Abstract
Epstein-Barr virus (EBV), a ubiquitous B-lymphotropic herpesvirus, has been associated with multiple sclerosis (MS), an inflammatory disease of the central nervous system (CNS), but direct proof of its involvement in the disease is still missing. To test the idea that MS might result from perturbed EBV infection in the CNS, we investigated expression of EBV markers in postmortem brain tissue from MS cases with different clinical courses. Contrary to previous studies, we found evidence of EBV infection in a substantial proportion of brain-infiltrating B cells and plasma cells in nearly 100% of the MS cases examined (21 of 22), but not in other inflammatory neurological diseases. Ectopic B cell follicles forming in the cerebral meninges of some cases with secondary progressive MS were identified as major sites of EBV persistence. Expression of viral latent proteins was regularly observed in MS brains, whereas viral reactivation appeared restricted to ectopic B cell follicles and acute lesions. Activation of CD8+ T cells with signs of cytotoxicity toward plasma cells was also noted at sites of major accumulations of EBV-infected cells. Whether homing of EBV-infected B cells to the CNS is a primary event in MS development or the consequence of a still unknown disease-related process, we interpret these findings as evidence that EBV persistence and reactivation in the CNS play an important role in MS immunopathology.
The etiology of multiple sclerosis (MS), a putative autoimmune disease affecting the central nervous system (CNS) and causing severe neurological disability, remains unknown but is likely to involve both heritable and nonheritable factors (1, 2). Among the latter, infectious agents are the most plausible candidates. Over the past 50 yr, several pathogens have been proposed as the potential causal factor in MS, but for most of them inconsistent results have been obtained (3). To date, EBV stands out as the infectious agent for which there is the most compelling evidence for an association with MS (3–6), as well as with other autoimmune diseases (7–9).
EBV is a B-lymphotropic human DNA herpesvirus that establishes asymptomatic latent infection in most individuals and can cause infectious mononucleosis in adolescents and young adults. Latently infected B cells can express three different programs of viral gene usage depending on the differentiation state of the infected B cell (10, 11). Analysis performed in the tonsils of healthy EBV carriers have shown that naive B cells, which are thought to be newly infected cells, express all nine known latency proteins (Epstein-Barr nuclear antigen [EBNA]1, 2, 3a, 3b, 3c, -LP, and latency membrane protein [LMP]1, 2a, and 2b; growth program), whereas germinal center and memory B cells express a more limited set of latency proteins (EBNA1, LMP1, and LMP2a; default program) (12). In the blood, the virus persists in rare memory B cells expressing no viral proteins, except perhaps EBNA1 and LMP2a (latency program) (12). Occasionally, viral replication occurs in tonsillar plasma cells, resulting in expression of lytic genes and shedding of low numbers of viral particles in the saliva (13). Host immune surveillance is essential both in limiting productive infection and in controlling latent infection. Infectious virus is destroyed by neutralizing antibodies, and all infected states, both lytic and latent, are the targets of cytotoxic CD8+ T cells, with the exception of latently infected, circulating memory B cells (14).
During the last two decades, sero-epidemiological studies have consistently shown that the EBV seropositivity rate in MS patients is higher than in controls (99% vs. 90–95% [4, 5, 15–17]; 83–99% in children with MS compared with 42–72% in age-matched controls [18, 19]). This high seroprevalence rate does not apply to other viruses (4, 17, 19), suggesting that EBV infection is a prerequisite for the development of MS. Furthermore, an increased risk of developing MS has been associated with infectious mononucleosis (20–22) and higher serum levels of anti-EBV antibodies (4, 16, 19, 23–25). Elevations in anti-EBV antibody titers, particularly IgG antibodies to EBNA complex and EBNA-1, have been detected up to 20 yr before the onset of MS symptoms (24, 25), suggesting a role for remote exposure to EBV in MS development. An increased humoral immune response to EBV was also demonstrated in the cerebrospinal fluid (CSF) of patients with MS (26), and oligoclonal IgG binding to EBNA1 (27, 28) and another less well characterized EBV antigen (BRRF2) (28) were found in the CSF of some MS patients. Recently, increased CD4+ and CD8+ T cell responses specific for EBV have been detected in the CSF (29, 30) and blood (28, 31, 32) of MS patients, consistent with abnormal immune activation toward the virus.
Several hypotheses have been addressed to explain the breaking of immune tolerance by EBV, including molecular mimicry between viral and myelin components (33–35), EBV-induced expansion of autoreactive B cells (36), and induction of heat-shock proteins (37) and superantigens (38), but evidence that these mechanisms are relevant to MS is not available yet. Because of its ability to establish a latent infection in B cells, to promote their proliferation and activation, and to reactivate periodically providing a constant antigenic challenge to the immune system (10, 11), EBV is well suited to be a trigger of chronic inflammatory states. However, in the absence of any data demonstrating the presence of EBV in the MS brain (39, 40) and a substantial increase of viral DNA load in the blood of MS patients compared with asymptomatic carriers (6, 32, 41), the link between EBV-related immune alterations and MS remains unclear.
Because intrathecal synthesis of Ig (predominantly oligoclonal IgG) is common to most MS patients (42), and clonally expanded memory B cells have been detected in MS brain lesions and CSF (43–45), we asked whether such an abnormal and compartmentalized humoral immune response could result from a perturbed EBV infection in the CNS. To approach this question, we investigated the expression of markers of EBV latent and lytic infection in postmortem brain specimens from eight cases with secondary progressive MS that we had previously characterized for the presence of prominent B cell/plasma cell infiltration in white matter lesions and the formation of ectopic B cell follicles in the meninges (46, 47). We then extended the search for EBV markers to 12 relapsing-remitting and progressive MS cases with less brain inflammation and to 2 cases with acute MS. Here, we show that intracerebral accumulation of EBV-infected B cells and plasma cells is a regular feature of MS and identify ectopic B cell follicles as main sites of viral persistence. We also provide evidence for a relationship between acute inflammation and EBV reactivation in the MS brain and for CD8+ T cell activation and cytotoxicity toward virally infected B cells/plasma cells. These findings support a role for EBV in MS pathogenesis through persistent infection of brain-infiltrating B cells and the induction of immunopathology.
RESULTS
Germinal center–like features of ectopic B cell follicles and B cell proliferation in acute lesions in the MS brain: first hint of a dysregulated EBV infection
Recently, we identified a subset of early-onset MS cases who died in the secondary progressive phase of the disease (which follows a relapsing-remitting course in 80–85% of the MS patient population) and were characterized by highly infiltrated white matter lesions, presence of lymphocytic aggregates resembling ectopic B cell follicles in the cerebral meninges, and extensive demyelination and axon loss in the adjacent cerebral cortex (Fig. 1 A) (47). Intrameningeal B cell follicles display features of germinal centers, as they are composed of CD20+ B cells clustered around a network of stromal/follicular dendritic cells that express the B cell–attracting chemokine CXCL13 (Fig. 1 B) and are the sites where B cell proliferation (Fig. 1 C) and differentiation into Ig-producing plasma cells occurs (Fig. 1 D). Here, we provide additional evidence that MS ectopic follicles are functional structures involved in B cell maturation, as they contain numerous cells expressing activation-induced cytidine deaminase (AID), the enzyme responsible for somatic hypermutation and Ig class-switch recombination in germinal center B cells (Fig. 1 E), and cells expressing activated caspase-3 (Fig. 1 F), which mediates apoptosis of B cells with low affinity variants of the B cell receptor. Intrameningeal B cell follicles are also enriched in cells expressing the antiapoptotic molecule bcl-2, which promotes survival of positively selected germinal center B cells (Fig. 1 G). In the same set of highly infiltrated MS brains, substantial numbers of bcl-2+ cells were present in some of the intraparenchymal B cell–enriched perivascular cuffs (Fig. 1 G, inset) and B cell proliferation was noted in acute lesions (Fig. 1 H). Because EBV can deliver proliferative, antiapoptotic and activation signals to B cells through expression of latency proteins (11), and because the lymphoid tissue is the main site of EBV persistence and replication (10), we postulated that EBV could promote intracerebral expansion and maturation of B cells and use ectopic lymphoid tissue to establish persistent infection in the MS brain.
Figure 1.
Immunohistochemical characterization of ectopic B cell follicles with germinal center–like features in the cerebral meninges of a subset of MS cases with secondary progressive disease. (A) Localization of an ectopic B cell follicle (asterisk) and a sparse inflammatory infiltrate in the subarachnoid space lined by the pial membrane (arrows) at the entrance of a cerebral sulcus (hematoxylin counterstaining; CD20 immunostaining in the inset). The adjacent cortical gray matter is extensively demyelinated, as indicated by loss of MOG immunoreactivity. (B–D) An ectopic B cell follicle located in the depth of a cerebral sulcus is shown that comprises the following: aggregated CD20+ B cells and a network of stromal/follicular dendritic cells expressing CXCL13 (B, double immunofluorescence staining for CD20 and CXCL13); proliferating B cells (C, double immunofluorescence staining for CD20 and the proliferation marker Ki67; the inset shows three double labeled CD20+/Ki67+ cells at higher magnification); and peripherally located Ig+ plasma cells (D). Immunostainings shown in B–D were performed in serial brain sections. (E–G) Different ectopic follicles are shown that contain the following: numerous cells expressing AID (E); fewer apoptotic cells expressing activated caspase-3 (F); and numerous cells expressing the antiapoptotic molecule bcl-2 (G). Perivascular bcl2+ cells in a white matter lesion (inset in G) and proliferating CD20+ B cells (arrows) in a large perivascular cuff of an acute lesion (H, double immunofluorescence staining for CD20 and Ki67) are shown. Bars: A, B, D–F, and H, 50 μm; C, G, and inset in G, 20 μm; inset in C, 10 μm.
