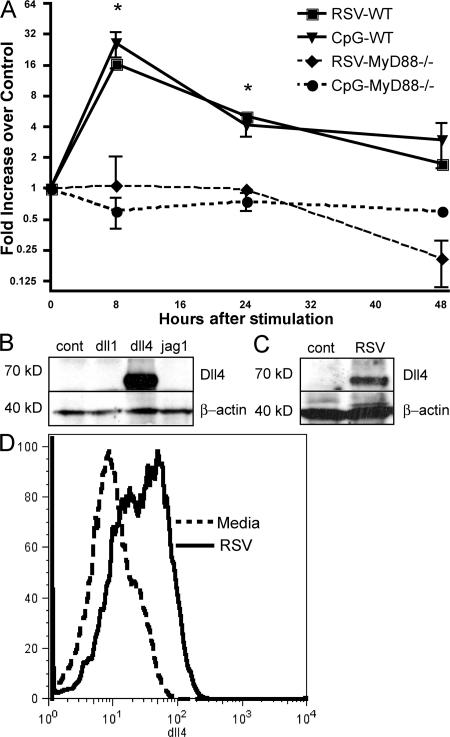
The identification of Notch ligand expression during viral infection provides initial data for justifying the role during disease. Freshly grown and isolated bone marrow–derived DCs (BMDCs) were infected with RSV (multiplicity of infection [MOI] = 1.0) and assessed for Notch ligand mRNA expression by quantitative PCR analyses (Fig. 1 A). The data indicates that, compared with uninfected DCs, RSV primarily induces the up-regulation of dll4 and causes little increase in the other Notch ligands, including dll1, dll3, Jagged1, and Jagged2, in BMDC populations (unpublished data) (18). To confirm previously published data that this up-regulation was MyD88 dependent, we compared the expression of dll4 during RSV infection in GM-CSF–grown BMDCs from MyD88 −/− mice and found no increase in dll4 expression when MyD88 was absent (Fig. 1 A). This result was also verified using a CpG/Toll-like receptor (TLR) 9 stimulus that specifically functions through MyD88. Thus, RSV specifically up-regulates dll4, but not other Notch ligands, and it is dependent on MyD88 activation pathways.
Figure 1.
dll4 up-regulation on DCs is MyD88 dependent, and occurs upon RSV infection. (A) BMDCs from wild-type or MyD88−/− mice were cultured for 6 h in the presence of CpG or RSV for 6 h. Fold increase in dll4 expression was determined by comparison to an unstimulated culture. *, P < 0.01 compared with cells from MyD88−/− mice. (B) Western blot demonstrating the specificity of our polyclonal dll4 antibody by using OP9 cells transfected with various Notch ligands. (C) Western blot demonstrating that dll4 is up-regulated on BMDCs after RSV infection. No nonspecific bands were observed on either of our blots after incubation with our polyclonal anti-dll4 antibody. (D) BMDCs from wild-type mice were stimulated with RSV for 24 h. Flow cytometry was performed using a specific polyclonal antibody to dll4. Error bars represent the mean ± the SEM.
After identifying that dll4 was the predominant ligand up-regulated by RSV infection, we generated a polyclonal rabbit anti–mouse antibody to dll4 using previously published protocols (19, 20). The specificity of this antibody was verified by using stably transfected OP-9 cell lines for Notch ligands Dll1, Dll4, and Jagged1, and the sera was found only to react with the cell line expressing dll4 (Fig. 1 B). The expression of dll4 protein on BMDCs was then assessed by Western blot analysis using cell lysates from BMDCs infected with RSV (Fig. 1 C). Flow cytometry of BMDCs infected with RSV using the specific antisera for dll4 demonstrated that this molecule was present on the surface of the cell after RSV infection (Fig. 1 D). Similar to the RNA expression pattern observed in Fig. 1 A, Fig. 1 (C and D) demonstrate that dll4 protein was substantially increased on BMDCs after they were infected with RSV.
Specific neutralization of dll4 during RSV infection induces airway hyperresponsiveness (AHR), increased tissue pathology, and Th2-type cytokines
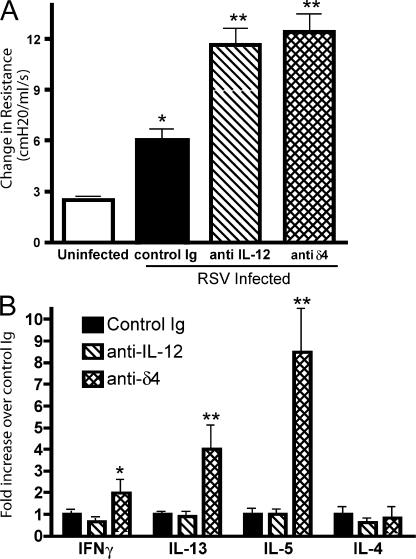
Recent results have suggested that Notch ligands can differentially alter immune responses. In particular, dll4 was identified as an important factor for skewing T cells toward a type I immune response. To test this hypothesis, animals were infected with RSV and treated with either the anti-dll4 or control antibody on day 0, 2, 4, and 6 of infection. Comparatively, a second group of animals were treated with anti–IL-12, which is an important factor in eliciting Th1 responses from T cells. We have previously shown that treatment with this antibody during RSV infection increases the pathology of RSV-induced disease. Animals were assessed for multiple parameters on day 8 of infection. In Fig. 2 A, we show that both anti–IL-12 and anti-dll4 treatment significantly increase airway hyperreactivity of mice 8 d after RSV infection compared with controls. To assess the immunologic mechanism through which increases in AHR occurred, mRNA from draining lymph nodes of mice 8 d after RSV infection was analyzed for cytokine expression. Although anti–IL-12 reduced the expression of IFNγ by ∼40%, the treatment did not have any effect on the levels of Th2 cytokines expressed in draining lymph nodes. In contrast, the treatment of mice with anti-dll4 led to increases in the Th2 cytokines IL-5 (8.5-fold) and -13 (4-fold) compared with control mice (Fig. 2 B). Thus, the initial studies comparing the role of these two instructive signals demonstrate a similar clinical outcome potentially resulting from a different cytokine profile. To determine if dll4 was required only during the initial phase of the viral infection or for the entire length of the immune response, we used a delayed administration of anti-dll4 antibody that began 4 d after infection, and compared mice receiving control serum or dll4 blockade from day 0 of infection. 8 d after infection, mice receiving delayed administration of dll4 antibody demonstrated an intermediate phenotype between those mice receiving control serum or dll4 blockade for the duration of the experiment. AHR readings from mice given anti-dll4 the entire time induced a 4-fold increase, whereas delayed anti-dll4 treatment induced a 2.5-fold increase over control IgG-treated animals after RSV infection. IL-13 mRNA expression levels in the lymph nodes were also examined. Compared with control mice anti-dll4 treatment for the entire RSV infection induced a 7.0 ± 1.2-fold increase, whereas delayed anti-dll4 induced a 1.64 ± 0.1-fold increase over control IgG treatment. These data indicate that dll4 is required to be present for the entire response, and not just during the initial viral infection.