Detection of EBV-encoded small nuclear mRNA (EBER) transcripts in B cells and plasma cells infiltrating the MS brain
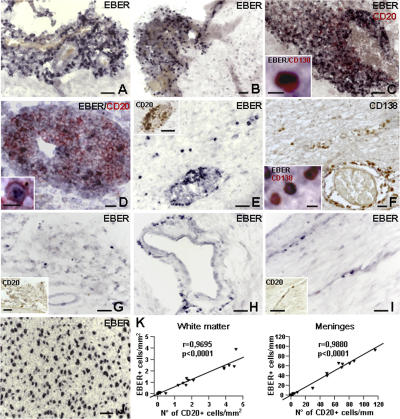
Using in situ hybridization, brain sections of eight MS cases with B cell/plasma cell–rich infiltrates and ectopic B cell follicles (Table S1, available at http://www.jem.org/cgi/content/full/jem.20071030/DC1) were stained for EBER transcripts, which are predominantly expressed during the latent phase of viral infection (11). Substantial accumulation of EBER+ cells was noted in the meninges, with maximal enrichment in ectopic follicles (n = 15) and in the perivascular cuffs of acute (n = 4) and chronic active (n = 16) white matter lesions of all MS cases analyzed (hereafter termed EBV-high) (Figs. 2, A–E, and 3). Less frequently, some EBER+ cells were found sparsely distributed in the lesioned parenchyma, particularly in the areas of plasma cell infiltration (Fig. 2, E and F). No or only occasional EBER+ cells were present in inactive white matter lesions and outside demyelinated areas; none was found inside the cortical gray matter. In all brain sections examined, EBER signals were confined to the cell nuclei, similarly to what was observed in a control, EBV-associated B cell lymphoma (Fig. 2 J). No EBER signals were detected in the B cell areas of a normal lymph node (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071030/DC1).
Figure 2.
Detection of EBER+ cells in the MS brain by in situ hybridization. (A–E) EBV-high MS cases. In situ hybridization for EBER shows enrichment of EBER+ cells (blue-black nuclei) in ectopic B cell follicles located in the meninges of two different MS cases (A and B). Combined in situ hybridization for EBER and immunostaining for CD20 (red surface staining) reveals a high frequency of EBER+ B cells in an ectopic B cell follicle (C) and a lower percentage of B cells expressing EBER in the large perivascular cuff of an acute white matter lesion (D; the same lesion stained for CD20 and Ki67 in an adjacent section is shown in Fig. 1 H). In the insets in C and D, an EBER+/CD138+ plasma cell and an EBER+/CD20+ B cell are shown, respectively, at higher magnification. Perivascular EBER+ (E), CD20+ B cells (inset in E), and CD138+ plasma cells (F) in a large periventricular white matter lesion (E), and some intraparenchymal infiltration by EBER+ cells (E) and plasma cells (F) in the same region. The inset in F shows CD138+ plasma cells positive for EBER inside the parenchyma. (G–I) EBV-low MS cases. EBER+ cells in the meninges (G) and around scarcely infiltrated blood vessels (H and I) in chronic active white matter lesions. The insets in G and I show CD20 immunostaining of the corresponding areas in adjacent sections. In situ hybridization for EBER in a control, EBV-associated B cell lymphoma (J). Bars: A–C, E, G, J, and insets in E, G, and I, 50 μm; D and F, 20 μm; insets in C, D, and F, 10 μm. (K) Statistically significant correlation between the number of CD20+ and EBER+ cells in the white matter (left) and meninges (right) of EBV-low (n = 12) and EBV-high (n = 8) MS cases. EBER+ and CD20+ cells were counted as described in Materials and methods.
By combining in situ hybridization for EBER with immunohistochemistry for the pan B cell marker CD20, 70–90% of EBER+ cells were identified as B cells, whereas 40–90% of B cells were positive for EBER transcripts (Fig. 2, C and D, and Table S1). The highest percentage of B cells expressing EBERs was detected inside and around ectopic B cell follicles (Fig. 2 C), suggesting that these structures may originate from the expansion of EBV-infected B cells. Such an enrichment of EBV-infected cells in ectopic follicles is in contrast with what has been observed in the tonsils of healthy EBV carriers and subjects with infectious mononucleosis in which most infected B cells are found outside B cell follicles (11). A substantial proportion of brain-infiltrating plasma cells (50–80%) was also positive for EBER transcripts (Fig. 2, C and F, insets), whereas no EBER+ cells with the morphological features of neurons, glial, endothelial cells, or meningeal fibroblasts were detected.
We next extended the analysis of EBERs to less infiltrated brain specimens from 12 MS cases with relapsing-remitting, progressive relapsing, primary progressive, and secondary progressive clinical courses (Table S1). Compared with EBV-high MS cases, fewer, often isolated, perivascular EBER+ cells were present in demyelinated lesions and/or meninges from 11 of these 12 MS cases (termed EBV-low) (Figs. 2, G–I, and 3). In one case only, two B cell follicles with EBER+ cells were identified in the meninges despite negligible parenchymal inflammation. Of note, the only EBV− MS case identified in this series (MS80 in Table S1) contained largely inactive lesions and no B cells/plasma cells in the brain tissue blocks analyzed, supporting a relationship between B cells, EBV gene expression, and inflammatory activity. The absence of EBV-infected in cells in immunologically silent, demyelinated lesions may suggest that in those brain regions a more robust EBV infection has occurred at earlier stages of the disease and that infected cells have been eliminated. The identification of EBV-infected cells as predominantly B cells was confirmed by the existence of a positive correlation between the number of EBER+ and CD20+ cells counted in serial brain sections of all the MS cases analyzed (Fig. 2 K).
Figure 3.
Quantification of CD20+, EBER+, and CD8+ cells in MS brain sections. MS cases with high and low brain inflammation were a posteriori classified as EBV-high and EBV-low, respectively. The graphs show significant differences between the two groups in the number of CD20+, EBER+, and CD8+ cells counted in the white matter (A) and meninges (B). Dot points represent values for each MS case. Each value represents the mean of cell counts performed as described in Materials and methods. The bars represent median values for each group (n = 12 for EBV-low MS cases; n = 8 for EBV-high MS cases).
To exclude that inflammation could act as a nonspecific trigger for the recruitment of EBV-infected B cells/plasma cells in the CNS, we performed in situ hybridization for EBER in autopsy brain specimens from seven cases with other neuroinflammatory diseases (two primary cerebral vasculitis, two viral encephalitis, two mycotic meningitis, and one encephalopathy with unknown etiology; Table S1). Each of these cases had B cell infiltrates in the brain parenchyma and/or meninges, but none was positive for EBER transcripts (Fig. S1). No EBER signals were detected in the brain of two nonneurological control cases, one case with Alzheimer's disease, and one case with non-EBV–related lymphoblastic leukemia (Table S1 and Fig. S1).
The above findings demonstrate that abnormal accumulation of EBV-infected cells in the CNS is a common feature of MS and is not linked to any particular disease course or other parameter, including gender, age at onset, disease duration, and immune suppressive therapy (Table S1).
Expression of EBV latent and lytic proteins in B-cells/plasma cells infiltrating the MS brain
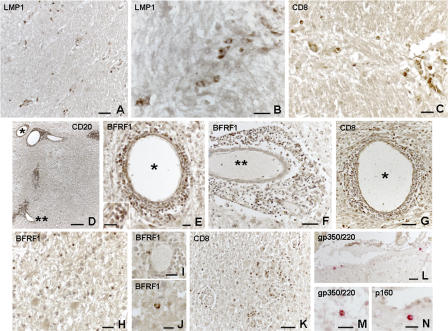
We further analyzed the status of EBV infection in the MS brain using immunohistochemical techniques with antibodies against latent and lytic viral proteins. As markers of viral latency, we studied EBNA2 and LMP1. EBNA2 is the first viral protein to be expressed in EBV-infected cells in vitro and acts as a transactivator that regulates the expression of viral and cellular genes involved in the initiation and maintenance of cell proliferation (growth program) (10, 11). LMP1 is an integral membrane protein that is expressed during the growth and default programs of EBV infection, acts as a constitutively acting mimic of CD40, a key receptor for B cell activation, and inhibits B cell apoptosis by up-regulating the expression of antiapoptotic molecules (10, 11). EBNA2+ and LMP1+ cells were detected perivascularly in active white matter lesions and in the meninges in 13 and 16 of 18 EBV+ MS cases analyzed, respectively (Table S1), the highest frequency being observed in EBV-high MS cases (Fig. 4, A–F). Ectopic B cell follicles contained numerous LMP1+ (Fig. 4 D), but no EBNA2+ cells (not depicted). This pattern of EBV gene expression is reminiscent of that observed in tonsillar germinal center and memory B cells of healthy EBV carriers where the virus expresses the default program, shutting off EBNA2 but still expressing LMP1 (12). Consistent with the absence of EBER transcripts, no EBNA2 and LMP1 immunoreactivity was detected in the brain tissue of cases with other inflammatory neurologic diseases (Table S1).
Figure 4.
Detection of EBV latent and early lytic proteins in the MS brain. In brain sections from EBV-high MS cases, cells expressing EBNA-2 (nuclear staining) are present in the perivascular inflammatory cell infiltrates in acute (A) and chronic active (B) white matter lesions. LMP1+ cells (membrane staining) in a sparse meningeal infiltrate (C) and in an intrameningeal ectopic B cell follicle (D; the inset shows the same follicle stained for EBER in an adjacent section) from EBV-high MS cases. Perivascular EBNA2+ and LMP1+ cells in chronic active lesions from an EBV-low MS case are shown in E and F, respectively. High frequency of BFRF1+ cells in an ectopic intrameningeal B cell follicle from an EBV-high MS case (G). In two different ectopic B cell follicles (H and I), double immunofluorescence for CD20 and BFRF1 shows that BFRF1 is expressed in a substantial proportion of CD20+ B cells, but its intensity is much stronger in CD20− cells (arrows). The insets in G and H highlight the typical perinuclear localization of BFRF1. Double immunofluorescence for Ig and BFRF1 shows that the lytic cycle–associated protein is strongly expressed in a proportion of Ig+ plasma cells in the same ectopic follicle shown in I (J) and in a large perivascular cuff in an acute lesion of an EBV-high MS case (K). Two double-labeled Ig+/BFRF1+ plasma cells are shown at high power magnification (L and M). Bars: K and inset in D, 50 μm; A–J and inset in B, 20 μm; L, M, and insets in G and H, 5 μm.
We then searched for evidence of EBV lytic replication in the MS brain. The lytic program consists of the sequential activation of three distinct classes of viral genes: immediate early, early, and late. BFRF1, a recently identified lytic protein (48), is expressed in the early phases of the EBV replicative cycle and is regulated by two transactivator proteins, ZEBRA and RTA (49). A high frequency of BFRF1+ cells was observed inside and around all intrameningeal B cell follicles analyzed (Fig. 4, G–I, and Table S1), indicating that these structures represent main sites of viral reactivation. No BFRF1 immunoreactivity was found in the B cell areas of a control, normal lymph node (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071030/DC1). Double immunostainings showed that BFRF1 immunoreactivity was present in a substantial proportion of intrameningeal B cells and plasma cells (30–55%) but was much stronger in plasma cells than in B cells (Fig. 4, H–J), in agreement with findings in healthy carriers where terminal differentiation into plasma cells initiates the viral replicative cycle (13). Some BFRF1+ cells (mainly plasma cells) were also detected in the large perivascular cuffs of two acute lesions in two EBV-high MS cases (Fig. 4, K–M), but not in chronic active lesions, indicating a relationship between viral reactivation and acute inflammation.
To evaluate the expression of structural EBV proteins, we stained MS brain sections with an antibody specific for gp350/220, the most abundant glycoprotein of the viral envelope, which is expressed on the membrane of EBV-infected cells (positive control staining is shown in Fig. S2). Except for two occasional gp350/220+ cells observed in one EBV-high MS case, no gp350/220 immunoreactivity was detected in the brain sections of 17 EBER+ MS cases analyzed (Table S1), indicating abortive EBV replication.
Widespread intracerebral EBV reactivation in acute MS
To test the idea that an acute clinical course, which is extremely rare in the MS population, might be associated with more robust EBV reactivation in the CNS, and to exclude the possibility that our findings are only relevant to the late chronic stages of MS, we investigated the distribution of EBV latent and lytic markers in cerebral brain sections from two MS cases with disease durations of 23 d and 10 mo (MSG9 and MSH6, respectively, in Table S1). Both cases had very severe pathology with extensive myelin loss in the periventricular regions of the cerebral hemispheres, multiple actively demyelinating lesions at all CNS levels (not depicted), and prominent B cell infiltration (Fig. 5 D). The meninges, when preserved, were scarcely or not at all infiltrated (not depicted). In situ hybridization for EBER could not be performed in the acute MS cases because of the long tissue fixation time in formalin. However, by immunohistochemistry, accumulation of LMP1+, but not of EBNA2+, cells was noted in all perivascular cuffs containing B cells, indicating expression of the latency default program (Fig. 5, A and B). Notably, numerous cells expressing the early lytic cycle–associated protein BFRF1 were present in large intraparenchymal perivascular cuffs (Fig. 5, D–F) as well as around small blood vessels disseminated throughout the white matter, and less frequently inside the parenchyma (Fig. 5, H–J). A few isolated cells (three to five cells/section) expressing the gp350/220 and the viral capside protein p160 were also present in the meninges and white matter lesions of both acute MS cases (Fig. 5, L–N), indicating viral replication, albeit at a low level. These findings suggest that seeding of the brain by EBV-infected cells is an early event in MS, and that widespread EBV reactivation in the white matter is associated with dramatic inflammatory tissue destruction and fast neurological deterioration.
Figure 5.
Diffuse infiltration of latently and lytically EBV-infected cells in the brain of acute MS cases. (A and B) Presence of LMP1+ cells around scarcely inflamed blood vessels throughout an active lesion of one acute MS case (MSG9; disease duration 23 d). In the same area, perivascular accumulation of CD8+ T cells is observed (C). A highly infiltrated lesion in the white matter of the second acute MS case examined (MSH6; disease duration 10 mo) contains large B cell–enriched perivascular cuffs (D). Immunostainings for BFRF1 and CD8 in adjacent sections show the presence of numerous perivascular BFRF1+ cells (E and F) and CD8+ T cells (G) in the same area. The blood vessels labeled with asterisks in D are shown at high power magnification in E, F, and G. The inset in E shows the typical perinuclear localization of BFRF1. Sparse infiltration of BFRF1+ cells in the white matter of case MSH6 is shown (H). Perivascular (I) and intraparenchymal (J) BFRF1+ cells are shown at high power magnification. Staining of an adjacent section with anti-CD8 mAb shows diffuse infiltration of CD8+ T cells in the same area (K). Presence of isolated cells expressing the structural viral proteins gp350/220 (L and M; membrane staining) and p160 (N; cytoplasmic staining) in the meninges of MSH6 (L and N) and in the white matter of MSG9 (M). Bars: D, 200 μm; A, F, G, K, and L, 50 μm; B, C, E, H, I, M, N, and inset in E, 20 μm; J, 10 μm.
Analysis of EBV DNA and of total and EBV-specific oligoclonal IgG in the MS CSF
We next investigated whether the frequency of EBV-infected cells and the status of EBV infection in the MS brain correlated with viral load and oligoclonal IgG bands (OCBs) in the corresponding postmortem CSF. This set of analyses included 16 MS cases with a relapsing-remitting or progressive clinical course (9 EBV-low and 7 EBV-high). Using real-time PCR, EBV DNA was generally undetectable, being found at low copy number in the CSF of only 2 of 16 MS cases (2 primary progressive MS with 1,310 and 4,900 copies/ml, respectively). None of the other viruses investigated (HSV-1/2, varicella-zoster virus, CMV, human herpesvirus 6 [HHV-6], and JC virus) was detected in the 16 CSF samples analyzed.
OCBs, which are generally CSF restricted and relatively constant throughout the disease (42), were found in the CSF of 15 of 16 MS cases. Interestingly, the number of CSF OCBs was significantly higher (P = 0.0079) in EBV-high MS cases (Fig. 6 A), supporting a relationship between EBV infection and intracerebral B cell expansion and activation. Consistent with previous findings (27, 28), EBV-specific OCBs were detected in some MS cases (7 of 16), but showed no preferential distribution in the EBV-high and EBV-low MS groups (Fig. 6 B). Except for one case, EBV-specific OCBs, when present, were few (median number, 2; range, 1–5) and generally faint (Fig. 6 C), indicating that persistent EBV infection in the MS brain does not yield a robust, virus-specific oligoclonal humoral response that is typical of CNS infections associated with viral replication (50).
Figure 6.
Analysis of total and EBV-specific OCBs in postmortem CSF from MS cases. The graphs show (A) significantly higher numbers of OCBs in the CSF of EBV-high MS cases (n = 7) versus EBV-low MS cases (n = 9) and (B) no difference in the number of EBV-specific OCBs between EBV-high and EBV-low MS cases. Dot points represent values for each MS case, and the bars represent median values for each group. p-values, calculated by the Mann-Whitney U test, are indicated where statistically significant. (C) Affinity-mediated immunoblotting on EBV antigen-coated nitrocellulose paper of isoelectrofocused CSF from two EBV-low MS cases (lanes 1 and 2 correspond to MS154 and MS102 in Table S1, respectively), anti-EBV EA-D monoclonal (mo) antibody used as positive control (lane 3), and serum (ser) from a patient with monoclonal IgG used as control for binding specificity (lane 4). An additional control for binding specificity included CSF from the MS102 case that was blotted onto casein-coated nitrocellulose paper (absence of reactivity; lane 5). Faint (lane 1) and both faint and strong (lane 2) EBV-specific OCBs are present in MS154 and MS102 cases, respectively (arrows indicate OCBs). Of note, MS102 was also positive for EBV DNA and had the highest frequency of EBER+ and CD8+ cells in white matter lesions and meninges among the EBV-low MS cases.