Figure 2.
Blockade of dll4 increases airway hyperreactivity and lymph node cytokines. (A) RSV-infected mice were treated on day 0, 2, 4, and 6 with antibody to either dll4 or IL-12. AHR was assessed 8 d after infection. *, P < 0.05 compared with uninfected; **, P < 0.001 compared with control-treated and infected mice. (B) Lymph nodes were isolated from mice subjected to the same treatments as in A. Quantitative real-time PCR was performed on lymph node mRNA for the cytokines IFNγ, IL-13, -5, and -4. *, P < 0.01 compared with control and anti–IL-12. **, P < 0.001 compared with control and anti–IL-12. Error bars represent the mean ± the SEM.
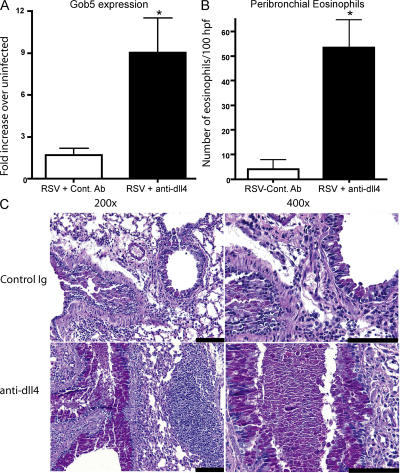
Mucus overproduction is one of the hallmarks of severe RSV-induced disease in infants. Examination of the mucus-associated gene gob5 in anti-dll4–treated animals demonstrated a substantial increase in expression compared with the control antibody-treated animals (Fig. 3 A). Several investigators have found RSV to be associated with increased pulmonary pathology, mucus hypersecretion, and eosinophil recruitment to the airways. Enumeration of peribronchial eosinophils associated with major airways revealed that although RSV-infected mice do not normally have significant numbers of eosinophils within or around their airways, animals treated with anti-dll4 displayed significant increases in eosinophil accumulation (Fig. 3 B). However, the majority of the infiltrating cells around the airway were mononuclear. Histologic examination of the lungs of mice 12 d after RSV infection that had received dll4 blockade demonstrated increased inflammatory cell infiltrate and goblet cell development, indicating a substantial change in the overall immune response (Fig. 3 C). In fact, as indicated by the histology, many of the airways were completely congested with mucus in the anti-dll4–treated animals. Thus, dll4 appears to provide signals that prevent the development of severe physiologic changes in the lung during RSV infection.
Figure 3.
dll4 blockade drives mucus expression in the lung. (A) Lungs from mice receiving Dll4 blockade were taken 8 d after RSV infection and assayed for mucus production by performing quantitative real-time PCR for the mucus gene GOB5. *, P = 0.02 compared with control. (B) Enumeration of peribronchial eosinophils by morphometric analysis of hemotoxylin and eosin–stained lung sections from control antibody or anti-dll4–treated animals after 12 d of RSV infection. Data represents the mean ± the SEM from three mice. *, P = 0.015. (C) periodic acid Schiff staining of formalin-fixed sections of lung showing the amount of mucus in the airways of control-treated and anti–Dll4–treated animals 12 d after RSV infection. Bars, 500 μm.
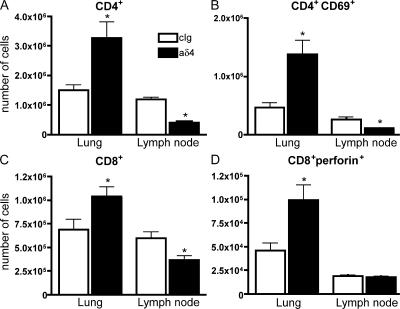
To further characterize the effect of the dll4 blockade during RSV infection, we enumerated T cells in the lung and draining lymph nodes of control and anti-dll4–treated mice 8 d after RSV infection by flow cytometry. There were notable changes in T cell subset numbers in both the lung and lymph node of mice receiving the dll4-specific antibody. As demonstrated in Fig. 4 A, there were significantly more total CD4+ T cells in the anti-dll4–treated animals compared to animals treated with control sera. Additionally, substantially more CD4+ T cells expressing the very early activation marker CD69 were found in the lungs of mice receiving dll4 blockade, suggesting that these cells were activated (Fig. 4 B). The total number of CD8+ T cells was also increased in the lungs of mice receiving anti-dll4 treatment (Fig. 4 C), as was the number of perforin+CD8+ T cells, indicating that these cells had also been activated. These data further imply that anti-dll4 blockade notably alters the immune environment of the lung. We also quantified the number of T cells in the draining lymph nodes of anti-dll4–treated mice. In contrast to our findings in the lung, we observed a substantial decrease in the number of CD4+ T cells in the lymph nodes of anti-dll4–treated mice. There appeared to be a decrease in the total number of CD8+ T cells, whereas the number of perforin+CD8+ T cells in the draining lymph nodes remained the same regardless of the absence of dll4. Given the aforementioned changes, separate studies examined viral clearance by performing plaque assays using the lungs of animals at 2, 4, and 5 d after RSV infection with specific anti-RSV antibodies to identify positive plaques in culture. No significant alteration in the viral clearance was observed in the anti-dll4–treated compared with control mice at any time point (D2 wild-type, 53 ± 1 pfu/g lung; anti-dll4, 95 ± 7 pfu/g lung; D4 wild-type, 3,609 ± 430 pfu/g lung; and anti-dll4, 1,578 ± 205 pfu/g lung).
Figure 4.
The absence of dll4 alters the number and activation of T cells in the lung and lymph nodes of RSV-infected mice. Flow cytometry was performed using whole-lung digests or dispersed lymph nodes taken from mice 8 d after RSV infection. (A) The total number of CD4+ T cells in the lung and lymph node. *, P = 0.0134 for lung and 0.0042 for lymph node. (B) The number of CD4+CD69+ T cells in the lung and lymph node. *, P = 0.0065 for lung and 0.0020 for lymph node. (C) The total number of CD8+ T cells in the lung and lymph node. *, P = 0.0349 for lung and 0.0113 for lymph node. (D) The number of CD8+perforin+ cells in the lung and lymph node. *, P = 0.02. Error bars represent the mean ± the SEM.
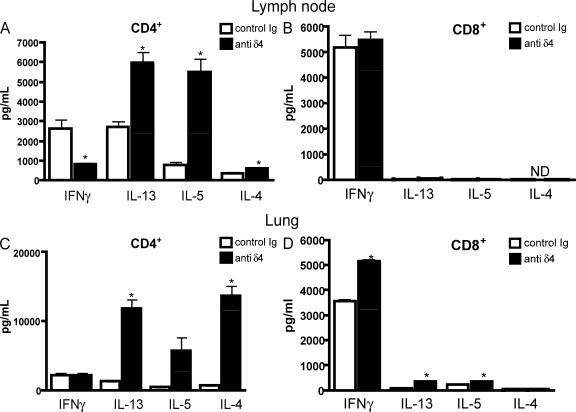
To further characterize the changes that occurred in vivo during the blockade of dll4, the production of Th1 and Th2 cytokines of treated mice was assessed after in vitro rechallenge with RSV. In these experiments CD4+ T cells from the lungs or draining lymph nodes of RSV-infected mice were isolated using a magnetic bead column and exposed to RSV-pulsed BMDCs for 48 h. The BMDCs were grown in GM-CSF for 6 d and purified so that >95% of the cells were CD11c+. These cells were infected with RSV (MOI = 1.0) for 2 h and washed before adding the T cells. Cytokine levels were measured using bioplex proteomic assays (Fig. 5, A and C). The data illustrate that there were considerable decreases in IFNγ production from T cells isolated from the lymph nodes of anti-dll4–treated animals compared with those from the control antibody-treated animals (Fig. 5 A). Additionally, the Th2 cytokines IL-4, -5, and -13 were significantly up-regulated in the anti-dll4–treated animals compared with control antibody–treated and infected animals. A similar cytokine profile was observed using CD4+ T cells isolated from the lung (Fig. 5 C). These data suggest that the blockade of the dll4 signal altered the cytokine profile of RSV-responsive T cells.
Figure 5.
Cytokine profile of T cells is altered in the absence of dll4. CD4+ and CD8+ T cells were isolated from the lymph nodes or lungs of control or anti–Dll4–treated mice and restimulated in vitro with RSV-pulsed BMDCs for 48 h. Cytokine levels were measured using a luminex system. (A) Lymph node CD4+ T cell cytokine production after restimulation. *, P = 0.02 for IFNγ, 0.005 for IL-13, 0.002 for IL-5, and 0.02 for IL-4. (B) Lymph node CD8+ T cell cytokine production after restimulation. (C) Lung CD4+ T cell cytokine profile after restimulation. *, P = 0.0137 for IL-4 and 0.0150 for IL-13. (D) Lung CD8+ T cell cytokine profile after restimulation. *, P = 0.0007 for IFNγ, 0.003 for IL-13, and 0.0150 for IL-5. Error bars represent the mean ± the SEM.
To assess the CD8+ T cell affect that was identified by increased activated CD8+ T cells in the lung and lymph node, we cultured CD8+ T cells isolated from both lung and draining lymph nodes with RSV-pulsed BMDCs, as described in the previous paragraph. We found that lymph node CD8+ T cells from mice receiving anti-dll4 blockade produced a level of IFNγ similar to those from CD8+ T cells from control-treated mice (Fig. 5 B). Interestingly, the CD8+ T cells isolated from the lungs of anti-Dll4–treated mice produced substantially more IFNγ and Th2 cytokines IL-13 and -5 than CD8+ T cells isolated from control-treated mice (Fig. 5 D).