Expansion and cytotoxic activity of CD8+ T cells at sites of accumulation of EBV-infected cells in the MS brain
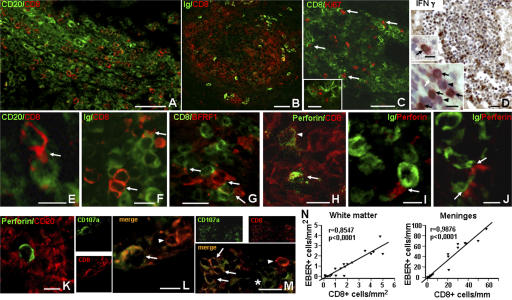
Because cytotoxic CD8+ T cells have a key role in controlling latent EBV infection and in preventing viral replication (14), we next investigated the distribution and activation of CD8+ T cells and their relationship with EBV-infected B cells/plasma cells in the MS brain. We found that the number of CD8+ T cells accumulating in MS meninges and white matter lesions was significantly higher in EBV-high than in EBV-low MS cases (Fig. 3), and that CD8+ T cells infiltrated all sites where virally infected B cells/plasma cells were located, including ectopic B cell follicles (Fig. 7, A and B). In addition, the frequency of CD8+ T cells strikingly correlated with that of EBER+ cells (Fig. 7 N). The highest frequency of CD8+ T cells was observed in the large intraparenchymal perivascular cuffs of EBV-high MS cases (Fig. 7 B). Prominent accumulation of CD8+ T cells was also observed in active lesions of the two acute MS cases, matching the distribution of EBV-infected cells (Fig. 5, C, G and K).
Figure 7.
CD8+ T cell activation and interaction with EBV-infected cells in the brain of EBV-high MS cases. Double immunofluorescence for CD8 and CD20 (A) and for CD8 and Ig (B) shows accumulation of CD8+ T cells in an intrameningeal ectopic B cell follicle (A) and in the perivascular cuff of an acute white matter lesion (B; same infiltrated blood vessel stained for BFRF1 in an adjacent section is shown in Fig. 4 K). The perivascular infiltrate shown in B contains numerous Ki67+ proliferating cells, of which some are CD8+ T cells (arrows) (C). Note the small cluster of three Ki67+/CD8+ cells shown at higher magnification in the inset in C. Immunohistochemistry with anti–IFN-γ antibody (D) reveals strong immunoreactivity (brown staining) in the cytoplasm of numerous cells in an intrameningeal B cell follicle (same follicle double stained for EBER and CD20 in Fig. 2 C). The insets in D show double stainings for CD8 (brown) and IFN-γ (purple). IFN-γ+/CD8+ cells (black arrows), as well as cells single stained for either CD8 (white arrow) or IFN-γ (arrowheads), are identified in the same follicle (lower inset). Two IFN-γ+ cells, one positive (arrow) and one negative (arrowhead) for CD8, are shown in the upper inset. Double immunofluorescence stainings for CD8 and CD20 (E), CD8 and Ig (F), and CD8 and BFRF1 (G) show that CD8+ T cells with an activated, lymphoblastoid morphology cluster around and extend cytoplasmic processes that contact CD20+ B cells, Ig+ plasma cells, and BFRF1+ cells, respectively. Double immunostaining for perforin and CD8 shows the presence of perforin granules in two out of several CD8+ T cells accumulating in the perivascular cuff of a chronic active lesion (H). Polarization of perforin granules is evident in one CD8+ T cell (arrow), but not in the other (arrowhead). Double immunostaining for perforin and Ig (I and J; inflamed meninges) or CD20 (K; intralesional perivascular cuff) shows polarization of perforin granules toward two Ig+ plasma cells, but not toward CD20+ B cells. Double immunostaining for CD8 and CD107a (L and M) reveals surface distribution of CD107a in some CD8+ T cells (arrows) accumulating in the meninges (L) and in an intraparenchymal perivascular cuff (M). Note the presence in the same microscopic fields of CD8+ T cells that do not express CD107a (arrowheads in L and M) and of a CD8− cell with a cytoplasmic distribution of CD107a (asterisk in M) . Bars: A–C, 50 μm; D, G, M, and inset in C, 20 μm; E, F, H–L, and lower inset in D, 10 μm; upper inset in D, 5 μm. (N) Statistically significant correlation between the number of CD8+ and EBER+ cells in the white matter (left) and meninges (right) of EBV-low (n = 12) and EBV-high (n = 8) MS cases. EBER+ and CD8+ cells were counted as described in Materials and methods.
Four EBV-high MS cases were analyzed for expression of CD8+ T cell activation markers and cytotoxic activity. We observed that the proliferation antigen Ki67 was expressed in a small percentage (2–3%) of CD8+ T cells accumulating in active intraparenchymal lesions and ectopic follicles (Fig. 7 C). Moreover, immunoreactivity for IFN-γ was detected in the cytoplasm of a discrete proportion (10–20%) of brain-infiltrating leukocytes (Fig. 7 D), many of which (60–80%) were identified as CD8+ T cells by double immunostaining (Fig. 7 D, insets). These findings indicate activation of CD8+ T cell biological responses that follow sustained antigenic stimulation. We also noted that several CD8+ T cells in the highly infiltrated MS brains had an activated lymphoblastoid morphology with cytoplasmic protrusions that contacted B cells and, more frequently, plasma cells and BFRF1+ cells (Fig. 7, E–G). By double immunostaining for CD8 and the lytic protein perforin, we found that 70–80% of the cells expressing perforin were CD8+ T cells, and that ∼3% of the CD8+ T cells were perforin+. Approximately 30% of the perforin-expressing cells showed polarization of perforin granules toward a target cell (Fig. 7 H), which was in most cases identified as a plasma cell (Fig. 7, I–K). This observation, together with the finding that a few CD8+ T cells infiltrating the MS brain displayed membrane expression of CD107a (Fig. 7, L and M), a component of cytotoxic granules that is exposed onto the cell surface after release of lytic granule contents (51), supports an ongoing cytotoxic activity at sites of EBV infection.
Consistent with the idea that an antiviral immune response occurs in the MS brain, blood dendritic cell antigen 2+ plasmacytoid dendritic cells, which are the main source of type I IFN and have a key role in antiviral immunity (52), were detected in many MS immune infiltrates. The highest frequency of plasmacytoid dendritic cells was found in intrameningeal B cell follicles and active lesions of EBV-high MS cases (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20071030/DC1). Prominent macrophage activation was also associated with intracerebral accumulation of EBV-infected cells and CD8+ T cells (not depicted).
DISCUSSION
Although an association between EBV and MS has been proposed for nearly 30 yr (15, 53), the mechanisms linking EBV infection to MS immunopathology have remained elusive. By analyzing postmortem brain tissue from MS cases with different disease courses, we show that accumulation of EBV-infected B cells/plasma cells in the meninges and perivascular compartment of white matter lesions is a common feature of MS and that the frequency of EBV-harboring cells correlates with the degree of brain inflammation. The absence of EBV in brain-infiltrating B cells in other inflammatory neurological diseases indicates that homing of EBV-infected B cells to the CNS is specific to MS and not a general phenomenon driven by inflammation. The other major finding of this study is that ectopic B cell follicles forming in the cerebral meninges of MS cases with secondary progressive disease are main sites of EBV persistence, substantiating a direct link between EBV infection and B cell dysregulation in MS. Methodological differences, as well as poor tissue preservation and absence of relevant inflammatory activity in the brain tissue blocks analyzed, may explain the failure of two previous studies to detect EBV in the MS brain using in situ hybridization for EBER and immunohistochemistry for viral proteins (39, 40).
The pattern of viral proteins expressed in the brain of most MS cases analyzed here is indicative of the growth (EBNA2, LMP1) and default (LMP1) programs of EBV latent infection. Because cells expressing EBNA2 and LMP1 are usually absent in the blood (10, 11), these findings indicate complete disruption of EBV regulation. This could be due to an underlying defect in the immune response or to the inability of T cells to eliminate latently infected cells in an immune-privileged site, such as the CNS, that is not as readily accessible to T cell surveillance. Consistent with a predominantly latent infection, in the MS CSF the presence of low titers of EBV DNA and of a strong, EBV-specific and oligoclonal humoral immune response was only occasional. However, detection of the early lytic cycle–associated protein BFRF1 in highly infiltrated brains of cases with secondary progressive MS and with acute, fatal MS indicates an attempt of EBV to enter the replicative cycle. These findings raise the possibility that periodic EBV reactivation is related to inflammatory activity in the brain parenchyma of MS patients, as assessed by gadolinium-enhanced magnetic resonance imaging (54). Such a scenario would be consistent with the results of a recent study showing higher numbers of gadolinium-enhanced lesions in relapsing-remitting MS patients with stable levels of serum IgG specific for EBV early antigens (which are considered markers of EBV reactivation) as compared with early antigen seronegative MS patients (55).
In MS patients, the intracerebral pool of virus-harboring B cells could be maintained by several mechanisms, including migration of infected B cells from the blood circulation, virus-driven B cell proliferation, and, perhaps, also local shedding of low numbers of viral particles due to occasional viral replication. B cells normally do not migrate into the CNS tissue and represent only a minority of the lymphocytes circulating through the CSF in physiological conditions. However, antigen-stimulated memory B cells (56) and abnormally activated B cells, including EBV-infected B cells (57) and malignant B cells (58), may home to the CNS through mechanisms that probably involve B cell–attracting chemokines, such as CXCL12 and CXCL13 (58, 59). Expression of the latency proteins EBNA2 and LMP1, both of which provide proliferative and prosurvival signals to B cells (10), and the presence of foci of B cell proliferation in the MS brain support a mechanism of EBV-driven B cell expansion. At advanced stages of the disease (secondary progressive MS), EBV-infected activated B cells may be induced to organize themselves into B cell follicles, thereby allowing viral persistence through exploitation of the B cell maturation process that takes place therein (12, 13). The question remains as to which antigens are involved in the activation of B cells undergoing germinal center reactions in ectopic follicles, or if EBV, through expression of the latency proteins LMP1 and LMP2a, which simulate activation of the CD40 receptor and B cell receptor, respectively (10), can make latently infected B cells potentially independent of T cell help and/or antigen. Viral reactivation, which was found to be predominantly associated with ectopic B cell follicles and acute MS lesions, most likely occurs as a consequence of B cell differentiation into Ig-producing plasma cells (13). The latter may be promoted by interactions between viral factors and inflammatory cytokines, like B cell–activating factor of the tumor necrosis factor family, which cooperates with LMP1 in inducing T cell–independent Ig heavy chain class switching (60, 61), and type I IFN and IL-6 released by activated plasmacytoid dendritic cells (62).
To date, the strongest evidence for a causative role of EBV in MS comes from sero-epidemiological studies (4, 5). Particularly, the marked increase in serum antibody titers to EBV antigens (mainly EBNA complex) several years before clinical onset suggests an involvement of EBV early in MS pathogenesis (24, 25). Such an early increase in anti-EBNA antibodies could be related to EBV spread in the CNS, which probably occurs long before clinical manifestations of MS. Furthermore, the fact that the risk of developing MS increases with infectious mononucleosis (20–22) suggests that a higher frequency of circulating infected B cells can predispose to viral invasion of the CNS and/or to immune hyperreactivity to EBV resulting in an immunopathologic response. The numerous similarities between epidemiology of MS and infectious mononucleosis, including occurrence in young adults, similar latitude gradient distribution, association with late EBV infection, increased frequency in individuals with high socioeconomic status, and earlier onset in women than in men, strengthen the concept that EBV infection is implicated in MS etiology (5). The demonstration of an abnormal accumulation of EBV-harboring B cells and plasma cells in the MS brain further supports this concept. However, it cannot be excluded that dysregulated EBV infection in the CNS, rather than being a primary trigger of MS, might be the consequence of an underlying, still unknown, disease process resulting in attraction of EBV-infected B cells to preexisting CNS lesions. In either case, a role for EBV in the initial stages of the disease is indicated by the diffuse viral infection observed in the brain of acute MS cases.
Persistent infection of CNS-infiltrating B cells with EBV provides a sound explanation for key features of MS pathology. Through expansion and activation of latently infected B cell clones, EBV would be directly responsible for the persistent intrathecal synthesis of oligoclonal IgG, which is common to most MS patients (42). This view is supported by the demonstration of an increased number of OCBs in the CSF of MS cases with a high frequency of brain-infiltrating EBV-infected B cells and plasma cells. EBV-driven expansion of postgerminal center B cells would also be consistent with enrichment of memory B cells and plasma blasts in the CSF of MS patients (63), and with the presence in MS lesions and CSF of B cell clones showing extensive somatic mutations in the Ig variable gene segments (43–45). The pattern of CSF OCBs is usually stable over time in MS patients (42), suggesting persistence of the same EBV-infected B cell clones. To date, the identity of the antigens recognized by OCBs has remained elusive. As shown in previous studies (27, 28) and confirmed here, CSF OCBs specific for EBV can be detected in a proportion of MS patients, but their presence does not appear to be related to the frequency of EBV-infected B cells in the brain tissue. The heterogeneous pattern of antibody reactivities toward CNS-restricted and ubiquitous self-antigens and toward infectious agents in the CSF of MS patients (64) may reflect a random, EBV-driven activation of B cell clones with different antigen specificities. Interestingly, production of autoantibodies is also a feature of infectious mononucleosis (65) and does not necessarily reflect a pathogenic immune response if an autoreactive T cell response is not induced.
One of the main implications of the present findings is that a chronic immune response toward latently and lytically infected B cells/plasma cells underlies the continuous recruitment and activation of inflammatory cells in the MS brain, resulting in an immunopathologic process. Evidence supporting an ongoing antiviral immune response at sites of accumulation of EBV-infected cells in the MS brain includes: (a) existence of a positive correlation between frequency and localization of CD8+ T cells and EBV-infected B cells/plasma cells; (b) presence of CD8+ T cells showing features of antigen-induced activation, such as proliferation, IFN-γ production, polarization of perforin granules, and surface expression of the degranulation marker CD107a, the latter two suggesting cytotoxicity; (c) establishment of direct interactions between CD8+ T cells and B cells/plasma cells, the latter apparently being the preferential target of a cytotoxic response; and (d) recruitment of plasmacytoid dendritic cells. Cross-presentation of viral antigens by dendritic cells present in MS-immune infiltrates probably plays a major role in the expansion and activation of EBV-specific CD8+ T cells (66, 67). This scenario would provide an explanation for the dominance and persistence of CD8+ T cell clones in MS lesions, as revealed by CDR3 spectratyping analysis (68, 69), and is consistent with the presence of EBV-specific CD8+ T cells in the CSF of MS patients (30).
CD8+ T cell–mediated immunopathology is the major determinant of tissue destruction in EBV-associated diseases (14) and may therefore contribute also to damage of myelin sheaths and axons in MS, probably in conjunction with deposition of antibody-complement complexes in the CNS tissue (1). The observation that in MS T cells, B cells, plasma cells, and myeloid and plasmacytoid dendritic cells preferentially accumulate in the meninges and perivascular compartment of white matter lesions (66, 70, and this study) suggests that the immune response targets antigens localized outside the neural parenchyma proper and that bystander tissue damage may be mainly caused by cytotoxic molecules diffusing from the above sites. This concept is highlighted in Fig. 1 A, which shows extensive myelin loss in the non-infiltrated cerebral gray matter adjacent to highly inflamed meninges containing a B cell follicle.
Because increased seroprevalence, higher frequency of EBV-infected B cells in the blood, and altered immune reactivity to EBV are common to other chronic inflammatory diseases with dysregulated B cell and autoimmune features, including systemic lupus erythematosus and rheumatoid arthritis (RA) (7–9, 71), comparison of EBV–host interactions and analysis of the relationship between EBV-infected cells and CD8+ T cells in affected tissues may provide clues for EBV-related, though disease-specific, immunopathologic mechanisms. The presence of EBV-infected cells in the RA synovium is controversial (72). However, several pathologic features in common with the MS brain, such as formation of ectopic germinal centers (73) and accumulation of CD8+ T cells (74) and plasmacytoid dendritic cells (75), raise the suspicion that EBV might be the trigger of B cell abnormalities and immunopathologic response in RA. This possibility is currently under investigation in RA and other autoimmune diseases.
Collectively, the strong association of EBV with MS indicated by epidemiological studies (4, 5), the increased EBV-specific humoral and cellular immune response to EBV in MS patients (6), and the present demonstration of a perturbed EBV infection in the MS brain provide strong support for the idea that EBV has a key role in MS pathogenesis. However, several questions remain open, particularly what attracts EBV-infected B cells and causes viral reactivation in the CNS, and if viral factors, coinfections, other environmental influences, and genetic factors increase the risk for dysregulated EBV infection in MS. Recently, an allelic variant of the gene encoding the interleukin 7 receptor α chain (IL7R) has been identified as a risk factor for MS (76–78) and shown to influence the amount of soluble and membrane-bound form of the receptor (77). In light of the present findings, this association is particularly intriguing because the IL-7–IL-7R pathway controls proliferation and survival of B cells and T cells, and is essential for the development and maintenance of memory T cells. It is envisaged that a better understanding of EBV–host interactions in susceptible individuals will be relevant for the design of new preventive and therapeutic strategies for MS.
MATERIALS AND METHODS
Postmortem brain tissue specimens and CSF.