Dll4 regulates activation of Th2 cytokines and IFN during immune responses
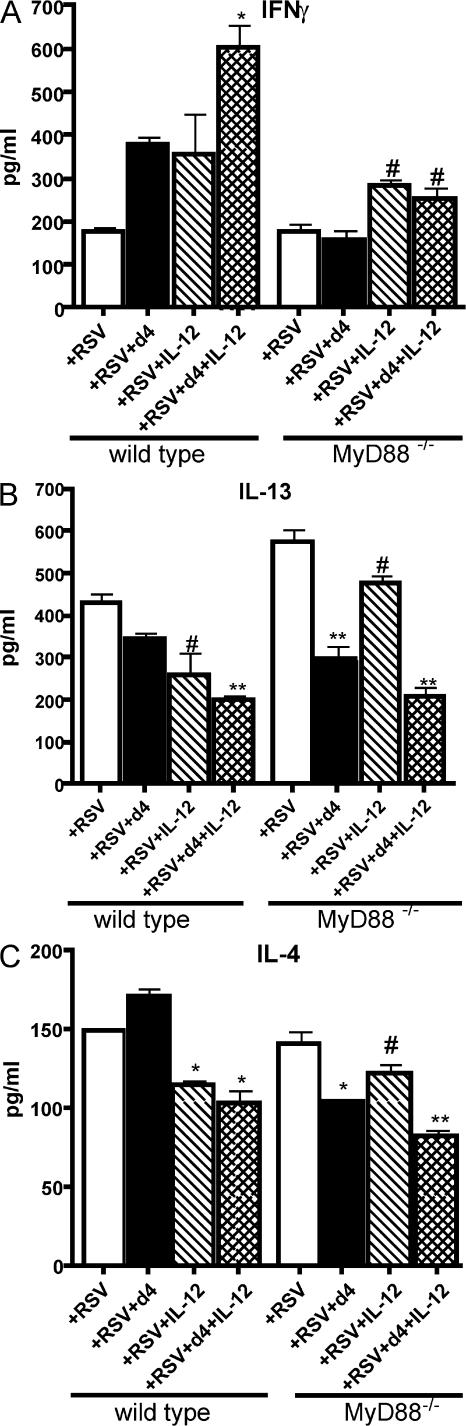
To further examine the distinct role that dll4 and IL-12 have in the generation of the immune response, we used BMDCs grown from MyD88−/− mice that do not express either IL-12 or dll4 after RSV infection (18). For these experiments, we again used T cells cocultured with BMDCs, as previously described. In these experiments, CD4+ T cells were isolated from the draining lymph nodes of RSV-infected wild-type or MyD88−/− mice 8 d after infection and cocultured with RSV-pulsed wild-type or MyD88−/− BMDCs. T cell cocultures also contained 10 μg/ml plate-coated recombinant dll4, 10 ng/ml rIL-12, or a combination of both. The data illustrate several important and novel findings regarding the potential individual roles for these two instructive signals (Fig. 6). First, when examining the ability of these two molecules to differentially block the enhanced Th2 cytokine production by CD4+ T cells, dll4 was able to considerably reduce the amount of IL-13 and -4 produced from MyD88−/− CD4+ T cells restimulated with RSV when compared with control cultures. A combination of these two signals produced the most striking reduction in Th2 cytokine levels, suggesting that the presence of both signals is necessary for the most appropriate T cell cytokine production in response to viral infection. Only with IL-12 present was there any increase in IFNγ production from T cells, with the most substantial increase occurring in the presence of both dll4 and IL-12. Although dll4 did little to significantly alter the response of wild-type T cells by itself, the combination of this signal with IL-12 again led to a significant reduction of Th2 cytokines and increased production of IFNγ. These data suggest that both of these signals are necessary for the strongest Th1 response. Altogether, these studies allow significant insight into the potential mechanisms of how these two instructive signals work in collaboration with one another. Together, the findings suggest that dll4 in combination with IL-12 can regulate both Th1 and Th2 cytokine production.
Figure 6.
IL-12 and dll4 differentially alter the cytokine profile of MyD88−/− T cells restimulated with RSV. CD4+ T cells were isolated from MyD88−/− mice 9 d after RSV infection and restimulated with MyD88−/− DC for 48 h. Cytokines were assayed by a luminex system, as in Fig. 5. (A) IL-12 regulates IFNγ production. *, P < 0.05 compared with control and dll4-treated wells. (B) Dll4 regulates IL-13 production. *, P < 0.05 compared with control. **, P < 0.01 compared with control cultures and those containing IL-12. (C) IL-4 cytokine production in the same cultures. #, P < 0.05 compared with control; *, P < 0.01 compared with control; **, P < 0.001 compared with control, and P < 0.05 compared with cultured with IL-12 or dll4 alone. Error bars represent the mean ± the SEM.
To verify that the addition of dll4 to cocultures of DC and T cells was activating Notch pathways, we examined HES1 expression by quantitative real-time PCR (21–23). Cultures receiving dll4 showed a 3.1 ± 0.28-fold increase in HES1 expression over cultures with DC and T cells alone. Together with the aforementioned data, these findings implicate that dll4 is able to skew T cell maturation via Notch signaling pathways.
DISCUSSION
Specific adaptive immunity is regulated by the host response to individual pathogens and dictated by numerous immunomodulatory molecules. This is of particular importance because the differentiation of CD4 into Th1 effector cells mediates host immunity against viruses via development of cell-mediated immunity. In general, a Th1 response is initiated by APCs that are stimulated via TLRs that recognize pathogen-associated molecular patterns (24–27). Specific pathogen–associated molecular patterns trigger the production of IL-12 and induce the differentiation of IFNγ-producing Th1 cells, as well as viral-specific Tc1 cells. Th2 responses tend to arise in response to pathogens in the absence of TLRs, leading many researchers to suggest that Th2 differentiation is a default pathway that arises in the absence of IL-12. This is supported by studies that show MyD88−/− animals were incapable of generating Th1 responses (28) and by studies using infectious agents in MyD88−/− mice (29–31). In addition, MyD88-mediated signals appear to provide a regulatory signal for Th2-type responses (28). Conversely, not all Th1-inducing pathogens default to Th2 responses in the absence of IL-12 (32). This suggests that other signals may exist on APCs to elicit a Th1 response. Recent studies suggest that the Notch ligands dll and jagged can provide previously unknown instructional signals for the development of Th1 and Th2 cells, respectively (1). However, little research has investigated the role of Notch–Notch ligand system in acquired viral immunity, and the requirement of Notch activation in development of Th1 and/or Th2 responses is still unclear. Importantly, dll4 was the primary Notch ligand up-regulated on BMDCs grown in culture after RSV infection (18). The data in these studies clearly identify a critical role for dll4 for the development of a Th1-mediated response, along with the requirement for IL-12 to optimally drive the production of IFNγ.