This study was performed on postmortem brain tissue from 22 MS patients, 7 patients with other inflammatory neurological diseases, 2 control patients without neurological disease, 1 patient with Alzheimer's disease, and 1 patient with non–EBV-related lymphoblastic leukemia. Patient details, including treatment status, are given in Table S1. MS brain and control tissue specimens were provided by the UK Multiple Sclerosis Tissue Bank at Imperial College London (confirmation of MS diagnosis provided by Drs F. Roncaroli and R. Nicholas), the Department of Neurosciences, Ophthalmology and Genetics, University of Genova, Italy, and the Institute of Pathological Anatomy, U.C.S.C. Policlinico A. Gemelli, Rome, Italy. The two acute MS cases were provided and characterized by J.W. Prineas (University of Sydney, Sydney, Australia). Tissue processing is described in the Supplemental Materials and methods. The postmortem delay ranged between 7 and 27 h (median time, 18 h). This study was approved by the Ethics Committee of the Istituto Superiore di Sanità.
For the MS cases, 34 tissue blocks (2 × 2 cm) from the cerebral hemispheres were analyzed and classified by histopathological methods or by immunohistochemistry using anti–myelin oligodendrocyte glycoprotein (MOG) antibody to identify areas of inflammation and demyelination (46, 47). Immunohistochemical staining for T and B cells, macrophages, and MHC class II molecule expression was performed as described previously (46, 47) to evaluate the degree of lesion inflammatory activity. Postmortem CSF samples were also available from some of the MS cases (n = 16) obtained from the UK MS Tissue Bank and were used for OCB determination and viral DNA analysis. The set of CSF samples used in this study was rather homogeneous (mean albumin concentration, 31.7 mg/dL, with a standard error of 2.1 and a 95% CI of 4.9; mean postmortem delay of CSF collection, 17.1 h, with a standard error of 1.2 and a 95% CI of 2.6).
EBER—in situ hybridization.
In situ hybridization experiments were performed using the Epstein-Barr virus (EBER) PNA Probe/Fluorescein and the PNA ISH detection kit (Dako), according to the manufacturer's instructions. Treatment with proteinase K was performed on frozen, PFA fixed-frozen, and paraffin-embedded sections at the following dilutions in TBS: 1:500 for 10 min, 1:100 and 1:10 for 20 min, respectively. An EBV-associated B cell lymphoma (paraffin-embedded sections provided by the Pathology Department of S. Andrea Hospital, Rome) was used as positive control tissue for EBER signals. Sections were sealed in aqueous medium and viewed and photographed with an Axiophot Zeiss microscope equipped with an Axiocam digital camera, using the Axiovision 4 AC software. Double staining for EBER and CD20 or CD138 was performed as described in the Supplemental Materials and methods.
Immunohistochemistry.
Deparaffinized 5-μm-thick and air-dried, acetone-fixed 10-μm-thick cryosections were rehydrated with PBS and immunostained with antibodies specific for human CD3, CD8, CD20, MHC class II, MOG, CD68, CD138, Ki67, IFN-γ, blood dendritic cell antigen 2, AID, bcl2, or activated caspase-3, and for the EBV proteins, EBNA2, LMP1, BFRF1, gp350/220, and p160 (Table S2, available at http://www.jem.org/cgi/content/full/jem.20071030/DC1). For single staining, postfixed sections were subjected to the antigen retrieval procedure and incubated for 20 min with 0.1% H2O2 in PBS to eliminate endogenous peroxidase activity for 1 h with 10% of normal sera and overnight at 4°C with the primary antibodies. The binding of biotinylated secondary antibodies was visualized with avidin-biotin horseradish peroxidase or alkaline phosphatase complex technique (ABC Vectastain Elite and Standard ABC-AP kit; Vector Laboratories) and 3,3′-diaminobenzidine containing 0.01% H2O2 as substrate or Fast Red (Sigma-Aldrich), respectively. All sections were counterstained with hematoxylin and analyzed as described above. Negative controls included the use of IgG isotype controls or preimmune serum, or omission of the primary antibody.
Indirect immunofluorescence and confocal analysis.
After an initial blockade with 10% normal sera in PBS, sections were incubated overnight at 4°C with unconjugated primary antibodies specific for CD8, CD20, Ig-A,-G,-M, Ki67, CXCL13, perforin, CD107a, or BFRF1, or with fluorescein-conjugated anti–Ig-A,-G, -M antibody (Table S2), alone or in different combinations. Sections were then incubated for 1 h at room temperature with fluorochrome-conjugated secondary antibodies and sealed in Vectashield (Vector Laboratories). For negative controls, primary antibodies were replaced with preimmune serum and IgG isotype controls. Images were analyzed and acquired with a laser scanning confocal microscope (LSM 5 Pascal; Carl Zeiss, Inc.). Further details about immunohistochemical and indirect immunofluorescence procedures are given in the Supplemental Materials and methods.
Quantitative analysis.
The number of EBER+, CD20+, and CD8+ cells was counted in six adjacent sections per tissue block (two 2 × 2-cm sections were analyzed for each immunohistochemical staining) at 20× by one investigator (B. Serafini) blinded to the case number. One to two blocks per MS case were analyzed. Values are expressed as the mean number of positive cells per mm2 in the white matter and per mm in the meninges.
Viral DNA detection.
Using the TaqMan technology (79), CSF samples were tested by real-time PCR for DNA of the following viruses: EBV, HSV-1, HSV-2, varicella-zoster virus, CMV, HHV6-A, HHV6-B, and JC virus, as described in the Supplemental Materials and methods. PCR primers and protocols are listed in Table S3, which is available at http://www.jem.org/cgi/content/full/jem.20071030/DC1.
Isoelectric focusing immunoblot.
The pattern of OCBs in autopsy CSF was investigated with agarose isoelectric focusing, pH 3.0–10.0 (Cambrex), and affinity immunoblotting using a standard protocol (80). Although the lack of paired serum samples, which were not available at autopsy, inherently affected the CSF analysis, in MS OCBs are typically restricted to the CSF, without corresponding serum bands (42). IgG and albumin were determined with turbidimetry (Cobas Integra). EBV-specific OCBs were determined using a previously published isoelectric focusing/affinity-mediated immunoblotting protocol (80), using nitrocellulose membranes that had been previously coated with highly pure EBV antigen (strain P3HR1), which contains all viral antigens, and a mouse monoclonal anti-EBV EA-D IgG as a positive control (both from Fitzgerald Industries International). The serum of a patient with a monoclonal gammapathy tested on EBV-precoated papers and EBV-specific OCB+ CSF samples tested on lanes coated with a blocking solution–containing casein (WesternBreeze; Invitrogen) only were used for assessing nonspecific binding. For each sample, 0.3 μg IgG was used.
Statistical analysis.
The Mann-Whitney U test was used to compare the median values of EBER+, CD20+, and CD8+ cells counted in adjacent MS brain sections and of CSF OCBs between EBV-low and EBV-high groups of MS cases. Spearman's rank correlation was calculated to analyze the relationship between the number of EBER+ cells and the number of CD20+ or CD8+ T cells in MS brain sections. A p-value lower than 0.05 was considered significant.
Online supplemental material.
Materials and methods for tissue processing and immunohistochemistry, and PCR protocols for viral DNA detection in the CSF are included in the supplemental material. Supplemental Materials and methods, Tables S1–S3, and Figs. S1–S3 are available at http://www.jem.org/cgi/content/full/jem.20071030/DC1.
Supplemental Material
Acknowledgments
We thank Dr. Egidio Stigliano (Policlinico A. Gemelli, Rome) for providing brain tissue from case MSG1, all the OIND, and two control cases; Prof. J.W. Prineas (University of Sydney, Australia) for providing brain tissue from two acute MS cases; Dr. E. Capello and Prof. G.L. Mancardi (University of Genova, Genova) for providing brain tissue from MS2 and MS5 cases; and Dr. J.M. Middeldorp (Vrije Universiteit Medical Center, Amsterdam) for the kind gift of anti-p160 mAb. We are grateful to Prof. Giulio Levi for helpful comments on the manuscript. We also thank Mrs E. Sansonetti for graphical work and Dr. Maria Puopolo for statistical analysis.
Most tissue samples were supplied by the UK Multiple Sclerosis Tissue Bank (www.ukmstissuebank.imperial.ac.uk), funded by the MS Society of Great Britain and Northern Ireland (registered charity 207495). This work was supported by 6th Framework Program of the European Union NeuroproMiSe LSHM-CT-2005-01863 (to F. Aloisi), Italian MS Foundation (fellowship to R. Magliozzi), and grant RC2006 of the Italian Ministry of Health to Mondino Foundation.
The authors have no conflicting financial interests.
Abbreviations used: AID, activation-induced cytidine deaminase; CNS, central nervous system; CSF, cerebrospinal fluid; EBER, EBV-encoded small nuclear mRNA; EBNA, Epstein-Barr nuclear antigen; HHV-6, human herpesvirus 6; LMP, latency membrane protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; OCB, oligoclonal IgG band; RA, rheumatoid arthritis.