Notch activation has been identified as an important signaling molecule in many cell populations, and regulates a wide variety of cell differentiation and proliferation parameters (4, 5, 33, 34). In the thymus, Notch and its ligands have been shown to be important in allowing DN T cell progenitors to mature to double-positive T cells (CD4/CD8), although the exact Notch receptor–ligand interactions are still not completely clear at each of the DN T cell stages (3, 6, 9, 10). The data in this study indicate that dll4 neutralization in vivo during RSV infection increased Th2 cytokine production. In addition, neutralization of dll4 during RSV infection altered CD4 and CD8 T cell accumulation and activation responses within the lung. Specifically, the increased CD4+ T cells in the lungs of anti-dll4–treated animals correlated with the changing cytokine profile toward increased Th2 type cytokines, as it is the CD4+ T cell population that primarily produces Th2 cytokines during RSV infection (unpublished data) (35–37). In vivo studies examined parameters associated with RSV-induced disease in infants, including airway hyperreactivity, mucus production, and goblet cell hyperplasia, and demonstrated a heightened pathogenic effect when dll4 was blocked. Numerous studies have previously examined these endpoints and found that they correspond to and depend on IL-13 production (38–41). The data in these studies suggest that the increased pathogenesis observed during dll4 blockade of RSV-infected mice appears to be associated with altered CD4+ T cell activation and not decreased viral clearance or CD8+ T cell activation. These data correspond well to earlier publications, demonstrating that different Notch ligands drive maturation of CD4+ T cells (11). However, the mechanism of how Notch regulates mature T cell differentiation is not yet clear. Although studies have demonstrated that individual Notch ligands can influence the direction of the T cell response, other studies using genetically manipulated mice have shown that Notch is required for Th2, but not Th1, cell development (11, 15). In the latter study, it may be that other Th1 signals, such as IL-12, may be able to compensate for the lack of Notch1, whereas Th2 development requires Notch1 signaling through alternative ligands, such as Jagged1. The requirement of Notch activation for Th2 development has more recently been validated in two independent studies using mice with defects in the Notch signaling pathway. The experiments performed assessed the role of Notch signaling in respect to the regulation of GATA3 expression and found that the Notch signaling protein RBPJκ can bind to a promoter region of GATA3 (42, 43). Furthermore, these studies demonstrate that the absence of the Notch signaling pathway impairs the ability of T cells to differentiate to a Th2 lineage. Although our data do not corroborate these latter observations, they do suggest that not all Notch signals are activating. Instead, some Notch signals may be regulatory in nature for specific genes in T cells that contain a functioning Notch signaling pathway. In addition, the outcome of Notch activation may also depend on the differentiation state of the T cell and the specific immune environment in which it receives Notch signals. In fact, dll4 has been described to specifically skew T cells to a Th1 phenotype upon initial activation.
In further support of the contention that Notch signals may be regulatory to certain genes, analysis of isolated CD4+ T cells that had been restimulated with RSV indicated that one role for dll4 was to suppress Th2 cytokine production. Several previous studies have identified other factors that are responsible for differentiation of T cells, such as specific costimulatory molecule use or IL-12 versus -10 production by APCs (24, 44, 45). Our previous studies with anti–IL-12 treatment during RSV infection demonstrated a significant reduction in IFNγ, along with increases in eosinophils and mucus overexpression. The development of AHR in the anti–IL-12–treated animals may result from an altered immune environment caused by a different mechanism, such as down-regulation of IFNγ, but leading to a similar physiologic endpoint as a blockade of dll4. The data outlined in these studies begin to define how Notch ligands can participate in concert with other factors, such as IL-12, to shape the immune response by regulating specific aspects of T cell activation. However, it is not entirely clear in our studies whether the development of the Th2 response is merely caused by the blockade of dll4, which directly affects Th2 cytokine production, or whether there was an up-regulation of other Th2-instructive signals that, until now, have been relatively undefined. The role of IL-13 on development of Th2 responses has been well established in multiple studies (46, 47), and it appears that dll4 expression on DCs is at least part of the mechanism that controls IL-13 production from CD4+ T cells. Although GATA3 activation is required for Notch1-induced Th2 cytokine activation (11), recent studies have highlighted that other binding elements are also important, such as the recently described CNS-2 that regulates Notch-induced Th2 cytokines in NK and memory T cells (48). These latter studies indicated that Notch and RBPJ activation regulate initial Th2 cytokine production, whereas STAT6 is necessary for secondary Th2 cytokine expression. Interestingly, deletion of GATA3 before primary activation of naive T cells diminished both IL-4-dependent and IL-4–independent Th2 differentiation, whereas deletion of GATA3 in established Th2 cells only reduced IL-5 and -13, but not IL-4 production (49). These latter results may best reflect our data with dll4-mediated regulation in RSV-induced responses, suggesting that perhaps dll4 activation of Notch regulates critical transcription factors during RSV infection. Overall, there is a paucity of data on how Notch activation regulates cytokine responses in T cells that will need to be examined carefully in future experiments.
The impact of these responses related to dll4 are defining for viral responses in the lung, as the data in this study indicate that the in vivo responses are profoundly effected by blocking dll4 function. The coordinated role of dll4 in cooperation with IL-12 provides a logical way to control Th1-type responses by regulating Th2 cytokines. Together, these studies outline several important observations and demonstrate the importance of dll4 in a clinically relevant infectious disease process.
MATERIALS AND METHODS
RSV propagation and titer determination.
RSV was derived from a clinical isolate at the University of Michigan (38, 39, 50). The virus was propagated in Hep2 cells (American Type Culture Collection) and titered using Vero cells (American Type Culture Collection), as previously described (51). Plaque assays were performed on RSV-infected lungs, as previously described (40). In brief, our plaque assessment uses a direct detection of plaques using RSV-specific polyclonal antibody to positively count each plaque.
Mice.
Female BALB/c mice were purchased from Jackson ImmunoResearch Laboratories and used in all antibody depletion studies and in vivo RSV infection studies. Female MyD88−/− mice were grown under SPF conditions at the University of Michigan and used for growing BMDCs for the in vitro analyses. All mice were maintained in specific pathogen–free facilities in the Unit for Laboratory Animal Medicine at the University of Michigan. Mice were anesthetized and infected intratracheally with RSV, as previously described (38, 39, 41). Animal work was overseen by the University Committee on Use and Care of Animals at the University of Michigan.
Quantification of cytokines.
RNA was isolated from the upper right lobes of lung, lymph nodes, and BMDCs using Trizol (Invitrogen). Levels of mRNA were assessed using qPCR analysis (Taqman; Applied Biosystems) with predeveloped primers and probe sets from PE Biosystems. Quantification of the genes of interests were normalized to GAPDH and expressed as fold increases over the negative control for each treatment at each time point, as previously described. Protein levels of cytokines were quantitated using a Bio-Plex bead-based cytokine assay purchased from Bio-Rad Laboratories.
Generation of rabbit anti–mouse polyclonal dll4-specific antibody.
Rabbit anti–mouse dll4 antibodies were prepared by multiple-site immunization of New Zealand white rabbits with recombinant mouse dll4 (R&D Systems) in CFA and boosted with dll4 in IFA, as in previously described procedures from our laboratory (19, 20, 52, 53). Polyclonal antibodies were titered by direct ELISA against dll4 coated onto 96-well plates and titered at 107. Serum from unimmunized rabbits was used for a control treatment group. Antibody specificity was verified by flow cytometry on Notch ligand expressing OP-9 cells stably transfected with the five different Notch ligands, dll1, 3, and 4, and jagged1 and 2. Only dll4-expressing cell lines were positive for staining using our polyclonal antibody.
Generation of BMDCs.
BM was harvested from uninfected, normal mice, filtered through nylon mesh, and depleted of erythrocytes with lysis buffer. BM cells were seeded in T-150 tissue culture flasks at 106 cells/ml in RPMI 1640–based complete media with GM-CSF/ml (R&D Systems). After 6 d, loosely adherent cells were collected and incubated with anti-CD11c coupled to magnetic beads for isolation of conventional DCs from the GM-CSF cultures (Miltenyi Biotec). BMDCs were purified using positive selection for the specific cells by running the cell suspension through a magnetic column and cells were plated overnight. The next day, DCs were infected with RSV (MOI = 1.0) for the indicated times.
In vitro CD4+ T cell treatments.
Magnetic bead isolated CD4+ lymph node T cells were obtained on day 8 after RSV infection using the MACS system. Lymph node cells were filtered through nylon mesh and depleted of erythrocytes with lysis buffer, and CD4+ T cells were isolated using anti-CD4+ magnetic beads (Miltenyi Biotec). For DC T cell cocultures, BMDCs were first plated in a flat-bottom 96-well plate and infected with RSV for 2 h. BMDCs were washed and resuspended in fresh media. CD4+ T cells from RSV-infected mice were added to wells at a DC:CD4+ cell ratio of 1:20, and supernatants were harvested 48 h later for cytokine protein analysis.
Flow cytometric analysis.
Lung cells were isolated after collagenase dispersion to obtain single-cell suspensions, as previously described (54), and lymph node cells were isolated as previously described. Cells were stained with the indicated antibodies after 5 min of preincubation with FC block (BD Biosciences), fixed overnight with 4% formalin, and run on a Cytomics FC-500 (Beckman Coulter). Samples were analyzed using FlowJo software (Tree Star, Inc.).
Statistics.
Statistical significance was determined by analysis of variance with a Student's Neuman Kuhl posttest, where relevant. Values of P < 0.05 were considered significant. In cases where two groups were being compared, a Student's t test was performed to determine significance; P ≤ 0.05 was considered significant.
Acknowledgments
This work was supported by National Institutes of Health grants AI036302 and AI073876.
The authors of this paper have no conflicting financial interests.
Abbreviations used: AHR, airway hyperresponsiveness; BMDC, bone marrow–derived DC; DN, double-negative; MOI, multiplicity of infection; RSV, respiratory syncytial virus; TLR, Toll-like receptor.