References
- 1.Compston, A., and A. Coles. 2002. Multiple sclerosis. Lancet. 359:1221–1231. [DOI] [PubMed] [Google Scholar]
- 2.Giovannoni, G., and G. Ebers. 2007. Multiple sclerosis: the environment and causation. Curr. Opin. Neurol. 20:261–268. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni, G., G.R. Cutter, J. Lunemann, R. Martin, C. Münz, S. Sriram, I. Steiner, M.R. Hammerschlag, and C.A. Gaydos. 2006. Infectious causes of multiple sclerosis. Lancet Neurol. 5:887–894. [DOI] [PubMed] [Google Scholar]
- 4.Haahr, S., and P. Höllsberg. 2006. Multiple sclerosis is linked to Epstein-Barr virus infection. Rev. Med. Virol. 16:297–310. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio, A., and K.L. Munger. 2007. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 61:288–299. [DOI] [PubMed] [Google Scholar]
- 6.Lünemann, J.D., T. Kamradt, R. Martin, and C. Münz. 2007. Epstein Barr virus: environmental trigger of multiple sclerosis? J. Virol. 13:6777–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, A.S., N.F. Rothfield, and J.C. Niederman. 1971. Raised antibody titres to E.B. virus in systemic lupus erythematosus. Lancet. 1:167–168. [DOI] [PubMed] [Google Scholar]
- 8.Harley, J.B., I.T. Harley, J.M. Guthridge, and J.A. James. 2006. The curiously suspicious: a role for Epstein-Barr virus in lupus. Lupus. 15:768–777. [DOI] [PubMed] [Google Scholar]
- 9.Balandraud, N., J. Roudier, and C. Roudier. 2004. Epstein-Barr virus and rheumatoid arthritis. Autoimmun. Rev. 3:362–376. [DOI] [PubMed] [Google Scholar]
- 10.Thorley-Lawson, D.A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75–82. [DOI] [PubMed] [Google Scholar]
- 11.Küppers, R. 2003. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 3:801–812. [DOI] [PubMed] [Google Scholar]
- 12.Babcock, G.J., D. Hochberg, and A.D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 13:497–506. [DOI] [PubMed] [Google Scholar]
- 13.Laichalk, L.L., and D.A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hislop, A.D., G.S. Taylor, D. Sauce, and A.B. Rickinson. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617. [DOI] [PubMed] [Google Scholar]
- 15.Bray, P.F., L.C. Bloomer, V.C. Salmon, M.H. Bagley, and P.D. Larsen. 1983. Epstein-Barr virus infection and antibody synthesis in patients with multiple sclerosis. Arch. Neurol. 40:406–408. [DOI] [PubMed] [Google Scholar]
- 16.Sumaya, C.V., L.W. Myers, G.W. Ellison, and Y. Ench. 1985. Increased prevalence and titer of Epstein-Barr virus antibodies in patients with multiple sclerosis. Ann. Neurol. 17:371–377. [DOI] [PubMed] [Google Scholar]
- 17.Wandinger, K., W. Jabs, A. Siekhaus, S. Bubel, P. Trillenberg, H. Wagner, K. Wessel, H. Kirchner, and H. Hennig. 2000. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 25:178–184. [DOI] [PubMed] [Google Scholar]
- 18.Alotaibi, S., J. Kennedy, R. Tellier, D. Stephens, and B. Banwell. 2004. Epstein-Barr virus in pediatric multiple sclerosis. JAMA. 291:1875–1879. [DOI] [PubMed] [Google Scholar]
- 19.Pohl, D., B. Krone, K. Rostasy, E. Kahler, E. Brunner, M. Lehnert, H.J. Wagner, J. Gartner, and F. Hanenfeld. 2006. High sereoprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology. 67:2063–2065. [DOI] [PubMed] [Google Scholar]
- 20.Operskalski, E.A., B.R. Visscher, R.M. Malmgren, and R. Detels. 1989. A case-control study of multiple sclerosis. Neurology. 39:825–829. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg, C., O. Andersen, A. Vahlne, M. Dalton, and B. Runmarker. 1991. Epidemiological investigation of the association between infectious mononucleosis and multiple sclerosis. Neuroepidemiology. 10:62–65. [DOI] [PubMed] [Google Scholar]
- 22.Thacker, E.L., F. Mirzaei, and A. Ascherio. 2006. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59:499–503. [DOI] [PubMed] [Google Scholar]
- 23.Sundström, P., P. Juto, G. Wadell, G. Hallmans, A. Svenningsson, L. Nystrom, J. Dillner, and L. Forsgren. 2004. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology. 62:2277–2282. [DOI] [PubMed] [Google Scholar]
- 24.Levin, L.I., K.L. Munger, M.V. Rubertone, C.A. Peck, E.T. Lennette, D. Spiegelmann, and A. Ascherio. 2005. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 293:2496–2500. [DOI] [PubMed] [Google Scholar]
- 25.DeLorenze, G.N., K.L. Munger, E.T. Lennette, N. Orentreich, J.H. Vogelman, and A. Ascherio. 2006. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch. Neurol. 63:839–844. [DOI] [PubMed] [Google Scholar]
- 26.Bray, P.F., J. Luka, K.W. Culp, and J.P. Schlight. 1992. Antibodies against Epstein-Barr nuclear antigen (EBNA) in multiple sclerosis CSF, and two pentapeptide sequence identities between EBNA and myelin basic protein. Neurology. 42:1798–1804. [DOI] [PubMed] [Google Scholar]
- 27.Rand, K.H., H. Houch, N.D. Denslow, and K.M. Heilman. 2000. Epstein-Barr virus nuclear antigen-1 (EBNA-1) associated oligoclonal bands in patients with multiple sclerosis. J. Neurol. Sci. 173:32–39. [DOI] [PubMed] [Google Scholar]
- 28.Cepok, S. D. Zhou, R. Srivastava, S. Nessler, S. Stei, K. Bussow, N. Sommer, and B. Hemmer. 2005. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J. Clin. Invest. 115:1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmøy, T., and F. Vartdal. 2004. Cerebrospinal fluid T cells from multiple sclerosis patients recognize autologous Epstein-Barr virus-transformed B cells. J. Neurovirol. 10:52–56. [DOI] [PubMed] [Google Scholar]
- 30.Scotet, E., M.A. Peyrat, X. Saulquin, C. Retiere, C. Couedel, F. Davodeau, N. Dulphy, A. Toubert, J.D. Bignon, A. Lim, et al. 1999. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 29:973–985. [DOI] [PubMed] [Google Scholar]
- 31.Höllsberg, P., H.J. Hansen, and S. Haahr. 2003. Altered CD8+ T-cell responses to selected Epstein-Barr virus immunodominant epitopes in patients with multiple sclerosis. Clin. Exp. Immunol. 132:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lünemann, J.D., N. Edwards, P.A. Muraro, S. Hayashi, J.I. Cohen, C. Munz, and R. Martin. 2006. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T-cells in multiple sclerosis. Brain. 129:1493–1506. [DOI] [PubMed] [Google Scholar]
- 33.Wucherpfennig, K.W., and J.L. Strominger. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 80:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang, H.L., H. Jacobsen, S. Ikemizu, C. Andersson, K. Harlos, L. Madsen, P. Hjort, L. Sondergaard, A. Svejgaard, K. Wucherpfennig, et al. 2002. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 3:940–943. [DOI] [PubMed] [Google Scholar]
- 35.Holmøy, T., E.O. Kvale, and F. Vartdal. 2004. Cerebrospinal fluid CD4+ T cells from a multiple sclerosis patient cross-recognize Epstein-Barr virus and myelin basic protein. J. Neurovirol. 10:278–283. [DOI] [PubMed] [Google Scholar]
- 36.Pender, M.P. 2003. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 24:584–588. [DOI] [PubMed] [Google Scholar]
- 37.van Sechel, A.C., J.J. Bajramovic, M.J. Stipdonk, C. Persoon-Deen, S.B. Geutskens, and J.M. van Noort. 1999. EBV-induced expression and HLA-DR-restricted presentation by human B cells of alpha-B-crystallin, a candidate autoantigen in multiple sclerosis. J. Immunol. 162:129–135. [PubMed] [Google Scholar]
- 38.Perron, H., J.P. Perin, F. Rieger, and P.M. Alliel. 2000. Particle-associated retroviral RNA and tandem RGH/HERV-W copies on human chromosome 7q: possible components of a ‘chain-reaction’ triggered by infectious agents in multiple sclerosis? J. Neurovirol. 6:S67–S75. [PubMed] [Google Scholar]
- 39.Hilton, D.A., S. Love, A. Fletcher, and H.J. Pringle. 1994. Absence of Epstein-Barr virus RNA in multiple sclerosis as assessed by in situ hybridisation. J. Neurol. Neurosurg. Psychiatry. 57:975–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opsahl, M.L., and P.G.E. Kennedy. 2007. An attempt to investigate the presence of Epstein Barr virus in multiple sclerosis and normal control brain tissue. J. Neurol. 254:425–430. [DOI] [PubMed] [Google Scholar]
- 41.Wagner, H.J., K.L. Munger, and A. Ascherio. 2004. Plasma viral load of Epstein-Barr virus and risk of multiple sclerosis. Eur. J. Neurol. 11:833–834. [DOI] [PubMed] [Google Scholar]
- 42.Link, H., and Y.-M. Huang. 2006. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J. Neuroimmunol. 180:17–28. [DOI] [PubMed] [Google Scholar]
- 43.Qin, Y., P. Duquette, Y. Zhang, P. Talbot, R. Poole, and J. Antel. 1998. Clonal expansion and somatic hypermutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J. Clin. Invest. 102:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colombo, M., M. Dono, P. Gazzola, S. Rondella, A. Valetto, N. Chiorazzi, G.L. Mancardi, and M. Ferrarini. 2000. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J. Immunol. 164:2782–2789. [DOI] [PubMed] [Google Scholar]
- 45.Owens, G.P., A.M. Ritchie, M.P. Burgoon, R.A. Williamson, J.R. Corboy, and D.H. Gilden. 2003. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J. Immunol. 171:2725–2733. [DOI] [PubMed] [Google Scholar]
- 46.Serafini, B., B. Rosicarelli, R. Magliozzi, E. Stigliano, and F. Aloisi. 2004. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magliozzi, R., O. Howell, A. Vora, B. Serafini, R. Nicholas, M. Puopolo, R. Reynolds, and F. Aloisi. 2007. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 130:1089–1104. [DOI] [PubMed] [Google Scholar]
- 48.Farina, A., R. Santarelli, R. Gonnella, R. Bei, R. Muraro, G. Cardinali, S. Uccini, G. Ragona, L. Frati, A. Faggioni, and A. Angeloni. 2000. The BFRF1 gene of Epstein-Barr virus encodes a novel protein. J. Virol. 74:3235–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Granato, M., A. Farina, R. Gonnella, R. Santarelli, L. Frati, A. Faggioni, and A. Angeloni. 2006. Regulation of the expression of the Epstein-Barr virus early gene BFRF1. Virology. 347:109–116. [DOI] [PubMed] [Google Scholar]
- 50.Sindic, C.J.M., P. Manteyne, and E.C. Laterre. 1994. The intrathecal synthesis of virus-specific oligoclonal IgG in multiple sclerosis. J. Neuroimmunol. 54:75–80. [DOI] [PubMed] [Google Scholar]
- 51.Betts, M.R., J.M. Brenchley, D.A. Price, S.C. De Rosa, D.C. Douek, M. Roederer, and R.A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 281:65–78. [DOI] [PubMed] [Google Scholar]
- 52.Cao, W., and Y.J. Liu. 2007. Innate immune functions of plasmacytoid dendritic cells. Curr. Opin. Immunol. 19:24–30. [DOI] [PubMed] [Google Scholar]
- 53.Sumaya, C.V., L.W. Myers, and G.W. Ellison. 1980. Epstein-Barr virus antibodies in multiple sclerosis. Arch. Neurol. 37:94–96. [DOI] [PubMed] [Google Scholar]
- 54.Filippi, M. 2000. Enhanced magnetic resonance imaging in multiple sclerosis. Mult. Scler. 6:320–326. [DOI] [PubMed] [Google Scholar]
- 55.Buljevac, D., G.J.J. van Doornum, H.Z. Flach, J. Groen, A.D.M.E. Osterhaus, W. Hop, P.A. van Doorn, F.G.A. van der Meché, and R.Q. Hintzen. 2005. Epstein-Barr virus and disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 76:1377–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knopf, P.M., C.J. Harling-Berg, H.F. Cserr, D. Basu, E.J. Sirulnick, S.C. Nolan, J.T. Park, G. Keir, E.J. Thompson, and W.F. Hickey. 1998. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J. Immunol. 161:692–701. [PubMed] [Google Scholar]
- 57.Schiff, J.A., J.A. Schaefer, and J.E. Robinson. 1982. Epstein-Barr virus in cerebrospinal fluid during infectious mononucleosis encephalitis. Yale J. Biol. Med. 55:59–63. [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, J.R., R.M. Braziel, S. Paoletti, M. Lipp, M. Uguccioni, and J.T. Rosenbaum. 2003. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 101:815–821. [DOI] [PubMed] [Google Scholar]
- 59.Krumbholz, M., D. Theil, S. Cepok, B. Hemmer, P. Kivisakk, R.M. Ransohoff, M. Hofbauer, C. Farina, T. Derfuss, C. Hartle, et al. 2006. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 129:200–211. [DOI] [PubMed] [Google Scholar]
- 60.He, B., N. Raab-Traub, P. Casali, and A. Cerutti. 2003. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J. Immunol. 171:5215–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krumbholz, M., D. Theil, T. Derfuss, A. Rosenwald, F. Schrader, C.-A. Monoranu, S. Kalled, D.M. Hess, B. Serafini, F. Aloisi, et al. 2005. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 201:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jego, G., A.K. Palucka, J.P. Blanck, C. Chalouni, V. Pascual, and J. Banchereau. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 19:225–234. [DOI] [PubMed] [Google Scholar]
- 63.Cepok, S., B. Rosche, V. Grummel, F. Vogel, D. Zhou, J. Sayn, N. Sommer, H.P. Hartung, and B. Hemmer. 2005. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 128:1667–1676. [DOI] [PubMed] [Google Scholar]
- 64.Reindl, M., M. Khalil, and T. Berger. 2006. Antibodies as biological markers for pathophysiological processes in MS. J. Neuroimmunol. 180:50–62. [DOI] [PubMed] [Google Scholar]
- 65.Rickinson, A.B., and E. Kieff. 2001. Epstein-Barr virus. In Fields Virology. D.M. Knipe and P.M. Howley, editors. Lippincott-Raven, Philadelphia. 2575–2627.
- 66.Serafini, B., B. Rosicarelli, R. Magliozzi, E. Stigliano, E. Capello, G.L. Mancardi, and F. Aloisi. 2006. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake and interaction with proliferating T cells. J. Neuropathol. Exp. Neurol. 65:124–141. [DOI] [PubMed] [Google Scholar]
- 67.Subklewe, M., C. Paludan, M.L. Tsang, K. Mahnke, R.M. Steinman, and C. Münz. 2001. Dendritic cells cross-present latency gene products from Epstein-Barr virus–transformed B cells and expand tumor-reactive CD8+ killer T cells. J. Exp. Med. 193:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babbe, H., A. Roers, A. Waisman, H. Lassmann, N. Goebels, R. Hohlfeld, M. Friese, R. Schroder, M. Deckert, S. Schmidt, et al. 2000. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skulina, C., S. Schmidt, K. Dornmair, H. Babbe, A. Roers, K. Rajewsky, H. Wekerle, R. Hohlfeld, and N. Goebels. 2004. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc. Natl. Acad. Sci. USA. 101:2428–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prineas, J.W., and R.G. Wright. 1978. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab. Invest. 38:409–421. [PubMed] [Google Scholar]
- 71.Gross, A.J., D. Hochberg, W.M. Rand, and D.A. Thorley-Lawson. 2005. EBV and systemic lupus erythematosus: a new perspective. J. Immunol. 174:6599–6607. [DOI] [PubMed] [Google Scholar]
- 72.Sawada, S., and M. Takei. 2005. Epstein-Barr virus etiology in rheumatoid synovitis. Autoimmun. Rev. 4:106–110. [DOI] [PubMed] [Google Scholar]
- 73.Weyand, C.M., and J.J. Goronzy. 2003. Ectopic germinal center formation in rheumatoid synovitis. Ann. NY Acad. Sci. 987:140–149. [DOI] [PubMed] [Google Scholar]
- 74.Wagner, U.G., P.J. Kurtin, A. Wahner, M. Brackertz, D.J. Berry, J.J. Goronzy, and C.M. Weyand. 1998. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J. Immunol. 161:6390–6397. [PubMed] [Google Scholar]
- 75.Lande, R., E. Giacomini, B. Serafini, B. Rosicarelli, G.D. Sebastiani, G. Minisola, U. Tarantino, V. Riccieri, G. Valesini, and E.M. Coccia. 2004. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J. Immunol. 173:2815–2824. [DOI] [PubMed] [Google Scholar]
- 76.Lundmark, F., K. Duvefelt, E. Jacobaeus, I. Kockum, E. Wallström, M. Khademi, A. Oturai, L.P. Ryder, J. Saarela, H.F. Harbo, et al. 2007. Variation in interleukin 7 receptor α chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 39:1108–1113. [DOI] [PubMed] [Google Scholar]
- 77.Gregory, S.G., S. Schmidt, P. Seth, J.R. Oksenberg, J. Hart, A. Prokop, S.J. Caillier, M. Ban, A. Goris, L.F. Barcellos, et al. 2007. Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 39:1083–1091. [DOI] [PubMed] [Google Scholar]
- 78.The International Multiple Sclerosis Genetics Consortium. 2007. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357:851–862. [DOI] [PubMed] [Google Scholar]
- 79.Bossolasco, S., P. Cinque, M. Ponzoni, M.G. Vigano, A. Lazzarin, A. Linde, and K.I. Falk. 2002. Epstein-Barr virus DNA load in cerebrospinal fluid and plasma of patients with AIDS-related lymphoma. J. Neurovirol. 8:432–438. [DOI] [PubMed] [Google Scholar]
- 80.Franciotta, D., E. Zardini, G. Bono, R. Brustia, L. Minoli, and V. Cosi. 1996. Antigen-specific oligoclonal IgG in AIDS-related cytomegalovirus and toxoplasma encephalitis. Acta Neurol. Scand. 94:215–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.