References
- 1.Dallman, M.J., E. Smith, R.A. Benson, and J.R. Lamb. 2005. Notch: control of lymphocyte differentiation in the periphery. Curr. Opin. Immunol. 17:259–266. [DOI] [PubMed] [Google Scholar]
- 2.Hoyne, G.F., M.J. Dallman, B.R. Champion, and J.R. Lamb. 2001. Notch signalling in the regulation of peripheral immunity. Immunol. Rev. 182:215–227. [DOI] [PubMed] [Google Scholar]
- 3.Harman, B.C., E.J. Jenkinson, and G. Anderson. 2003. Microenvironmental regulation of Notch signalling in T cell development. Semin. Immunol. 15:91–97. [DOI] [PubMed] [Google Scholar]
- 4.Iso, T., Y. Hamamori, and L. Kedes. 2003. Notch signaling in vascular development. Arterioscler. Thromb. Vasc. Biol. 23:543–553. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki, T., and S. Chiba. 2005. Notch signaling in hematopoietic stem cells. Int. J. Hematol. 82:285–294. [DOI] [PubMed] [Google Scholar]
- 6.Jenkinson, E.J., W.E. Jenkinson, S.W. Rossi, and G. Anderson. 2006. The thymus and T-cell commitment: the right niche for Notch? Nat. Rev. Immunol. 6:551–555. [DOI] [PubMed] [Google Scholar]
- 7.Dallman, M.J., B. Champion, and J.R. Lamb. 2003. Notch signalling in the peripheral immune system. Novartis Found. Symp. 252:268–276. [PubMed] [Google Scholar]
- 8.McKenzie, G.J., L.L. Young, E. Briend, J.R. Lamb, M.J. Dallman, and B.R. Champion. 2003. Notch signalling in the regulation of peripheral T-cell function. Semin. Cell Dev. Biol. 14:127–134. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt, T.M., and J.C. Zuniga-Pflucker. 2005. Thymus-derived signals regulate early T-cell development. Crit. Rev. Immunol. 25:141–159. [DOI] [PubMed] [Google Scholar]
- 10.Basson, M.A., and R. Zamoyska. 2000. The CD4/CD8 lineage decision: integration of signalling pathways. Immunol. Today. 21:509–514. [DOI] [PubMed] [Google Scholar]
- 11.Amsen, D., J.M. Blander, G.R. Lee, K. Tanigaki, T. Honjo, and R.A. Flavell. 2004. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 117:515–526. [DOI] [PubMed] [Google Scholar]
- 12.Minter, L.M., D.M. Turley, P. Das, H.M. Shin, I. Joshi, R.G. Lawlor, O.H. Cho, T. Palaga, S. Gottipati, J.C. Telfer, et al. 2005. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 6:680–688. [PubMed] [Google Scholar]
- 13.Rutz, S., B. Mordmuller, S. Sakano, and A. Scheffold. 2005. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur. J. Immunol. 35:2443–2451. [DOI] [PubMed] [Google Scholar]
- 14.Maekawa, Y., S. Tsukumo, S. Chiba, H. Hirai, Y. Hayashi, H. Okada, K. Kishihara, and K. Yasutomo. 2003. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 19:549–559. [DOI] [PubMed] [Google Scholar]
- 15.Tu, L., T.C. Fang, D. Artis, O. Shestova, S.E. Pross, I. Maillard, and W.S. Pear. 2005. Notch signaling is an important regulator of type 2 immunity. J. Exp. Med. 202:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostroukhova, M., Z. Qi, T.B. Oriss, B. Dixon-McCarthy, P. Ray, and A. Ray. 2006. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J. Clin. Invest. 116:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bheeshmachar, G., D. Purushotaman, H. Sade, V. Gunasekharan, A. Rangarajan, and A. Sarin. 2006. Evidence for a role for notch signaling in the cytokine-dependent survival of activated T cells. J. Immunol. 177:5041–5050. [DOI] [PubMed] [Google Scholar]
- 18.Rudd, B.D., M.A. Schaller, J.J. Smit, S.L. Kunkel, R. Neupane, L. Kelley, A.A. Berlin, and N.W. Lukacs. 2007. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 178:5820–5827. [DOI] [PubMed] [Google Scholar]
- 19.Lukacs, N.W., R.M. Strieter, P.M. Lincoln, E. Brownell, D.M. Pullen, H.J. Schock, S.W. Chensue, D.D. Taub, and S.L. Kunkel. 1996. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. J. Immunol. 156:3945–3951. [PubMed] [Google Scholar]
- 20.Campbell, E.M., I.F. Charo, S.L. Kunkel, R.M. Strieter, L. Boring, J. Gosling, and N.W. Lukacs. 1999. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J. Immunol. 163:2160–2167. [PubMed] [Google Scholar]
- 21.Tan, J.B., I. Visan, J.S. Yuan, and C.J. Guidos. 2005. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat. Immunol. 6:671–679. [DOI] [PubMed] [Google Scholar]
- 22.Kawamata, S., C. Du, K. Li, and C. Lavau. 2002. Overexpression of the Notch target genes Hes in vivo induces lymphoid and myeloid alterations. Oncogene. 21:3855–3863. [DOI] [PubMed] [Google Scholar]
- 23.Deftos, M.L., E. Huang, E.W. Ojala, K.A. Forbush, and M.J. Bevan. 2000. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity. 13:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146. [DOI] [PubMed] [Google Scholar]
- 25.Zanoni, I., M. Foti, P. Ricciardi-Castagnoli, and F. Granucci. 2005. TLR-dependent activation stimuli associated with Th1 responses confer NK cell stimulatory capacity to mouse dendritic cells. J. Immunol. 175:286–292. [DOI] [PubMed] [Google Scholar]
- 26.Xu, D., H. Liu, and M. Komai-Koma. 2004. Direct and indirect role of Toll-like receptors in T cell mediated immunity. Cell. Mol. Immunol. 1:239–246. [PubMed] [Google Scholar]
- 27.Dabbagh, K., and D.B. Lewis. 2003. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr. Opin. Infect. Dis. 16:199–204. [DOI] [PubMed] [Google Scholar]
- 28.Sun, J., M. Walsh, A.V. Villarino, L. Cervi, C.A. Hunter, Y. Choi, and E.J. Pearce. 2005. TLR ligands can activate dendritic cells to provide a MyD88-dependent negative signal for Th2 cell development. J. Immunol. 174:742–751. [DOI] [PubMed] [Google Scholar]
- 29.Pearce, E.J., C.M. Kane, and J. Sun. 2006. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem. Immunol. Allergy. 90:82–90. [DOI] [PubMed] [Google Scholar]
- 30.Liu, N., R.R. Montgomery, S.W. Barthold, and L.K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debus, A., J. Glasner, M. Rollinghoff, and A. Gessner. 2003. High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect. Immun. 71:7215–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jankovic, D., M.C. Kullberg, S. Hieny, P. Caspar, C.M. Collazo, and A. Sher. 2002. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 16:429–439. [DOI] [PubMed] [Google Scholar]
- 33.Mack, J.A., S. Anand, and E.V. Maytin. 2005. Proliferation and cornification during development of the mammalian epidermis. Birth Defects Res. C Embryo Today. 75:314–329. [DOI] [PubMed] [Google Scholar]
- 34.Nye, J.S., and R. Kopan. 1995. Developmental signaling. Vertebrate ligands for Notch. Curr. Biol. 5:966–969. [DOI] [PubMed] [Google Scholar]
- 35.Tang, Y.W., and B.S. Graham. 1997. T cell source of type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J. Clin. Invest. 99:2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srikiatkhachorn, A., W. Chang, and T.J. Braciale. 1999. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J. Virol. 73:6590–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tripp, R.A., D. Moore, and L.J. Anderson. 2000. TH(1)- and TH(2)-TYPE cytokine expression by activated T lymphocytes from the lung and spleen during the inflammatory response to respiratory syncytial virus. Cytokine. 12:801–807. [DOI] [PubMed] [Google Scholar]
- 38.Smit, J.J., B.D. Rudd, and N.W. Lukacs. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 203:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudd, B.D., J.J. Smit, R.A. Flavell, L. Alexopoulou, M.A. Schaller, A. Gruber, A.A. Berlin, and N.W. Lukacs. 2006. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176:1937–1942. [DOI] [PubMed] [Google Scholar]
- 40.Miller, A.L., T.L. Bowlin, and N.W. Lukacs. 2004. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 189:1419–1430. [DOI] [PubMed] [Google Scholar]
- 41.Tekkanat, K.K., H.F. Maassab, D.S. Cho, J.J. Lai, A. John, A. Berlin, M.H. Kaplan, and N.W. Lukacs. 2001. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J. Immunol. 166:3542–3548. [DOI] [PubMed] [Google Scholar]
- 42.Amsen, D., A. Antov, D. Jankovic, A. Sher, F. Radtke, A. Souabni, M. Busslinger, B. McCright, T. Gridley, and R.A. Flavell. 2007. Direct regulation of gata3 expression determines the T helper differentiation potential of notch. Immunity. 27:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang, T.C., Y. Yashiro-Ohtani, C. Del Bianco, D.M. Knoblock, S.C. Blacklow, and W.S. Pear. 2007. Notch Directly Regulates Gata3 Expression during T Helper 2 Cell Differentiation. Immunity. 27:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jong, E.C., H.H. Smits, and M.L. Kapsenberg. 2005. Dendritic cell-mediated T cell polarization. Springer Semin. Immunopathol. 26:289–307. [DOI] [PubMed] [Google Scholar]
- 45.Banchereau, J., S. Paczesny, P. Blanco, L. Bennett, V. Pascual, J. Fay, and A.K. Palucka. 2003. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann. N. Y. Acad. Sci. 987:180–187. [DOI] [PubMed] [Google Scholar]
- 46.McKenzie, G.J., C.L. Emson, S.E. Bell, S. Anderson, P. Fallon, G. Zurawski, R. Murray, R. Grencis, and A.N. McKenzie. 1998. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 9:423–432. [DOI] [PubMed] [Google Scholar]
- 47.Mattes, J., M. Yang, A. Siqueira, K. Clark, J. MacKenzie, A.N. McKenzie, D.C. Webb, K.I. Matthaei, and P.S. Foster. 2001. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J. Immunol. 167:1683–1692. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, S., J. Tsukada, W. Suzuki, K. Hayashi, K. Tanigaki, M. Tsuji, H. Inoue, T. Honjo, and M. Kubo. 2006. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 24:689–701. [DOI] [PubMed] [Google Scholar]
- 49.Zhu, J., B. Min, J. Hu-Li, C.J. Watson, A. Grinberg, Q. Wang, N. Killeen, J.F. Urban Jr., L. Guo, and W.E. Paul. 2004. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 5:1157–1165. [DOI] [PubMed] [Google Scholar]
- 50.Lukacs, N.W., M.L. Moore, B.D. Rudd, A.A. Berlin, R.D. Collins, S.J. Olson, S.B. Ho, and R.S. Peebles Jr. 2006. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 169:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller, A.L., R.M. Strieter, A.D. Gruber, S.B. Ho, and N.W. Lukacs. 2003. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J. Immunol. 170:3348–3356. [DOI] [PubMed] [Google Scholar]
- 52.Hogaboam, C.M., C.S. Gallinat, D.D. Taub, R.M. Strieter, S.L. Kunkel, and N.W. Lukacs. 1999. Immunomodulatory role of C10 chemokine in a murine model of allergic bronchopulmonary aspergillosis. J. Immunol. 162:6071–6079. [PubMed] [Google Scholar]
- 53.Thomas, M.S., S.L. Kunkel, and N.W. Lukacs. 2004. Regulation of cockroach antigen-induced allergic airway hyperreactivity by the CXCR3 ligand CXCL9. J. Immunol. 173:615–623. [DOI] [PubMed] [Google Scholar]
- 54.Lundy, S.K., S.A. Lira, J.J. Smit, D.N. Cook, A.A. Berlin, and N.W. Lukacs. 2005. Attenuation of allergen-induced responses in CCR6−/− mice is dependent upon altered pulmonary T lymphocyte activation. J. Immunol. 174:2054–2060. [DOI] [PubMed] [Google Scholar]